Abstract
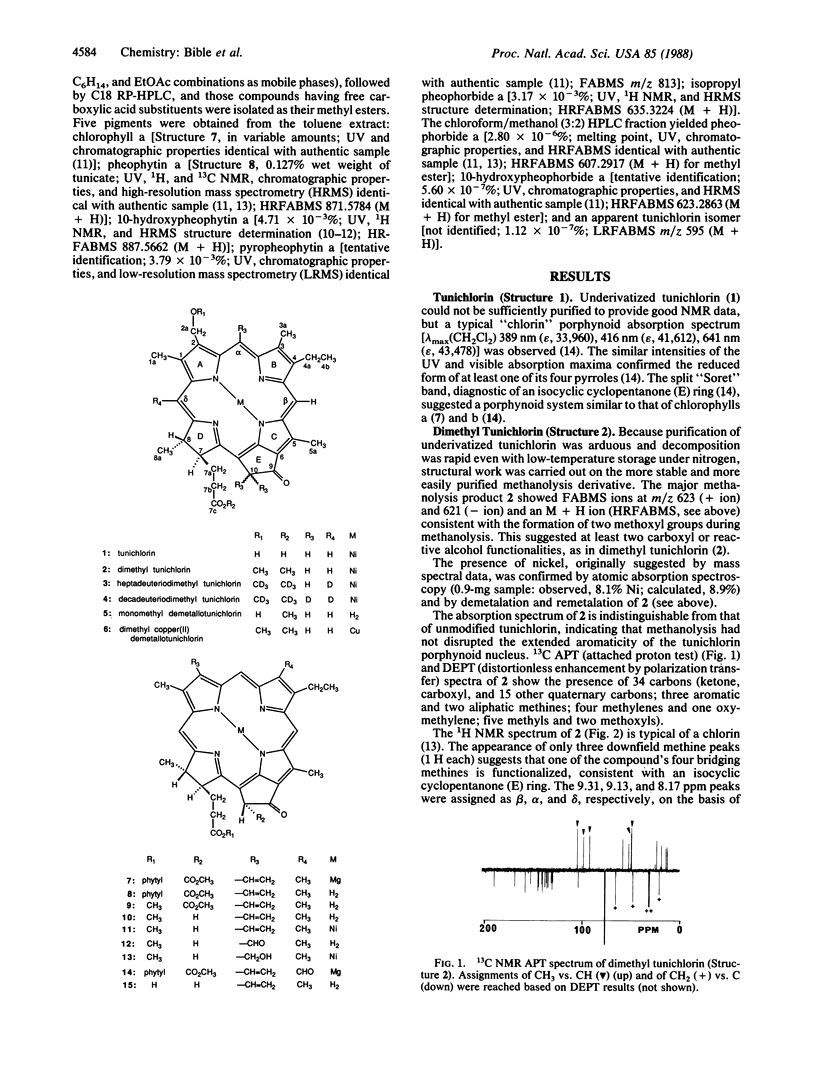
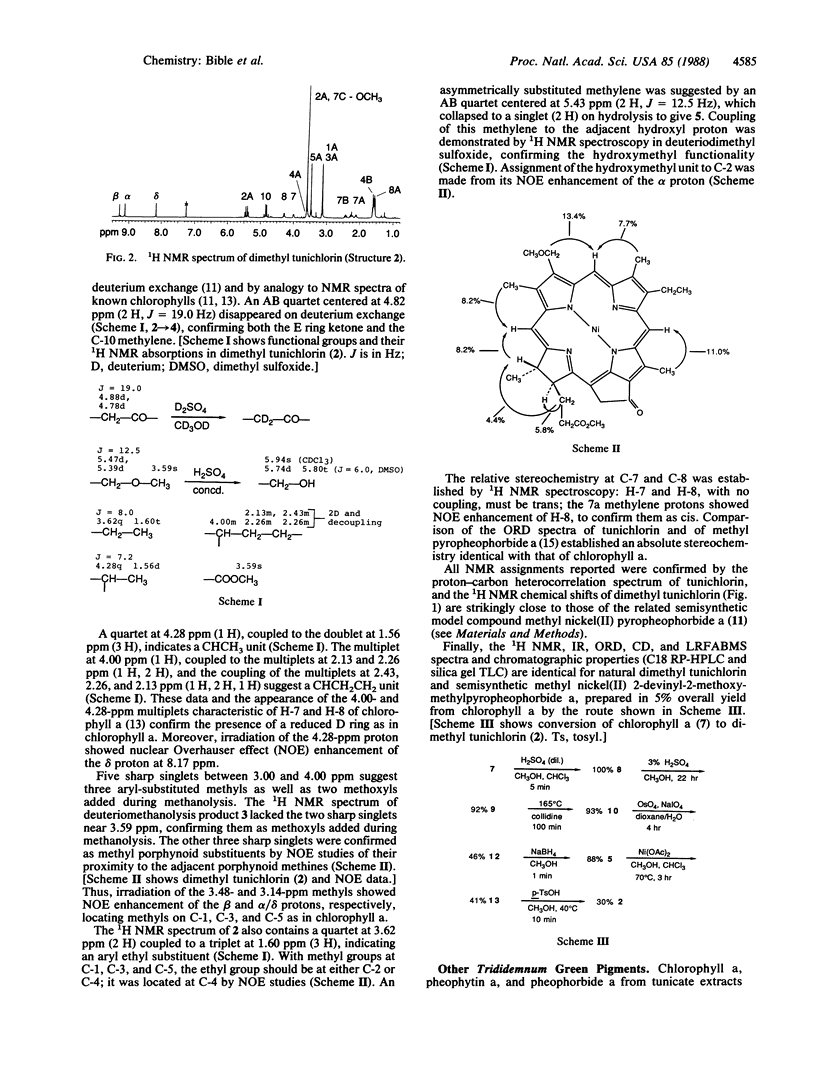
Tunichlorin, a blue-green pigment isolated from the Caribbean tunicate Trididemnum solidum, has been identified as nickel(II) 2-devinyl-2-hydroxymethylpyropheophorbide a by chemical and spectroscopic methods, with confirmation by partial synthesis of dimethyl tunichlorin from chlorophyll a. Nickel chlorins have been reported from geological sources but not from living organisms. Its occurrence in a living system suggests a metabolic role for tunichlorin and may clarify the selective accumulation of nickel by marine tunicates. Because Trididemnum tunicates are associated with algal symbionts, tunichlorin may arise directly from the tunicate, from symbiotic algae, or from tunicate modification of an algal chlorin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruening R. C., Oltz E. M., Furukawa J., Nakanishi K., Kustin K. Isolation of tunichrome B-1, a reducing blood pigment of the sea squirt, Ascidia nigra. J Nat Prod. 1986 Mar-Apr;49(2):193–204. doi: 10.1021/np50044a001. [DOI] [PubMed] [Google Scholar]
- Eskew D. L., Welch R. M., Cary E. E. Nickel: an essential micronutrient for legumes and possibly all higher plants. Science. 1983 Nov 11;222(4624):621–623. doi: 10.1126/science.222.4624.621. [DOI] [PubMed] [Google Scholar]
- Hausinger R. P. Nickel utilization by microorganisms. Microbiol Rev. 1987 Mar;51(1):22–42. doi: 10.1128/mr.51.1.22-42.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz W. The essential trace elements. Science. 1981 Sep 18;213(4514):1332–1338. doi: 10.1126/science.7022654. [DOI] [PubMed] [Google Scholar]
- Patterson G. M., Withers N. W. Laboratory cultivation of prochloron, a tryptophan auxotroph. Science. 1982 Sep 10;217(4564):1034–1035. doi: 10.1126/science.217.4564.1034. [DOI] [PubMed] [Google Scholar]
- Timkovich R., Cork M. S., Taylor P. V. Proposed structure for the noncovalently associated heme prosthetic group of dissimilatory nitrite reductases. Configuration of substituents of acrylochlorin. J Biol Chem. 1984 Dec 25;259(24):15089–15093. [PubMed] [Google Scholar]
- Timkovich R., Cork M. S., Taylor P. V. Proposed structure for the noncovalently associated heme prosthetic group of dissimilatory nitrite reductases. Identification of substituents. J Biol Chem. 1984 Feb 10;259(3):1577–1585. [PubMed] [Google Scholar]
- Witten J. L., Schaffer M. H., O'Shea M., Cook J. C., Hemling M. E., Rinehart K. L., Jr Structures of two cockroach neuropeptides assigned by fast atom bombardment mass spectrometry. Biochem Biophys Res Commun. 1984 Oct 30;124(2):350–358. doi: 10.1016/0006-291x(84)91560-2. [DOI] [PubMed] [Google Scholar]



