Abstract
Significant effort has been applied to discover and develop vehicles which can guide small interfering RNAs (siRNA) through the many barriers guarding the interior of target cells. While studies have demonstrated the potential of gene silencing in vivo, improvements in delivery efficacy are required to fulfill the broadest potential of RNA interference therapeutics. Through the combinatorial synthesis and screening of a different class of materials, a formulation has been identified that enables siRNA-directed liver gene silencing in mice at doses below 0.01 mg/kg. This formulation was also shown to specifically inhibit expression of five hepatic genes simultaneously, after a single injection. The potential of this formulation was further validated in nonhuman primates, where high levels of knockdown of the clinically relevant gene transthyretin was observed at doses as low as 0.03 mg/kg. To our knowledge, this formulation facilitates gene silencing at orders-of-magnitude lower doses than required by any previously described siRNA liver delivery system.
Keywords: lipidoid, siRNA delivery, multiple gene silencing, primates
Since the discovery of RNA interference (RNAi) by Fire and Mello in 1998 (1) and siRNAs by Tuschl and coworkers in 2001 (2), considerable effort has been directed towards their therapeutic application in humans (3). The most significant challenges to delivery include the relatively large size (∼13 kDa) and negative charge of siRNA molecules as well as their susceptibility to enzymatic degradation in vivo (4, 5). In some applications, effective delivery of naked siRNAs, without a carrier, may be possible (6, 7). However, systemic delivery to many tissues, including liver, requires a vehicle to provide protection and transport of siRNA to the cells of interest. To this end, a variety of carrier systems utilizing both natural and synthetic materials have been developed (8–15). Cationic lipids represent one of the most well-studied classes of synthetic materials for siRNA delivery. To date, the most advanced examples of these materials demonstrate the ability to bind and condense siRNA into nanocomplexes through electrostatic interactions and to deliver the payload across the cellular membrane into the cytoplasm of target cells (16, 17).
Previously, Akinc et al., (2008) reported a high-throughput combinatorial approach to new material synthesis and discovery for siRNA delivery applications (15). Michael addition chemistry was utilized to create a structurally diverse library of amino-alkyl-acrylate and -acrylamide materials termed ‘lipidoids’, which were then analyzed for their ability to transfect cells both in vitro and in vivo. The lead candidate from the initial study was demonstrated to facilitate sequence-specific knockdown in a variety of cellular targets and animal species, including mice, rats, and nonhuman primates. While promising, delivery with these materials requires siRNA doses greater than 1 mg/kg to achieve high levels of gene silencing in vivo (18). Such doses are comparable to those required by stable nucleic acid lipid particle (SNALP) formulations, another delivery system which has shown utility for siRNA delivery in nonhuman primates (14). To significantly expand the therapeutic potential of lipid-based formulations, different materials with improved efficacy would be of great utility.
Results and Discussion
Synthesis of a Combinatorial Epoxide-Derived Lipidoid Library.
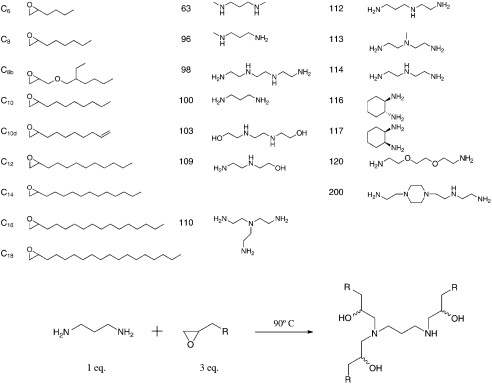
In an effort to identify increasingly efficacious delivery materials, we used a unique synthetic strategy to rapidly build a library of lipid-like compounds based on epoxide chemistry. The library is composed of nondegradable amino alcohols consisting of polar amine-containing head groups and nonpolar hydrocarbon tails. Synthesis of the compounds was achieved through efficient ring-opening of epoxides by amine substrates (Fig. 1). This synthetic strategy is particularly well suited to parallel synthesis and high-throughput screening in that reactions can be carried out without solvent, do not require protection/deprotection steps, and resultant materials can be used in cell-based screens without purification. One advantage of this synthetic strategy over the previous scheme (15) is that the reactions were typically complete within 3 d. Using this one-step approach, a library of 126 lipid-like compounds was created and the reaction products were tested in cells without further processing.
Fig. 1.
Synthesis of Epoxide-Derived Lipidoid Library (A) Epoxide terminated alkyl chains and amine-containing monomers were used in synthesis of combinatorial library, (B) addition of epoxides to amines by efficient ring-opening enables parallel synthesis of library members
Lipidoid-Mediated siRNA Delivery In Vitro.
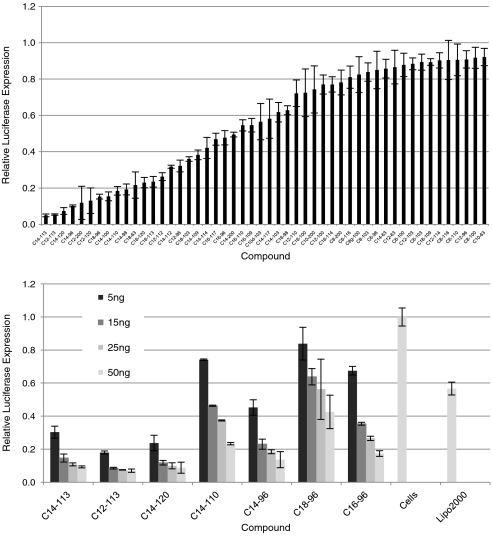
Lipid-like materials were screened in vitro in a high-throughput fashion in a luciferase-expressing Hela-derived cell line. These cells are genetically modified to stably express both reporter proteins firefly Photinus pyralis and Renilla reniformis luciferase (15). In these experiments, antifirefly luciferase siRNA was complexed with lipidoid at weight ratios of 2.5∶1, 5∶1, 10∶1, and 15∶1 lipidoid∶siRNA and incubated with cells in the presence of growth media. Reduction in firefly luciferase expression in the absence of Renilla reduction was considered siRNA-mediated silencing. Renilla expression was monitored as an internal control for lipidoid-related toxicity. Cytotoxicity assays were also performed with no evidence of adverse effects (Fig. S1) In this screen, numerous compounds were identified which facilitated luciferase silencing, including three which silenced greater than 90% (Fig. 2A). For ease of graphical representation, only 5∶1 weight ratio data is shown. Interestingly, from these results a number of structure-activity relationships emerged. With respect to tail length, seven of the top 15 performing structures possessed tails 14 carbons in length. Additionally, no compounds with tails less than 12-carbons in length mediated silencing greater than 30%. Regarding amine head groups, amine 113 was present in the top two performing compounds and three of the top 15. While the convergence upon C14 tails and amine 113 is evident, not all compounds containing these structures show silencing activity. For example, neither C8-113 nor C14-116 facilitated gene silencing in vitro, suggesting an optimized combination of amine group and tail length is necessary to impart delivery activity.
Fig. 2.
In vitro screening of lipidoid library. Lipidoids were screened in luciferase-expressing Hela-derived cell line. (A) Antifirefly luciferase siRNA was complexed with lipidoids and incubated with cells in presence of growth media. Relative firefly luciferase expression determined by comparison of detected protein levels in treated groups vs. untreated control. (B) Luciferase silencing at low doses of siRNA (s.d., n = 4)
To investigate in vitro efficacy at low doses of siRNA, a dose response was conducted in which cells were exposed to titrated concentrations of siRNA-containing lipidoid complexes. siRNA was incubated with cells at doses between 5 and 50 nanogram (ng), with the ratio of lipidoid∶siRNA held constant at 5∶1 (wt∶wt). From these experiments, three compounds were identified which facilitated greater than 70% silencing at an siRNA dose of 5 ng per well (Fig. 2B).
In Vivo Delivery of siRNA to Hepatocytes in Mice.
While in vitro delivery experiments are useful for identifying compounds with in vivo delivery potential, we find they are not highly predictive for identifying most effective compound for in vivo delivery. To evaluate the utility of the epoxide-based lipidoids in facilitating siRNA delivery in vivo, the mouse Factor VII gene silencing model was employed (15, 18). Lipidoids formulated with siRNA-directed against the blood clotting Factor VII were delivered intravenously. Factor VII is a useful gene target to evaluate hepatocyte-specific delivery in that the protein is produced only in the cells of the liver parenchyma, is secreted into the blood enabling facile protein quantitation, and possesses a relatively short plasma half-life (15).
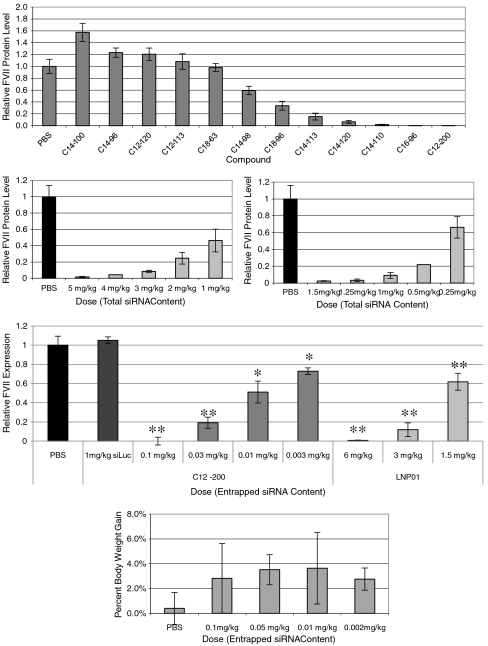
Twelve of the top-performing lipidoids from the initial in vitro screen were purified and evaluated for in vivo performance. For in vitro experiments, simple complexation of siRNA and lipidoid is sufficient for particle formation and cellular delivery. However, during intravenous administration, barriers such as electrostatic interactions with serum proteins, uptake by cells of the immune system, and aggregation in areas such as the spleen and lung can inhibit delivery to hepatocytes. To improve serum stability of lipidoid particles, distearoyl phosphatidylcholine (DSPC), cholesterol and polyethylene glycol (PEG) were used in the formulations (15). For in vivo screening experiments, lipidoids were formulated at a constant weight ratio of lipidoid∶DSPC∶cholesterol∶PEG and mice were administered a single bolus dose of 3 mg/kg total siRNA via tail vein injection. Mouse body-weight was also monitored over the duration of the experiment, as body-weight loss can indicate toxicity associated with lipidoid particle treatment. Mean particle diameter varied between formulations and ranged from 65 nm to 250 nm.. From this screen, three compounds were found to facilitate complete silencing at the administered dose (Fig. 3A). While these results demonstrate the ability of epoxide lipidoids to effectively reduce protein levels in hepatocytes, dose response experiments were conducted with the top three compounds, C16-96, C14-110, and C12-200, to investigate potency of silencing at lower doses (Fig. 3B–D). Dose-dependent gene silencing was achieved with each of the three lipidoids tested, and one compound in particular, C12-200, demonstrated over two orders-of-magnitude higher potency than LNP01 (18), the optimized liver delivery formulation from the previous acrylamide- and acrylate-based library of lipid-like materials. A formulated control siRNA was administered at a dose of 1 mg/kg to confirm the specificity of gene silencing (Fig. 3D).
Fig. 3.
In vivo silencing of Factor VII in mice. A) Top-performing lipidoids from in vitro screen were purified, formulated for serum stability and delivered intravenously to C57BL/6 mice. Mice received a single bolus administration of 3 mg/kg total siRNA via tail-vein injection and Factor VII levels were quantified 72 h postinjection. B–D) Dose response experiments with top three performing lipidoids from in vivo screen; C16-96 (B), C14-110 (C), and C12-200 (D). No lipidoid-related toxicity is observed as measured by body-weight loss (E). (s.d.,n = 3 or 4, * P < 0.005, **P < 0.001; t-test, single tailed)
Perhaps the most important advantage of low-dose delivery is the significantly reduced amount of carrier material required to transport the siRNA to its target. The several hundredfold improvement in potency of C12-200 over LNP01 translates to a reduction in both administered siRNA drug and lipidoid formulation materials. If a similar improvement in potency were to hold true in humans, this would result in a reduction in the injected siRNA dose from over 100 mg to lower than 1 mg, along with a concomitant reduction in dosed formulation excipients. By effectively lowering the dosage of siRNA and formulation materials required to induce silencing, the resulting lipidoid-related tolerability should be greatly increased. This concept is supported by tolerability analysis in mice in which no indication of toxicity is observed at 1 mg/kg, several orders-of-magnitude above the efficacious dosage (Fig. 3E, Table 1).
Table 1.
| Dose | ALT (U/L) | AST (U/L) | ALP (U/L) | Bilirubin (dL |
| 0 mg/kg | 42.3 ± 6.0 | 90.7 ± 15.0 | 183.0 ± 8.9 | 2 ± 0.0 |
| 0.06 mg/kg | 40.7 ± 4.6 | 74.0 ± 12.8 | 219.3 ± 35.3 | 2 ± 0.0 |
| 0.02 mg/kg | 47.3 ± 10.1 | 103.3 ± 11.8 | 178.7 ± 46.5 | 2 ± 0.0 |
| 0.2 mg/kg | 52.0 ± 6.2 | 161.0 ± 66.2 | 229.0 ± 46.4 | 2 ± 0.0 |
| 0.6 mg/kg | 40.7 ± 6.8 | 102.3 ± 15.1 | 201.3 ± 37.4 | 2 ± 0.0 |
| 1 mg/kg | 37.0 ± 7.8 | 77.7 ± 25.5 | 123.3 ± 25.6 | 2 ± 0.0 |
Clinical chemistry parameters following single injection of formulated C12-200-siRNA particles. ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase
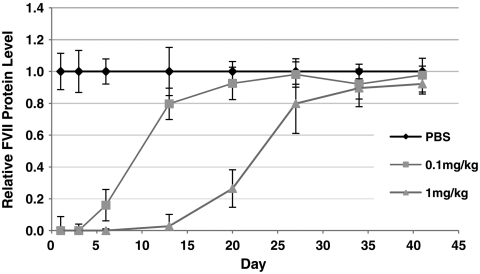
Next, we investigated the durability of gene silencing mediated by C12-200-formulated siRNA. Mice received a single i.v. injection of formulated siRNA at either 0.1 or 1 mg/kg and serum Factor VII levels were monitored for over 40 d (Fig. 4). At both doses, complete silencing was observed at 24 h and protein levels returned to baseline within 20 and 35 d for the 0.1 and 1 mg/kg doses, respectively. These results indicate that larger siRNA doses may lead to a longer duration of effect, further highlighting the potential for dosing at higher multiples of the efficacious dose.
Fig. 4.
In vivo persistence of C12-200-mediated silencing was investigated by monitoring Factor VII protein levels for a period of over 40 days. Mice we administered a single dose of either 1 or 0.1 mg/kg siRNA and blood samples were drawn at varying timepoints for quantitation of serum protein levels. (s.d.,n = 3)
Low-Dose Efficacy Enables Multiple Gene Silencing in Vivo.
As a result of the highly efficient gene silencing achieved by C12-200, we hypothesized that silencing of multiple genes in the liver with a single i.v. administration should be possible while remaining well within the range of tolerability established in previous experiments. It could be envisioned that the ability to regulate multiple genes may provide a powerful therapeutic approach to diseases in which multiple gene targets have already been identified (14, 19). To investigate the feasibility of this approach, siRNA sequences against liver targets of possible therapeutic interest, Factor VII, ApoB, PCSK9, Xbp1, and SORT1, were pooled and formulated with C12-200. ApoB, PCSK9, XBP-1 and SORT1 are all genes implicated in metabolic pathways involved in cholesterol homeostasis, and mutations in these genes have been linked to altered cholesterol levels either in knock-out mouse models or in human genetic association studies (20–23). ApoB has a role in cholesterol trafficking from the liver to the plasma, PCSK9 a role in cholesterol clearance from plasma back into the liver, XBP-1 has been implicated in cholesterol synthesis, while the mechanistic role of SORT1 is less clear (20–23).
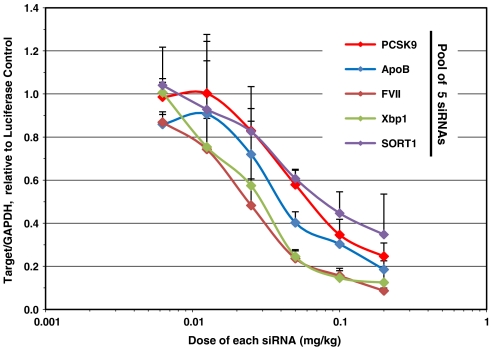
Silencing of these particular five genes is not expected to provide any cooperative therapeutic effect per se; however silencing all five genes simultaneously serves as a proof of principle that multiple genes involved in similar or divergent signaling pathways, could selectively be silenced with a single administration of a single drug product. In this experiment, mice received a single i.v. injection of a C12-200-formulated pool of siRNAs, and at 72 h postadministration, liver tissue was harvested for analysis of mRNA transcript levels. Dose-dependent silencing effects were investigated by titrating dosage of each siRNA from 0.2 to 0.005 mg/kg. Greater than 65% silencing of all five genes was observed at a dose of 0.2 mg/kg per siRNA (1 mg/kg total siRNA dose) (Fig. 5). Consistent with the tolerability studies described above, no adverse side effects were observed.
Fig. 5.
Five hepatocellular gene targets were simultaneously silenced by a single injection of pooled siRNAs formulated with C12-200. Mice we administered a single dose and dosage was titrated from 0.2 and .005 mg/kg per siRNA. 72 h postinjection, liver tissue was harvested for analysis of gene transcript levels. (s.d.,n = 5).
To our knowledge, this is the earliest report of the simultaneous siRNA-mediated silencing of five hepatic targets in vivo. Given the potency of C12-200-mediated delivery, we hypothesize that even more genes could be simultaneously silenced by a pooled siRNA product. From a therapeutic standpoint, this could enable more complex therapeutic approaches, where silencing of multiple targets achieve an enhanced therapeutic effect (24). For example, this strategy may be particularly useful in treating viral infections such as Hepatitis C virus (HCV) in which rapidly evolving viral genomes have proven elusive to siRNAs of a single sequence. In fact, this idea has been shown previously in vitro utilizing delivery of endoribonuclease-prepared siRNAs and retroviral vectors encoding short hairpin RNAs against multiple regions of the HCV genome (25). This multitarget approach may also allow for different strategies to treat multifactoral diseases such as metabolic syndrome, cancer, or infectious disease where multiple genes and pathways have been implicated.
Investigating the Mechanism of C12-200-mediated Cellular Delivery.
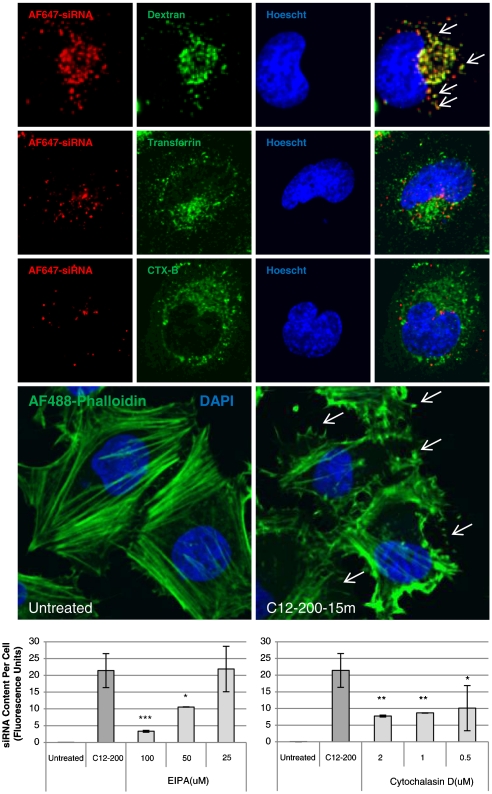
To investigate the mechanism by which C12-200 particles are internalized, nonspecific antiGFP siRNA labeled with Alexa-Fluor® 647 was delivered to HeLa cells in order to observe siRNA uptake and intracellular trafficking. In these experiments, C12-200-formulated siRNA was incubated with HeLa cells in the presence of labeled cargo known to enter cells by different endocytic pathways. As shown in Fig. 6A, the labeled siRNA colocalized with a fluid phase marker dextran but not with transferrin or Cholera toxin B, markers of clathrin and caveolae mediated endocytosis, respectively. This suggests that C12-200 particles may be internalized through a macropinocytosis mechanism (26, 27). One of the hallmarks of such an uptake pathway is membrane ruffling and actin rearrangement (28), which was observed in HeLa cells within 15 min of the application of the particles (Fig. 6B). Furthermore, the effects of the macropinocytosis inhibitor, 5-N-ethyl-N-isoproamiloride (EIPA), and the actin polymerization inhibitor, Cytochalasin D, on particle uptake were examined with our delivery system (26). Both compounds dose-dependently inhibited siRNA uptake (Figs. 6C,D), which further suggested that the majority of C12-200-formulated siRNA likely entered cells via macropinocytosis. It has been reported that in some cell types the fluid content of macropinosomes does not merge with the degradative pathway (29, 30). We hypothesize that that C12-200-facilitated silencing may be enhanced by avoidance of lysosomal degradation which is a common problem encountered with drug delivery vehicles that enter cells through the classical endocytic pathway (31).
Fig. 6.
C12-200-siRNA particles elicit cellular uptake by macropinocytosis. (A) Fluorescently labeled siRNA formulated with C12-200 was incubated with HeLa cells in the presence of labeled markers of various endocytic pathways. siRNA-containing particles colocalize with labeled dextran, a fluid phase marker known to enter cells via macropinocytosis (White arrows). (B) Fluorescence labeling of actin fibers reveals membrane ruffling (White arrows) and actin rearrangement, hallmark indicators of uptake by macropinocytosis, within 15 min of exposure of HeLa cells to C12-200-siRNA particles. (C,D) Prior exposure of cells to EIPA and Cytochalasin D, inhibitors of macropinocytosis and actin polymerization, respectively, reduce uptake of C12-200-siRNA particles in dose-dependent fashion. (s.d., n = 3, *** P < 0.005; ** P < 0.01; * P < 0.05
Investigating the C12-200-Mediated Silencing in Nonhuman Primates.
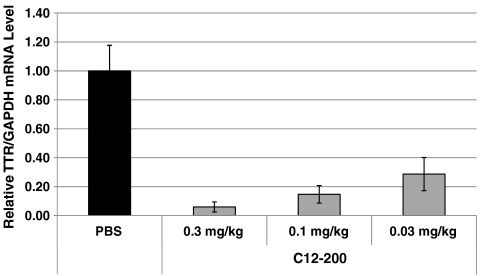
To further investigate the potential of these materials, C12-200 was formulated with siRNA specific to transthyretin (TTR), and silencing was evaluated in nonhuman primates. TTR is a serum protein synthesized primarily in hepatocytes. Although amyloidogenic TTR mutations are rare, they are endemic to certain populations and can affect both the peripheral nerves and heart, leading to familial amyloidotic polyneuropathy and familial amyloid cardiomyopathy, respectively. Currently, the only disease modifying therapy is liver transplantation. C12-200-siRNA-mediated silencing of mutant TTR is a potential approach for the treatment of TTR amyloidosis (32). Dosing of nonhuman primates with C12-200-TTR siRNA resulted in high levels of specific knockdown at 0.3 mg/kg, 0.1 mg/kg, and 0.03 mg/kg (Fig. 7). To our knowledge this formulation provides for the most efficacious knockdown yet reported in primates.
Fig. 7.
Efficacy of C12-200 in nonhuman primates. Cynomolgus monkeys (n = 3 per group) received either PBS or 0.03, 0.1, or 0.3 mg/kg siTTR formulated in C12-200 as 15 min intravenous infusions (5 mL/kg) via the cephalic vein. Liver biopsies were collected from animals at 48 h postadministration. TTR mRNA levels relative to GAPDH mRNA levels were determined in liver samples. Data points represent group mean ± s.d
We believe that the development of safe and effective siRNA delivery vehicles is an important part of the continued advancement of RNAi-based therapeutics. With the identification of highly efficacious materials such as C12-200, widened therapeutic indices, persistent gene silencing, and multitarget approaches to treatment of disease may be achieved.
Methods
Lipidoid Synthesis.
Compounds in the library were synthesized by reacting alkyl epoxides with a selection of amines. Substoichiometric amounts of epoxide were added to increase the proportion of products with one less tail than the total possible for a given amine monomer. The amine (1 equiv, typically 1 millimoles (mmol)) and epoxide ([N−1] equiv, where N is the number of secondary amines plus 2× number of primary amines in the amine starting material) were added to a 2 mL glass vial containing a magnetic stir bar. The vial was sealed, and the reaction was heated to 90 °C with stirring for 2.5 d. A selection of crude reaction mixtures were characterized by MALDI-TOF mass spectroscopy (Table S1); the spectra revealed that the mixtures contained predominately N and [N−1] tailed products, as expected. Crude reaction products were used for in vitro screening; groups of products could be separated by number of lipid tails by chromatography on silica with gradient elution from CH2Cl2 to 75∶22∶3 CH2Cl2/MeOH/NH4OH (aq).
Lipidoid-siRNA Formulations.
Lipidoid-siRNA formulations for in vivo screening were made from lipidoid, cholesterol, and a polyethylene glycol modified lipid as previously described (15, 18). Stock solutions of lipidoid, cholesterol (MW 387, Sigma-Aldrich), and mPEG2000-DMG (MW 2660, synthesized by Alnylam) (15) were made in absolute ethanol at concentrations of 100, 20, and 100 mg/mL, respectively. Components were combined to yield weight fractions of 52∶20∶28. Ethanol mixture was then added to 200 mM sodium acetate buffer (pH 5) while stirring to spontaneously form empty liposomes. siRNA at a concentration of 10 mg/mL in 50 mM sodium acetate was added to empty liposomes at a weight ratio of 10∶1 total lipids∶siRNA and the mixture was incubated at 37 °C for 30 min. Formulations were then dialyzed against PBS in 3,500 MWCO dialysis cassettes (Pierce) for 75 min. Following buffer exchange, a sample of each formulation was used for particle characterization. A modified Ribogreen assay (Invitrogen) was performed to quantify degree of siRNA entrapment (33) and mean particle diameter was measured by dynamic light scattering (ZetaPALS, Brookhaven Instruments).
C12-200-siRNA formulations were prepared using a method adapted from Jeffs et al. (34) Briefly, C12-200, distearoyl phosphatidylcholine (DSPC), cholesterol and mPEG2000-DMG were solubilized in 90% ethanol at a molar ratio of 50∶10∶38.5∶1.5. The siRNA (or pool of siRNAs) was solubilized in 10 mM citrate, pH 3 buffer at a concentration of 0.4 mg/mL. The ethanolic lipid solution and the aqueous siRNA solution were pumped by means of a peristaltic pump fitted with dual pump heads at equivalent volumetric flow rates and mixed in a “T”-junction. Lipids were combined with siRNA at a total lipid to siRNA ratio of 7∶1 (wt∶wt). The spontaneously formed C12-200-siRNA formulations were dialyzed against PBS (155 mM NaCl, 3 mM Na2HPO4, 1 mM KH2PO4, pH 7.5) to remove ethanol and exchange buffer. This formulation yields a mean particle diameter of 80 nm with approximately 90% siRNA entrapment efficiency.
In Vivo Factor VII and Multiple Gene Silencing in Mice.
All procedures used in animal studies were approved by the Institutional Animal Care and Use Committee and were consistent with local, state and federal regulations as applicable. C57BL/6 mice (Charles River Labs) were used for siRNA silencing experiments. Prior to injection, formulations were diluted in PBS at siRNA concentrations such that each mouse was administered a dose of 0.01 mL/g body-weight. Formulations were administered intravenously via tail vein injection. After 48 or 72 h, body-weight gain/loss was measured and mice were anaesthetized by isofluorane inhalation for blood sample collection by retroorbital eye bleed. Serum was isolated with serum separation tubes (Falcon tubes, Becton Dickinson) and Factor VII protein levels were analyzed by chromogenic assay (Biophen FVII, Aniara Corporation). A standard curve was constructed using samples from PBS-injected mice and relative Factor VII expression was determined by comparing treated groups to untreated PBS control.
In the multiple gene silencing study Factor VII, ApoB, PCSK9, XBP-1, and SORT1 mRNA levels were assessed in livers harvested from mice treated with C12-200 formulated pool of five siRNAs or control unrelated siRNA targeting luciferase. Frozen liver tissue was ground and tissue lysates were prepared. Factor VII, ApoB, PCSK9, XBP-1, and SORT1 mRNA levels normalized to those of GAPDH were determined in the lysates by using a branched DNA assay (QuantiGene Reagent System, Panomics). Target/GAPDH levels in mice treated with C12-200 formulated pool of five siRNAs were plotted after normalization to the corresponding Target/GAPDH levels in mice treated with C12-200 formulated luciferase control siRNA.
siRNA Uptake and Microscopy.
HeLa cells were purchased from ATCC. Alexa-Fluor® 488-labeled dextran, transferrin, cholera toxin, and phalloidin were purchased from Invitrogen. Cells were seeded in 96-well plates (Grenier) overnight, then incubated with C12-200 formulated Alexa-647 tagged siRNA for durations ranging from 15 min to 3 h. Labeled cargo was added during the final 15 min of nanoparticle incubation prior to nuclear staining with Hoescht. In some experiments, EIPA or Cytochalasin D (Sigma-Aldrich) were preincubated with cells for 1 h prior to incubation with C12-200 particles where drug was continually present. For examination of actin ruffling, cells were serum starved for 1 h, followed by addition of particles for 15 min in serum-free media. Cells were fixed in 4% paraformaldehyde, permeabilized with 0.1% saponin and stained with Alexa-Fluor® 488 phalloidin. All images were acquired using an Opera spinning disc confocal system (Perkin Elmer), and the data was analyzed using Acapella Software (Perkin Elmer).
Supplementary Material
Acknowledgments.
The authors would like to thank John Maraganore for helpful comments on this manuscript. This work was supported by a grant from Alnylam Pharmaceuticals, and the National Institutes of Health Grant (EB000244).
Footnotes
Confllict of interest statement: The Sponsor declares a conflict of interest. R.L. is a shareholder and member of the Scientific Advisory Board of Alnylam. D.G.A. is a consultant with Alnylam Pharmaceuticals. R.L and D.G.A have sponsored research grants from Alnylam. Alnylam also has a license to certain intellectual property invented at Massachusetts Institute of Technology by Drs. Anderson, Langer, and colleagues. W.Q., J.R.D., J.Q., W.C., L.L.Q., T.R., M.F.-K., K.F., V.K., A.F., R.A., D.W.Y.S., and A.A. are employed by Alnylam. The authors declare a conflict of interest. R.L. is a shareholder and member of the Scientific Advisory Board of Alnylam. D.G.A. is a consultant with Alnylam Pharmaceuticals. R.L and D.G.A have sponsored research grants from Alnylam. Alnylam also has a license to certain intellectual property invented at Massachusetts Institute of Technology by Drs. Anderson, Langer, and colleagues. W.Q., J.R.D., J.Q., W.C., L.L.Q., T.R., M.F.-K., K.F., V.K., and A.A. are employed by Alnylam.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910603106/DCSupplemental.
References
- 1.Fire A, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21- and 22- nucleotide RNAs. Genes Dev. 2001;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: Advances in siRNA delivery. Nat Rev Drug Discovery. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vargason JM, Szittya G, Burgyan J, Hall TM. Size selective recognition of siRNA by an RNA silencing suppressor. Cell. 2003;115:799–811. doi: 10.1016/s0092-8674(03)00984-x. [DOI] [PubMed] [Google Scholar]
- 5.Judge AD, et al. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- 6.Kleinman ME, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bitko V, Musiyenko A, Shulyayeva O, Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nat Med. 2005;11:50–55. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]
- 8.Tiscornia G, Singer O, Ikawa M, Verma IM. A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proc Natl Acad Sci USA. 2003;100:1844–1848. doi: 10.1073/pnas.0437912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malone RW, Felgner PL, Verma IM. Cationic liposome-mediated RNA transfection. Proc Natl Acad Sci USA. 1989;86:6077–6081. doi: 10.1073/pnas.86.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santel A, et al. A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene Ther. 2006;13:1222–1234. doi: 10.1038/sj.gt.3302777. [DOI] [PubMed] [Google Scholar]
- 11.Takeshita F, et al. Efficient delivery of small interfering RNA to bone-metastatic tumors by using atelocollagen in vivo. Proc Natl Acad Sci USA. 2005;102:12177–12182. doi: 10.1073/pnas.0501753102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu-Lieskovan S, Heidel JD, Bartlett DW, Davis ME, Triche TJ. Sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing’s sarcoma. Cancer Res. 2005;65:8984–8992. doi: 10.1158/0008-5472.CAN-05-0565. [DOI] [PubMed] [Google Scholar]
- 13.Rozema DB, et al. Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc Natl Acad Sci USA. 2007;104:12982–12987. doi: 10.1073/pnas.0703778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmerman TS, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 15.Akinc A, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26:561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S, Zhao B, Jiang H, Wang B, Ma B. Cationic lipids and polymers mediated vectors for delivery of siRNA. J Controlled Release. 2007;123:1–10. doi: 10.1016/j.jconrel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Lu JJ, Langer R, Chen J. A novel mechanism is involved in cationic lipid-mediated functional siRNA delivery. Mol Pharmaceutics. 2009;6:763–771. doi: 10.1021/mp900023v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akinc A, et al. Development of lipidoid-siRNA formulations for systemic delivery to the liver. Mol Ther. 2009;17:872–879. doi: 10.1038/mt.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frank-Kamenetsky M, et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci USA. 2008;105:11915–11920. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abifadel M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 21.Rashid S, et al. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc Natl Acad Sci USA. 2005;102:5374–5379. doi: 10.1073/pnas.0501652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tybjærg-Hansen A, et al. Association of mutations in the Apolipoprotein B gene with hypercholesterolemia and the Risk of Ischemic Heart Disease. N Engl J Med. 1998;338:1577–1584. doi: 10.1056/NEJM199805283382203. [DOI] [PubMed] [Google Scholar]
- 23.Lee A, et al. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2009;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimm D, Kay MA. Combinatorial RNAi: A winning strategy for the race against evolving targets? Mol Ther. 2007;15:878–888. doi: 10.1038/sj.mt.6300116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kronke J, et al. Alternative approaches for efficient inhibition of hepatitis C virus RNA replication by small interfering RNAs. J Virol. 2004;78:3436–3446. doi: 10.1128/JVI.78.7.3436-3446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- 27.Schnatwinkel C, et al. The Rab5 effector Rabankyrin-5 regulates and coordinates different endocytic mechanisms. PLoS Biol. 2004;2:1363–1379. doi: 10.1371/journal.pbio.0020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Araki N, Egami Y, Watanabe Y, Hatae T. Phosphoinositide metabolism during membrane ruffling and macropinosome formation in EGF-stimulated A431 cells. Exp Cell Res. 2007;313:1496–1507. doi: 10.1016/j.yexcr.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Hamasaki M, Araki N, Hatae T. Association of early endosomal autoantigen 1 with macropinocytosis in EGF-stimulated A431 cells. Anat Rec. 2004;277:298–306. doi: 10.1002/ar.a.20027. [DOI] [PubMed] [Google Scholar]
- 30.Hewlett LJ, Prescott AR, Watts C. The coated pit and macropinocytic pathways serve distinct endosome populations. J Cell Biol. 1994;124:689–703. doi: 10.1083/jcb.124.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hillaireau H, Couvreur P. Nanocarrier’s entry into the cell: Relevance to drug delivery. Cell Mol Life Sci. 2009;66(17):2873–2896. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sekijima Y, Kelly JW, Ikeda S. Pathogenesis of and therapeutic strategies to ameliorate the transthyretin amyloidoses. Curr Pharm Des. 2008;14:3219–3230. doi: 10.2174/138161208786404155. [DOI] [PubMed] [Google Scholar]
- 33.Heyes J, Palmer L, Bremner K, Maclachlan I. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J Controlled Release. 2005;107:276–287. doi: 10.1016/j.jconrel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Jeffs LB, et al. A scalable, extrusion-free method for efficient liposomal encapsulation of plasmid DNA. Pharm Res. 2004;22:362–372. doi: 10.1007/s11095-004-1873-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.