Abstract
Background
The clinical course of chronic pancreatitis is still unpredictable, which relates to the lack of the availability of a clinical classification. Therefore, patient populations cannot be compared, the course and the outcome of the disease remain undetermined in the individual patient, and treatment is not standardized.
Aim
To establish a clinical classification for chronic pancreatitis which is user friendly, transparent, relevant, prognosis- as well as treatment-related and offers a frame for future disease evaluation.
Methods
Diagnostic requirements will include one clinical criterion, in combination with well defined imaging or functional abnormalities.
Results
A classification system consisting of three stages (A, B and C) is presented, which fulfils the above-mentioned criteria. Clinical criteria are: pain, recurrent attacks of pancreatitis, complications of chronic pancreatitis (e.g. bile duct stenosis), steatorrhea, and diabetes mellitus. Imaging criteria consist of ductal or parenchymal changes observed by ultrasonography, ERCP, CT, MRI, and/or endosonography.
Conclusion
A new classification of chronic pancreatitis, based on combination of clinical signs, morphology and function, is presented. It is easy to handle and an instrument to study and to compare the natural course, the prognosis and treatment of patients with chronic pancreatitis.
Introduction
Chronic pancreatitis is a heterogeneous disorder with a clinical spectrum that encompasses pain, loss of exocrine pancreatic function, diabetes mellitus and various complications usually involving organs adjacent to the pancreas[1,2]). The disease may present clinically either with an individual symptom or a combination of symptoms associated with loss of pancreatic function. The single most frequent symptom of chronic pancreatitis is pain, either in the form of intermittent episodes or in a more chronic or persistent pattern[3-5].
The natural history of chronic pancreatitis is usually characterized by progression of tissue damage and various degrees of exocrine and endocrine pancreatic insufficiency, which will become apparent over time[4,6,7]. Various well-defined complications may occur at any stage during the course of the disease. However, independent of the underlying etiology, chronic pancreatitis evolves toward the same end stage, i.e., pancreatic fibrosis[8,9].
It is general knowledge that the terminal stage of chronic pancreatitis results in a loss of organ function, with the clinical manifestations of maldigestion and diabetes mellitus[10]. However, there is little knowledge of the early structural and functional abnormalities because the current diagnostic imaging procedures are not sensitive enough to visualize them and histology is normally not available in these early stages[11-13].
The presentation of very distinct clinical symptoms and the frequent complexity of their associations require a well-differentiated therapeutic approach. Current therapies in the management of chronic pancreatitis include conservative measures (analgesics, anti-inflammatory agents, enzyme replacement), endoscopic interventions as well as surgical procedures[14-18]. However, there are no guidelines for the treatment of various alterations of chronic pancreatitis, and therefore therapy is generally individualized, depending on the personal experience of the treating clinician and local logistics. Evidence-based recommendations for the treatment of chronic pancreatitis are currently lacking, but need to be implemented in the future to address the key question of whether the natural course of chronic pancreatitis can be positively influenced by different interventions, including surgery.
There is considerable controversy, for example, over the management of pain in chronic pancreatitis, with some clinicians preferring long-term conservative management, others advocating interventional endoscopies, and still others making a plea for surgical procedures[6,14,19,20]. More than half a century after the first and classical description of chronic pancreatitis by Comfort and coworkers, we are still searching for an instrument which will allow us to test and compare different therapeutic options for this disease[21].
Attempts to classify chronic pancreatitis
The main reason for the lack of guided strategies in the therapeutic management of chronic pancreatitis is the absence of a clinically applicable classification of chronic pancreatitis. In the past, several classifications have certainly contributed to a better understanding of the pathogenesis and pathophysiology of chronic pancreatitis. The meetings in Marseilles 1963 and 1984 and in Rome 1985 added a great deal of information to our knowledge of the pathogenesis and evolution of chronic pancreatitis[22-24]. However, other than the designation of chronic alcoholic pancreatitis as a special entity of chronic obstructive pancreatitis of various etiologies, no further attempt to design a clinically relevant system of staging was undertaken[23]. In another meeting, in Cambridge 1984, the participants focused on using the emerging imaging procedure of that time, ERCP, to stage the changes in chronic pancreatitis, but again there was no staging of the clinical manifestations and no attempt was made to correlate clinical aspects with imaging[25,26].
In recent years it has become quite evident that clinical decisions cannot be based on the type and the degree of morphological abnormalities, but need to be based on clinical findings (pain, complications, pseudocysts, etc.) in combination with findings in the functional, diagnostic and imaging procedures[27].
The concept of selecting an endoscopic or a surgical procedure based primarily on the type of morphological complications has contributed significantly to the current controversy over management of pain in chronic pancreatitis[28,29].
Amman et al., organizing a symposium 1996, attempted to introduce clinical aspects into a classification of chronic pancreatitis, with special consideration of acute and chronic alcoholic pancreatitis[30]. The main result of that meeting was a better insight into different evolutional patterns of alcoholic pancreatitis, but again it was limited to alcoholic etiology and did not attempt to classify the disease in different clinical stages. Ramesh published 2002 a classification which was based on a ABC Classification system too, but included too many and redundant sub-classification parameters and therefore the classification did not gained widespread use[31]. The Manchester classification which was presented in 2006, used the ABC grading system as well but had difficulties in resembling the natural course of chronic pancreatitis and therefore needs to be further evaluated[32]. The M-ANNHEIM classification presented by Schneider et al. in 2007 tried to describe chronic pancreatitis very detailed and therefore it might be difficult to use in clinical practice[33].
The need for a new clinical classification of chronic pancreatitis
In order to combine clinical experience in the field of chronic pancreatitis with progress in diagnostic methods and new molecular technologies for the assessment of chronic pancreatitis, a classification of chronic pancreatitis based on key clinical aspects is crucial[34-36]. This classification would not only allow us to develop a common language for the description of this disease, but also to study the dynamics of disease evolution and to compare the role of different etiologies in the appearance of chronic pancreatitis.
Such a classification needs to be applicable and transparent in all parts of the world, and would allow for comparison of experiences and serve as a basis for disease staging in clinical trials in chronic pancreatitis. A new classification should first be validated to determine whether it is suitable to be applied to the majority of patients with chronic pancreatitis, and then the value of such a classification needs to be tested in our understanding of the natural course in different etiologies (progression, arrest, regression) and most importantly, to study the clinical outcome when different therapeutic strategies are applied.
In suggesting a new classification of CP, we propose a definition of chronic pancreatitis and disease staging based on key clinical features in association with various findings obtained in current imaging techniques. Our concept for a new classification will include a rationale adopted from a liver-pancreas analogy, and will take into account established knowledge as well as the differing treatment approaches commonly used today.
Liver-pancreas analogy in fibrogenesis
From a standpoint of morphology/histology, chronic pancreatitis evolves in a similar manner as chronic hepatitis, i.e. liver fibrosis/liver cirrhosis. In both diseases, independent of the etiology, the chronic inflammatory process results in a final morphologic picture of fibrosis/cirrhosis[37,38]. Fibrosis/cirrhosis leads to increasing functional impairment over time and finally to complete functional loss in both organs. Also, the progression from fibrosis/cirrhosis to cancer has been described in both conditions[39,40]. However, the clinical picture differs inasmuch as chronic pancreatitis is dominated by pain in addition to exocrine and endocrine functional loss.
As a hypothesis we suggest that chronic pancreatitis begins with episodes of acute inflammation with or without clinical appearance, analagous to the acute, often also clinically in apparent evolution of hepatitis from an acute into a chronic disease state (i.e., hepatitis C).
Recently, almost identical mechanisms have been described in the pathogenetic evolution of pancreatic and hepatic fibrogenesis, with a central role assigned to stellate cells for remodeling and repair of the inflammatory damage[41-44]. For staging of chronic liver disease, i.e., liver cirrhosis, the Child-Pugh classification [30], based on functional impairment and complications, has received world-wide validation and acceptance[45]. However, such a grading system has not yet been proposed in chronic pancreatitis and/or end-stage pancreatic fibrosis.
Facts and state-of-the-art knowledge in chronic pancreatitis
1) Different etiologies lead to chronic pancreatitis with the end result of pancreatic fibrosis.
2) There are no serologic or blood markers available to diagnose/stage (grade) the disease.
3) Pathognomonic lesions of the ductular system and parenchyma are detectable by imaging.
4) Loss of exocrine and endocrine function develops during progression of the disease.
5) The end stage is characterized by steatorrhea and insulin-dependent diabetes mellitus.
6) Several characteristic complications of chronic pancreatitis are known such as common bile duct, duodenal, main pancreatic duct and vascular obstruction/stenosis.
7) Chronic pancreatitis represents a risk factor for pancreatic cancer.
8) Overall life expectancy is reduced.
Unresolved issues in chronic pancreatitis
1) The relationship between acute pancreatitis and chronic pancreatitis is not completely defined.
2) The issues whether in chronic pancreatitis disease progression, arrest and regression of functional and morphologic findings occur is debated.
3) Diagnosis of early chronic pancreatitis by imaging is not established.
4) The role and validity of exocrine pancreatic function tests in the diagnosis is not established.
5) The pathogenesis of pain is at least multifactorial and not defined.
6) The burn-out hypothesis is still debated and not defined with regard to time evolution in different etiologies.
7) There is disagreement over whether to use enzyme treatment to influence pain.
8) The role of endoscopic intervention is not defined under evidence-based criteria.
9) The role of surgery is not defined under evidence-based criteria.
Proposal for a clinically-based classification of chronic pancreatitis
Definition of chronic pancreatitis (diagnosis)
For the diagnosis of chronic pancreatitis we require at least one clinical criterion (Table 1) such as pain, (recurrent) attacks of acute pancreatitis, steatorrhea, diabetes mellitus or well-defined complications (Table 2) of chronic pancreatitis. These clinical criteria must be accompanied by well-defined abnormalities in imaging findings (Table 3) or in a direct pancreatic function test.
Table 1.
Clinical criteria
| Clinical Criteria |
|---|
| ▪pain |
| ▪attacks of acute pancreatitis |
| ▪complications of CP (see Table 2) |
| ▪steatorrhea |
| ▪diabetis mellitus |
Table 2.
Definition of complications
| Definition of complications |
|---|
| bile duct obstruction/stenosis with cholestasis or jaundice |
| ▪duodenal obstruction/stenosis with clinical signs |
| ▪vascular obstruction/stenosis with clinical or morphological signs of portal/splenic vein hypertension |
| ▪pancreatic pseudocysts with clinical signs (compression of adjacent organs, infection, bleeding, etc.) |
| ▪pancreatic fistula (internal or external) |
| ▪pancreatogenic ascites |
| ▪other rare complications related to organs in vicinity (i.e., colonic stenosis, splenic pseudocyst, etc.) |
Table 3.
Imaging criteria for chronic pancreatitis
| Imaging criteria for chronic pancreatitis | |
|---|---|
| Ductal changes: | Irregularity of the main pancreatic duct or side branches ± intraductal filling defects, calculi, duct obstruction (stricture), duct dilatation (>3 mm) |
| Parenchymal changes: | General or focal enlargement of the gland, cysts, calcifications, heterogenous reflectivity. |
The etiology of chronic pancreatitis needs to be specified according to Table 4.
Table 4.
Etiology of chronic pancreatitis
| Etiology of chronic pancreatitis |
|---|
| ▪alcohol |
| ▪idiopathic (unknown origin) |
| ▪hereditary |
| ▪autoimmune or in combination with specific diseases (Crohn's, PBC) |
| ▪tropical |
| ▪cystic fibrosis |
| ▪obstructive (pancreatic duct) |
| ▪drugs |
Staging/classification of chronic pancreatitis (Stages A, B, C)
Specific definition of chronic pancreatitis stage A
Stage A is the early stage of chronic pancreatitis where complications have not yet appeared and the clinical exocrine and endocrine function is preserved. Subclinical signs (impaired glucose tolerance, reduced exocrine function but without steatorrhea) might already be apparent.
Stage A is accepted under the following conditions:
Pain of any type and degree and/or attacks of acute pancreatitis, no complications (Table 2), no steatorrhea, no insulin-dependent diabetes mellitus.
Specific definition of chronic pancreatitis stage B
Stage B is the intermediate stage where chronic pancreatitis has led to complications but clinical exocrine and endocrine function is still preserved.
The type of complication is specified (e.g., stage B, bile duct)
Stage B is accepted under the following conditions:
Patients with complications (Table 2) but without steatorrhea or diabetes mellitus.
Specific definition of chronic pancreatitis stage C
Stage C is the end stage of chronic pancreatitis, where pancreatic fibrosis has led to clinical exocrine and/or endocrine pancreatic function loss (steatorrhea and/or diabetes mellitus). Complications of chronic pancreatitis might or might not be present.
The type of exocrine and/or endocrine pancreatic function loss is specified (e.g., stage C, steatorrhea).
Stage C can be sub classified into three categories:
C1: Patients with endocrine function impairment
C2: Patients with exocrine function impairment
C3: Patients with exocrine/endocrine function impairment and/or complications as they are defined in table 2.
Stage C is accepted under the following conditions:
Patients with clinical manifestation of end-stage functional impairment with or without complications.
Classification arrangement
The diagnosis of chronic pancreatitis is defined by the requested criteria (Table 1 + 3 ). The diagnosis is supplemented by the etiology (Table 4). This determination is then supplemented by the staging, potential complications (e.g., bile duct) and potential function loss (e.g., steatorrhea).
For example:
chronic pancreatitis (alcohol), stage A
chronic pancreatitis (ideopathic), stage B, bile duct
chronic pancreatitis (tropical), stage C
Clinical evaluation of the classification
To get a first impression of the clinical practicability of the new classification we have evaluated 191 patients who were operated at the Department of General Surgery, University of Heidelberg and followed up for a period up to 3 years. The study was performed in line with the guidelines of the Declaration of Helsinki, and each patient was asked to give written informed consent for data collection as well as for publication of data in an anonymous manner.
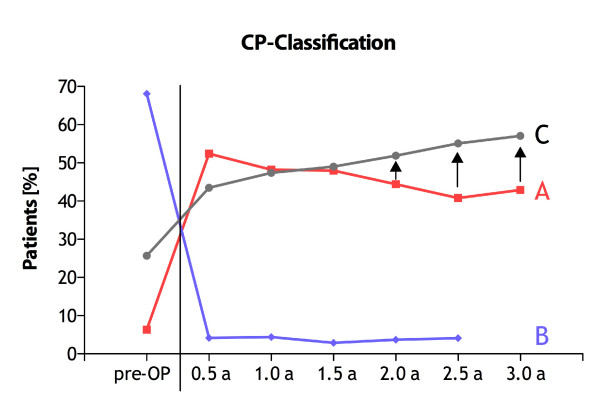
The median age of the patients was 49 years (40a/58a) (25th/75th quarter). The demographic details of the patients are summarized in table 5. The type of operations are given in table 6. Patients were followed-up and restaged according to the new classification every 6 months. The classification could be applied to all patients and was easy to use. The majority of the patients were operated because of a complication (Grade B) of chronic pancreatitis (68.1%), whereas 25.7% of the patients had pancreatic insufficiency (Grade C). Interestingly, only 12 patients (6.3%) had as the only indication for operation pain, which could not be handled by medical therapy (Grade A). This finding is supported by a recent study which demonstrated a comparable incidence of complications leading to surgery[46]. A quite stable rate of 3-4.5% of the patients developed additional complications within the 6 months follow-up intervals. With a longer duration of the disease more patients developed a pancreatic insufficiency - 25.7% preoperative to 57.1% after 3a. (Table 7; Figure 1)
Table 5.
Patients with chronic pancreatitis who were operated at the Department of General Surgery, University of Heidelberg
| N = 191 | N | [%] |
| Gender | ||
| male | 135 | 70.7 |
| female | 56 | 29.3 |
| Etiology | ||
| alcohol | 55 | 33.7 |
| idiopathic | 48 | 29.4 |
| biliary | 16 | 9.8 |
| other | 44 | 27.0 |
| Mortality | ||
| alive | 184 | 96.3 |
| mortality (other disease) | 5 | 2.6 |
| mortality (reason CP) | 2 | 1.0 |
Table 6.
Indication and type of operation of patients with chronic pancreatitis who were operated at the Department of General Surgery, University of Heidelberg
| N = 191 | N | [%] |
| Indication for operation | ||
| pain | 110 | 57.5 |
| stenosis of Choledochus | 22 | 11.5 |
| inflam. Tumor | 54 | 28.3 |
| pseudocyst | 26 | 13.6 |
| duodenal Stenosis | 5 | 2.6 |
| rez. Pankreatitis | 26 | 13.6 |
| stenosis of pancreatic duct | 23 | 12.0 |
| other complications | 15 | 7.9 |
| Type of operation | ||
| duodenum preserving pancreatic head resection | 86 | 45.0 |
| classical Whipple | 12 | 6.3 |
| pp-Whipple | 47 | 24.6 |
| segmental resection | 11 | 5.8 |
| pancreatic left resection | 11 | 5.8 |
| pancreatico-jejunostomy | 10 | 5.2 |
| bili-digestive anastomosis | 4 | 2.1 |
| other | 10 | 5.2 |
Table 7.
Classification of patients with chronic pancreatitis throughout the follow-up period
| [pre-OP] | [0.5a] | [1a] | [1.5a] | [2a] | [2.5a] | [3a] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| A | 12 | 6,3% | 100 | 52,4% | 66 | 48,2% | 49 | 48,0% | 36 | 44,4% | 20 | 40,8% | 9 | 42,9% |
| B | 130 | 68,0% | 8 | 4,1% | 6 | 4,4% | 3 | 2,9% | 3 | 3,7% | 2 | 4,1% | ||
| C | 49 | 25,7% | 83 | 43,5% | 65 | 47,4% | 50 | 49,1% | 42 | 51,9% | 27 | 55,1% | 12 | 57,1% |
| 191 | 100,0% | 191 | 100,0% | 137 | 100,0% | 102 | 100,0% | 81 | 100,0% | 49 | 100,0% | 21 | 100,0% | |
Figure 1.
Clinical course of chronic pancreatitis according to the classification.
Discussion
The present article proposes a new clinical classification of chronic pancreatitis, which is practically easy to use and applicable for all etiologies of chronic pancreatitis.
The key concern in the management of CP arises from the inability to compare CP patients with different etiologies on different continents and from the absence of a staging/disease evolution system of chronic pancreatitis. For decades debate has continued, centering on the optimal therapeutic options. Conservative, endoscopic and surgical treatment concepts compete without being able to provide evidence of their validity at any time in the course of the disease[19,47-49].
Patients with CP are treated according to local expertise and bias, and not based on evidence-based studies. Therefore, a new classification for the definition and staging of CP is urgently needed and should serve as a basis to learn about and better understand the natural course of the disease and the effects of different interventions.
As an example of the current confusion, depending upon the different interpretations of pain mechanisms in CP, several authors advocate endoscopic interventions, i.e., stenting of the common bile duct or the main pancreatic duct or extracorporeal shock wave lithotripsy (ESWL) of pancreatic stones, whereas others deny this approach[12,28,50-52]. Furthermore, in the surgical world the debate continues about drainage operations[35-37] versus resection [17,38-41] in the management of pain and complications of CP[20,29,53-58]. In the interest of the patient, we need to carefully select, analyze and compare these measures. However, in order for a new classification to be applied, it needs to be applicable in centers around the world, and reliable assessment and comparison of the patients should be possible.
The new classification, which we propose, seems to fulfill these requirements. It is simple, reproducible and based upon clinical signs and modern imaging techniques as well as pancreatic function, and most importantly, it is also based upon our current still limited knowledge about the natural course of CP. We have taken into account the liver cirrhosis classification (Child-Pugh) as a successful model, an analogy that seems applicable in CP as well, because most recently, several authors have described common mechanisms for liver and pancreatic fibrogenesis with a resulting loss in organ function at the end stage of both diseases[41-43,59,60].
The future task should be to establish an international working group and to prospectively evaluate the clinical significance of the proposed classification. This procedure should enable us both to validate this classification and to study the course of CP with and without treatment aspects.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MWB and PM carried out the idea of the classification and drafted the manuscript. HF and MEM contributed to the final version of the classification and carried out the clinical study. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Markus W Büchler, Email: markus.buechler@med.uni-heidelberg.de.
Marc E Martignoni, Email: martignoni@chir.med.tu-muenchen.de.
Helmut Friess, Email: friess@chir.med.tu-muenchen.de.
Peter Malfertheiner, Email: peter.malfertheiner@medizin.uni-magdeburg.de.
Acknowledgements
We wish to thank Jeannie Wurz for editing the manuscript.
References
- DiMagno MJ, Dimagno EP. Chronic pancreatitis. Curr Opin Gastroenterol. 2006;22:487–97. doi: 10.1097/01.mog.0000239862.96833.89. [DOI] [PubMed] [Google Scholar]
- Layer P, DiMagno EP. Early and late onset in idiopathic and alcoholic chronic pancreatitis. Different clinical courses. Surg Clin North Am. 1999;79:847–60. doi: 10.1016/S0039-6109(05)70047-5. [DOI] [PubMed] [Google Scholar]
- Ammann RW. Pain profile in alcoholic and nonalcoholic chronic pancreatitis (CP) Pancreas. 1996;12:315–8. doi: 10.1097/00006676-199604000-00018. [DOI] [PubMed] [Google Scholar]
- Ammann RW, Muellhaupt B. The natural history of pain in alcoholic chronic pancreatitis. Gastroenterology. 1999;116:1132–40. doi: 10.1016/S0016-5085(99)70016-8. [DOI] [PubMed] [Google Scholar]
- Malfertheiner P, Mayer D, Büchler MW. et al. Treatment of pain in chronic pancreatitis by inhibition of pancreatic secretion with octreotide. Gut. 1995;36:450–4. doi: 10.1136/gut.36.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMagno EP. Toward understanding (and management) of painful chronic pancreatitis. Gastroenterology. 1999;116:1252–7. doi: 10.1016/S0016-5085(99)70031-4. [DOI] [PubMed] [Google Scholar]
- Pezzilli R, Bini L, Fantini L. et al. Quality of life in chronic pancreatitis. World J Gastroenterol. 2006;12:6249–51. doi: 10.3748/wjg.v12.i39.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrikhande SV, Martignoni ME, Shrikhande M. et al. Comparison of histological features and inflammatory cell reaction in alcoholic, idiopathic and tropical chronic pancreatitis. Br J Surg. 2003;90:1565–1572. doi: 10.1002/bjs.4353. [DOI] [PubMed] [Google Scholar]
- Spicak J, Poulova P, Plucnarova J. et al. Pancreas divisum does not modify the natural course of chronic pancreatitis. J Gastroenterol. 2007;42:135–9. doi: 10.1007/s00535-006-1976-x. [DOI] [PubMed] [Google Scholar]
- Toskes PP. Medical management of chronic pancreatitis. Scand J Gastroenterol Suppl. 1995;208:74–80. doi: 10.3109/00365529509107766. [DOI] [PubMed] [Google Scholar]
- Manes G, Kahl S, Glasbrenner B. et al. Chronic pancreatitis: diagnosis and staging. Ann Ital Chir. 2000;71:23–32. [PubMed] [Google Scholar]
- Toskes PP. Update on diagnosis and management of chronic pancreatitis. Curr Gastroenterol Rep. 1999;1:145–53. doi: 10.1007/s11894-996-0014-8. [DOI] [PubMed] [Google Scholar]
- Nichols MT, Russ PD, Chen YK. Pancreatic imaging: current and emerging technologies. Pancreas. 2006;33:211–20. doi: 10.1097/01.mpa.0000227912.71202.2c. [DOI] [PubMed] [Google Scholar]
- Dumonceau JM, Vonlaufen A. Pancreatic endoscopic retrograde cholangiopancreatography (ERCP) Endoscopy. 2007;39:124–30. doi: 10.1055/s-2006-945096. [DOI] [PubMed] [Google Scholar]
- Dominguez-Munoz JE. Pancreatic enzyme therapy for pancreatic exocrine insufficiency. Curr Gastroenterol Rep. 2007;9:116–22. doi: 10.1007/s11894-007-0005-4. [DOI] [PubMed] [Google Scholar]
- van Esch AA, Wilder-Smith OH, Jansen JB. et al. Pharmacological management of pain in chronic pancreatitis. Dig Liver Dis. 2006;38:518–26. doi: 10.1016/j.dld.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Alexakis N, Halloran C, Raraty M. et al. Current standards of surgery for pancreatic cancer. Br J Surg. 2004;91:1410–27. doi: 10.1002/bjs.4794. [DOI] [PubMed] [Google Scholar]
- Andren-Sandberg A, Ansorge C, Eiriksson K. et al. Treatment of pancreatic pseudocysts. Scand J Surg. 2005;94:165–75. doi: 10.1177/145749690509400214. [DOI] [PubMed] [Google Scholar]
- Lamme B, Boermeester MA, Straatsburg IH, Early versus late surgical drainage for obstructive pancreatitis in an experimental model. Br J Surg. 2007. [DOI] [PubMed]
- Beger HG, Schlosser W, Friess HM. et al. Duodenum-preserving head resection in chronic pancreatitis changes the natural course of the disease: a single-center 26-year experience. Ann Surg. 1999;230:512–9. doi: 10.1097/00000658-199910000-00007. discussion 519-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comfort M, Gambill E, Baggenstoss A. Chronic relapsing pancreatitis. Gastroenterology. 1946;6:46. [PubMed] [Google Scholar]
- Sarles H. Pancreatitis Symposium. 1965.
- Singer MV, Gyr K, Sarles H. Revised classification of pancreatitis. Report of the Second International Symposium on the Classification of Pancreatitis in Marseille, France, March 28-30, 1984. Gastroenterology. 1985;89:683–5. [PubMed] [Google Scholar]
- Singer MV, Chari ST. In: The Pancreas. Beger HG, Warshaw AL, Büchler MW, et al, editor. Oxford: Blackwell Science; 1998. Classification of chronic pancreatitis. [Google Scholar]
- Axon AT, Classen M, Cotton PB. et al. Pancreatography in chronic pancreatitis: international definitions. Gut. 1984;25:1107–12. doi: 10.1136/gut.25.10.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axon AT. Endoscopic retrograde cholangiopancreatography in chronic pancreatitis. Cambridge classification. Radiol Clin North Am. 1989;27:39–50. [PubMed] [Google Scholar]
- Malfertheiner P, Büchler MW. Correlation of imaging and function in chronic pancreatitis. Radiol Clin North Am. 1989;27:51–64. [PubMed] [Google Scholar]
- Dumonceau JM, Deviere J, Le Moine O. et al. Endoscopic pancreatic drainage in chronic pancreatitis associated with ductal stones: long-term results. Gastrointest Endosc. 1996;43:547–55. doi: 10.1016/S0016-5107(96)70189-X. [DOI] [PubMed] [Google Scholar]
- Büchler MW, Friess H, Bittner R. et al. Duodenum-preserving pancreatic head resection: Long-term results. J Gastrointest Surg. 1997;1:13–9. doi: 10.1007/s11605-006-0004-z. [DOI] [PubMed] [Google Scholar]
- Ammann RW. A clinically based classification system for alcoholic chronic pancreatitis: summary of an international workshop on chronic pancreatitis. Pancreas. 1997;14:215–21. doi: 10.1097/00006676-199704000-00001. [DOI] [PubMed] [Google Scholar]
- Ramesh H. Proposal for a new grading system for chronic pancreatitis: the ABC system. J Clin Gastroenterol. 2002;35:67–70. doi: 10.1097/00004836-200207000-00014. [DOI] [PubMed] [Google Scholar]
- Bagul A, Siriwardena AK. Evaluation of the Manchester classification system for chronic pancreatitis. Jop. 2006;7:390–6. [PubMed] [Google Scholar]
- Schneider A, Lohr JM, Singer MV. The M-ANNHEIM classification of chronic pancreatitis: introduction of a unifying classification system based on a review of previous classifications of the disease. J Gastroenterol. 2007;42:101–19. doi: 10.1007/s00535-006-1945-4. [DOI] [PubMed] [Google Scholar]
- Whitcomb DC, Gorry MC, Preston RA. et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–5. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- Friess H, Yamanaka Y, Büchler MW. et al. A subgroup of patients with chronic pancreatitis overexpress the c-erb B-2 protooncogene. Ann Surg. 1994;220:183–92. doi: 10.1097/00000658-199408000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrikhande SV, Friess H, di Mola FF. et al. NK-1 receptor gene expression is related to pain in chronic pancreatitis. Pain. 2001;91:209–17. doi: 10.1016/S0304-3959(00)00436-X. [DOI] [PubMed] [Google Scholar]
- Klöppel G, Maillet B. In: The Pancreas. Beger HG, Warshaw AL, Büchler MW, et al, editor. Oxford: Blackwell Science; 1998. Pathology of chronic pancreatitis. [Google Scholar]
- Kapoor D, Kumar N. Hepatic fibrosis: pathobiology and management. Trop Gastroenterol. 2000;21:114–7. [PubMed] [Google Scholar]
- Lowenfels AB, Maisonneuve P, Cavallini G. et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–7. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- Colombo M, de Franchis R, Del E Ninno. et al. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325:675–80. doi: 10.1056/NEJM199109053251002. [DOI] [PubMed] [Google Scholar]
- Bachem MG, Schneider E, Gross H. et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421–32. doi: 10.1016/S0016-5085(98)70209-4. [DOI] [PubMed] [Google Scholar]
- Schmid-Kotsas A, Gross HJ, Menke A. et al. Lipopolysaccharide-activated macrophages stimulate the synthesis of collagen type I and C-fibronectin in cultured pancreatic stellate cells. Am J Pathol. 1999;155:1749–58. doi: 10.1016/S0002-9440(10)65490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte MV, Haber PS, Darby SJ. et al. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut. 1999;44:534–41. doi: 10.1136/gut.44.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary MB, Lugea A, Lowe AW. et al. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50–9. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- Keck T, Marjanovic G, Fernandez-del C Castillo. et al. The inflammatory pancreatic head mass: significant differences in the anatomic pathology of German and American patients with chronic pancreatitis determine very different surgical strategies. Ann Surg. 2009;249:105–10. doi: 10.1097/SLA.0b013e31818ef078. [DOI] [PubMed] [Google Scholar]
- Gupta V, Toskes PP. Diagnosis and management of chronic pancreatitis. Postgrad Med J. 2005;81:491–7. doi: 10.1136/pgmj.2003.009761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha JE, Penteado S, Jukemura J. et al. Surgical and interventional treatment of chronic pancreatitis. Pancreatology. 2004;4:540–50. doi: 10.1159/000081560. [DOI] [PubMed] [Google Scholar]
- Weber A, Schneider J, Neu B. et al. Endoscopic stent therapy for patients with chronic pancreatitis: results from a prospective follow-up study. Pancreas. 2007;34:287–94. doi: 10.1097/mpa.0b013e3180325ba6. [DOI] [PubMed] [Google Scholar]
- Binmoeller KF, Jue P, Seifert H. et al. Endoscopic pancreatic stent drainage in chronic pancreatitis and a dominant stricture: long-term results. Endoscopy. 1995;27:638–44. doi: 10.1055/s-2007-1005780. [DOI] [PubMed] [Google Scholar]
- Sauerbruch T, Holl J, Sackmann M. et al. Extracorporeal lithotripsy of pancreatic stones in patients with chronic pancreatitis and pain: a prospective follow up study. Gut. 1992;33:969–72. doi: 10.1136/gut.33.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMagno EP. Conservative Management of chronic pancreatitis. Dig Surg. 1994;11:300–303. doi: 10.1159/000172270. [DOI] [Google Scholar]
- Nealon WH, Thompson JC. Progressive loss of pancreatic function in chronic pancreatitis is delayed by main pancreatic duct decompression. A longitudinal prospective analysis of the modified puestow procedure. Ann Surg. 1993;217:458–66. doi: 10.1097/00000658-199305010-00005. discussion 466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealon WH, Matin S. Analysis of surgical success in preventing recurrent acute exacerbations in chronic pancreatitis. Ann Surg. 2001;233:793–800. doi: 10.1097/00000658-200106000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconi M, Valerio A, Caldiron E. et al. Changes in pancreatic resection for chronic pancreatitis over 28 years in a single institution. Br J Surg. 2000;87:428–33. doi: 10.1046/j.1365-2168.2000.01391.x. [DOI] [PubMed] [Google Scholar]
- Sohn TA, Campbell KA, Pitt HA. et al. Quality of life and long-term survival after surgery for chronic pancreatitis. J Gastrointest Surg. 2000;4:355–64. doi: 10.1016/S1091-255X(00)80013-X. discussion 364-5. [DOI] [PubMed] [Google Scholar]
- Ozawa F, Friess H, Kondo Y. et al. Duodenum-preserving pancreatic head resection (DPPHR) in chronic pancreatitis: its rationale and results. J Hepatobiliary Pancreat Surg. 2000;7:456–65. doi: 10.1007/s005340070015. [DOI] [PubMed] [Google Scholar]
- Büchler MW, Friess H, Wagner M. et al. Pancreatic fistula after pancreatic head resection. Br J Surg. 2000;87:883–9. doi: 10.1046/j.1365-2168.2000.01465.x. [DOI] [PubMed] [Google Scholar]
- Haber PS, Keogh GW, Apte MV. et al. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol. 1999;155:1087–95. doi: 10.1016/S0002-9440(10)65211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaster R. Molecular regulation of pancreatic stellate cell function. Mol Cancer. 2004;3:26. doi: 10.1186/1476-4598-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]