Abstract
Thyroid disease is a common paediatric disorder that affects up to 3.7% of school-aged children, and it usually presents with a goitre. By far, the most frequent cause of a goitre is autoimmune thyroid disease, although a benign colloid goitre is also a common cause. The present review focuses on the diagnostic approach to a child with a hypothyroid, hyperthyroid or euthyroid goitre.
Keywords: Goitre, Hyperthyroidism, Hypothyroidism, Thyroid nodule
Abstract
La maladie thyroïdienne est un trouble pédiatrique courant qui touche jusqu’à 3,7 % des enfants d’âge scolaire, et elle s’accompagne généralement d’un goitre. La cause la plus fréquente de goitre est, de loin, une maladie thyroïdienne auto-immune, bien qu’un goitre colloïde bénin constitue également une cause courante. Le présent examen porte sur la méthode diagnostique face à un enfant présentant un goitre hypothyroïdien, hyperthyroïdien ou euthyroïdien.
Thyroid disorders are one of the most common endocrine problems in children and adolescents. One American study showed thyroid disorders to be present in 3.7% of children between the ages of 11 and 18 years (1). Children with thyroid disorders usually present with an enlargement of the thyroid gland (goitre), with or without symptoms of thyroid hormone deficiency or excess. These symptoms are generally insidious in onset, which may delay diagnosis by several weeks to several months. Because a goitre is often the first sign of thyroid disease, the diagnostic considerations can be approached from the perspective of the goitre (Table 1).
TABLE 1:
Differential diagnosis of thyromegaly
| Autoimmune thyroid disease |
| Chronic lymphocytic thyroiditis (Hasmimoto thyroiditis) |
| Graves disease |
| Colloid (simple) goitre |
| Goitrogen exposure |
| Dyshormonogenesis |
| Infectious |
| Subacute (viral) thyroiditis |
| Chronic suppurative thyroiditis |
| Anatomic abnormalities |
| Thyroglossal duct cyst |
| Hemiagenesis of the thyroid |
| Nodular goitre |
| Solitary nodule (adenoma, carcinoma, cyst) |
| Multinodular goitre secondary to autoimmune thyroid disease |
The most common finding related to thyroid disease is a diffuse enlargement of the thyroid, with the right lobe being frequently larger than the left lobe. The enlargement of the thyroid is generally mediated by an increase in the pituitary-derived thyroid stimulating hormone (TSH) or in antibodies that bind to the TSH receptor, such as the thyroid stimulating immunoglobulins (TSIs) found in Graves disease. Inflammation or infiltration may cause diffuse, symmetrical enlargement, although the gland is usually asymmetric and nodular. The most common inflammatory process is autoimmune thyroiditis (2).
Chronic lymphocytic thyroiditis (CLT), also known as Hashimoto thyroiditis, is an autoimmune, inflammatory process that causes up to 55% to 65% of all euthyroid goitres (3), and almost all cases of thyroiditis in childhood and adolescence. A large North American prevalence study showed that as many as 1.2% of children aged 11 to 18 years have CLT, as defined by an enlarged thyroid gland and detectable serum thyroid antibodies (4), including thyroid peroxidase antibodies (formerly known as antimicrosomal antibodies) and antithyroglobulin antibodies. These antibodies, which are directed toward intracellular antigens, are generally associated with Hashimoto disease. Other antibodies to the TSH receptor (TRAb) can either block antibodies that cause hypothyroidism, or stimulate antibodies (TSI) that cause hyperthyroidism or Graves disease. TSH receptor antibodies are not measured easily, and are generally reserved for research purposes or specific clinical indications.
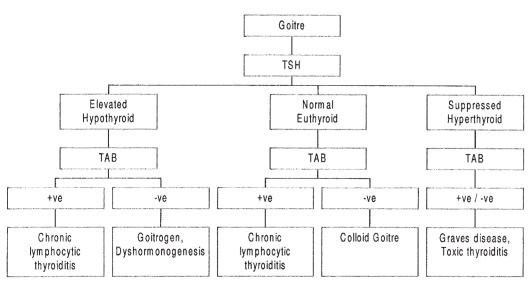
In determining the cause of thyromegaly, the initial diagnostic evaluation should be limited to measurements of TSH and thyroid antibodies. The T4 level is required only if the TSH level is elevated. Free T4 measurement is preferred to the total T4 level because the former is not influenced by thyroid binding globulin levels and, therefore, better reflects the active thyroid hormone level. Thyroid antibody titres greater than 1:2000 or 10 mIU/L most likely indicate autoimmune thyroiditis (5,6). Lower levels may simply reflect a nonspecific inflammation of the thyroid gland. Imaging is only required if a solitary nodule is suspected. A diagnostic approach to the most common causes of goitres that is based on the results of the above tests is shown in Figure 1.
Figure 1).
Algorithm for the most common causes of a goitre. −ve Negative; +ve Positive; TAB Thyroid antibodies; TSH Thyroid stimulating hormone
GOITRE AND HYPOTHYROIDISM
Hashimoto thyroiditis is uncommon in children younger than four years of age. The peak age of onset in the first two decades of life is in early to midpuberty. Although the ratio is 2:1 in favour of females versus males (4), this ratio is less skewed than in adulthood when 90% of cases occur in females (5). Although there is no defined pattern of inheritance, a family history is reported in 30% of affected children (2). It is well established that the risk of CLT is higher in individuals with chromosomal abnormalities such as Turner syndrome, Klinefelter syndrome and Down syndrome. There is also an increase in association with other autoimmune diseases. For instance, the prevalence of thyroid autoantibodies in children and adolescents with type 1 diabetes has been reported to be as high as 20% to 40% (7,8), while abnormal thyroid function is reported in approximately 7% of these patients (7,9).
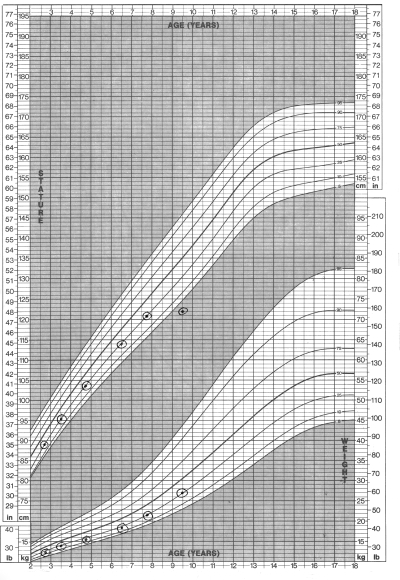
Autoimmune thyroid disease may be associated with a euthyroid state, hypothyroidism or hyperthyroidism. In the early stages, the gland will be smooth and soft, progressing to a granular or pebbly texture, and then becoming firm and irregular. Most children are euthyroid at diagnosis. Hypothyroidism will be present in 3% to 13% of patients, and they will show typical symptoms (Table 2) and have a growth chart as shown in Figure 2. An additional 3% to 35% of patients have elevated TSH concentrations and normal T4 levels (3), a state termed subclinical or compensated hypothyroidism. Rarely, children will present with transient thyrotoxicosis with suppressed TSH, and elevated serum T3 and T4 levels, a condition known as toxic thyroiditis or Hashitoxicosis.
TABLE 2:
Symptoms and signs of hypothyroidism
| Symptoms | Signs |
|---|---|
| Slow growth | Short stature, mild obesity |
| Pubertal delay | Delayed secondary sexual development |
| Puffy features | Myxedematous facies |
| Neck swelling | Goitre |
| Cold intolerance | Bradycardia |
| Constipation | Narrow pulse pressure |
| Dry skin, brittle hair | Dry, carotenemic skin, brittle hair |
| Fatigue | Delayed relaxation of deep tendon reflex |
| Decline in school performance |
Figure 2).
Growth chart of children with hypothyroidism. Of note is the decline in growth velocity with weight sparing
The decision to treat with l-thyroxine is clear for overt hypothyroidism (with decreased T4 levels), but controversial for a euthyroid goitre or compensated hypothyroidism. What percentage of individuals with these conditions will develop hypothyroidism? A subset of the cohort in the North American prevalence study mentioned above (4) was examined from 1965 to 1968 and again 20 years later (1). Of the 61 patients with chronic thyroiditis on initial examination, 27% had a normal thyroid assessment, 33% remained unchanged and 33% were hypothyroid. Another long term, prospective study conducted in England (10) showed a similar rate of conversion to hypothyroidism in a cohort of adults that was followed for 20 years. Researchers found the rate of hypothyroidism to be 2.1% to 2.6% per year in individuals with either elevated TSH levels or positive thyroid antibodies, and 4.3% per year in those with both criteria. If the TSH was greater than 20 mIU/L or if the thyroid antibodies were highly positive at more than 1:100,000, then the rate of developing hypothyroidism was 25% per year. The two studies emphasize the point that to treat all individuals with evidence of chronic thyroiditis would result in treating many patients unnecessarily.
The approach used by the author is to monitor patients with mild elevations of TSH level (less than 10 mIU/L) six months after the diagnosis is confirmed and then annually. Repeat levels of TSH are often normal. In those patients with a confirmed TSH level that is greater than 10 mIU/L, treatment with thyroxine is usually continued until growth is complete. Thyroid status is then reassessed to determine the need for ongoing treatment. Patients with a TSH that is greater than 20 mIU/L have a high rate of progression to hypothyroidism, and are treated with thyroxine.
The author’s practice is not to treat euthyroid goitres unless they are cosmetically significant. Even then, the use of thyroxine is controversial.
An endemic goitre is the most common cause of hypothyroidism worldwide (11), and it is the most common preventable cause of mental retardation (12). However, because of the routine addition of iodine to salt in the Western world, it is not seen in that area. Goitrogen exposure should be considered in patients with a goitre and negative thyroid antibodies, and it will generally be evident from the patient’s history. In addition to prescribed medications, it is important to ask about the use of over-the-counter preparations, such as iodide-containing expectorants and natural remedies. Dyshormonogenesis occurs in 1:30,000 infants, and usually presents as congenital hypothyroidism. However, milder forms may present later as acquired hypothyroidism with a goitre and negative thyroid antibodies. A family history should be sought because this is an autosomal recessive condition. While hypothyroidism may also occur if a patient has hypothalamic and pituitary lesions (ie, tertiary and secondary hypothyroidism, respectively), a goitre is not a clinical feature of these cases.
GOITRE AND HYPERTHYROIDISM
Graves disease
Graves disease is unquestionably the most common cause of hyperthyroidism in children and adolescents. Other causes are rare and should be suspected only when there is an atypical presentation (13). As with Hashimoto thyroiditis, females are predominantly affected, and the ratio of affected females versus males is less pronounced than in adulthood. A family history of autoimmune thyroid disease is common.
Presenting symptoms and signs of Graves disease are shown in Table 3. Declining school performance and behavioural manifestations often predominate. Thyromegaly is almost invariably present and the thyroid has a smooth, rubbery texture. Although lid retraction and lid lag are common findings at diagnosis, exopthalmos occurs in only one-third of children, and it is generally mild (14).
TABLE 3:
Symptoms and signs of Graves disease in adolescents
| Symptoms | % | Signs | % |
|---|---|---|---|
| Nervousness | 80 | Goitre | 99 |
| Increased appetite | 60 | Tachycardia | 83 |
| Weight loss | 54 | Wide pulse pressure | 77 |
| Increased sweating | 49 | Systolic hypertension | 71 |
| Hyperactivity | 44 | Tremor | 61 |
| Palpitations | 34 | Thyroid bruit | 53 |
| Heat intolerance | 33 | Heart murmur | 43 |
| Restless sleep | 27 | Exopthalmos | 33 |
| Fatigue | 16 | Lid lag | 22 |
| Headache | 15 | ||
| Diarrhea | 13 |
The diagnosis of Graves disease is made by finding a suppressed TSH level with elevated T4 and/or T3 levels. When the patient’s clinical presentation is mild, free T4 levels may be high normal, with an inappropriately suppressed TSH, in which case T3 levels should be measured. Antithyroglobulin antibodies and thyroid peroxidase antibody levels may be positive but they are not pathogenic. TSI, if measured, will be positive. However, this test generally does not change management, and measurement of TSI is not clinically indicated.
Hashitoxicosis can present in a fashion that is similar to Graves disease. However, Hashitoxicosis is a self-limiting condition and lacks opthalmopathy. It is caused by auto-immune damage to follicular cells, resulting in the release of preformed T4 and T3 into circulation. Transient or permanent hypothyroidism may follow. If the hyperthyroidism lasts more than a few weeks, this diagnosis of Hashitoxicosis is unlikely.
Subacute thyroiditis is generally caused by a viral infection. It is reported to be uncommon in the paediatric age group. However, it may well be underdiagnosed in an age group where viral illnesses are particularly common. Children with subacute thyroiditis typically have an enlarged, tender thyroid, malaise, fatigue and weakness that develop after an upper respiratory tract infection. There are two distinct phases in the disease’s progression. During the acute stage (which lasts from two to six weeks), preformed T4 and T3 are released from the inflamed thyroid follicles, resulting in biochemical hyperthyroidism with or without clinical symptoms. For the next two to seven months, the damaged thyroid is less effective at synthesizing hormone, resulting in low to normal T3 and T4 concentrations with a compensatory elevation of TSH. Nearly all patients recover from hypothyroidism. If this diagnosis is suspected, treatment with thyroxine should be re-evaluated after three to six months.
Autonomously hyperfunctioning adenomas tend to secrete T3 and cause mild hyperthyroidism (15). The thyroid will be of small or normal size with a palpable nodule. Symptoms of hyperthyroidism generally occur once the nodule is greater than 2.5 cm in diameter. The nodules are rarely malignant (16), and surgery is curative. Exogenous thyroxine will cause hyperthyroidism in patients who are overtreated for hypothyroidism or who take thyroxine surreptitiously, most commonly for weight control.
TSH dependent causes
TSH dependent causes, such as a TSH secreting pituitary adenoma, are very rare. This is easily distinguished from Graves disease in that the TSH is high in the face of an elevated T4. Another potential cause of such a biochemical picture is a syndrome of resistance to thyroid hormone, which, again, is very rare.
EUTHYROID GOITRE
The most common causes of a euthyroid goitre in childhood are CLT and a colloid goitre. Thyroid enlargement that is not caused by inflammatory, infectious or neoplastic causes is termed a colloid goitre, also referred to as a sporadic or idiopathic simple goitre. Histological findings include enlarged thyroid follicles filled with abundant colloid. The cause remains controversial. Although TSH is the principal growth-stimulating factor for the thyroid, TSH levels are normal in patients with colloid goitre. Other as yet unknown growth factors may play a role in the condition. A preponderance of female patients with this condition and familial clustering have raised the possibility of an autoimmune cause. Onset is generally in adolescence, and a family history of CLT is found in one-half of all patients. There is no reported association between a colloid goitre and thyroid malignancy. The prospective study of 11- to 18-year-old school children (1) found simple goitres in 1.9% of students. The natural history is for spontaneous reduction with time, and treatment with thyroxine is not indicated. On re-examination 20 years later, 60% of patients were normal, 20% were unchanged and a few (10%) developed thyroiditis (4).
NODULAR GOITRE
Thyroid nodules are relatively common in adolescents; they are usually asymptomatic and often discovered inadvertently, but they raise the fear of cancer for the family and physician (17). In the survey cited above, thyroid nodules were present in 1.8% of school children 11 to 18 years of age (1). Many nodules that seem solitary on physical examination are discovered to be one of several nodules following ultrasound examinations or radionucleotide scanning. A multinodular goitre is almost invariably caused by Hashimoto thyroiditis and it carries a good prognosis. The asymptomatic, solitary thyroid nodule is a thyroid adenoma, thyroid carcinoma or a thyroid cyst. Thyroid carcinoma occurs in approximately one per one million persons/year in the first two decades of life (18). Certain features of the history and physical examination increase the risk of thyroid cancer, including male sex; a history of irradiation to the head, neck or chest area; exposure to nuclear fallout; and a family history of thyroid cancer, especially medullary carcinoma of the thyroid. A large-sized nodule (more than 4 cm), rapid growth, a hard texture to the nodule with fixation to adjacent structures, regional lymphadenopathy and hoarseness or dysphagia are also suspicious clinical features, increasing the probability of malignancy.
Investigation of a solitary nodule should begin with thyroid function tests and thyroid antibodies. These are usually both negative, and thyroid imaging studies are then indicated. Thyroid cancer is unlikely in the presence of hypothyroidism, hyperthyroidism or autoimmune thyroiditis (17). The approach to assessing thyroid nodules is somewhat controversial. The least invasive test is a thyroid ultrasound. It will identify other cervical masses that can be confused with a thyroid nodule, such as a thyroglossal dust cyst, and determine whether the nodule is cystic, solid or mixed. If a purely cystic thyroid nodule is identified, no further investigations are required and the patient may be followed conservatively. If, however, the lesion is solid or of mixed density, then radionuclide scanning (99mTc-pertechnetate, 123I or 131I) is indicated to differentiate a hyperfunctioning (hot) from a hypofunctioning (cold) nodule. A hyperfunctioning nodule is, most likely, a benign hyperfunctioning adenoma. A cold nodule in a paediatric patient has a higher likelihood of malignancy than in adults. Ten per cent to 24% of solitary nodules in children and adolescents are malignant. In general, hypofunctioning, solid, solitary nodules undergo surgical excision unless they have demonstrated benign cytology on fine-needle aspiration. The overall accuracy of fine-needle aspiration is 70% to 97%, and it depends on the experience of the operator and cytopathologist (17).
CONCLUSION
Thyroid disorders, in particular, CLT and simple colloid goitres, are common in children and adolescents. The majority of patients with chronic CLT are euthyroid and present with an asymptomatic goitre. The only investigations required to establish the diagnosis in most individuals with a goitre are measuring TSH and thyroid antibody levels. Thyroid hormone (T4) should be measured if the TSH level is abnormal. Further studies, such as imaging, are not necessary unless there is a specific concern, such as a single palpable nodule.
REFERENCES
- 1.Rallison ML, Dobyns BM, Meikle AW, Bishop M, Lyon JL, Stevens W. Natural history of thyroid abnormalities: Prevalence, incidence, and regression of thyroid diseases in adolescents and young adults. Am J Med. 1991;91:363–70. doi: 10.1016/0002-9343(91)90153-o. [DOI] [PubMed] [Google Scholar]
- 2.Lafranchi S. Thyroiditis and acquired hypothyroidism. Pediatr Ann. 1992;21:29–39. doi: 10.3928/0090-4481-19920101-07. [DOI] [PubMed] [Google Scholar]
- 3.Bachrach LK, Foley TP. Thyroiditis in children. Pediatr Rev. 1989;11:184–91. doi: 10.1542/pir.11-6-184. [DOI] [PubMed] [Google Scholar]
- 4.Rallsion M, Dobyns BM, Keating FR, et al. Occurrence and natural history of chronic lymphocytic thyroiditis in childhood. J Pediatr. 1975;86:675–82. doi: 10.1016/s0022-3476(75)80350-7. [DOI] [PubMed] [Google Scholar]
- 5.Weetman AP. Autoimmune thyroiditis: Predisposition and pathogenesis. Clin Endocrinol. 1992;36:307–23. doi: 10.1111/j.1365-2265.1992.tb01453.x. [DOI] [PubMed] [Google Scholar]
- 6.Beever K, Bradbury J, Phillips D, et al. Highly sensitive assays of autoantibodies to thyroglobulin and to thyroid peroxidase. Clin Chem. 1989;35:1949–54. [PubMed] [Google Scholar]
- 7.McKenna MJ, Herskowitz R, Wolfsdorf JI. Screening for thyroid disease in children with IDDM. Diabetes Care. 1990;13:801–3. doi: 10.2337/diacare.13.7.801. [DOI] [PubMed] [Google Scholar]
- 8.Neufeld M, Maclaren NK, Riley WJ, et al. Islet cell and other organ-specific antibodies in US Caucasians and blacks with insulin-dependent diabetes mellitus. Diabetes. 1980;29:589–92. doi: 10.2337/diab.29.8.589. [DOI] [PubMed] [Google Scholar]
- 9.Riley WJ, Maclaren NK, Lezotte DC, Spillar RP, Rosenbloom AL. Thyroid autoimmunity an insulin-dependent diabetes mellitus: The case for routine screening. J Pediatr. 1981;99:350–4. doi: 10.1016/s0022-3476(81)80316-2. [DOI] [PubMed] [Google Scholar]
- 10.Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: A twenty year follow up of the Whickham survey. Clin Endocrinol. 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 11.Boyages SC. Iodine deficiency disorders. J Clin Endocrinol Metab. 1993;77:587–91. doi: 10.1210/jcem.77.3.8370679. [DOI] [PubMed] [Google Scholar]
- 12.Cao XY, Jiang XM, Dou ZH, et al. Timing of vulnerability of the brain to iodine deficiency in endemic cretinism. N Engl J Med. 1994;331:1739–44. doi: 10.1056/NEJM199412293312603. [DOI] [PubMed] [Google Scholar]
- 13.Foley TP., Jr Thyrotoxicosis in childhood. Pediatr Ann. 1992;21:43–6. doi: 10.3928/0090-4481-19920101-08. [DOI] [PubMed] [Google Scholar]
- 14.Dallas JS, Foley TP., Jr . Hyperthyroidism. In: Lifshitz F, editor. Pediatric Endocrinology: A Clinical Guide. 3rd edn. New York: Marcel Dekker; 1995. pp. 401–14. [Google Scholar]
- 15.Namba H, Ross JL, Goodman D, Fagin JA. Solitary polyclonal autonomous thyroid nodule: A rare cause of childhood hyperthyroidism. J Clin Endocrinol Metab. 1991;72:1108–12. doi: 10.1210/jcem-72-5-1108. [DOI] [PubMed] [Google Scholar]
- 16.Sussman L, Labrik L, Clayton GW. Hyperthyroidism attributable to a hyperfunctioning thyroid carcinoma. J Pediatr. 1968;72:208–13. doi: 10.1016/s0022-3476(68)80310-5. [DOI] [PubMed] [Google Scholar]
- 17.LaFranchi S. Adolescent thyroid disorders. Adolesc Med. 1994;5:65–86. [PubMed] [Google Scholar]
- 18.Foley TP. Disorders of the thyroid in children. In: Sperling MA, editor. Pediatric Endocrinology. 1st edn. Philadelphia: WB Saunders Company; 1996. pp. 171–94. [Google Scholar]