Abstract
We reviewed 25 randomized clinical trials that assessed the effect of peer-based interventions on health-related behaviors in adults. Effect sizes were calculated as odds ratios or standardized mean differences. We grouped most of the studies by 7 measured outcomes, with effect sizes ranging from −0.50 to 2.86. We found that peer-based interventions facilitated important changes in health-related behaviors, including physical activity, smoking, and condom use, with a small- to medium-sized effect. However, the evidence was mixed, possibly because of the heterogeneity we found in methods, dose, and other variables between the studies. Interventions aimed at increasing breastfeeding, medication adherence, women's health screening, and participation in general activities did not produce significant changes.
Peer-based interventions have become a common method to effect important health-related behavior changes.1,2 However, no generally accepted definition of peer or peer-based intervention has been established to date. Peers often share a common culture, language, and knowledge about the problems that their community experiences.3 Moreover, in the research context, peers must share a health problem (e.g., newly diagnosed tuberculosis) or a potential for change in their health status (e.g., breastfeeding for new mothers). For this review, we defined peer-based interventions as a method of teaching or facilitating health promotion that asks people to share specific health messages with members of their own community.3
Over the past 20 years a growing body of literature has examined the efficacy of peer-based interventions to improve health care. These studies have examined a variety of illnesses, conditions, populations, and interventions to determine what can be done at the community level to facilitate positive health care outcomes. In addition, numerous studies have tested new ways to reach minority populations and to decrease health care spending. Outcomes measures in these studies have included improved quality of life, improved self-efficacy, increased self-care and symptom management, and reduction in harmful behaviors.1 These studies have concluded that peer-based interventions have the potential to enhance health equity in persons living with disease.1,3–5 Examples of behaviors targeted include physical activity,6 smoking,7 and breast self-exams.8 Peer-based interventions have been found to improve access to health care services, provide support, improve self-efficacy and self-confidence, facilitate involvement in self-care activities, and increase cost effectiveness.1
Despite the importance of these outcomes to individuals' health and the rapidly increasing use of such interventions, no systematic review of the effect of peer-based interventions on health-related behavior change in adults has been published. Systematic reviews synthesize the evidence on the effectiveness and appropriateness of interventions in specific circumstances.9 These reviews can guide individual health care practitioners and health policymakers to select the most effective intervention. We therefore reviewed randomized clinical trials to assess the effect of peer-based interventions on health-related behaviors in adults.
METHODS
We searched for prospective, experimental studies assessing any health-related behavior change in adults resulting from a peer-led intervention. Criteria for studies' inclusion in our analysis were (1) participants older than 18 years; (2) randomization to intervention and control groups; (3) a primary outcome of health-related behavior change, defined as any measurable behavior change related to a disease or change in an individual's health; (4) independence from other studies; (5) a quality rating greater than 12 out of 18 possible points10,11; (6) sufficient information to allow adequate estimation of odds ratios (ORs) or standardized mean differences and 95% confidence intervals (CIs); and (7) a primary population of lay participants, rather than health care providers.
We combined all health-related behavior outcomes for our systematic review. Prominent theories about health behavior change state that it is not the particular behavior that is affected by an intervention but rather the process of behavior change.12–14 Although the outcomes of different peer-based interventions vary, the process of behavior change and the factors that facilitate that change are similar for each outcome. Therefore, it is reasonable to examine health-related behavior changes as a group.
We searched for clinical trials, applying no language or time restrictions, in MEDLINE, CINAHL, EMBASE, PSYCHInfo, and the Cochrane Library. We conducted our searches between August and November 2007; we found relevant articles published through October 1, 2007, as well as unpublished studies (none of the latter were included in our analysis). To search for a peer-based intervention, we entered the following keywords: “peer-based interventions,” “peer-led interventions,” “peer education,” “peers,” “peer support,” “peer counseling,” “group support,” “group education,” “peer leader,” and “opinion leader.” We used the following keywords to search for methods: “intervention,” “control trial,” “randomized control trial,” and “experiment.” We applied “adult” as a limit and conducted a general search with combinations of keywords for intervention and method.
Data Extraction and Analysis
We used standardized coding forms to abstract data from the published articles identified by our searches. Each study was blinded and coded for study, sample, and intervention characteristics. Two reviewers independently assessed inclusion criteria for the review and rated the quality of each study, according to the established criteria.10,11 We retrieved 909 abstracts and reviewed each (and, if necessary, the full study) for inclusion in our analysis. We determined that 27 articles met the inclusion criteria, but 2 did not provide the data necessary to calculate effect sizes. We contacted the corresponding authors to ask for this information, but they were unable to provide it, so we removed those articles from the final analysis,15,16 leaving 25 articles for review.
Several studies reported multiple behavior change outcomes with varying endpoints. When several outcomes were measured in 1 study, we selected the most relevant and clinically meaningful outcome that correlated with outcomes from other studies in the review. We used the outcome data from the final endpoint, when available, because it represented the most conservative estimate of the effect. We recorded dose information verbatim from the article. If this was not provided in the published article or was unclear, we contacted the author and asked for precise information on dose.
Effect Size Calculation
We calculated each study-specific effect size and, if appropriate, a group effect size. All the studies reported outcomes either as dichotomous, yielding ORs, or as continuous, yielding standardized mean difference scores.
For studies reporting dichotomous outcomes in ORs, we calculated the study effect and group effect (for studies grouped by outcome) sizes by applying the random effects of the DerSimonian and Laird analytic model.17–19 This model assumes that the findings from the individual studies are estimates of a true effect size and have some random error. We calculated the study effect sizes as ORs from the raw data provided in each article. An OR greater than 1 indicated that participants in the intervention group were more likely than participants in the control group to achieve a health-related behavior change. We calculated the summary effect sizes across different studies as the weighted mean of the individual ORs, with weights equal to the inverse of the study variance and the summary effect estimate.17–19
For studies reporting continuous outcomes as mean difference scores, we calculated the study effect sizes as standardized mean difference, again applying a random-effects, DerSimonian and Laird analytic model. We calculated this effect as the mean outcome between the 2 groups divided by the standard deviation of the outcome measure in the study. We calculated the summary effect size across the studies grouped by outcome as the weighted average of the study-specific effect sizes, with weights equal to the inverse of the estimated variance.17–19
We evaluated the homogeneity of the individual study effect sizes by calculating the Q statistic (the weighted average of the squared difference between the summary and study-specific effect sizes17) and comparing it with an appropriate χ2 distribution. We selected a significance value of .10 to determine whether heterogeneity was present.20 We evaluated homogeneity for each outcome subgroup and the overall grouped studies. We also analyzed subgroups by intervention model, intervention setting, sample size, and publication year. This analysis still yielded statistically heterogeneous effects and thus could not explain the heterogeneity in the overall effect size. All analyses were completed with Stata SE version 9.0.21 We assessed publication bias by inspection of a funnel plot of the standard error estimates versus effect size estimates from individual samples and by a linear regression test.22
RESULTS
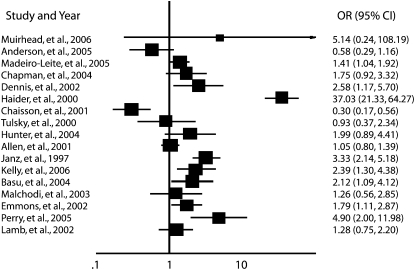
We retrieved 909 abstracts; 25 of these met our inclusion criteria. Although the studies documented 27 individual health-related behavior outcomes, the assumption of independence for statistical analysis required that each study be counted only once in the calculation. To address this, we grouped the studies by outcome. These outcomes were (1) an increase in breastfeeding, (2) an increase in physical activity, (3) an increase in medication adherence, (4) an increase in women's health preventative behaviors (cancer screenings), (5) an increase in self-care activities, (6) smoking cessation, and (7) an increase in condom use. We separated 23 of the outcomes into these groups with no repeated studies. Two studies measured outcomes not shared with any other study (completion of advance directives and a decrease in weekly drinking); these were analyzed separately.23,24 The sample sizes of the included articles ranged from 56 to 2757 participants; the total sample size for our analysis was approximately 8942 participants. The studies took place in 8 countries (Table 1).
TABLE 1.
Characteristics of 25 Randomized Clinical Trials of Peer-Based Health Behavior Change Interventions: 8 Countries, 1997–2007
| Outcome Measure and Date of Study | Sample Size, No. | Intervention Modela | Setting | Dose of Interventionb | Duration of Intervention | OR or Standardized Mean Difference | Country |
| Breastfeeding | |||||||
| 200625 | 225 | Dyad | Telephone call | Varied by pair | 1 mo; if requested, 4 mo | 5.14 | Scotland |
| 200526 | 135 | Dyad | Home and hospital | Average: 2.6 ±1.9 h/prenatal visit; average: 2.2 ±2.0 h/in-hospital visit | 6 wk | 0.58 | United States |
| 200527 | 859 | Dyad | Home | Average: 30–40 min/session | Maximium of 6 visits over 4 mo | 1.41* | Brazil |
| 200428 | 157 | Dyad | Home and hospital | 20 min/session | 1 mo | 1.75 | United States |
| 200229 | 258 | Dyad | Home | Mean: 16.2 min ±12.22 min/session | Mean 53.1 ±30.90 d | 2.58* | Canada |
| 200030 | 573 | Dyad | Home | 20–40 min/session | 15 sessions | 37.03* | Bangladesh |
| Physical activity | |||||||
| 200631 | 89 | Dyad | Telephone call | Average: 1 call/wk | 3 mo | −0.10 | United States |
| 200432 | 352 | Combination | Church | Varied by church | 9 mo | 0.56* | United States |
| 200333 | 725 | Group | … | 150 min/session | 6 wk | 0.16* | China |
| 200334 | 443 | Group | Outpatient clinics | 150 min/session | 6 wk | 0.21* | United States |
| 200235 | 250 | Combination | Telephone calls | Up to 3 calls | 12 mo | 1.28 | United Kingdom |
| Medication adherence | |||||||
| 200736 | 136 | Combination | Home and clinics | 12 h | 3 mo | −0.20 | United States |
| 200137 | 201 | Dyad | Clinic | 2 connections in first month; 1 connection/mo thereafter | Six mo | 0.30* | United States |
| 200038 | 73 | Dyad | … | Varied by pair | 6 mo | 0.93 | United States |
| Women's health cancer screening | |||||||
| 200439 | 101 | Dyad | Home | 1 home visit | … | 1.99 | United States |
| 200140 | 2757 | Combination | Workplace | 20 min/session | 16 mo | 1.05 | United States |
| 199741 | 460 | Dyad | Telephone call | Mean: 5.3 min/call | Once | 3.33* | United States |
| Smoking cessation | |||||||
| 200542 | 796 | Dyad | Home | ≤ 6 calls | 7 mo | 1.79* | United States |
| 20037 | 118 | Dyad | Home and telephone call | Mean: 45 ±15 min/session | Median: 6 contacts | 1.26 | United States |
| Condom use | |||||||
| 200643 | 275 | Dyad | Community | Mean: 6.8 min/session | 3 mo | 2.39* | Bulgaria |
| 200444 | 172 | Dyad | Workplace | 1 session/month | 16 mo | 2.12* | India |
| Participation in general activities | |||||||
| 200445 | 88 | Dyad | Telephone call | Varied by pair | 3 mo | −0.49 | United States |
| 200046 | 56 | Dyad | Hospital | 3 visits | 1 mo | 0.54 | United States |
| Completion of advance directives | |||||||
| 200523 | 203 | Dyad | Home and hospital | Varied by pair | 4 mo | 4.90* | United States |
| Weekly drinking | |||||||
| 200424 | 357 | Group | University health center | 2 h/session | 2 sessions | 0.35* | United States |
Note. OR = odds ratio. Ellipses indicate data was not specified in published study.
Dyad refers to a one-on-one peer counseling method. Group refers to group peer education sessions. Combination is blend of both types.
All information on dose was recorded verbatim from the article. If it was not provided in the published article or was unclear, the author was contacted and asked to provide precise information.
P < .05.
Intervention Models and Doses
Our review identified 3 common models of peer-based interventions in health care. The group-based peer education intervention used peers as group leaders to guide people with a related health care concern or similar demographics to adopt a new behavior that would facilitate healthy outcomes. This model was often used in research about increasing physical activity and decreasing weekly drinking.24,33–34 The second, more popular model used peers as buddies for individuals, who were matched for the health care concern of interest and demographics (dyads). In this model, peers provided one-on-one advice and support about how to achieve a particular health care goal. This model was also used in interventions aiming to increase breastfeeding, medication adherence, and mammography screening and to decrease smoking.29,37,41,42
Some studies adopted a combination of the group and dyad models. Allen et al. held workplace events led by lay advisers to increase mammogram rates. The lay advisers then called the women who were unable to attend the larger events.40 Table 1 has additional information on the model types used in the interventions in our analysis.
The length of peer–participant sessions varied from 5.3 minutes41 to 150 minutes.33,34 Two studies reported only 1 contact between the peer leader and the participants.39,41 In 1 study, the peer adviser spent varying amounts of time with the participants.38 The maximum number of contacts by the peer leader was 48.38 More complete information on the dose and duration of the interventions can be found in Table 1.
Outcomes
Breastfeeding.
Six studies used a dyad peer-based intervention to increase breastfeeding among new mothers. The ORs for these studies ranged from 0.58326 to 37.0330; 5 reported positive results, of which 3 were statistically significant. The overall effect size for this outcome was 2.857 (95% CI = 0.769, 10.61). The heterogeneity χ2 statistic for this outcome was 126.84 (df = 5; P < .001), indicating significant heterogeneity among these studies.
Physical activity.
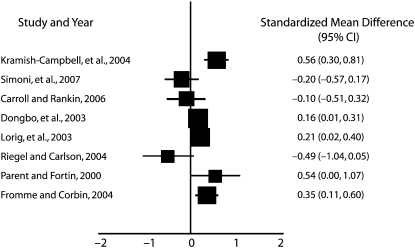
Five studies used a combination of all 3 types of peer-based interventions to increase physical activity in adults. These interventions were employed in a variety of settings, and the results were mixed. Three studies reported their outcomes as mean differences between the intervention and control groups, and these scores ranged from −0.095131 to 0.208.34 The overall effect size for this outcome was 0.1578 (95% CI = 0.047, 0.269). The heterogeneity statistic for this analysis was 1.699 (df = 3; P = .57), indicating no heterogeneity among the 3 studies.
Medication adherence.
Three studies used a combination of peer-based interventions to increase medication adherence. Two studies reported ORs that included 0.305 (a statistically significant difference)37 and 0.926.38 In addition, Simoni et al. reported a mean difference of −0.1972 between the intervention and control groups,36 indicating that the control group had better medication adherence than the peer-based intervention group. The overall effect size for this outcome was 0.502 (95% CI = 0.17, 1.48). The heterogeneity χ2 statistic for this outcome was 3.88 (df = 1; P = .049), indicating significant heterogeneity between these 2 studies.
Women's health cancer screening.
Three articles reported women's health outcomes, including an increase in mammography screening40,41 and an increase in gynecological cancer screening.39 These studies reported their outcomes in ORs ranging from 1.05 to 3.33, all of which indicated an increase in women's health activities in the peer-based intervention groups. The overall effect size for this outcome was 1.88 (95% CI = 0.82, 4.30). The heterogeneity χ2 statistic for this outcome was 19.25 (df = 2; P < 001), indicating significant heterogeneity among these 3 studies.
Smoking cessation.
Two studies used dyad peer-based interventions to promote smoking cessation. Their reported ORs were 1.267 and 1.79,42 indicating that the intervention group had higher levels of smoking cessation than did the control group. The overall effect size for this outcome was 1.64 (95% CI = 1.09, 2.46). The heterogeneity χ2 statistic for this outcome was 0.470 (df = 1; P = .470), indicating no heterogeneity between these studies.
Participation in general activities.
Two studies examined whether peer-based interventions could increase general activities in participants with chronic disease. They both used dyad interventions and reported the mean differences between the groups as 0.5381 and −0.476. These opposing scores yielded an overall effect size of 0.3043 (95% CI = −0.339, 0.424), indicating that the intervention did not have a significant effect on whether the participants from either the control or intervention groups engaged in more general activities in the course of the study. The heterogeneity statistic for this analysis was 6.788 (df = 3; P = .991), indicating no heterogeneity between these 2 studies.
Condom use.
Two studies used dyad peer-based interventions to increase condom use and reported ORs of 2.1244 and 2.39.43 Both articles reported statistically significant positive outcomes, indicating that participants in the peer-based intervention reported more condom use than did those in the control group. The overall effect size for condom use was 2.266 (95% CI = 1.145, 3.54). The heterogeneity statistic for this outcome was 0.07 (df = 1; P = .797), indicating no heterogeneity between these studies.
Other outcomes.
Two studies assessed behavior change outcomes not shared with any other study in our analysis. Perry et al. employed a dyad peer-based intervention to increase completion of advance directives among dialysis patients.23 They reported a statistically significant OR of 4.89 (95% CI = 2.00, 11.98), indicating that those who participated in the intervention were more likely than members of the control group to complete advance directives.
Fromme and Corbin used a group peer-based intervention to decrease alcohol intake among university students.24 They reported mean differences between the intervention and control groups, and the standardized mean difference was 0.3521 (95% CI = 0.11, 0.59), indicating that participants exposed to the intervention, but not members of the control group, significantly, though moderately, changed their drinking behavior. More information on reported effect sizes can be found in Figures 1 and 2.
FIGURE 1.
Effect size in studies reporting a dichotomous outcome: 8 countries, 1997–2007.
Note. CI = confidence interval; OR = odds ratio.
FIGURE 2.
Effect size in studies reporting a continuous outcome: 8 countries, 1997–2007.
Note. CI = confidence interval.
Summary effect size.
After testing the data for heterogeneity and publication bias, we decided not to report the overall group effect size for all of the studies because we found significant heterogeneity. The χ2 statistic for the articles with dichotomous outcomes was 214.65 (df = 16; P < .005). The Q statistic was 23.26 (df = 7; P < .001) for the articles reporting continuous outcomes. Our subgroup analyses by intervention model, intervention setting, sample size, and publication year still yielded statistically heterogeneous effects and similar effect sizes and thus could not explain the heterogeneity in the overall effect size.
DISCUSSION
Use of peer-based interventions to facilitate health-related behavior changes in adults is increasing around the world, but the effectiveness of this type of intervention has not yet been systematically evaluated. Our systematic review is the first to examine the effect of peer-based interventions on health-related behavior outcomes in adults.
We analyzed 25 different studies to assess the effectiveness of peer-based interventions in promoting health-related behavior changes in adults. We identified 3 distinct models of peer-based interventions: dyads, groups, and a combination of both. Subgroup analyses by intervention model did not reveal significant differences in outcomes. The majority (72%) of studies employed the dyad model, perhaps because it is the simplest to implement. This model allows the peers to individualize the intervention to meet participants' needs. It allows for flexibility with participants' schedules and logistical concerns. It can also facilitate a more personal bond between a participant and peer leader, increase the peer's legitimacy with the participant, and increase the participant's willingness to sustain the proposed behavior. Nevertheless, it is difficult to analytically control for these factors, and the wide variation in results reported by studies of this type of intervention limited our analysis of the model's effectiveness.
Analysis of outcome groups showed that the studies in 3 of the 7 groups had significant positive findings (increasing physical activity, decreasing smoking, and increasing condom use). One study reported significantly increasing advance directive completion. These are desirable health outcomes that will positively affect the health of individual participants, their families, and the public health care system. The success of peer-based interventions in producing these outcomes suggests that they may be effective for other outcomes.
However, the evidence is mixed. For the remaining 4 outcomes (breastfeeding, medication adherence, women's health, and participation in general activities), the studies we analyzed did not report a significant difference between participants exposed to the peer-based intervention and members of the control group. This may be related to the variability among interventions that we grouped together because of their similar outcomes of interest; for example, the training and experience level of the peers may have varied, which could lead to varied results. The dose of the interventions also varied in the studies we grouped together. Among the breastfeeding intervention studies, for example, the peers spent from 16 minutes to more than 2 hours with the participants. This variability in dose may have led to the heterogeneity among the studies.
Our systematic review had several strengths, including the use of explicit eligibility criteria, reviewers who independently assessed eligibility, and a random-effects analytic model. We included only randomized clinical trials to help eliminate confounding from other variables. Yet, even when studies used similar intervention models and outcomes, we found little standardization. We used subgroup analyses to explore the effect of the varied intervention models, follow-up times, settings, training of peer leaders, and doses of intervention among the different studies but did not detect any variables that could explain the differences in outcomes.
Limitations
The main limitation of our review was our use of study quality as an exclusion criterion. To address concerns about study rigor, 2 reviewers assessed quality with a standardized instrument. Quality-rating scores are controversial, but only 18 of 909 studies were eliminated from the analysis because of low quality scores.47 We used this score to limit our analysis to more generalizable studies. For example, 1 study that was excluded had a sample size of 8. Other excluded studies did not explain how the investigators collected their data or which instruments they used.
Another limitation was the heterogeneity of some outcomes. Health behavior change theories contend that it is appropriate to combine the varied behavior change outcomes, but the statistical assumptions of systematic reviews suggest otherwise. We found significant heterogeneity in our overall analysis and among some of our grouped outcomes. Although it is possible that much of this heterogeneity is related to the clinical diversity of the studies, we did not have enough evidence to confirm this, which limited our ability to answer our primary research question: are peer-based interventions effective in changing health-related behaviors in adults? Subgroup analyses did not reveal the source of the heterogeneity but did eliminate variables such as intervention model, intervention setting, sample size, and publication year. However, future research might alleviate this problem by using a commonly agreed-upon definition of peer-based intervention and a common evaluation protocol, including follow-up time.
Most of the outcomes were ascertained by self-report. The nature of behavior change renders it difficult to efficiently assess by other methods, but self-reporting should be complemented with a direct measure of behavior change whenever possible. Complementary outcomes in various studies we analyzed could have included, for example, pediatric disease rates, changes in weight, HIV viral control, or cancer rates, but few reported such outcomes. The low prevalence of such outcomes may make adequately powered sample sizes difficult to assemble, but future research would be enhanced by inclusion of such clinical outcomes or an accepted proxy in their protocols.
We were unable to assess the effect of the dose of intervention on behavior outcomes because each study reported dose differently, in ways that could not readily be compared (e.g., some reported the number of sessions but not total time spent). A great strength of peer-based interventions is their flexibility, which can be applied to real-world problems, but the variability in both dose and how it was reported precluded quantitative statistical analysis of the studies we reviewed. We recommend that future studies report dose in standardized units of time so that the effects of dose can be determined.
Conclusions
Health-related behavior changes are increasingly important because many health conditions are becoming more chronic and less susceptible to biomedical interventions. It is therefore important to learn more about how large numbers of people can be induced to modify their activity level and decrease unhealthy behaviors.
Our systematic review showed that peer-based interventions facilitated positive outcomes, but evidence was mixed. The studies reported significant effects on physical activity, smoking, and condom use but no significant effects on breastfeeding, medication adherence, women's health, or participation in general activities. Evaluation of the effectiveness of peer-based interventions would be facilitated by future research conducted with rigorous methods, including the quantification of dose in time.
Acknowledgments
This project was funded by the National Institutes of Health (training grants 1T32RR023259 and 1F31NR009910).
We acknowledge Gloria Won at the Fishbon Memorial library for her assistance in developing the search strategy and Chris Longenecker for his editorial assistance.
Human Participant Protection
No protocol approval was required because the data were obtained from secondary sources.
References
- 1.Doull M, O'Connor AM, Wells GA, Tugwell P, Welch V. Peer-based interventions for reducing morbidity and mortality in HIV-infected women (protocol). Cochrane Database Syst Rev 2004;2:CD004774 [Google Scholar]
- 2.Posavac EJ, Kattapong KR, Dew DE. Peer-based interventions to influence health-related behaviors and attitudes: a meta-analysis. Psychol Rep 1999;85(3, pt 2):1179–1194 [DOI] [PubMed] [Google Scholar]
- 3.Szilagyi T. Peer education of tobacco issues in Hungarian communities of Roma and socially disadvantaged children. Cent Eur J Public Health 2002;10(3):117–120 [PubMed] [Google Scholar]
- 4.Turner G, Shepherd J. A method in search of a theory: peer education and health promotion. Health Educ Res 1999;14(2):235–247 [DOI] [PubMed] [Google Scholar]
- 5.Campbell C, MacPhail C. Peer education, gender and the development of critical consciousness: participatory HIV prevention by South African youth. Soc Sci Med 2002;55(2):331–345 [DOI] [PubMed] [Google Scholar]
- 6.Sallis JF, Calfas KJ, Nichols JF, et al. Evaluation of a university course to promote physical activity: project GRAD. Res Q Exerc Sport 1999;70(1):1–10 [DOI] [PubMed] [Google Scholar]
- 7.Malchodi CS, Oncken C, Dornelas EA, Caramanica L, Gregonis E, Curry SL. The effects of peer counseling on smoking cessation and reduction. Obstet Gynecol 2003;101(3):504–510 [DOI] [PubMed] [Google Scholar]
- 8.Navarro AM, Senn KL, McNicholas LJ, Kaplan RM, Roppé B, Campo MC. Por La Vida model intervention enhances use of cancer screening tests among Latinas. Am J Prev Med 1998;15(1):32–41 [DOI] [PubMed] [Google Scholar]
- 9.The Cochrane Collaboration An introduction to Cochrane reviews and the Cochrane Library. Available at: http://www.cochrane.org/reviews/clibintro.htm. Accessed June 9, 2008
- 10.Wantland DJ, Portillo CJ, Holzemer WL, Slaughter R, McGhee EM. The effectiveness of Web-based vs. non-Web-based interventions: a meta-analysis of behavioral change outcomes. J Med Internet Res 2004;6(4):e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352(9128):609–613 [DOI] [PubMed] [Google Scholar]
- 12.Bandura A. Social Foundations of Thought and Action Upper Saddle River, NJ: Prentice Hall; 1986 [Google Scholar]
- 13.Bandura A. Health promotion by social cognitive means. Health Educ Behav 2004;31(2):143–164 [DOI] [PubMed] [Google Scholar]
- 14.Champion VL, Skinner CJ. The health belief model. Glanz K, Rimer BK, Lewis, Health Behavior and Health Education San Francisco, CA: Jossey-Bass; 2002:46–66 [Google Scholar]
- 15.Auslander W, Haire-Joshu D, Houston C, Rhee CW, Williams JH. A controlled evaluation of staging dietary patterns to reduce the risk of diabetes in African-American women. Diabetes Care 2002;25(5):809–814 [DOI] [PubMed] [Google Scholar]
- 16.Merewood A, Chamberlain LB, Cook JT, Philipp BL, Malone K, Bauchner H. The effect of peer counselors on breastfeeding rates in the neonatal intensive care unit: results of a randomized controlled trial. Arch Pediatr Adolesc Med 2006;160(7):681–685 [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Smith GD, Altman DG. Systematic Reviews in Health Care: Meta-Analysis in Context 2nd ed.London, UK: British Medical Journal Publishing Group; 2001 [Google Scholar]
- 18.Mosteller F, Colditz GA. Understanding research synthesis (meta-analysis). Annu Rev Public Health 1996;17(1):1–23 [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7(3):177–188 [DOI] [PubMed] [Google Scholar]
- 20.The Cochrane Collaboration Diversity and heterogeneity. Available at: http://www.cochrane-net.org/openlearning/HTML/mod13-3.htm. Accessed May 3, 2008
- 21.StataCorp Stata Statistical Software [computer program]. Version 9. College Station, TX: StataCorp LP; 2005 [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry E, Swartz J, Brown S, Smith D, Kelly G, Swartz R. Peer mentoring: a culturally sensitive approach to end-of-life planning for long-term dialysis patients. Am J Kidney Dis 2005;46(1):111–119 [DOI] [PubMed] [Google Scholar]
- 24.Fromme K, Corbin W. Prevention of heavy drinking and associated negative consequences among mandated and voluntary college students. J Consult Clin Psychol 2004;72(6):1038–1049 [DOI] [PubMed] [Google Scholar]
- 25.Muirhead PE, Butcher G, Rankin J, Munley A. The effect of a programme of organised and supervised peer support on the initiation and duration of breastfeeding: a randomised trial. Br J Gen Pract 2006;56(524):191–197 [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson AK, Damio G, Young S, Chapman DJ, Perez-Escamilla R. A randomized trial assessing the efficacy of peer counseling on exclusive breastfeeding in a predominantly Latina low-income community. Arch Pediatr Adolesc Med 2005;159(9):836–841 [DOI] [PubMed] [Google Scholar]
- 27.Madeiro Leite AJ, Fiorini Puccini R, Atalah AN, Alves Da Cunha AL, Tavares Machado M. Effectiveness of home-based peer counselling to promote breastfeeding in the northeast of Brazil: a randomized clinical trial. Acta Paediatr 2005;94(6):741–746 [DOI] [PubMed] [Google Scholar]
- 28.Chapman DJ, Damio G, Young S, Perez-Escamilla R. Effectiveness of breastfeeding peer counseling in a low-income, predominantly Latina population: a randomized controlled trial. Arch Pediatr Adolesc Med 2004;158(9):897–902 [DOI] [PubMed] [Google Scholar]
- 29.Dennis CL. Breastfeeding peer support: maternal and volunteer perceptions from a randomized controlled trial. Birth 2002;29(3):169–176 [DOI] [PubMed] [Google Scholar]
- 30.Haider R, Ashworth A, Kabir I, Huttly SR. Effect of community-based peer counsellors on exclusive breastfeeding practices in Dhaka, Bangladesh: a randomised controlled trial. Lancet 2000;356(9242):1643–1647 [DOI] [PubMed] [Google Scholar]
- 31.Carroll DL, Rankin SH. Comparing interventions in older unpartnered adults after myocardial infarction. Eur J Cardiovasc Nurs 2006;5(1):83–89 [DOI] [PubMed] [Google Scholar]
- 32.Kramish Campbell M, James A, Hudson MA, et al. Improving multiple behaviors for colorectal cancer prevention among African American church members. Health Psychol 2004;23(5):492–502 [DOI] [PubMed] [Google Scholar]
- 33.Fu D, Fu H, McGowan P, et al. Implementation and quantitative evaluation of chronic disease self-management programme in Shanghai, China: randomized controlled trial. Bull World Health Organ 2003;81(3):174–182 [PMC free article] [PubMed] [Google Scholar]
- 34.Lorig KR, Ritter PL, Gonzalez VM. Hispanic chronic disease self-management: a randomized community-based outcome trial. Nurs Res 2003;52(6):361–369 [DOI] [PubMed] [Google Scholar]
- 35.Lamb SE, Bartlett HP, Ashley A, Bird W. Can lay-led walking programmes increase physical activity in middle aged adults? A randomised controlled trial. J Epidemiol Comm Health 2002;56(4):246–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simoni JM, Pantalone DW, Plummer MD, Huang B. A randomized controlled trial of a peer support intervention targeting antiretroviral medication adherence and depressive symptomatology in HIV-positive men and women. Health Psychol 2007;26(4):488–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaisson RE, Barnes GL, Hackman J, et al. A randomized, controlled trial of interventions to improve adherence to isoniazid therapy to prevent tuberculosis in injection drug users. Am J Med 2001;110(8):610–615 [DOI] [PubMed] [Google Scholar]
- 38.Tulsky JP, Pilote L, Hahn JA, et al. Adherence to isoniazid prophylaxis in the homeless: a randomized controlled trial. Arch Intern Med 2000;160(5):697–702 [DOI] [PubMed] [Google Scholar]
- 39.Hunter JB, de Zapien JG, Papenfuss M, Fernandez ML, Meister J, Giuliano AR. The impact of a promotora on increasing routine chronic disease prevention among women aged 40 and older at the U.S.–Mexico border. Health Educ Behav 2004;31(Suppl 4):18S–28S [DOI] [PubMed] [Google Scholar]
- 40.Allen JD, Stoddard AM, Mays J, Sorensen G. Promoting breast and cervical cancer screening at the workplace: results from the Woman to Woman Study. Am J Public Health 2001;91(4):584–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janz NK, Schottenfeld D, Doerr KM, et al. A two-step intervention of increase mammography among women aged 65 and older. Am J Public Health 1997;87(10):1683–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emmons KM, Puleo E, Park E, et al. Peer-delivered smoking counseling for childhood cancer survivors increases rate of cessation: the partnership for health study. J Clin Oncol 2005;23(27):6516–6523 [DOI] [PubMed] [Google Scholar]
- 43.Kelly JA, Amirkhanian YA, Kabakchieva E, et al. Prevention of HIV and sexually transmitted diseases in high risk social networks of young Roma (Gypsy) men in Bulgaria: randomised controlled trial. BMJ 2006;333(7578):1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basu I, Jana S, Rotheram-Borus MJ, et al. HIV prevention among sex workers in India. J Acquir Immune Defic Syndr 2004;36(3):845–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riegel B, Carlson B. Is individual peer support a promising intervention for persons with heart failure? J Cardiovasc Nurs 2004;19(3):174–183 [DOI] [PubMed] [Google Scholar]
- 46.Parent N, Fortin F. A randomized, controlled trial of vicarious experience through peer support for male first-time cardiac surgery patients: impact on anxiety, self-efficacy expectation, and self-reported activity. Heart Lung 2000;29(6):389–400 [DOI] [PubMed] [Google Scholar]
- 47.Balk EM, Bonis PA, Moskowitz H, et al. Correlation of quality measures with estimates of treatment effect in meta-analyses of randomized controlled trials. JAMA 2002;287(22):2973–2982 [DOI] [PubMed] [Google Scholar]