Abstract
CMV reinfections have been associated with damaging congenital infection and adverse outcomes in transplant recipients. To determine the frequency and risk factors for CMV reinfections, 205 seropositive women were followed prospectively. The appearance of new antibody specificity against one of four polymorphic epitopes was considered as evidence of CMV reinfection. About a third of study women (29%) were noted to have CMV reinfection during follow-up. None of the exposures were associated with CMV reinfection. Women with antibodies against ≥1 of the four antigens at baseline had a 63% decreased risk of reinfection suggesting a protective role for strain-specific immunity.
Keywords: cytomegalovirus, reinfection, seroimmune, strain-specific immunity
Cytomegalovirus is a frequent cause of congenital infection and an important cause of sensorineural hearing loss (SNHL) in children worldwide [1, 2]. Preconceptional immunity against CMV only provides incomplete protection against intrauterine transmission and adverse outcomes can occur in infected children born to women seropositive prior to pregnancy [2-6]. CMV reinfections were also associated with adverse outcomes in renal transplant recipients [7].
It is not clear whether transplacental transmission of CMV in women with preexisting seroimmunity is secondary to virus reactivation or infection with a new or different CMV strain (reinfection) during pregnancy. We undertook a prospective study to determine the frequency of CMV reinfections in healthy seropositive women and to understand the various factors associated with such reinfections. Serial serum specimens from the study women were analyzed for strain-specific IgG antibodies against the polymorphic determinants on the envelope glycoproteins gH and gB of CMV using an ELISA method [3, 8].
Methods
Of the 258 CMV IgG seropositive postpartum women enrolled in the study between February 2000 and June 2004, 205 participants had serum samples from at least two visits, and these women constituted the study population. A standardized interview was administered at baseline to obtain demographic characteristics and exposure factors. Standardized prenatal summary information was abstracted onto standard case report forms. The study participants were followed at six-month intervals for up to three years and at each visit, serum samples were obtained and a standard questionnaire was administered to obtain an interval history of STIs, sex partner information, and caring for children. Serum specimens obtained at each visit were stored at -20°C until analysis. The study was approved by the University of Alabama at Birmingham Institutional Review Board for Human Use, and informed consent was obtained from the participants prior to study enrollment.
CMV strain-specific antibody responses were determined based on polymorphisms in antibody binding sites within envelope glycoproteins, gH and gB, between the two prototypic laboratory strains of CMV, AD169 and Towne [8-10]. The detection of new antibody specificities to either epitope (AD169 or Towne) on gH or gB in follow-up serum samples was considered evidence of infection with a new virus strain (reinfection) during the study. One of the 258 women had antibodies to all four antigens at enrolment in the study and was excluded from the analysis. To approximate the average time to reinfection with a new virus strain from study entry, we measured the time from the baseline study visit until the visit during which new antibody specificities were detected.
Recombinant peptides containing antibody combining sites within the amino terminal regions of gH and gB genes present in AD169 and Towne strains of CMV were synthesized and used as antigens to determine strain-specific IgG reactivity as described previously [3, 8]. Strain-specific antibodies against the polymorphic gH and gB regions of CMV were determined using an ELISA method that has been validated in a recent report [8].
The demographic and exposure characteristics were compared between the groups of women with and without CMV reinfection. Statistical significance was determined using Chi-square, Fisher exact or Wilcoxon rank-sum test where appropriate. Univariate odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using the exact method. Multivariate unconditional logistic regression using backward stepwise selection with P<0.10 was used as a cutoff for retention in the model to assess whether exposure factors were associated with CMV reinfection. All data analyses were performed using SAS 9.1 software (SAS Institute, Inc, Cary, NC).
Results
The demographic characteristics of women with CMV reinfection were not different from those without evidence of reinfection. Both groups were predominantly single, African American women and had one previous pregnancy. The mean age for the study women in both groups was 18 years and the study participants on average had 11 years of education. None of the study participants were HIV positive. Twenty-nine percent (59/205) of study women acquired new antibody specificities against gH or gB epitopes, thus were considered to be reinfected. The average time for the appearance of new strain-specific antibodies was 17.8 ± 10.3 months. The median follow-up duration was 35.4 months (range 11-50) for women with reinfection and 30.6 months (range 6-58) for those without reinfection (p=0.15). Forty-nine percent of the reinfection group completed 6 study visits compared with 34% of those without reinfection (p=0.05). A higher proportion of white women had serological evidence of reinfection (10/19, 53%) compared with 26% (48/185) of African American women (p=0.02).
Baseline exposure characteristics for women with and without CMV reinfection were similar. The median number of persons living in the household was 5 and 4 for women with and without reinfection, respectively (p=0.72). The median age of sexual debut was 15 years in both groups. There were no differences between the two groups with regard to the number of lifetime sexual partners (median 3) or the number of sexual partners in the year prior to study enrollment (median 1). About half of the women in each group had a history of sexually transmitted infection (STI). The frequency of gonococcal infection was higher in the group of women without reinfection (25%) compared to those with reinfection (12%, p=0.04). Sixty-eight percent of the women with reinfection and 60% of those without reinfection were involved in the direct care of young children.
The data on various exposure factors during the study were compared between the group of women identified to have been reinfected with new CMV strains and those without reinfection (Table 1). Only white race was significantly associated with CMV reinfection. To further examine the association between potential risk factors and CMV reinfection, we evaluated exposures during the 12 month period prior to the detection of new antibody specificities in women with reinfection and in the year prior to the final study visit for those without reinfection. Again, none of the exposures were found to be associated with reinfection (data not shown).
Table 1. Selected exposures and characteristics with unadjusted ORs and 95% CIs for CMV seropositive women according to the development of new antibody specificity against CMV epitopes (CMV reinfection).
| Variables | CMV Reinfection (n=59), n% |
No CMV Reinfection (n=146), n% |
Unadjusted OR (95% CI) |
|---|---|---|---|
| White Race | 10 (16.9) | 9 (6.2) | 3.11 (1.05-9.15) |
| Maternal age < 19 years | 42 (71.2) | 103 (70.6) | 1.03 (0.51-2.15) |
| Sexual partners > 1 | 57 (96.6) | 143 (98.6) a | 0.39 (0.03-5.65) |
| STIs > 1 | 13 (22.0) | 31 (21.2) | 1.05 (0.46-2.28) |
| Bacterial Vaginosis | 6 (10.2) | 24 (16.4) | 0.58 (0.18-1.56) |
| Chlamydia | 3 (5.1) | 6 (4.1) | 1.25 (0.20-6.09) |
| Herpes Simples Virus | 1 (1.7) | 3 (2.1) | 0.82 (0.01-10.49) |
| Trichomoniasis | 3 (5.1) | 3 (2.1) | 2.55 (0.33-19.54) |
| Syphilis | 0 (0) | 0 (0) | --- |
| Gonorrhea | 0 (0) | 4 (2.7) | 0 (0-2.75) |
| Direct care of children | 41 (69.5) | 95 (65.1) | 1.22 (0.61-2.50) |
| More than 2 Children <6y of age living in the household | 16 (27.1) | 34 (23.3) | 1.23 (0.57-2.56) |
Data available for 145 subjects
Race along with age, new sexual partners and direct care of children in the year prior to reinfection or year prior to the final study visit were entered into a logistic regression model. Race remained the only factor associated with reinfection (aOR 3.11; 95% CI: 1.1-9.2).
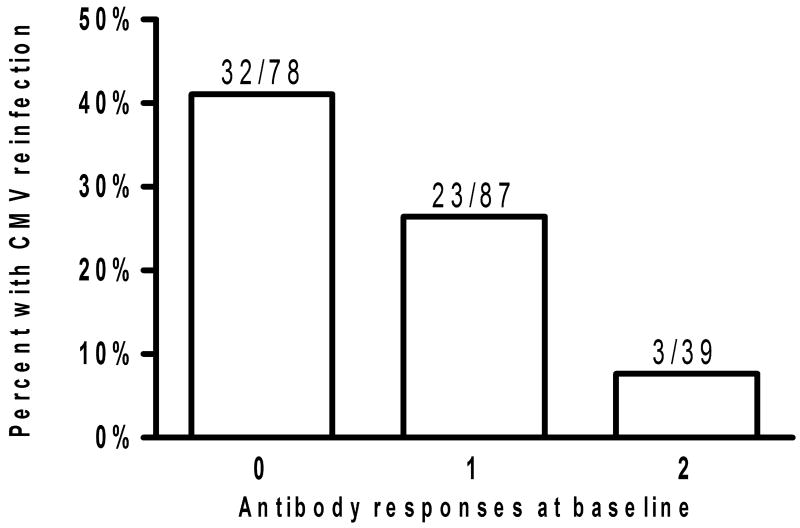
The association between serological responses to the four antigens (AP86, TO86, AP55, TO55) at baseline and the likelihood of CMV reinfection during the study period was determined. Reactivity to strain specific epitopes was shown to persist on average for 21 months. As illustrated in figure 1, women with antibodies against 1 or more antigens at baseline were less likely to be reinfected with a new CMV strain during the study period (OR 0.37; 95% CI: 0.19-0.73).
Figure 1.
Graph showing frequency of CMV reinfection in 205 CMV seropositive women based on number of strain specific antibodies present to the four antigens (AP86, TO86, AP55, TO55) at study enrollment. Women with ≥1 antibody present at baseline were less likely to undergo CMV reinfection during the study period than women with no antibodies at baseline (OR 0.37; 95% CI: 0.19-0.73).
Discussion
The results of this prospective study demonstrate that about a third of CMV seroimmune women (59/205) were infected with a new or different CMV strain during the study period as evidenced by the appearance of new antibody specificities against the linear polymorphic epitopes on gB and gH of CMV. The study women were followed for almost three years and, therefore, the annualized rate of CMV reinfection was about 10%, a rate similar to the frequency of primary CMV infection in the population [11]. The ELISA assay was adapted from the previously described radioimmunoassay to detect strain-specific antibodies against the envelope glycoprotein gH [3] and was validated in a recent study of 96 seropositive and 51 seronegative individuals [8]. A similar strain-specific ELISA assay was employed in a recent study of CMV reinfections in renal transplant recipients [7]. However, about a third of CMV seropositive individuals in the previous study [8] and 46/204 women in the present study did not have detectable serum antibodies against any of the four antigens tested using this assay. This finding suggests that these women are infected with viruses containing gH and/or gB epitope variants not represented in the ELISA assay used in this study. Alternatively, our ELISA might lack the sensitivity to detect low levels of strain-specific antibodies in these women. Therefore, it could be argued that our study may have underestimated the actual frequency of CMV reinfections in the population. It is also possible that the appearance of new antibody specificities could be due to reactivation of endogenous virus. However, this is unlikely as there is no data in the literature in support of this phenomenon and the stability of CMV hypervariable genes has been shown in vitro in renal transplant recipients [12].
We were unable to identify an association between CMV reinfection and any of the known exposure factors for acquisition of CMV including STIs, sexual practices and caring for young children [13]. The demographic and baseline exposure characteristics were similar between the groups of women with and without reinfection. Although more women without CMV reinfection had a history of Gonorrhea at enrollment than those with CMV reinfection, the number of women with Gonorrhea was small and thus, this finding should be interpreted with caution. This study may have underestimated the number of sexually transmitted infections and sexual partners in the population because this information was obtained through interval questionnaires relying on participant recall. To minimize recall bias, the study women were interviewed alone at each visit using a standardized questionnaire. Prenatal medical records were reviewed at enrollment for the results of laboratory studies and dates of sexually transmitted infections. The smaller sample size and study population with similar demographic and exposure characteristics may have led to our inability to identify an association between any of the exposure factors and CMV reinfection.
We did observe that women with a more broadly reactive antibody response at baseline were less likely to be reinfected during the study. Women with strain-specific antibodies to ≥ 1 antigen at baseline had a 63% decreased risk of CMV reinfection during the study (OR 0.37; 95% CI: 0.19-0.73), compared with subjects having no antibodies against any of the four antigens. This reduced risk of reinfection in women with antibodies to ≥1 antigens indicates that individuals infected with multiple CMV strains prior to the study entry were less likely to be reinfected and that strain specific immunity may play an important protective role in infection with new virus strains in seroimmune individuals. A recent study of recombinant CMV gB vaccine suggested that prevention of maternal infection and intrauterine transmission to offspring of previously non-immune women could represent a feasible approach [14]. However, other studies have revealed that natural infection produces higher neutralizing antibody titers and higher titers against epithelial cell entry than sera from recipients of Towne or gB/MF59 vaccine [15]. This could be due to the fact that individuals with natural infection are more likely to develop antibody response against multiple CMV strains whereas vaccines may only induce antibody responses with narrow specificity. Therefore, the traditional vaccine approaches may have a limited success in reducing intrauterine transmission and CMV disease in congenitally infected children in populations with high maternal seroprevalence.
In the present study, we observed a higher frequency of reinfections (10/20) among white women. However, the number of white participants in our study is small and therefore, this association could be due to a sampling bias. In addition, when the exposure factors were examined for Caucasian and African American women independently, we did not detect differences between women with CMV reinfection and without reinfection in either racial group. Since the group of women with CMV reinfection were followed longer than those without serological evidence of reinfection, it is possible that more women in the non-reinfection group could acquire new antibody specificities if they are monitored for longer duration. However, it is unlikely to have had an affect on the lack of an association between various exposure factors and CMV reinfection because of similar demographic and exposure characteristics between the groups.
In summary, the results of the study demonstrate that CMV reinfections are frequent in young, low income African American seroimmune women. Our findings also suggest that in addition to exposure, strain-specific immunity and possibly other yet undefined factors may play an important role in providing protection from infection with new CMV strains in seroimmune individuals.
Acknowledgments
Financial Support: National Institute on Deafness and other Communication Disorders (R01 DC04163, S.B., K23 DC008539, S.R.), the General Clinical Research Center (M01 R00032), and the National Institute of Child Health and Human Development (K12 HD043997).
Footnotes
Potential conflict of interest: None
Presented in part: 33rd International Herpesvirus Workshop, Estoril, Portugal, July 27-August 1, 2008; 11th International CMV and Betaherpes virus workshop, May, 2007. Toulouse, France
References
- 1.Stagno S. Cytomegalovirus. In: Remington JS, Klein JO, Wilson CB, Baker CJ, editors. Infectious Diseases of the Fetus and Newborn Infant. 6th. Philadelphia: W.B. Saunders Company; 2006. pp. 389–424. [Google Scholar]
- 2.Mussi-Pinhata MM, Yamamoto AY, Moura Brito RM, et al. Birth prevalence and natural history of congenital cytomegalovirus infection in a highly seroimmune population. Clin Infect Dis. 2009;49:522–8. doi: 10.1086/600882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. Intrauterine Transmission of Cytomegalovirus to Infants of Women with Preconceptional Immunity. N Engl J Med. 2001;344:1366–71. doi: 10.1056/NEJM200105033441804. [DOI] [PubMed] [Google Scholar]
- 4.Ahlfors K, Ivarsson SA, Harris S. Secondary maternal cytomegalovirus infection--A significant cause of congenital disease. Pediatrics. 2001;107:1227–8. doi: 10.1542/peds.107.5.1227. [DOI] [PubMed] [Google Scholar]
- 5.Ross SA, Fowler KB, Ashrith G, et al. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J Pediatr. 2006;148:332–6. doi: 10.1016/j.jpeds.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Dar L, Pati SK, Patro AR, et al. Congenital cytomegalovirus infection in a highly seropositive semi-urban population in India. Pediatr Infect Dis J. 2008;27:841–3. doi: 10.1097/INF.0b013e3181723d55. [DOI] [PubMed] [Google Scholar]
- 7.Ishibashi K, Tokumoto T, Tanabe K, et al. Association of the outcome of renal transplantation with antibody response to cytomegalovirus strain-specific glycoprotein H epitopes. Clin Infect Dis. 2007;45:60–7. doi: 10.1086/518571. [DOI] [PubMed] [Google Scholar]
- 8.Novak Z, Ross SA, Patro RK, et al. Enzyme-linked immunosorbent assay method for detection of cytomegalovirus strain-specific antibody responses. Clin Vaccine Immunol. 2009;16:288–90. doi: 10.1128/CVI.00281-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer H, Sundqvist VA, Pereira L, Mach M. Glycoprotein gp116 of human cytomegalovirus contains epitopes for strain-common and strain-specific antibodies. J Gen Virol. 1992;73(Pt 9):2375–83. doi: 10.1099/0022-1317-73-9-2375. [DOI] [PubMed] [Google Scholar]
- 10.Urban M, Britt W, Mach M. The dominant linear neutralizing antibody-binding site of glycoprotein gp86 of human cytomegalovirus is strain specific. J Virol. 1992;66:1303–11. doi: 10.1128/jvi.66.3.1303-1311.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colugnati FA, Staras SA, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis. 2007;7:71. doi: 10.1186/1471-2334-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanton D, Westmoreland D, Fox JD, Davison AJ, Wilkinson GW. Stability of human cytomegalovirus genotypes in persistently infected renal transplant recipients. J Med Virol. 2005;75:42–6. doi: 10.1002/jmv.20235. [DOI] [PubMed] [Google Scholar]
- 13.Fowler KB, Pass RF. Risk factors for congenital cytomegalovirus infection in the offspring of young women: exposure to young children and recent onset of sexual activity. Pediatrics. 2006;118:e286–92. doi: 10.1542/peds.2005-1142. [DOI] [PubMed] [Google Scholar]
- 14.Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009;360:1191–9. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui X, Meza BP, Adler SP, McVoy MA. Cytomegalovirus vaccines fail to induce epithelial entry neutralizing antibodies comparable to natural infection. Vaccine. 2008;26:5760–6. doi: 10.1016/j.vaccine.2008.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]