Abstract
Objectives. To compare potential population-wide benefits and risks, we examined the potential impact of increased nicotine replacement therapy (NRT) use for smoking cessation on future US mortality.
Methods. We developed a simulation model incorporating a Monte Carlo uncertainty analysis, with data from the 2005 National Health Interview Survey and Cancer Prevention Study II. We estimated the number of avoided premature deaths from smoking attributable to increased NRT use, before and after incorporating assumptions about NRT harm.
Results. We estimate that a gradual increase in the proportion of NRT-aided quit attempts to 100% by 2025 would lead to 40 000 (95% credible interval = 31 000, 50 000) premature deaths avoided over a 20-year period. Most avoided deaths would be attributable to lung cancer and cardiovascular disease. After we incorporated assumptions about potential risk from long-term NRT, the estimate of avoided premature deaths from all causes declined to 32 000.
Conclusions. Even after we assumed some harm from long-term NRT use, the benefits from increased cessation success far outweigh the risks. However, the projected reduction in premature mortality still reflects a small portion of the tobacco-related deaths expected over a 20-year period.
Cigarette smoking is one of the leading modifiable causes of death in the United States, accounting for more than 400 000 deaths1 and 5.5 million years of life lost annually.2 It has been estimated that up to half of persistent smokers will be killed by their habit, and lifelong smokers lose, on average, 10 years of life compared with nonsmokers.3 Despite a decline in smoking prevalence in the United States, there were still approximately 36 million daily smokers in 2005.4 Further, although the majority of smokers express a desire to quit,5 the average smoker makes several quit attempts before succeeding.6
The use of pharmacotherapy, including nicotine replacement therapy (NRT), has been shown to increase the likelihood of a successful quit attempt.7 Smoking cessation has numerous health benefits,6 including an increase in longevity, even among smokers who quit later in life.3 Effective smoking cessation policies, including increased NRT availability and use, would be expected to reduce smoking-attributable deaths in the United States.
Some concerns have arisen about the safety of long-term NRT use, which could reduce the cessation-related benefits of NRT-aided quit attempts. Hemodynamic effects of nicotine intake have been described, which may have implications for cardiovascular disease risk.8–10 However, tobacco smoke contains many toxic compounds that can damage the cardiovascular system, including combustion products such as carbon monoxide and nitrogen oxides8,11,12; thus, it is not clear what fraction of smoking-related cardiovascular risk may be attributable to nicotine intake. Further, clinical trials have generally shown NRT use to be safe.13,14 Concerns have also been raised about increased risk for cancer on the basis of evidence from in vitro and in vivo studies showing that nicotine can result in tumor promotion through increased cell proliferation, inhibition of apoptosis, and angiogenesis.15–17
To quantitatively compare the risks and benefits of NRT use, we developed a Monte Carlo simulation model to estimate future mortality patterns associated with changing patterns of NRT use and subsequent success in smoking cessation. We also incorporated assumptions about long-term NRT use and its potential harms to weigh the risks and benefits of NRT use for smoking cessation.
METHODS
We used data from the 2005 National Health Interview Survey (NHIS) to estimate prevalence of smoking among adults (aged 18 years or older). The NHIS is a nationally representative survey of the US population that is conducted annually by the National Center for Health Statistics.18 We estimated the number of smokers, by age group and gender, as defined by the following questions and answers: “Have you smoked at least 100 cigarettes in your entire life?” (yes) and “Do you now smoke cigarettes every day, some days or not at all?” (every day and some days).
We incorporated several assumptions regarding smoking initiation into the model. First, we assumed that initiation occurs prior to age 25 years. Second, we modeled smoking initiation at a constant rate into the future, such that the number of smokers in the group aged 18 to 24 years remained constant. The model was designed so that assumptions regarding initiation would have a minimal impact on the results because excess mortality from smoking does not begin to be observed until early middle age, which is beyond the timeframe for the population of new smokers in our simulation.
We estimated the fraction of smokers who made a quit attempt in the past year by using the 2005 NHIS data. The total number of smokers who made a quit attempt was equal to the number of current smokers who made a quit attempt and the number of exsmokers who successfully quit in the past year. The total number of smokers “at-risk” for a quit attempt was equal to all current smokers and exsmokers who quit in the past year. We calculated the proportion of smokers making a quit attempt in the past year by dividing these 2 quantities within strata defined by 5-year age group and gender.
We estimated current NRT use in the United States, by age group and gender, by using the adult cancer module of the 2005 NHIS. Nicotine replacement therapy use included any of the following products: nicotine gum, patch, spray, inhaler, lozenge, or tablet. Use of NRT during the past year was calculated among current smokers who attempted to quit in the past year and exsmokers who successfully quit in the past year.
The probability of long-term abstinence for a given quit attempt with and without the use of NRT was obtained from peer-reviewed literature. A recent review suggests that long-term abstinence rate for self-quitters with no assistance ranges from 3% to 5%.19 A recent systematic review characterized the increased chance of smoking abstinence when using NRT versus no therapy during a quit attempt. We used the overall estimate of NRT effectiveness from this review in our model (odds ratio [OR] = 1.77; 95% confidence interval = 1.66, 1.88).7
We obtained mortality rates among nonsmokers and smoking relative risks from the Cancer Prevention Study II (CPS-II) cohort study (Michael Thun, American Cancer Society, written communication, 2006). CPS-II is a nationwide, prospective cohort study begun in 1982, that comprises 1.2 million US adults aged 30 years and older.20 We obtained results for follow-up from 1984 to 1991. Mortality rates were calculated for never-smokers by 5-year age group and gender for the following causes of death: lung cancer, smoking-related cancers (lip, oral cavity, or pharynx; esophagus; pancreas; larynx; and kidney, bladder, or other urinary cancer), cardiovascular disease, and all-cause mortality. Unadjusted relative risks were calculated for current smokers and exsmokers by age group, gender, and age at cessation. We limited our simulation to individuals aged between 35 and 84 years because of lack of reliable risk estimates outside these age groups.
In CPS-II, women have a lower relative risk of smoking-related mortality compared with men. Much of this difference is likely because of differences in smoking patterns between men and women during the decades leading up to the study.21 We modeled future mortality by using the overall current smoking prevalence in the United States, without stratifying on smoking frequency. Smoking patterns between men and women are more similar now than in the past and more similar to historical patterns for men.22 As a result, we applied the relative risks estimated for men to both men and women.
Because there are limited data to quantify the potential risks from long-term nicotine use, we used an estimate from the smokeless tobacco literature. A study of male Swedish construction workers reported an excess cardiovascular and all-cause mortality risk of 40% for users of smokeless tobacco compared with non–tobacco users.23 In our analysis, we used this estimate as an upper bound on the impact of prolonged nicotine use on all causes of mortality, assuming that the excess risk is solely because of nicotine intake. To estimate the potential population-level risks of NRT use, we assumed that 5% of NRT-aided quitters would become long-term users and experience an increased risk relative to unaided or nonpersistent NRT-aided quitters.
Simulation Model
Using these data sources, we developed a simulation model with a Monte Carlo uncertainty analysis to predict future mortality patterns associated with changing patterns of NRT use among smokers. The simulation begins with current smokers in the United States, estimated as of year 2005. As the population of smokers ages, their smoking status either remains current or transitions to former and mortality is estimated for current smokers and exsmokers (see the figure available as a supplement to the online version of this article at http://www.ajph.org). The extent to which current smokers transition to exsmokers depends on quit attempts, use of NRT, long-term quitting success in the absence of NRT, and the effectiveness of NRT in maintaining smoking abstinence.
We modeled a set of future scenarios associated with 2 different patterns of NRT use among smokers making a quit attempt: (1) a constant increase until a doubling of use is achieved by year 2025 and (2) a constant increase until 100% use is achieved by year 2025. The first scenario was chosen as a reasonable possibility for increased use, whereas the second scenario was chosen as an upper bound. For each iteration of the model, a “baseline” scenario assumes the status quo, i.e., no future changes in NRT use among the US smoking population, and an “alternative” scenario assumes an increase in future NRT use. The difference in total mortality between the baseline and alternative scenarios is the estimate of avoided premature mortality associated with increased use of NRT.
For the simulation, we estimated relevant parameters from the underlying data and specified distributional assumptions for each data input in the model (Table 1). Relative risks and odds ratios were assumed to be lognormally distributed. We used the normal approximation of the binomial distribution to model smoking prevalence, the proportion of smokers making a quit attempt, and the proportion of smokers using NRT for a given quit attempt. Mortality rates among nonsmokers were assumed to follow a Poisson distribution. The long-term quitting success without NRT use was assumed to be uniformly distributed.
TABLE 1.
Data Inputs and Distributional Assumptions in the Simulation Model of the Risks and Benefits of Nicotine Replacement Therapy for Smoking Cessation in the United States
| Data Input | Distributional Shape | Parameters | Source |
| Nonsmoker mortality rates | Poisson | Varies by cause, age group, and gender | CPS-IIa |
| Smoking relative risks | Lognormal | Varies by cause, smoking status, age group, and gender | CPS-IIa |
| Smoking prevalence | Normal | Varies by age group and gender | 2005 NHIS18 |
| Quit attempt percentage | Normal | Varies by age group and gender | 2005 NHIS18 |
| NRT usage | Normal | Varies by age group | 2005 NHIS18 |
| NRT effectiveness | Lognormal | OR = 1.77; 95% CI = 1.66, 1.88 | Silagy et al., 20047 |
| Long-term cessation success rate without NRT | Uniform | 3%–5% | Hughes, 200419 |
Note. CPS-II = Cancer Prevention Study II; NHIS = National Health Interview Survey; NRT = nicotine replacement therapy; OR = odds ratio; CI = confidence interval.
Written communication, Michael Thun, American Cancer Society, 2006.
We created the simulated data by generating 1000 random values from the corresponding distributions for each variable of interest. For each iteration, we predicted future mortality patterns over a 20-year projection and estimated the number of premature deaths avoided associated with increased NRT use. We generated 95% credible intervals from the resultant distribution, defined as the range between the 2.5th and the 97.5th percentiles. Central estimates of avoided premature deaths were based on the point estimates from the original data sources described previously.
We performed statistical analyses with SAS version 9.1 (SAS Institute, Cary, NC). We used Taylor linear variance estimation with SAS-Callable SUDAAN software version 9.01 (RTI Institute, Research Triangle Park, NC) to obtain standard errors for smoking prevalence, quit attempt proportions, and NRT. The simulation model was also developed in SAS version 9.1. We generated the graphical output from the model with the R statistical software (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
In 2005, approximately 1 in 5 adults in the United States was a smoker and close to 50% reported a quit attempt in the past year (see the table available as a supplement to the online version of this article at http://www.ajph.org). The proportion of smokers making a quit attempt was greatest among young adults (56%) and women (50%). Additionally, close to 25% of the most recent quit attempts were NRT-aided. The use of NRT during a quit attempt was lowest among young adults (13%) and greater in women than in men (28%).
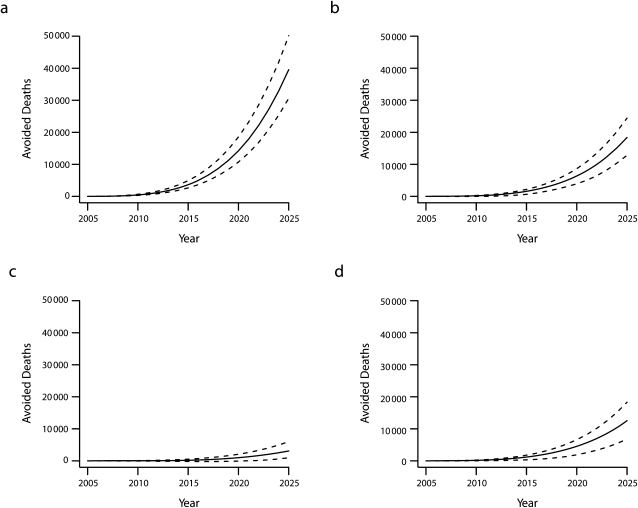
We estimated that a doubling of NRT use prevalence during quit attempts would lead to approximately 19 000 (95% credible intervals = 14 000, 24 000) avoided premature deaths from all causes over the next 20 years (Table 2). Of these avoided premature deaths, approximately 9000 (47%) would be attributable to lung cancer and 6000 (32%) to cardiovascular disease. Assuming 100% NRT use by 2025, we estimated 40 000 (95% credible intervals = 31 000, 50 000) cumulative premature deaths avoided (Table 2). The cumulative number of premature deaths avoided under the 100% NRT use scenario is shown in Figure 1.
TABLE 2.
Estimated Cumulative Premature Deaths Avoided Over 20 Years With Increased Nicotine Replacement Therapy (NRT) Use in the US Population
| Premature Deaths Avoided |
||
| Cause | Doubling of NRT Use,a No. (95% CI) | 100% NRT Use,b No. (95% CI) |
| All cause | ||
| No NRT risk | 19 000 (14 000, 24 000) | 40 000 (31 000, 50 000) |
| NRT risk included | 15 000 (11 000, 20 000) | 32 000 (25 000, 42 000) |
| Lung cancer | ||
| No NRT risk | 9000 (6000, 12 000) | 18 000 (13 000, 24 000) |
| NRT risk included | 8000 (6000, 11 000) | 17 000 (11 000, 23 000) |
| Other smoking-related cancers | ||
| No NRT risk | 1000 (<500, 3000) | 3000 (< 500, 6000) |
| NRT risk included | 1000 (<500, 2000) | 3000 (< 500, 5000) |
| Cardiovascular disease | ||
| No NRT risk | 6000 (3000, 9000) | 13 000 (7000, 18 000) |
| NRT risk included | 5000 (3000, 8000) | 11 000 (5000, 16 000) |
Note. CI = credible interval.
A constant increase in NRT use among smokers making a quit attempt until a doubling of use is achieved by year 2025.
A constant increase in NRT use among smokers making a quit attempt until 100% use is achieved by year 2025.
FIGURE 1.
Estimated cumulative premature deaths avoided in the United States with 100% nicotine replacement therapy use by year 2025 from (a) all causes, (b) lung cancer, (c) other smoking-related cancers, and (d) cardiovascular diseases.
Note. Solid line denotes central estimate and dashed line denotes 2.5th and 97.5th percentiles from Monte Carlo uncertainty analysis.
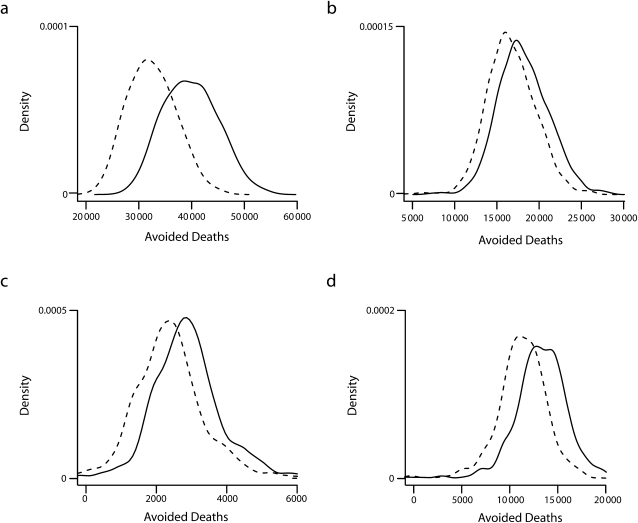
Figure 2 shows the distribution of estimates of avoided premature deaths under the 100% NRT use scenario before and after incorporating assumptions about long-term NRT use and associated harm. As expected, the distributions from the model are shifted lower with the assumption of NRT-related risk. For all-cause mortality, the central estimate of premature deaths avoided was reduced by 20% after inclusion of NRT-related risk (40 000 vs 32 000; Table 2). For all causes of death, the credible intervals exclude zero, suggesting a net benefit after including assumptions regarding NRT-related risk. Similar results were observed in the scenario in which NRT use was doubled (Table 2).
FIGURE 2.
Density plots of estimated cumulative premature deaths avoided in the United States with 100% nicotine replacement therapy use by year 2025 from (a) all causes, (b) lung cancer, (c) other smoking-related cancers, and (d) cardiovascular diseases.
Note. Solid line denotes scenario without assumptions of NRT harm and dashed line denotes scenario with assumption of NRT harm.
DISCUSSION
We estimated the number of premature deaths that could be avoided over a 20-year period with increased NRT use and subsequent cessation. The upper-bound estimate, based on 100% NRT use in 20 years, would result in about 40 000 avoided premature deaths. The majority of these premature deaths would be attributable to lung cancer and cardiovascular disease. It should be noted that data were not available to model chronic obstructive lung disease, which also makes a significant contribution to the burden of tobacco-related disease. After we made some bounding assumptions of increased risk attributable to long-term NRT use, a favorable risk–benefit profile was still observed, although the estimate of avoidable deaths declined by about 20%. These findings were based on a model that included data from the largest US studies of smoking behavior and health and incorporated the statistical uncertainty underlying the model inputs. Although we used NRT as an example, the methodology was general for comparing benefits and risks and could be applied to other cessation approaches.
Relationship to Overall Tobacco-Related Mortality Burden
In the context of the number of annual premature deaths in the United States caused by smoking (approximately 400 000 annually),1 the estimates of avoidable deaths over a 20-year period were relatively small (40 000). If we ran the simulation out over the lifetime of the population of smokers, the avoidable mortality would be significantly greater. In addition, we incrementally increased the proportion of smokers using NRT for a quit attempt so that doubling or 100% use was not achieved until the final year of the simulation. Even if all quit attempts were aided by NRT, the long-term quit rate would still reach less than 10% in our simulation. Further, only about half of the smokers in the model were expected to make a quit attempt in a given year as estimated for the US smoking population from the 2005 NHIS. It should be noted that we assumed only 1 quit attempt per year among these smokers, which would underestimate the overall population benefits based on the extent to which multiple quit attempts are made in a given year. We also assumed that the fraction of smokers making a quit attempt would remain constant into the future. Factors that may affect NRT use, such as reduced cost or increased availability, may also positively impact the likelihood of making a quit attempt, thus increasing the population-level health benefits. This highlights the need for effective approaches that influence both the likelihood of making a quit attempt and the probability that such an attempt will be successful.
Safety of Nicotine Replacement Therapy Use
Concerns about product safety may influence the extent of NRT use among smokers. Survey data suggest a large amount of misinformation exists regarding the relative safety and harm of NRT and smoking. In a recent survey of adult smokers, about two thirds of respondents believed that nicotine patches were more likely to cause a heart attack than was cigarette smoking, and two thirds believed that nicotine was a cause of cancer.24,25 Not surprisingly, those who had used NRT in the past tended to be more knowledgeable regarding safety and effectiveness.25 More effort should be made to increase awareness of the full spectrum of harm from tobacco use to put into context the potential risks from NRT use.
We also modeled the possible population health impact of NRT-related harm by using a bounding estimate of risk. Nicotine may play a role in smoking-related cardiovascular disease through hemodynamic effects8–10 and possibly through the acceleration of atherosclerosis.8,15,26 However, tobacco smoke contains many chemical constituents that can harm the cardiovascular system, including combustion products such as carbon monoxide and nitrogen oxides.8,11,12 It is not clear what fraction of cardiovascular morbidity is attributable to nicotine intake; however, it is generally believed that the benefits of nicotine pharmacotherapy use in terms of smoking cessation outweigh the risks, even among smokers with stable heart disease.11,12,27–29 Cigarette smoking produces a higher peak and average dose of nicotine than does NRT,28 suggesting that smoking would lead to greater nicotine-related risk than would NRT use. Further, the dose–response relationship between nicotine intake and hemodynamic effects appears to be flat,11 suggesting that concomitant use of cigarette smoking and NRT would not result in increased risk attributable to nicotine. It has also been shown that smokers tend to titrate their nicotine intake to achieve a relative constant dose,30 which could lead to a reduced intake of combustion products and a favorable risk–benefit profile. Thus, concomitant use may lead to lower exposure to the harmful combustion products of tobacco smoke.
Several short-term clinical trials of NRT use among smokers with coronary heart disease13,14 and a meta-analysis of nicotine patch randomized trials found no excess of adverse cardiovascular events among participants, although the authors noted that large studies would be necessary to identify risks for these outcomes.31 These studies do not necessarily prove a lack of harm, but they are consistent with a favorable risk–benefit ratio of NRT use for smoking cessation. The few observational studies conducted both among the general population and those with coronary heart disease have also failed to identify excess cardiovascular risk among NRT users.32–34 These studies, however, are harder to interpret because of potential confounding caused by self-selection of NRT use, lack of documented NRT use, or, in some cases, low statistical power.
Nicotine is not considered to be a carcinogen, but in vitro and animal studies have suggested that nicotine may play a role in tumor promotion through processes including angiogenesis15,16 and inhibition of apoptosis.16 Apoptosis is important for the normal regulation of cell growth and the inhibition of apoptosis is a key component of cancer progression.35 Angiogenesis is necessary for tumor growth and metastasis.36 Although these data suggest that nicotine may act as a tumor promoter, nicotine supplementation is undoubtedly safer than the continuation of smoking. In addition to the many known carcinogens in tobacco smoke, the typical dose of nicotine received from supplementation does not exceed that received by an active cigarette smoker.28
Studies of smokeless tobacco products provide some insight into the potential harms of nicotine. These products provide a nicotine dose similar to that from cigarette smoking,37 but without the combustion products of smoking. Although smokeless tobacco products may contain many toxins that are associated with a host of adverse health effects,38 there is evidence that some smokeless tobacco products may pose less cardiovascular risk than cigarette smoking.39,40 In particular, snus (Swedish moist snuff) has garnered attention as a potential harm reduction product because of its lower concentration of tobacco-specific nitrosamines and other contaminants and because of ecological observations regarding the trends in tobacco-related diseases in Sweden.41 A study of male Swedish construction workers, however, reported an excess cardiovascular and all-cause mortality risk of 40% among snus users compared with never-users of tobacco.23 In a follow-up study, long-term snus use was associated with an increased risk of fatal, but not nonfatal, myocardial infarction.42 Additional studies in similar populations have reported no association between snus use and oral or lung cancer,43 but have reported an excess risk for esophageal squamous cell carcinoma (relative risk = 3.5; 95% confidence interval = 1.6, 7.6),44 noncardia stomach cancer (relative risk = 1.4; 95% confidence interval = 1.1, 1.9),44 and pancreatic cancer (relative risk = 2.1; 95% confidence interval = 1.2, 3.6).43
In our analysis, we used the 40% increase in all-cause mortality risk from Bolinder et al.23 as a conservative estimate of the impact of prolonged nicotine intake on mortality. We considered this estimate to be conservative because it assumed that the excess risk was caused solely by nicotine intake. Additionally, although this risk estimate was based on a comparison of snus users to never-users of tobacco, we applied the estimate to exsmokers, for which the baseline risk would be higher and the relative risk lower. Even after incorporating this assumption of NRT risk, we still found that the benefits in terms of reduced premature mortality outweighed the risks.
Strengths and Limitations
We used a Monte Carlo approach to account for the statistical uncertainty in the underlying model parameters. In addition, any attempt to predict future mortality patterns will, of necessity, require many assumptions to bridge evidence gaps and will come with related uncertainties. We assumed the current proportion of smokers making a quit attempt in a given year remained constant into the future. If the quit attempt proportions were to increase over time, our model would underestimate the population benefits of increased cessation success.
We also assumed that mortality rates among never-smokers remained constant into the future. However, numerous factors may result in changing rates over time, including improvements in detection and treatment and trends in the prevalence of risk factors over time. Diseases such as lung cancer, which has very few nonsmoking-related risk factors, no effective screening modalities,45 and has shown little change in survival rates over time,46 were not likely to be affected by this assumption. However, changes in risk factor prevalence may be especially important for multifactorial diseases, such as cardiovascular disease. We specifically did not address how the modeled scenarios would be achieved, instead choosing to use a realistic and upper-bound scenario to demonstrate an approach to modeling future health impacts. Further, we did not examine cost-effectiveness, but many studies have shown that pharmacotherapy is among the most cost-effective clinical interventions.47
Several other simplifying assumptions were made in this model. We assumed that the smoking relative risks from CPS-II would reflect the disease risk of current smokers into the future, irrespective of smoking frequency and duration. Over the past several decades, changes have been observed in the average age at initiation and the average number of cigarettes consumed per day.21,48,49 Both of these factors influence the magnitude of the effect of smoking on mortality risk, along with other temporal changes, such as the constituents of cigarettes and the depth of smoke inhalation.
In addition, mortality rates were not available for each year of age, but instead were aggregated by 5-year age groups and 10-year age-at-cessation groups. In the model, changes in risk occurred discretely when individuals transitioned to a new age group or successfully quit. This likely overestimated the magnitude of avoidable disease because risk reduction does not occur immediately after cessation, specifically for cancer. We did not consider competing causes of death in our model, which would impact the estimate of avoided cause-specific deaths but not all-cause mortality. Finally, we assumed the distributions for each parameter were independent of one another. Future approaches using Monte Carlo simulation should attempt to account for the dependency of parameters, specifically the relative risks among current smokers and exsmokers.
Despite these uncertainties, this analysis shows that considerable reductions in premature mortality can be achieved through a modest increase in cessation rates, although this still reflects a small proportion of smoking-related deaths in the United States. Although there is no clear evidence on the role of long-term nicotine intake on disease risk, we have shown that the long-term benefits of increased smoking cessation still far outweigh the risks from long-term NRT use. In addition to premature mortality, smoking is a cause of many nonfatal adverse health conditions that lead to poorer overall health and more days of missed work for smokers than for comparable nonsmokers.50 If we include the gain in healthy and productive life, the public health impact of increased success in smoking cessation would be significantly greater than the results reported here.
Acknowledgments
This research was supported by a grant from GlaxoSmithKline.
The authors would like to thank the American Cancer Society for providing age-, gender-, and disease-specific death rates from the Cancer Prevention Study II.
Note. The funding organization had no role in the outcome of this study or the preparation of the article.
Human Participant Protection
This study is not considered human subjects research because only aggregate data were used in the simulation from preexisting and publicly available sources.
References
- 1.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA 2004;291(10):1238–1245 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Annual smoking-attributable mortality, years of potential life lost, and productivity losses–United States, 1997-2001. MMWR Morb Mortal Wkly Rep 2005;54(25):625–628 [PubMed] [Google Scholar]
- 3.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ 2004;328(7455):1519–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Summary Health Statistics for U.S. Adults: National Health Interview Survey, 2005 Provisional Report. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2006. DHHS publication no. 2007-1560 [Google Scholar]
- 5.Centers for Disease Control and Prevention Cigarette smoking among adults–United States, 2000. MMWR Morb Mortal Wkly Rep 2002;51(29):642–645 [PubMed] [Google Scholar]
- 6.The Health Benefits of Smoking Cessation. A Report of the Surgeon General Washington, DC: US Department of Health and Human Services; 1990 [PubMed] [Google Scholar]
- 7.Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2004;(3):CD000146. [DOI] [PubMed] [Google Scholar]
- 8.Benowitz NL. The role of nicotine in smoking-related cardiovascular disease. Prev Med 1997;26(4):412–417 [DOI] [PubMed] [Google Scholar]
- 9.Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol 1997;29(7):1422–1431 [DOI] [PubMed] [Google Scholar]
- 10.Khosla S, Laddu A, Ehrenpreis S, Somberg JC. Cardiovascular effects of nicotine: relation to deleterious effects of cigarette smoking. Am Heart J 1994;127(6):1669–1672 [DOI] [PubMed] [Google Scholar]
- 11.Balfour D, Benowitz N, Fagerstrom K, Kunze M, Keil U. Diagnosis and treatment of nicotine dependence with emphasis on nicotine replacement therapy. A status report. Eur Heart J 2000;21(6):438–445 [DOI] [PubMed] [Google Scholar]
- 12.Haustein KO. Smoking tobacco, microcirculatory changes and the role of nicotine. Int J Clin Pharmacol Ther 1999;37(2):76–85 [PubMed] [Google Scholar]
- 13.Joseph AM, Norman SM, Ferry LH, et al. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. N Engl J Med 1996;335(24):1792–1798 [DOI] [PubMed] [Google Scholar]
- 14.Tzivoni D, Keren A, Meyler S, Khoury Z, Lerer T, Brunel P. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovasc Drugs Ther 1998;12(3):239–244 [DOI] [PubMed] [Google Scholar]
- 15.Cooke JP, Bitterman H. Nicotine and angiogenesis: a new paradigm for tobacco-related diseases. Ann Med 2004;36(1):33–40 [DOI] [PubMed] [Google Scholar]
- 16.Catassi A, Servent D, Paleari L, Cesario A, Russo P. Multiple roles of nicotine on cell proliferation and inhibition of apoptosis: implications on lung carcinogenesis. Mutat Res 2008;659(3):221–231 [DOI] [PubMed] [Google Scholar]
- 17.Zeidler R, Albermann K, Lang S. Nicotine and apoptosis. Apoptosis 2007;12(11):1927–1943 [DOI] [PubMed] [Google Scholar]
- 18.2005 National Health Interview Survey (NHIS) public use data release: NHIS survey description Hyattsville, MD: National Center for Health Statistics, Division of Health Interview Statistics; 2006 [Google Scholar]
- 19.Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004;99(1):29–38 [DOI] [PubMed] [Google Scholar]
- 20.Stellman SD, Garfinkel L. Smoking habits and tar levels in a new American Cancer Society prospective study of 1.2 million men and women. J Natl Cancer Inst 1986;76(6):1057–1063 [PubMed] [Google Scholar]
- 21.Changes in Cigarette-Related Disease Risks and Their Implication for Prevention and Control Burns DM, Garfinkel L, Samet JM, Bethesda, MD: US Department of Health and Human Services, Public Health Service, National Cancer Institute; 1997. NIH publication no. 97-4213. Smoking and Tobacco Control Monograph [Google Scholar]
- 22.Centers for Disease Control and Prevention Cigarette smoking among adults–United States, 2006. MMWR Morb Mortal Wkly Rep 2007;56(44):1157–1161 [PubMed] [Google Scholar]
- 23.Bolinder G, Alfredsson L, Englund A, de Faire U. Smokeless tobacco use and increased cardiovascular mortality among Swedish construction workers. Am J Public Health 1994;84(3):399–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cummings KM, Hyland A, Giovino GA, Hastrup JL, Bauer JE, Bansal MA. Are smokers adequately informed about the health risks of smoking and medicinal nicotine? Nicotine Tob Res 2004; 6Suppl 3:S333–S340 [DOI] [PubMed] [Google Scholar]
- 25.Bansal MA, Cummings KM, Hyland A, Giovino GA. Stop-smoking medications: who uses them, who misuses them, and who is misinformed about them? Nicotine Tob Res 2004; 6Suppl 3:S303–S310 [DOI] [PubMed] [Google Scholar]
- 26.Kilaru S, Frangos SG, Chen AH, et al. Nicotine: a review of its role in atherosclerosis. J Am Coll Surg 2001;193(5):538–546 [DOI] [PubMed] [Google Scholar]
- 27.Joseph AM, Fu SS. Safety issues in pharmacotherapy for smoking in patients with cardiovascular disease. Prog Cardiovasc Dis 2003;45(6):429–441 [DOI] [PubMed] [Google Scholar]
- 28.Benowitz NL. Smoking-induced coronary vasoconstriction: implications for therapeutic use of nicotine. J Am Coll Cardiol 1993;22(3):648–649 [DOI] [PubMed] [Google Scholar]
- 29.Van Gilder TJ, Remington PL, Fiore MC. The direct effects of nicotine use on human health. Wis Med J 1997;96(2):43–48 [PubMed] [Google Scholar]
- 30.US Department of Health and Human Services, National Cancer Institute. Risks Associated With Smoking Cigarettes With Low Machine-Measured Yields of Tar and Nicotine Bethesda, MD: National Institutes of Health; 2001 [Google Scholar]
- 31.Greenland S, Satterfield MH, Lanes SF. A meta-analysis to assess the incidence of adverse effects associated with the transdermal nicotine patch. Drug Saf 1998;18(4):297–308 [DOI] [PubMed] [Google Scholar]
- 32.Meine TJ, Patel MR, Washam JB, Pappas PA, Jollis JG. Safety and effectiveness of transdermal nicotine patch in smokers admitted with acute coronary syndromes. Am J Cardiol 2005;95(8):976–978 [DOI] [PubMed] [Google Scholar]
- 33.Hubbard R, Lewis S, Smith C, et al. Use of nicotine replacement therapy and the risk of acute myocardial infarction, stroke, and death. Tob Control 2005;14(6):416–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimmel SE, Berlin JA, Miles C, Jaskowiak J, Carson JL, Strom BL. Risk of acute first myocardial infarction and use of nicotine patches in a general population. J Am Coll Cardiol 2001;37(5):1297–1302 [DOI] [PubMed] [Google Scholar]
- 35.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature 2001;411(6835):342–348 [DOI] [PubMed] [Google Scholar]
- 36.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000;407(6801):249–257 [DOI] [PubMed] [Google Scholar]
- 37.Benowitz NL, Jacob P, III, Yu L. Daily use of smokeless tobacco: systemic effects. Ann Intern Med 1989;111(2):112–116 [DOI] [PubMed] [Google Scholar]
- 38.Smokeless Tobacco or Health: An International Perspective Rockville, MD: National Cancer Institute; 1992 [Google Scholar]
- 39.Gupta R, Gurm H, Bartholomew JR. Smokeless tobacco and cardiovascular risk. Arch Intern Med 2004;164(17):1845–1849 [DOI] [PubMed] [Google Scholar]
- 40.Asplund K. Smokeless tobacco and cardiovascular disease. Prog Cardiovasc Dis 2003;45(5):383–394 [DOI] [PubMed] [Google Scholar]
- 41.Daniel Roth H, Roth AB, Liu X. Health risks of smoking compared to Swedish snus. Inhal Toxicol 2005;17(13):741–748 [DOI] [PubMed] [Google Scholar]
- 42.Hergens MP, Alfredsson L, Bolinder G, Lambe M, Pershagen G, Ye W. Long-term use of Swedish moist snuff and the risk of myocardial infarction amongst men. J Intern Med 2007;262(3):351–359 [DOI] [PubMed] [Google Scholar]
- 43.Luo J, Ye W, Zendehdel K, et al. Oral use of Swedish moist snuff (snus) and risk for cancer of the mouth, lung, and pancreas in male construction workers: a retrospective cohort study. Lancet 2007;369(9578):2015–2020 [DOI] [PubMed] [Google Scholar]
- 44.Zendehdel K, Nyren O, Luo J, et al. Risk of gastroesophageal cancer among smokers and users of Scandinavian moist snuff. Int J Cancer 2008;122(5):1095–1099 [DOI] [PubMed] [Google Scholar]
- 45.Manser RL, Irving LB, Stone C, Byrnes G, Abramson M, Campbell D. Screening for lung cancer. Cochrane Database Syst Rev 2004;(1):CD001991. [DOI] [PubMed] [Google Scholar]
- 46.Cancer trends progress report–2005 update. Bethesda, MD: National Cancer Institute; 2005 [Google Scholar]
- 47.AHCPR Supported Clinical Practice Guidelines. 18 Treating tobacco use and dependence: 2008 update. Rockville, MD: Agency for Healthcare Research and Quality, US Department of Health and Human Services; 2008 [Google Scholar]
- 48.Centers for Disease Control and Prevention Incidence of initiation of cigarette smoking—United States, 1965-1996. MMWR Morb Mortal Wkly Rep 1998;47(39):837–840 [PubMed] [Google Scholar]
- 49.National Cancer Institute. Those Who Continue to Smoke: Is Achieving Abstinence Harder and Do We Need to Change Our Interventions? Rockville, MD: US Department of Health and Human Services; 2003. Smoking and Tobacco Control Monograph 15 [Google Scholar]
- 50.The Health Effects of Active Smoking: A Report of the Surgeon General Washington, DC: US Department of Health and Human Services; 2004 [Google Scholar]