Abstract
Canadian statistics show that children from birth to four years of age are more likely to be reported with an infection from Campylobacter, Giardia, Salmonella and Shigella species, and verotoxigenic Escherichia coli than any other age group. A review of the Canadian and international literature, and an analysis of case and outbreak data suggest that the risk factors for infection in young children (ages birth to four years) are different from the risk factors for older children and adults. In children from birth to four years of age, infant formula, fast foods, snacks and candies have caused major outbreaks of enteric and foodborne diseases; however, the contamination of a child’s environment or the presence of ill individuals in a household may be highly significant to disease expression. Contact with animals (including family pets) and contaminated surfaces, together with experimental touching and testing behaviours, are important routes of infection for infants and preschool children. Risk factors for enteric infections in children appear to be related, occasionally, to specific foods that are particularly attractive to all children (all age groups from infants up to and including elementary school-aged childen), to an infected person or pet in the same household, or to the contamination of a child’s environment. Nonfood-related risk factors may be of particular significance in infection in infants and very young children. Contact with animals, particularly exotic pets and farm animals, or their environments should be considered to be a potential source of infection in children in situations in which there is an absence of other risk factors. The evidence presented in the current paper emphasizes the importance of personal and home hygiene practices in limiting children’s exposure to enteric pathogens. Strict hand washing practices and restrictions on touching birds, reptiles and other animals at petting zoos or in nursery and primary school facilities are recommended to avoid widespread infection. Public health authorities should consider the development of guidelines on the provision of hand washing facilities and instruction notices in settings where the public may come into contact with farm or other animals in jurisdictions where such guidelines do not already exist.
Keywords: Children, Enteric pathogens, Environment, Foodborne disease, Risk factors
Abstract
Les statistiques canadiennes démontrent que les enfants de la naissance à quatre ans sont plus susceptibles d’être déclarés atteints d’une infection au Campylobacter, au Giardia, à la salmonelle, aux espèces de Shigella et à l’Escherichia coli vérotoxinogène que ceux de tout autre groupe d’âge. Un examen de la documentation scientifique canadienne et internationale et une analyse des données sur les cas et les issues indiquent que les facteurs de risque d’infection chez les jeunes enfants (de la naissance à quatre ans) diffèrent de ceux des enfants plus âgés et des adultes. Chez les enfants de la naissance à quatre ans, les préparations lactées pour nourrisson, le prêt-à-manger, les grignotines et les bonbons ont provoqué d’importantes flambées de maladies entériques et d’origine alimentaire. Cependant, la contamination du milieu de l’enfant ou la présence de personnes malades au foyer peuvent jouer un rôle très important dans l’expression de la maladie. Le contact avec des animaux (y compris les animaux domestiques) et avec des surfaces contaminées de même que l’expérimentation par le toucher et les essais constituent d’importantes voies d’infection chez les nourrissons et les enfants d’âge préscolaire. Les facteurs de risque d’infections entériques chez les enfants semblent parfois liés à des aliments précis qui attirent tous les enfants (dans tous les groupes d’âge, des nourrissons jusqu’aux enfants de l’école primaire), à la personne infectée ou à l’animal domestique au foyer ou à la contamination du milieu de l’enfant. Les facteurs de risque non alimentaires peuvent contribuer davantage à l’infection chez les nourrissons et les toutpetits. Le contact avec des animaux ou avec leur milieu, surtout s’il s’agit d’animaux exotiques ou de la ferme, devrait être perçu comme une source potentielle d’infection chez les enfants dans des situations où il n’existe pas d’autres facteurs de risque. Les observations exprimées dans le présent article soulignent l’importance de l’hygiène personnelle et dans le domicile pour limiter l’exposition des enfants aux entéropathogènes. Un lavage des mains strict et l’interdiction de toucher aux oiseaux, aux reptiles et aux autres animaux dans les zoos pour enfants, les services de garde et les écoles primaires est recommandé pour éviter une infection généralisée. Aux endroits où il n’existe pas de réglementation en ce sens, les organismes de santé publique devraient envisager d’élaborer des directives sur l’installation de lavabos et des notes explicatives dans les lieux où le public peut entrer en contact avec des animaux de la ferme ou d’autres animaux.
Enteric diseases, including foodborne pathogens, are among the most frequently reported infections in Canadians, and are recognized as being a significant public health issue in both industrialized and developing countries. Children are at greater risk of infection and serious illness from these organisms than other age groups, excluding elderly individuals. However, it is well recognized that, even in countries with structured reporting systems, national statistics may considerably underestimate the actual incidence of human enteric diseases in all ages. A recent national study of infectious intestinal diseases in England, a nation with a highly developed mechanism for the central reporting of notifiable diseases and laboratory identified infection, found that enteric infections were most frequently reported in children (1). The study also indicated that for every case of infectious intestinal diseases reported to national surveillance authorities, 136 cases were not reported (1). Reporting ratios varied markedly by organism, for example, from 1:3.2 for Salmonella species to 1:1500 for gastroenteritis due to small round structured viruses. The same study found no etiology in a high proportion of cases that were investigated.
Although no comparable national study has been reported for Canada, Todd (2) extrapolated from studies in the United States, and estimated that there are in excess of two million cases of enteric and foodborne illness in Canada each year. Todd’s study further suggested that the socioeconomic burden of these illnesses is high (over CDN$1.2 billion annually); is consistent with estimates for other industrialized countries; and is, probably, a considerable underestimate of the actual disease burden (2–5). In particular, these data underestimate the ongoing medical and social costs of the long term sequelae of enteric infections. These sequelae may include chronic kidney disease and neurological impairment resulting from hemolytic uremic syndrome (HUS) after an infection with verocytotoxin-producing Escherichia coli (VTEC). In a study of VTEC cases predominantly among children in the United States, Marks and Roberts (6) estimated that annual medical costs and productivity losses were between US$216 million and US$580 million. Between US$13 million and US$35 million of these costs and losses were associated with chronic kidney diseases. The various reported sequelae of enteric infections include bone-joint infections, reactive arthritis, irritable bowel syndrome, nutritional deficiencies, vascular problems, neurological conditions such as Guillain-Barré syndrome and Miller-Fisher syndrome linked to Campylobacter species infection, and possibly some carcinomas (7–11). The implications of these sequelae have not been explored fully.
The present paper reviews reports on risk factors for enteric disease in children, particularly in situations in which such infections are related to food and the environment. The relevance of specific categories of risk are compared among children’s age groups, and the public health significance of the risk factors is discussed.
AGE-SPECIFIC REPORTING RATES
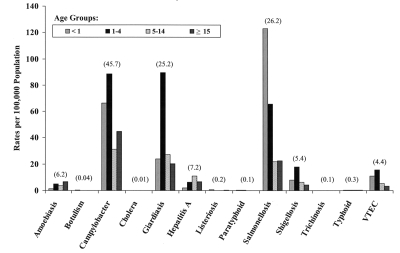
Issues relating to enteric and foodborne diseases are of particular relevance to paediatricians because the age-specific incidence rates for many of the most commonly reported enteric and foodborne pathogens are highest among infants and young children (12,13). Analysis of aggregate data on selected enteric and foodborne pathogens reported in Canada from 1989 to 1998 indicates that risk factors associated with children vary by age group and pathogen. The average annual reporting rates from 1989 to 1998 for nationally notifiable enteric and foodborne diseases are shown by age group in Figure 1. The pattern indicated is consistent over the 10-year period, and the average reporting rate for all cases of each disease (including patients for whom age was unknown) is shown above each pathogen. In terms of overall incidence within the population, three groups of conditions caused by individual pathogens stand out: Campylobacter, Giardia and Salmonella species are the most commonly reported causes of infection (25 to 46 cases/100,000 population). A middle group of infections consists of amoebiasis, hepatitis A, shigellosis and VTEC (four to seven cases/100,000 population). The remaining pathogens are relatively rare, but include causes of potentially severe disease.
Figure 1).
Age-specific reporting rates for enteric pathogens in Canadian children (10-year average) from 1989 to 1998. The 10-year average rate (all reported cases) for each pathogen is shown in parentheses. Data extracted from the National Notifiable Disease Reporting System database (1989 to 1998, inclusive). VTEC Verocytotoxin-producing Escherichia coli
Incidence rates for the 10-year period indicate that the association with particular pathogens may be, to some extent, age-related, at least for the more common diseases shown in Figure 1. Reporting rates are highest for infants (children younger than one year of age) for Salmonella species infection, and comparatively high for Campylobacter species and VTEC infections. Reporting rates for children aged one to four years are highest for Campylobacter, Giardia and Shigella species, and VTEC, whereas hepatitis A is more commonly reported in older children between the ages of five and 15 years. The age distribution of VTEC cases, which shows the highest incidence in children under five years of age, is also reflective of the age distribution of cases of HUS in Canada (14). In addition to immunological factors that increase susceptibility to infection, specific behavioural risk factors may increase the likelihood of exposure of children to enteric and foodborne pathogens. These risk factors are considered below under the headings of food and environment-related risk factors.
FOOD-RELATED RISK FACTORS
There is evidence that specific categories of foods play an occasional role in exposing infants and young children to enteric and foodborne disease; the association of dried milk products with Salmonella and Clostridium species and staphylococcal food poisoning has been documented since the 1950s in Canada, the United States, the United Kingdom and elsewhere (15–19). A small number of reported investigations have associated the use of infant feeding formula to Salmonella species infection; the risk from some formulas may be increased if they contain elevated levels of iron (20). In 1977, a national outbreak of Salmonella bredeney infection in Australia was linked to popular brands of infant formula that was produced at the same factory (21). A similar outbreak of Salmonella ealing in the United Kingdom in 1985 affected 48 infants and family contacts, and was linked to dried infant milk formula (22). In both of the outbreaks, persistent contamination of the product was traced to damage to the inner lining of a spray drier and the contamination of insulation material. These incidents, although dramatic, are rare, and direct foodborne infection of infants is probably relatively unusual because of the limitations associated with diet. This conclusion is supported by case control studies of infant salmonellosis in Italy and on the island of Guam, as well as with investigations of infant feeding practices and their relationship to diarrheal disease in Peru. All of these studies identified breastfeeding as being protective (20,23,24). This protective effect may be related to decreased exposure to enteric pathogens or anti-infective factors in breast milk (24).
The protective effect of breastfeeding was also found in a study of infectious intestinal diseases in England (25). Furthermore, the study indicated that the increased risk of infection associated with bottle feeding was related to the method of cleaning the bottle. The risk of infection increased in babies whose bottles were cleaned with hot water, and was less marked if the bottles were cleaned in steam, or if cold water and chemical disinfectants were used (25).
Older children may share many of the same foodborne infection risks as adults. Infections in children are evident but not predominant in, for example, national case control studies of risk factors related to Salmonella and Campylobacter species infection, and investigations of outbreaks linked to foods that appeal to all ages (26–28). However, of greater significance for the risk of infection in children, other than infants, is the contamination of snack foods or food products that are particularly attractive to these age groups (29–36). In each of the selected outbreaks listed in Table 1, children younger than 15 years of age were the predominant group affected. Chocolate candies were linked to three national outbreaks, including two outbreaks in Canada. A particular feature of these outbreaks associated with chocolate consumption is the apparently very low infective doses that were linked to illness. This may be a result of the protective effects of components in the chocolate enabling Salmonella species to survive the gastric acids or to reduced levels of stomach acidity in young children permitting survival of the organisms (37). Other products included flavoured corn snacks and dried salami sticks. Probably the largest outbreak of food poisoning ever recorded in Canada was associated with prepacked lunch products that contained contaminated cheese. About 800 persons were affected nationwide, and over 73% of the recorded patients investigated were between six and 15 years of age (35). Other foods particularly linked to enteric disease and that are well liked by children include fast food products such as hamburgers. In one of the largest reported outbreaks of enteric disease linked to hamburgers in North America, about 500 people in the state of Washington were infected. Seventy-five per cent of the patients were younger than 18 years of age, 45 people developed HUS, and three deaths occurred (36).
TABLE 1:
Foods causing illness predominantly in children: Selected outbreaks*
| Organism | Country | Food | Number of cases recorded† | Age groups of patients particularly affected |
|---|---|---|---|---|
| Salmonella manchester (29) | United Kingdom | Savoury corn snacks | 40 | 77.5% younger than 5 years |
| Salmonella typhimurium DT124 (30) | United Kingdom | Salami sticks | 101 | Median age 6 years |
| Salmonella napoli (31) | United Kingdom | Chocolate bars | 245 | 58% younger than 15 years |
| Salmonella nima (32,33) | Canada, United States | Chocolate coins | 33 | 62% younger than 5 years |
| Salmonella eastbourne (34) | Canada, United States | Christmas chocolate balls | 119 | Median age about 3 years |
| Salmonella enteritidis (35) | Canada | Prepackaged lunches | >800 | 73% aged 6 to 15 years |
| Escherichia coli 0157 (36) | United States | Hamburgers from fast food restaurant | 501 | 75% younger than 18 years of age (median age 8 years) |
Outbreaks selected from the predominance of cases in children;
Either total symptomatic cases recorded or total laboratory confirmed cases
ENVIRONMENT-RELATED RISK FACTORS
The environment has been increasingly recognized as being important to the development of enteric disease in children, including salmonellosis and Campylobacter species infections. Environmental factors may be of greater significance than food as a source of infection in infants and very young children (20,38–40). Potential risk factors include direct contact with a floor and other surfaces, and the presence of an infected person or pet in the home. In a detailed microbiological survey of the homes of 50 cases of enteric disease among children younger than four years of age in Arkansas, investigators found extensive microbial contamination of multiple surfaces. Animals and insects were also found to carry Salmonella species (40). In three of the homes, infected contacts had diarrheal illness before the onset of illness in the index case recruited into the study. Investigators concluded that the environment or other infected individuals in the household had a more significant role than food in the spread of disease (41).
The potential importance of infected individuals in spreading infection was emphasized in an earlier report by Schutze et al (42), who suggested that this route of infection could explain the observation that patients were infected by identical organisms that occurred in a community but were separated by both distance and time. Persistent excretion of Salmonella species and the extended and intimate handling of younger children and infants by caregivers provide an ample opportunity for cross-infection to occur.
The possibility that young children may become infected as a direct result of contact with their environment cannot be understated. Pathogens, once introduced into the household via contaminated food or some other mechanism, may persist for considerable periods of time; the survival of Salmonella species in floor dust and on work surfaces has been well documented (38,43–45). Crawling, experimental tasting and touching behaviours in young children, petting animals, licking by pets, chewing and finger sucking may be important mechanisms for the ingestion of organisms from the environment by young children. This particular scenario may have played a key role in a national outbreak of Salmonella infantis in Canada in 1999. The bacteria were probably introduced into the home by contaminated pig ear dog treats, but the high incidence of salmonellosis in infants and young children suggested that infection was linked to environmental contact (46 and unpublished data).
Plaut et al (47) reviewed the health hazards to humans associated with household pets, and identified the importance of animals in the introduction of enteric pathogens into households. Isolates referred to the National Laboratory for Enteric Pathogens, Winnipeg, Manitoba for strain characterization indicated a variety of Salmonella species serotypes identified in domestic and exotic pets (Table 2). Contact with pets was demonstrated to be a risk factor for illness in a review of Campylobacter species enteritis by Healing et al (48). In addition, a case control study in Darlington in the United Kingdom showed a significant relationship between Campylobacter species enteritis in children from birth to five years of age and the presence of a young dog in the household (49). The role of dogs and, to some extent, cats as a vehicle for the introduction of enteric pathogens into the home is well documented. Reports indicate that dogs, in particular, may harbour multiple serotypes of Salmonella species and may shed the organism without symptomatic illness (50–54). Consumption of carrion and coprophagic activities provide ample opportunity for pet dog infection. However, the possibility that contaminated pet products, such as the pig ear dog treats described above, may serve as a source of infection cannot be ruled out (55). Once infected, a dog may act as a mechanism for the amplification of a pathogen and may shed the organism for prolonged periods.
TABLE 2:
Salmonella species serotypes* isolated from domestic pets (1993 to 1999) and exotic pets (1991 to 2000) in Canada
| Source | Salmonellaserotypes |
|---|---|
| Birds (exotic) | Salmonella bareilly, Salmonella typhimurium |
| Cats | Salmonella heidelberg, Salmonella javiana, Salmonella muenster, S typhimurium |
| Chameleons | Salmonella farmsen, Salmonella houten, S typhimurium |
| Dogs |
Salmonella agona, Salmonella anatum, Salmonella anatum var 15+, Salmonella berta, Salmonella brandenburg, Salmonella cubana, Salmonella derby, Salmonella emek, Salmonella enteritidis, Salmonella give var 15+, Salmonella hadar, Salmonella havana, S heidelberg, Salmonella london, Salmonella montevideo, Salmonella ohio, Salmonella orion var 15+, Salmonella rissen, Salmonella schwarzengrund, Salmonella senftenberg, Salmonella tennessee, Salmonella thompson, S typhimurium, Salmonella worthington |
| Frogs | Salmonella ealing |
| Geckos | Salmonella eastbourne, Salmonella 50:b:z6 ssp II |
| Hedgehogs | S enteritidis, Salmonella ituri, Salmonella tilene, S typhimurium |
| Iguanas |
Salmonella abaetetuba, S anatum, Salmonella bonaire, Salmonella cerro, Salmonella chameleon, Salmonella florida, Salmonella fluntern, S houten, Salmonella infantis, Salmonella irumu, S javiana, Salmonella kirkee, Salmonella kua, Salmonella kralendyk, Salmonella matadi, Salmonella marina, Salmonella muenchen, Salmonella newport, Salmonella poona, Salmonella phoenix, Salmonella rubislaw, Salmonella urbana, Salmonella wassenaar, Salmonella 45:g,z51:-ssp.IV, Salmonella 48:g,z51:-ssp.IV, S 50:b:z6 sspII |
| Lizards | S ealing, Salmonella gaminara, Salmonella kisarawe, S muenchen, Salmonella panama, S poona, S typhimurium, S urbana, S wassenaar, S 50:b:z6 sspII |
| Snakes |
Salmonella adelaide, S bareilly, Salmonella carrau, Salmonella lome, S newport, S panama, S typhimurium, Salmonella uzaramo |
| Sugar gliders | Salmonella tilene |
| Turtles and turtle water |
Salmonella agbeni, Salmonella blockley, Salmonella dugbe, S ealing, S enteritidis, S hadar, Salmonella jangwani, Salmonella java, S javiana, Salmonella litchfield, Salmonella miami, Salmonella monschaui, S muenchen, S muenster, S newport, Salmonella oranienburg, S panama, S pomona, S poona, S phoenix, S rubislaw, Salmonella stanley, S thompson, S wandsworth, S 50:b:z6 ssp II |
Many other Salmonella arizonae species IIIa and IIIb, and Salmonella species IV have been found to be associated with exotic pets; these animals serve as a reservoir for infections among children.
Isolates were referred to the National Laboratory for Enteric Pathogens, Winnipeg, Manitoba for characterization from 1993 to1999. The data are not necessarily representative of all such infections in Canada and were used only to indicate the links between infections in humans and associated pets
The popularity of exotic pets has increased the opportunities for the introduction of salmonellae into the home environment, and a number of incidents linked to infection in children have been recorded in Canada (Table 3). Investigation of Salmonella species infection in families in Connecticut in 1970 showed an association between disease and keeping pet turtles. Although all age groups were involved, 79% of patients were younger than five years of age. This supports earlier findings that linked pet turtles to Salmonella infection in children and ultimately led to restrictions on the importation of these pets (56,57).
TABLE 3:
Salmonella*species infections in children in Canada associated with exotic pets (1994 to 2000)
| Salmonellaserotype | Patient(s) | Epidemiological link to pet |
|---|---|---|
| Salmonella poona | 3-year-old boy | Iguana |
| Salmonella wassenaar species IV | 5 in one family | Iguana |
| Salmonella tilene | 3 in one family | Sugar glider |
| S tilene | 4-month-old boy | Hedgehog |
| S tilene | 2-year-old boy | Hedgehog |
| Salmonella marina species IV | 11-year-old boy | Iguana |
| S marina species IV | Twin baby boys | Iguana |
| S marina species IV | Infant boy | Iguana |
| Salmonella typhimurium | 6-year-old boy | Hedgehog |
| Salmonella urbana | Infant boy | Bearded dragon lizard |
Isolates were referred to the National Laboratory for Enteric Pathogens, Winnipeg, Manitoba for characterization from 1994 to 2000. The data were not necessarily representative of all infections in Canada, and were used only to indicate the links between Salmonella species infections in humans and exposure to an exotic household pet
Published studies have recorded enteric infections due mostly to salmonellae that were linked to a variety of exotic pets, including reptiles and amphibians; frequently, children were the population most at risk (58–60). The information shown in Tables 2 and 3 emphasizes the wide variety of salmonella serotypes isolated from domestic pets. Interestingly, the serotypes identified in dogs and cats are markedly similar to the types commonly isolated from the human population in Canada. A higher proportion of isolates from exotic pets are of unusual serotypes. The frequency of infection linked to iguanas and hedgehogs in Table 2 may reflect the popularity of these animals as pets. Of greater concern is the number of infants and very young children who were affected.
Further hazards have been linked to petting zoos, and visits to farms and agricultural events where extensive contact with a variety of animals and their environment occurs. In autumn 1999, 159 individuals reported diarrheal illness after visits to the Agricultural Pavilion at a fair in Ontario. Seven primary cases of E coli 0157:H7 infection were identified; six patients were younger than 15 years of age, and a further four secondary cases were recorded in family contacts. Epidemiological and microbiological studies identified a petting zoo and contact with goats as being significant risk factors for the predominantly child cases (61). The report of this outbreak in Ontario and similar incidents elsewhere have implicated farm animals and the farm environment as potential sources of enteric infections, including VTEC and cryptosporidium, in child visitors and adults. Specific risk factors include poor personal and environmental hygiene; poor hand washing facilities; and close contact with and petting of animals, particularly young animals, and, even, tasting animal feeds (62–65). A recent report of a Salmonella species outbreak in the United Kingdom among children who handled chicks and ducklings has served to emphasize the potential hazards of hatchery projects in nursery and elementary school environments (66). In this incident, up to 40% of the children exposed were affected, and four children were admitted to hospital. The chicks and ducklings were kept in a classroom where the children ate their meals, and although the children were not meant to handle the birds, they were known to have done so. Similar hazards were recently reported in the United States, and have prompted some states to introduce legislative controls (67).
CONCLUSIONS
Enteric and foodborne diseases are a major and almost certainly under-reported cause of morbidity in the child population of Canada. The severity of disease in some cases and the long term sequelae of infection in others have yet to be fully described, but such cases probably constitute a significant health burden for some Canadian children. Further risks extend to the young with pre-existing or underlying conditions that affect their natural immunity. Significant risk factors for enteric infection in children appear to be related to specific foods for, or particularly attractive to, these age groups, to the presence of infected persons in the household, or to the contamination of the child’s environment.
The consumption of snack products, prepackaged meals for children and fast foods is ever popular. While outbreaks associated with these categories of products are rare, they do have the potential to affect large numbers of individuals over wide geographic areas due to large scale manufacturing and distribution practices. Recent publications by Scott (68) and Sattar et al (69) have re-emphasized the importance of the home environment in the transmission of enteric disease, and the study of infectious intestinal diseases in England indicated the possible importance of the cleaning method for feeding bottles in decreasing risk of infection in infants (25). These groups of researchers have highlighted the role of cross-contamination, as well as pointed to changes in eating practices and personal and home hygiene practices as being potential contributory factors to the spread of infection within families, especially among children. The following practices are of particular importance in limiting the risk of infection:
thorough hand washing after touching pets or
their litter, especially before preparing food; and
keeping pets out of the kitchen and off food
preparation surfaces.
Exposure to farm animals can be an enriching experience for young children, but there is also the need to protect children from enteric diseases. Clear guidelines on the maintenance of petting farms and zoos should be available to proprietors and should identify the following:
the provision of adequate hand washing facilities;
the need to post highly visible and clear notices about
the need for hand washing after touching animals, as
well as the location of these facilities; and
separate areas for food consumption.
Similarly, hatchery projects in nursery and elementary schools can be exciting and fun, but should also be carefully managed to minimize any risk of infection to children. Guidelines on the management of such projects should do the following:
Emphasize that young children should not touch the chicks or ducklings, or their litter, if at all possible.
Ensure that food is not kept or consumed in the same room as the room where the chicks or ducklings are kept.
Ensure that children wash their hands thoroughly, under supervision, if they do touch the chicks or ducklings or their environment, especially before eating.
Ensure that chicks and ducklings are kept in a cage that minimizes the spread of their litter to the environment.
Keeping exotic pets, particularly reptiles, has become increasingly popular. However, the risk of infection, particularly in young children, suggests that this practice may be inappropriate in households with infants. Paediatricians and public health officials should consider the possibility of a pet as a source of infection in situations in which there is an absence of other risk factors, and particularly, when an unusual serotype is concerned.
Acknowledgments
The authors thank Carole Scott, Marielle Pauzé and David Woodward for their help and patience in compiling data, and searching the literature for the preparation of this article. The authors also thank Sheila Herman for her help in preparing the manuscript.
REFERENCES
- 1.Wheeler JG, Sethi D, Cowden JM, et al. Study of infectious intestinal disease in England: Rates in the community, presenting to general practice, and reported to national surveillance. The Infectious Intestinal Disease Study Executive. BMJ. 1999;318:1046–50. doi: 10.1136/bmj.318.7190.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Todd ECD. Preliminary estimates of costs of foodborne disease in Canada and costs to reduce salmonellosis. J Food Prot. 1989;52:586–94. doi: 10.4315/0362-028X-52.8.586. [DOI] [PubMed] [Google Scholar]
- 3.Roberts T. Human illness costs of foodborne bacteria. Am J Agric Econ. 1989;71:468–74. [Google Scholar]
- 4.Sockett P. Social and economic aspects of food-borne disease. Food Policy. 1993;18:110–9. [Google Scholar]
- 5.Sockett PN, Todd ECD. The economic costs of foodborne disease In: Lund BM, Baird-Parker TC, Gould GW The Microbiological Safety and Quality of Food. Vol. 2. Maryland: Aspen Inc; 2000. pp. 1563–88. [Google Scholar]
- 6.Marks S, Roberts T. Escherichia coli 0157:H7 ranks as the fourth most costly foodborne disease. Food Rev. 1993;16:1–8. [Google Scholar]
- 7.Archer DL. Diarrheal episodes and diarrheal disease: Acute disease with chronic implications. J Food Prot. 1984;47:321–8. doi: 10.4315/0362-028X-47.4.321. [DOI] [PubMed] [Google Scholar]
- 8.Archer DL. Enteric microorganisms in rheumatoid diseases: Causative agents and possible mechanisms. J Food Prot. 1985;48:538–45. doi: 10.4315/0362-028X-48.6.538. [DOI] [PubMed] [Google Scholar]
- 9.Archer DL. Foodborne Gram-negative bacteria and atherosclerosis: Is there a connection? J Food Prot. 1987;50:783–7. doi: 10.4315/0362-028X-50.9.783. [DOI] [PubMed] [Google Scholar]
- 10.Rees JH, Soudain SE, Gregson NA, Hughes RA. Campylobacter jejuni infection and Guillain-Barré Syndrome. N Engl J Med. 1995;333:1374–9. doi: 10.1056/NEJM199511233332102. [DOI] [PubMed] [Google Scholar]
- 11.Neal KR, Hebden J, Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: Postal survey of patients. BMJ. 1997;314:779–82. doi: 10.1136/bmj.314.7083.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Communicable Disease Control Section, Seattle-King County Department of Public Health . Surveillance of the flow of Salmonella and Campylobacter in a community. Food and Drug Administration; 1984. For: United States Department of Health and Human Services, Public Health Services (Contract Number: 223-81-7041) [Google Scholar]
- 13.Wright J. Gastrointestinal illness: What are the causative organisms? NZ Public Health Rep. 1996;3:9–11. [Google Scholar]
- 14.Rowe PC, Orrbine E, Wells GA, McLaine PN. Epidemiology of hemolytic-uremic syndrome in Canadian children from 1986 to 1988. The Canadian Pediatric Kidney Disease Reference Centre. J Pediatr. 1991;119:218–24. doi: 10.1016/s0022-3476(05)80730-9. [DOI] [PubMed] [Google Scholar]
- 15.Anderson PHR, Stone DM. Staphylococcal food poisoning associated with spray-dried milk. J Hyg Camb. 1955;53:387–97. doi: 10.1017/s0022172400000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins RN, Treger MD, Goldsby JB, Boring JR, Coohon DB, Barr RN. Interstate outbreak of Salmonella newsbrunswick infection traced to powdered milk. JAMA. 1968;203:838–44. [PubMed] [Google Scholar]
- 17.Fardy PW, Butler R. Salmonellosis in Newfoundland. Epidemiol Bull. 1968;12:14. [Google Scholar]
- 18.Weissmap JB, Deen AD, Williams M, Swanston N, Ali S. An island-wide epidemic of salmonellosis in Trinidad traced to contaminated powdered milk. West Indian Med J. 1977;26:135–43. [PubMed] [Google Scholar]
- 19.Galbraith NS, Pusey JJ. Milkborne infectious disease in England and Wales 1938–82. In: Freed DJ, editor. Health Hazards of Milk. London: Baillière Tindall; 1984. pp. 27–29. [Google Scholar]
- 20.Haddock RL, Cousens SN, Guzman CC. Infant diet and salmonellosis. Am J Public Health. 1991;81:997–1000. doi: 10.2105/ajph.81.8.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craven JA. Salmonella contamination of dried milk products. Victoria Vet Proc. 1978;9:56–7. [Google Scholar]
- 22.Rowe B, Begg NT, Hutchinson DN, et al. Salmonella ealing infections associated with consumption of infant dried milk. Lancet. 1987;ii:900–3. doi: 10.1016/s0140-6736(87)91384-5. [DOI] [PubMed] [Google Scholar]
- 23.Brown KH, Black RE, Lopez de Romana G, Creed-de-Kanashiro H. Infant-feeding practices and their relationship with diarrhoeal and other diseases in Huascar (Lima), Peru. Pediatr. 1989;83:31–40. [PubMed] [Google Scholar]
- 24.Borgnolo G, Barbone F, Scornavacca G, Franco D, Vinci A, Iuculano F. A case-control study of salmonella gastrointestinal infection in Italian children. Acta Paediatr. 1996;85:804–8. doi: 10.1111/j.1651-2227.1996.tb14155.x. [DOI] [PubMed] [Google Scholar]
- 25.Joint Food Safety and Standards Group . The Infectious Intestinal Diseases (IID) Study Summary Report. London: Ministry of Agriculture, Fisheries and Food, and the Department of Health; 2000. [Google Scholar]
- 26.Cowden JM, Lynch D, Joseph CA, et al. Case-control study of infections with Salmonella enteritidis phage type 4 in England. BMJ. 1989;299:771–3. doi: 10.1136/bmj.299.6702.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Mahony M, Cowden J, Smyth B, et al. An outbreak of Salmonella saint-paul infection associated with beansprouts. Epidemiol Infect. 1990;104:229–33. doi: 10.1017/s0950268800059392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adak GK, Cowden JM, Nicholas S, Evans HS. The Public Health Laboratory Service national case-control study of primary indigenous sporadic cases of Campylobacter infection. Epidemiol Infect. 1995;115:15–22. doi: 10.1017/s0950268800058076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joseph CA, Mitchell EM, Cowden JM, et al. A national outbreak of salmonellosis from yeast flavoured products. CDR (Lond Engl Rev) 1991;1:R16–9. [PubMed] [Google Scholar]
- 30.Cowden JM, O’Mahony M, Bartlett CL, et al. A national outbreak of Salmonella typhimurium DT 124 caused by contaminated salami sticks. Epidemiol Infect. 1989;103:219–25. doi: 10.1017/s0950268800030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gill ON, Sockett PN, Bartlett CL, et al. Outbreak of Salmonella napoli infection caused by contaminated chocolate bars. Lancet. 1983;i:574–7. doi: 10.1016/s0140-6736(83)92822-2. [DOI] [PubMed] [Google Scholar]
- 32.Laboratory Centre for Disease Control Salmonella nima in Canada. Can Dis Wkly Rep. 1986;12:97–8. [Google Scholar]
- 33.Hockin JC, D’Aoust J-Y, Bowering D, et al. An international outbreak of Salmonella nima from imported chocolate. J Food Prot. 1989;52:51–4. doi: 10.4315/0362-028X-52.1.51. [DOI] [PubMed] [Google Scholar]
- 34.Craven PC, Mackel DC, Baine WB, Barker WH, Gangarosa EJ. International outbreak of Salmonella eastbourne infection traced to contaminated chocolate. Lancet. 1975;i:788–93. doi: 10.1016/s0140-6736(75)92446-0. [DOI] [PubMed] [Google Scholar]
- 35.Ratnam S, Stratton F, O’Keefe C, et al. Salmonella enteritidis outbreak due to contaminated cheese – Newfoundland. Can Commun Dis Rep. 1999;25:17–9. [PubMed] [Google Scholar]
- 36.Bell BP, Goldoft M, Griffin PM, et al. A multistate outbreak of Escherichia coli O157:H7-associated bloody diarrhea and hemolytic uremic syndrome from hamburgers. The Washington experience. JAMA. 1994;272:1349–53. [PubMed] [Google Scholar]
- 37.D’Aoust JY. Salmonella and the chocolate industry. A review. J Food Prot. 1977;40:718–27. doi: 10.4315/0362-028X-40.10.718. [DOI] [PubMed] [Google Scholar]
- 38.Haddock RL, San Nicolas A. Infant salmonellosis: Vacuum cleaners used to investigate an outbreak. J Environ Health. 1989;52:106–7. [Google Scholar]
- 39.Haddock RL. The origins of infant salmonellosis. Am J Public Health. 1993;83:772. doi: 10.2105/ajph.83.5.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schutze GE, Sikes JD, Stefanova R, Cave MD. The home environment and salmonellosis in children. Pediatr. 1999;103:E1. doi: 10.1542/peds.103.1.e1. [DOI] [PubMed] [Google Scholar]
- 41.Wilson R, Feldman RA, Davis J, LaVenture M. Salmonellosis in infants: the importance of intrafamilial transmission. Pediatrics. 1982;69:436–8. [PubMed] [Google Scholar]
- 42.Schutze GE, Kirby RS, Flick EL, Stefanova R, Eisenach KD, Cave D. Epidemiology and molecular identification of Salmonella infections in children. Arch Pediatr Adolesc Med. 1998;152:659–64. doi: 10.1001/archpedi.152.7.659. [DOI] [PubMed] [Google Scholar]
- 43.Robertson MH. Survival of Salmonella typhimurium in floor dust –a possible reservoir of infection in institutions. Public Health. 1972;87:39–45. doi: 10.1016/s0033-3506(72)80034-9. [DOI] [PubMed] [Google Scholar]
- 44.Haddock RL. Salmonella in vacuum cleaners. Lancet. 1986;ii:637. doi: 10.1016/s0140-6736(86)92467-0. [DOI] [PubMed] [Google Scholar]
- 45.Humphrey TJ, Martin KW, Whitehead A. Contamination of hands and work surfaces with Salmonella enteritidis PT4 during the preparation of egg dishes. Epidemiol Infect. 1994;113:403–9. doi: 10.1017/s0950268800068412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Human health risk from exposure to natural dog treats. Can Commun Dis Rep. 2000;26:41–2. [PubMed] [Google Scholar]
- 47.Plaut M, Zimmerman EM, Goldstein RA. Health hazards to humans associated with domestic pets. Annu Rev Public Health. 1996;17:221–45. doi: 10.1146/annurev.pu.17.050196.001253. [DOI] [PubMed] [Google Scholar]
- 48.Healing TD, Greenwood MH, Pearson AD. Campylobacters and enteritis. Rev Med Microbiol. 1992;3:159–67. [Google Scholar]
- 49.Salfield NJ, Pugh EJ. Campylobacter enteritis in young children living in households with puppies. Br Med J. 1987;294:21–2. doi: 10.1136/bmj.294.6563.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galton MM, Scatterday JE, Hardy AV. Salmonellosis in dogs. J Infect Dis. 1952;91:1–5. doi: 10.1093/infdis/91.1.1. [DOI] [PubMed] [Google Scholar]
- 51.Morse EV, Duncan MA. Canine salmonellosis: Prevalence, epizootiology, signs, and public health significance. J Am Vet Med Assoc. 1975;167:817–20. [PubMed] [Google Scholar]
- 52.Morse EV, Duncan MA, Estep DA, Riggs WA, Blackburn BO. Canine salmonellosis: A review and report of dog to child transmission of Salmonella enteritidis. Am J Public Health. 1976;66:82–4. doi: 10.2105/ajph.66.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimi A, Keyhani M, Bolurchi M. Salmonellosis in apparently healthy dogs. Vet Rec. 1976;98:110–1. doi: 10.1136/vr.98.6.110. [DOI] [PubMed] [Google Scholar]
- 54.Wall PG, Davis S, Threlfall EJ, Ward LR, Ewbank AJ. Chronic carriage of multidrug resistant Salmonella typhimurium in a cat. J Small Anim Pract. 1995;36:279–81. doi: 10.1111/j.1748-5827.1995.tb02919.x. [DOI] [PubMed] [Google Scholar]
- 55.Galbraith NS, Taylor CE, Cavanagh P, Hagan JG, Patton JL. Pet foods and garden fertilizers as sources of human salmonellosis. Lancet. 1962;i:372–4. doi: 10.1016/s0140-6736(62)91321-1. [DOI] [PubMed] [Google Scholar]
- 56.Williams LP, Helsdon HL. Pet turtles as a cause of human salmonellosis. JAMA. 1965;192:347–51. doi: 10.1001/jama.1965.03080180005001. [DOI] [PubMed] [Google Scholar]
- 57.Armentano T, Bruce A, Redys J, Hart JC. Pet turtle-associated salmonellosis. MMWR Morb Mortal Wkly Rep. 1971;20:45–6. [Google Scholar]
- 58.Lipsky S, Tanino T, Lewis JH. African pygmy hedgehog-associated salmonellosis – Washington, 1994. MMWR Morb Mortal Wkly Rep. 1995;44:462–3. [PubMed] [Google Scholar]
- 59.Dalton C, Hoffman R, Pape J. Iguana-associated salmonellosis in children. Pediatr Infect Dis J. 1995;14:319–20. doi: 10.1097/00006454-199504000-00014. [DOI] [PubMed] [Google Scholar]
- 60.Woodward DL, Khakhria R, Johnson WM. Human salmonellosis associated with exotic pets. J Clin Microbiol. 1997;35:2786–90. doi: 10.1128/jcm.35.11.2786-2790.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Middlesex-London Health Unit . An E coli 0157:H7 outbreak associated with an animal exhibit. London: The Middlesex-London Health Unit Investigation and Recommendations; 1999. [Google Scholar]
- 62.Shield J, Baumer JH, Dawson JA, Wilkinson PJ. Cryptosporidiosis – an educational experience. J Infect. 1990;21:297–301. doi: 10.1016/0163-4453(90)94053-3. [DOI] [PubMed] [Google Scholar]
- 63.Evans MR, Gardner D. Cryptosporidiosis outbreak associated with an educational farm holiday. Bur. 1996;6:R50–1. [PubMed] [Google Scholar]
- 64.Sayers GM, Dillon MC, Connolly E, et al. Cryptosporidiosis in children who visited an open farm. Bur. 1996;6:R140–4. [PubMed] [Google Scholar]
- 65.Shukla R, Slack R, George A, Cheasty T, Rowe B, Sculter J. Escherichia coli O157 infection associated with a farm visitor centre. Bur. 1995;5:R86–90. [PubMed] [Google Scholar]
- 66.Public Health Laboratory Service Outbreak of salmonellosis associated with chicks and ducklings at a children’s nursery. Commun Dis Wkly Rep. 2000;10:149, 152. [PubMed] [Google Scholar]
- 67.US Department of Health and Human Services Salmonellosis associated with chicks and ducklings – Michigan and Missouri, Spring 1999. MMWR Morb Mortal Wkly Rep. 2000;49:297–9. [PubMed] [Google Scholar]
- 68.Scott E. Hygiene issues in the home. Am J Infect Control. 1999;27:S22–5. doi: 10.1016/s0196-6553(99)70038-6. [DOI] [PubMed] [Google Scholar]
- 69.Sattar SA, Tetro J, Springthorpe VS. Impact of changing societal trends on the spread of infections in American and Canadian homes. Am J Infect Control. 1999;27:S4–21. doi: 10.1016/s0196-6553(99)70037-4. [DOI] [PubMed] [Google Scholar]