Abstract
The health impact of environmental toxins has gained increasing recognition over the years. Polycyclic aromatic hydrocarbons (PAHs) and environmental tobacco smoke (ETS) are known to affect nervous system development in children, but no studies have investigated how polymorphisms in PAH metabolic or detoxification genes affect child cognitive development following PAH exposure during pregnancy. In two parallel prospective cohort studies of nonsmoking African American and Dominican mothers and children in New York City and of Caucasian mothers and children in Krakow, Poland, we explored the effect of gene-PAH interaction on child mental development index (MDI), as measured by the Bayley Scales of Infant Development-Revised (BSID-II). Genes known to play important roles in the metabolic activation or detoxification of PAHs were selected. Genetic variations in these genes could influence susceptibility to adverse effects of PAHs in polluted air. We explored the effects of interactions between prenatal PAH exposure and 21 polymorphisms or haplotypes in these genes on MDI at 12, 24, and 36 months among 547 newborns and 806 mothers from three different ethnic groups: African Americans, Dominicans, and Caucasians. PAHs were measured by personal air monitoring of mothers during pregnancy. Significant interaction effects between haplotypes and PAHs were observed in mothers and their newborns in all three ethnic groups after Bonferroni correction for multiple comparisons. The strongest and most consistent effect observed was between PAH and haplotype ACCGGC of the CYP1B1 gene.
Introduction
There is growing evidence that exposure to ambient and indoor air pollutants adversely affects fetal growth and early childhood neural development (Dejmek et al., 2000; Perera et al., 1999; Perera et al., 2003; Perera et al., 2004; Perera et al., 2006). Polycyclic aromatic hydrocarbons (PAHs) are widespread pollutants commonly found in air, food, and drinking water (International Agency for Research on Cancer, 1983). Airborne PAHs (outdoor or indoor) mainly result from combustion of fossil fuels, tobacco, and other organic materials. Emissions from motor vehicles, electricity generation, and residential heating are generally the major source of PAHs in outdoor urban air, whereas environmental tobacco smoke (ETS) is a major indoor source (Donnenfeld et al., 1993; Lewtas 1994). Perera et al. recently studied the effect of prenatal exposure to airborne PAHs on child development and found that high prenatal exposure to PAHs was associated with a lower mental development index (MDI) and a higher incidence of developmental delay at age three (Perera et al., 2006). Studies on prenatal exposure to ETS have also associated PAH exposure with reduced fetal growth and cognitive function (Windham et al., 1999; Rauh et al., 2004).
The cytochrome P450 genes CYP1A1, CYP1A2 and CYP1B1 have been shown to play important roles in the metabolic activation of PAHs, while PAH detoxification is partially controlled by the glutathione S-transferase (GST) genes GSTM1 and GSTT2 (Kawajiri et al., 1990; Bennett et al., 1999; Abnet et al., 2005). P450 (CYP1A1) is involved in conversion of PAH into DNA-binding intermediates (Chisolm 1981; Hanazawa et al., 2000) and is part of an inducible enzyme system that transforms PAH into epoxide-containing metabolites, some of which are mutagenic and carcinogenic (Nebert 1991). GST genes participate in antioxidant defense within the airways. Oxidative stress in pregnant women can be modified by heritable polymorphisms in several genes, including GSTM1 (Thomas et al., 1997). To our knowledge, no studies have examined the role of genetic polymorphisms in metabolic activation or detoxification of PAHs on child mental development. Here, we use data from two prospective cohort studies of nonsmoking African American and Dominican mothers and children in New York City and nonsmoking Caucasian mothers and children in Krakow, Poland to assess the association of airborne PAH exposure levels child MDI at 12, 24, and 36 months, measured by Bayley Scales of Infant Development-Revised (BSID-II) (Bayley 1993), with the genotype or haplotype of selected candidate genes.
Materials and Methods
Study populations
Subjects were chosen from two independent, parallel studies. One is currently being conducted in New York City (NYC) and the details on the study design have been previously published (Perera et al., 2003). Nonsmoking, self-identified African American and Dominican women residing in Washington Heights, Central Harlem, NYC, and the South Bronx were recruited through the obstetrical services of New York Presbyterian Hospital, Harlem Hospital, or satellite clinics between February 1998 and February 2003. The women carried a backpack containing a portable personal exposure air monitor during the day and kept it at their bedsides at night during a consecutive 48 hr period during the third trimester of pregnancy for PAH measurements. The institutional review board of New York Presbyterian Medical Center approved the study, and informed consent was obtained from all study participants.
The second cohort study is being conducted in Krakow, Poland, a region known to have higher levels of air pollution than NYC, presumably due to coal burning and vehicle emission (Choi et al., 2006). Details on the study design for the Krakow cohort have been previously published (Choi et al., 2006; Jedrychowski et al., 2003). Nonsmoking, pregnant women residing in the Srodmiescie and the Krowodrza-Nowa Huta areas were recruited between November 2000 and March 2003 (Choi et al., 2006). The women carried a backpack containing a portable personal exposure air monitor during the day and kept it at their bedsides at night during a consecutive 48 hr period between the 20th and 30th week of pregnancy for PAH measurements. The study was approved by the ethics committee of the Jagiellonian University, and informed consent was obtained from all subjects.
In Polish study, personal indoor and outdoor PAH exposure was monitored during all three trimesters on a representative subset of 80 women using the same monitoring instrument. High crude correlation coefficients between the second- and third-trimester personal monitoring values were observed, reflecting the short temporal gap between the monitoring periods (mean, 6 weeks; range, 5–10 weeks). In contrast, lower crude correlation coefficients for the nine PAHs were observed between the first and second trimester due to the longer gap between the two monitoring periods (mean, 19 weeks; range, 17–23 weeks).
The seasonal effect of personal PAH monitoring was further investigated in Choi et al. (2008). In the NYC study, PAH exposure was only monitored during the 3rd trimester for the reasons of cost. However, we have found that the seasonal variation in PAH levels is far smaller in NYC than in Poland. In order to further document prenatal exposure to PAHs and to test whether the prenatal personal air concentrations were correlated with prenatal residential PAH levels, we measured PAH levels of indoor air samples collected in sequential two-week periods from a representative subset of homes over six weeks during the final trimester of pregnancy in a parallel cohort in NYC. The levels of pollutants measured in weeks 1–2 correlated significantly with the levels measured in week 2–6. The indoor air levels of the pollutants over the six weeks of monitoring were significantly associated with the levels measured in the single 48 hour prenatal personal air samples. Thus, our single PAHs/pyrene measurements from prenatal personal air are reasonable indicators of chronic prenatal exposure via inhalation (Choi et al., 2006; Choi et al., 2008).
In both cohort studies, maternal blood (30–35 mL) was collected within 1 day postpartum, and umbilical cord blood (30–60 mL) was collected at delivery.
Subjects included in the present analysis are mothers with genotype data (n=178 African Americans, n=282 Dominicans, and n=381 Polish Caucasians) and their newborns with genotype data (n=116 African Americans, n=167 Dominicans, and n=294 Polish Caucasians) (Table 1). Among those are 98 African American mother-newborn pairs, 138 Dominican mother-newborn pairs, and 275 Caucasian mother-newborn pairs. The subset selected based on genotype availability did not differ significantly from the original cohort with respect to the selected demographic and exposure characteristics. The same cohort was previously investigated to explore the effect of airborne PAH exposure on DNA adducts in mothers and their newborns in the three ethnic groups (Wang et al., 2008).
Table 1.
Exposure, biomarker and demographic characteristics of the study population that have maternal or baby genotyped.*†
| AA# | D # | C # | P-value AA vs. D | P-value AA vs. C | P-value D vs. C | |
|---|---|---|---|---|---|---|
| Prenatal PAHs in air (ng/m3)a | 3.32 ± 3.50(n=166) | 3.48 ± 4.10(n=255) | 36.75 ± 46.19(n=328) | 0.58i | <.001i | <.001i |
| Maternal age (year) | 24.21 ± 4.98(n=166) | 25.45 ± 5(n=260) | 27.95 ± 3.72(n=345) | 0.006iv | <.001iv | <.001iv |
| Dietary PAH2 | 46.62 ± 9.63(n=172) | 39.29 ± 6.75(n=257) | 42.54 ± 5.97(n=345) | <.001i | <.001i | <.001i |
| Maternal ETSb | 49% (n=175) | 27% (n=279) | 23% (n=345) | <.001iii | <.001iii | 0.25iii |
| Maternal BMI | 27.13 ± 6.72(n=159) | 25.03 ± 5.85(n=218) | 21.41 ± 3.14(n=345) | 0.003i | <.001i | <.001i |
| >=High schoolc | 56% (n=178) | 64% (n=281) | 90% (n=345) | 0.09iii | <.001iii | <.001iii |
| Genderd | 57% (n=116) | 56% (n=167) | 49%(n=263) | 0.87iii | 0.15iii | 0.16iii |
| MDI_12e | 96.30 ± 10.03(n=111) | 96.52 ± 9.58(n=147) | 100.61 ± 9.95(n=284) | 0.86ii | <.001ii | <.001ii |
| MDI_24e | 88.53 ± 12.28(n=106) | 84.46 ± 11.96(n=143) | 101.04 ± 12.84(n=271) | 0.009ii | <.001ii | <.001ii |
| MDI_36e | 94.22 ± 11.91(n=93) | 88.79 ± 10.38(n=138) | 103.52 ± 10.58(n=255) | <.001ii | <.001ii | <.001ii |
Subjects included in the present analysis are those with genotype data in mothers (178 AA, 282 D, and 381 C) or in their newborns (116 AA, 167 D, and 294 C). Among those, there are 98 mother-baby pairs in AA, 138 mother-baby pairs in D, and 275 mother-baby pairs in C.
No significant difference between the subset and the total population with respect to selected demographic and exposure characteristic.
AA: African American; D: Dominican; C: Caucasian;
mean± sd (n);
Maternal ETS (Environmental Tobacco Smoke): percentage who report a smoker in household;
>=High school: percent of mothers with >=12 years of education;
Gender: percentage of female;
MDI_12, MDI_24, MDI_36: mental development index at months 12, 24, and 36.
two sample t test after natural log transformation;
two sample t test with original scale;
two sample z test;
Wilcoxon signed-rank test;
Dietary PAH is measured through questionnaire on meat consumptions.
Selection of Polymorphisms for Genotyping
Twenty-one genetic polymorphisms from genes CYP1A1, CYP1A2, CYP1B1, GSTM3 GSTM1, and GSTT2 were selected from the SNP500Cancer resource (Packer et al., 2006). For CYP1A1, CYP1A2, and CYP1B1, haplotype-tagging SNPs (ht-SNPs) were selected based on the genomic analysis of these genes (seven in CYP1A1: CYP1A1-78 (rs2198843), CYP1A1-109 (rs1456432), CYP1A1-06 (rs4646903), CYP1A1-15 (rs4646421), CYP1A1-14 (rs2606345), CYP1A1-83 (rs7495708), and CYP1A1-81 (rs2472299); three in CYP1A2: CYP1A2-03 (rs762551), CYP1A2-12 (rs2472304), and CYP1A2-52 (rs4886406); six in CYP1B1): CYP1B1-66 (rs162549), CYP1B1-06 (rs1056837), CYP1B1-05 (rs1056836), CYP1B1-74 (rs162560), CYP1B1-04 (rs10012), and CYP1B1-03 (rs2617266)) by the Breast and Prostate Cancer Cohort Consortium (BPC3) (Hunter et al., 2005) using the approach of Stram et al. (2003). Three SNPs were chosen from GSTT2 (GSTT2-02 (rs2719), GSTT2-01 (rs1622002), and GSTT2-03 (rs140194)), which cover less than 50% of the common genetic variations in this gene. A known nonsynonymous SNP with both functional data and preliminary association in several cancers (Closas et al., 2005) was chosen from GSTM3 (GSTM3-01 (rs7483). A real-time PCR assay (TaqMan) was performed for genotyping (Moore et al., 2005).
As in our previous work (Wang et al., 2008), genotyping calls for individual genetic markers with genotyping rates <75% were deleted for quality control (including three African American mothers, 25 African American newborns, one Dominican mother, 56 Dominican newborns, 14 Polish Caucasian mothers, and 82 Polish Caucasian newborns). All genetic variants had minor allele (defined as the less common allele in the cohort) frequencies greater than 0.05 in all three populations. A goodness-of-fit test for Hardy-Weinberg equilibrium indicated that there were no significant deviations in each population.
Measures of child mental development index (MDI)
The Bayley Scales of Infant Development – Revised (BSID-II) (Bayley, 1993) was used to assess cognitive development at 12, 24, and 36 months of age in all three populations. The BSID-II is the most widely used norm-referenced developmental test for young children. It can be used to diagnose developmental delays and is clinically useful for the identification of children performing in the subnormal range (Burchinal et al., 2000; Sternberg et al, 2001). The BSID-II is known to be sensitive to the developmental effects of toxic exposure. Although cognitive assessments during the first few years of life have limited stability, the predictive power of BSID-II increases after two years. A developmental quotient measured by raw score/chronologic age is yielded with each test, and a mental development index (MDI) is generated based on these data.
Interactions between single genetic markers and PAH on MDI
As previously described (Perera et al., 2004), a composite PAH variable was computed based on eight PAH air concentration measures. Benz[a]anthracene, chrysene, benzo[b]fluroanthene, benzo[k]fluroanthene, B[a]P, indeno[1,2,3-cd]pyrene, disbenz[a,h]anthracene and benzo[g,h,i]perylene were significantly correlated (Geno et al., 1993; Majumdar et al, 1993; Camann et al., 1995). A binary high/low PAH exposure measure was defined by dichotomizing this summed measure at the median (2.45 ng/m3 for NYC African Americans; 2.30 ng/m3 for NYC Dominicans; and 16.89 ng/m3 for Polish Caucasians). Given the significantly higher PAH level in the Polish cohort than in the NYC cohort, the direct comparison of the two cohorts using the continuous PAH measures is not appropriate. In order to examine the genetic effects on MDI at different levels of PAHs within each population, we defined a high PAH and a low PAH exposure group for each ethnic group using the median of the continuous PAH measure. We assessed baby gene-PAH interactions and maternal gene-PAH interactions on MDIs independently at 12, 24, and 36 months, adjusting for significant confounders such as ETS, gender, and whether the mother had high school education, using the following multiple linear regression models:
The possible confounders for which we controlled were independently and significantly associated with MDI measurements, in models not including genetic information, in at least one of the ethnic groups (Supplementary Table S3). We will consider a p-value of <0.00013 as significant for the examination of the 378 single-marker-PAH interactions tested (21 markers, three populations, three outcomes, and maternal and newborn genotypes analyzed separately).
Interactions between haplotype and PAH on MDI
Analysis of haplotypes composed of several genetic markers can sometimes be more powerful than the analysis of single genetic markers for the detection of effects (Akey and Xiong, 2001). We examined haplotypes in CYP1A1, CYP1A2, CYP1B1, and GSTT2. Because inferring the most likely pair of haplotypes and treating them as if they were being directly observed results in information loss and biased confidence intervals for parameter estimates (Morris et al., 2004), we modeled the probabilities of all possible haplotype pairs per subject to account for unphased haplotypes. Linkage disequilibrium (LD) patterns and haplotype structures of CYP1A1, CYP1A2, CYP1B1 and GSTT2 in African Americans, Dominicans, and Caucasians were first analyzed using Haploview software (http://www.broad.mit.edu/mpg/haploview/) (Barrett et al., 2005), then we used the standardized linkage disequilibrium coefficient D′ to measure pairwise LD between any two genetic markers. Haplotype blocks were defined based on the method of Gabriel et al. (2002). Different haplotype blocks might be defined in different populations, and different haplotype frequencies might be obtained for the same haplotype block in different populations. To ease interpretation of the results, for the analysis of interaction between haplotypes and PAH, we disregarded the haplotype block structures and defined the same reference haplotypes for all three groups. We assessed baby haplotype-PAH interactions and maternal-haplotype-PAH interactions on MDI independently at 12, 24, and 36 months, adjusting for the same set of covariates as in the analysis of interaction between single genetic markers and PAHs using the following generalized linear model (GLM):
We will consider a p-value of <0.00038 as significant for the examination of the 132 haplotype-PAH interactions tested (four haplotypes of the CYP1A1 gene tested in African Americans and Dominicans on three MDIs with mother and newborn haplotypes separately, 2 haplotypes of the CYP1A1 gene tested in Caucasians on 3 MDIs with mother and newborn haplotypes separately, 5 haplotypes of the CYP1B1 gene tested in African Americans and Dominicans on 3 MDIs with mother and newborn haplotypes separately, and 2 haplotypes of the CYP1B1 gene tested in Caucasians on 3 MDIs with mother and newborn haplotypes separately). The analysis was conducted using the R package haplo.stats (Schaid, 2004).
Results
Descriptive Analysis
Demographic and exposure characteristics of mothers and their newborns are summarized in Table 1. The subset analyzed in this study is not significantly different from the total population with respect to the selected demographic and exposure characteristics. Prenatal PAH exposure did not differ significantly between NYC African Americans and Dominicans, but was more than 10-fold higher in Polish Caucasians, consistent with the higher levels of air pollution in Poland (Perera et al., 2003).
The genotype frequency distributions of the tested common genetic variants are summarized for African Americans, Dominicans, and Caucasians for mothers and newborns separately (Supplementary Tables S1, S2). Note that for some genetic markers, different ethnic groups have different minor alleles. Supplementary Table S2 shows that the genotype frequency distributions of most genetic markers differ significantly among the three ethnic groups.
Interactions between single genetic markers and PAH on MDI
Assuming a dominant genetic model (minor allele considered as the risk allele) and with a one degree of freedom (df) association test, we identified a number of significant interactions between maternal genetic markers and PAHs, as well as interactions between newborn genetic markers and PAHs, on MDIs at 12, 24, and 36 months in the three populations separately before adjusting for multiple comparisons with Bonferroni correction. However, little consistency was observed, and no single-marker-PAH interaction remains significant at the 0.05 significance level after Bonferroni correction. The results highlighted in Table 2 are before Bonferroni correction, and thus are only suggestive. Only one significant gene-PAH interaction (before Bonferroni correction) at marker GSTT2-02 was observed in both Dominicans and Caucasians on MDI at 24 months and in the same direction. The interaction suggests that MDI at 24 months is lower in newborns whose mothers have a genotype of TT/TG at GSTT2-02 than those whose mothers have a genotype of GG within the low PAH exposure group, but is higher within the high PAH exposure group (interaction β=8.1, unadjusted-p=0.009, n=227 in Dominicans; interaction β= 7.96, unadjusted p=0.015, n=301 in Caucasians).
Table 2.
Significant single-marker-PAH interactions on three year MDIs†
| AA | D | C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genetic marker | Minor allele(%) | βa | P-value | N | Minor allele(%) | βa | P-value | N | Minor allele(%) | βa | P-value | N |
| Part I: Interaction of PAHs and maternal genetic markers on MDIs | ||||||||||||
| Interaction of PAHs and maternal genetic markers on MDI_12 | ||||||||||||
| CYP1A1-14 | T (14%) | 2.57 | 0.49 | 164 | T(36%) | −2.58 | 0.333 | 231 | G(32%) | −4.98 | 0.036 | 316 |
| CYP1A1-78 | G (44%) | 4.26 | 0.211 | 163 | C(36%) | 5.5 | 0.038 | 231 | C(12%) | −2.76 | 0.32 | 316 |
| CYP1A1-109 | A(46%) | 5.38 | 0.121 | 161 | G(36%) | 6 | 0.021 | 231 | G(12%) | −2.58 | 0.356 | 315 |
| CYP1B1-05 | C(27%) | 1.25 | 0.693 | 163 | C(41%) | 1.53 | 0.576 | 230 | G(43%) | −5.5 | 0.033 | 315 |
| CYP1B1-06 | C(29%) | 1.51 | 0.628 | 165 | C(42%) | 2.08 | 0.446 | 232 | T(43%) | −5.62 | 0.03 | 313 |
| GSTT2-03 | A(6%) | −9.6 | 0.046 | 161 | A(17%) | 4.78 | 0.094 | 221 | A(19%) | 2.59 | 0.306 | 313 |
| Interaction of PAHs and maternal genetic markers on MDI_24 | ||||||||||||
| GSTT2-02 | T(12%) | 1.18 | 0.797 | 149 | T(33%) | 8.1 | 0.009 | 227 | G(49%) | 7.96 | 0.015 | 301 |
| GSTT2-03 | A(6%) | −11 | 0.091 | 148 | A(17%) | 2.85 | 0.42 | 217 | A(19%) | 6.86 | 0.023 | 298 |
| Interaction of PAHs and maternal genetic markers on MDI_36 (none) | ||||||||||||
| Part II: Interaction of PAHs and newborn genetic markers on MDIs | ||||||||||||
| Interaction of PAHs and newborn genetic marker on MDI_12 | ||||||||||||
| GSTM3-01 | A(8%) | 12.5 | 0.041 | 99 | A (24%) | −4.39 | 0.195 | 123 | A(31%) | −3.6 | 0.179 | 215 |
| Interaction of PAHs and newborn genetic marker on MDI_24 | ||||||||||||
| CYP1A1-83 | G(49%) | −15 | 0.02 | 51 | G(34%) | −2.38 | 0.633 | 93 | G(15%) | 1.05 | 0.824 | 131 |
| CYP1B1-03 | T(23%) | −4.6 | 0.34 | 92 | T(20%) | −9 | 0.038 | 123 | T(32%) | −0.5 | 0.889 | 227 |
| GSTM3-01 | A(8%) | 3.91 | 0.63 | 95 | A(24%) | −9.3 | 0.021 | 121 | A(31%) | 4.22 | 0.231 | 205 |
| GSTT2-03 | A(6%) | NA* | NA | NA | A(15%) | 10.5 | 0.029 | 122 | A(19%) | 0.75 | 0.829 | 227 |
| Interaction of PAHs and newborn genetic marker on MDI_36 | ||||||||||||
| CYP1B1-66 | T(18%) | 1.75 | 0.747 | 90 | T(14%) | 3.44 | 0.407 | 123 | T(19%) | 5.72 | 0.041 | 217 |
| CYP1A1-06 | C(26%) | 10 | 0.035 | 87 | C(19%) | −8.6 | 0.023 | 124 | C(9%) | −2.2 | 0.568 | 215 |
| CYP1A1-15 | T(37%) | 6.27 | 0.196 | 90 | T(25%) | −7.8 | 0.029 | 123 | T(9%) | −2.4 | 0.521 | 217 |
| CYP1A1-81 | T(41%) | −6.2 | 0.21 | 89 | T(43%) | 8 | 0.031 | 125 | T(33%) | −1.6 | 0.549 | 213 |
| CYP1A2-03 | C(42%) | −5.3 | 0.294 | 88 | C(42%) | 8.5 | 0.023 | 123 | C(33%) | −0.8 | 0.77 | 211 |
| CYP1B1-03 | T(23%) | −5.6 | 0.254 | 91 | T(20%) | −8.3 | 0.027 | 122 | T(32%) | 1.3 | 0.641 | 215 |
| GSTM3-01 | A(8%) | 17.6 | 0.009 | 84 | A(24%) | −1.77 | 0.639 | 115 | A(31%) | 3.48 | 0.231 | 194 |
| GSTT2-03 | A(6%) | NA* | NA | NA | A(15%) | 10.4 | 0.011 | 121 | A(19%) | −4.2 | 0.137 | 216 |
Unadjusted p-values are listed; the highlighted ones are the significant ones BEFORE Bonferroni correction (the Bonferroni adjusted significance level is 0.00013, which adjusted for number of single markers tested, number of populations tested, and number of outcomes tested, please refer to the test for more detail).
Regression coefficients of the gene-PAH interactions from the linear regression models.
No estimation can be found because of collinearity between genetic polymorphism and other covaraites in the model.
We found both maternal markers and newborn markers to interact with PAH on MDIs when there is no genetic main effect. For example, in Caucasians, maternal markers CYP1A1-14, CYP1B1-05 and CYP1B1-06 significantly interact with PAH on MDI at 12 months, but do not have main effects on MDI at 12 months; maternal markers GSTT2-02 and GSTT2-03 significantly interact with PAH on MDI at 24 months, but do not have main effects on MDI at 24 months. We also observed some genetic markers to have main effects on MDIs but do not interact with PAH on MDIs. For example, in Caucasians, maternal markers CYP1A1-15 and CYP1B1-66 have significant main effects but do not significantly interact with PAH on MDI at 12 months; newborn marker CYP1B1-174 has significant main effects but does not significantly interact with PAH on MDI at 36 months. However, as gene-environment interactions are the focus of this study, we do not formally report the genetic main effects here but summarize the main effects of genetic markers in the Supplementary Materials (Supplementary Tables S4 and S5).
Interactions between haplotype and PAH on MDI
Although the analysis of LD structure and haplotype blocks (Supplementary Materials) suggested different haplotype block structures in the three populations, CYP1A1 and CYP1B1 were considered to have the same haplotype structure in the haplotype-PAH interaction analysis to facilitate the interpretation of the results. For CYP1A2 and GSTT2, either completely different haplotype blocks were defined or no haplotype block was defined in the three populations. Thus, we did not consider haplotype-PAH interactions for these two genes. Note that the same reference haplotype was selected for all three populations for analysis of CYP1A1 and CYP1B1 to facilitate interpretation. Haplotypes frequencies of the CYP1A1 and CYP1B1 genes were summarized separately for the three populations (Supplementary Tables S6, S7). Significant effects of interactions between haplotypes of the CYP1A1 gene (Table 3) or haplotypes of the CYP1B1 gene (Table 4) and PAHs on MDIs at three years were observed after Bonferroni correction.
Table 3.
| AA | D | C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Part I: Interaction between PAHs and maternal haplotypes of CYP1A1 gene on MDIs | |||||||||
| Haplotype | βa | P-value | N | βa | P-value | N | βa | P-value | N |
| MDI_12 | MDI_12 | MDI_12 | |||||||
| CGCTGG | −2.60 | 0.20 | 165 | 4.42 | 0.00145 | 233 | −3.49 | <.0001 | 318 |
| GATCGA | −1.35 | 0.46 | 165 | 2.12 | 0.16 | 233 | −4.45 | <.0001 | 318 |
| CGTTGG | −10.64 | <.0001 | 165 | 1.66 | 0.046 | 233 | NA§ | NA | NA |
| MDI_24 | MDI_24 | MDI_24 | |||||||
| CGCTGG | −1.24 | 0.53 | 152 | 5.75 | <.0001 | 229 | 2.24 | 0.0041 | 303 |
| MDI_36 | MDI_36 | MDI_36 | |||||||
| CGCTGG | −2.68 | 0.25 | 149 | 3.57 | <.0001 | 225 | −1.11 | 0.069 | 289 |
| GATCGA | −3.23 | 0.15 | 149 | 3.62 | <.0001 | 225 | 0.30 | 0.61 | 289 |
| CGTTGG | −7.51 | <.0001 | 149 | 1.89 | 0.053 | 225 | NA | NA | NA |
| CGTCGG | −7.40 | 0.0021 | 149 | 6.68 | <.0001 | 225 | NA | NA | NA |
| Part II: Interaction between PAHs and newborn haplotypes of CYP1A1 gene on MDIs | |||||||||
| Haplotype | MDI_12 | MDI_12 | MDI_12 | ||||||
| CGTTGG | −7.67 | 0.038 | 106 | 5.78 | <.0001 | 133 | NA | NA | NA |
| CGTCGG | −0.31 | 0.91 | 106 | 9.89 | <.0001 | 131 | NA | NA | NA |
| MDI_24 | MDI_24 | MDI_24 | |||||||
| CGCTGG | 2.00 | 0.50 | 101 | −4.81 | 0.14 | 131 | −5.32 | <.0001 | 230 |
| CGTTGG | −6.90 | <.0001 | 101 | −0.64 | 0.80 | 131 | NA | NA | NA |
| MDI_36 | MDI_36 | MDI_36 | |||||||
| CGCTGG | 11.95 | 0.00062 | 91 | −5.29 | 0.08 | 126 | −4.24 | <.0001 | 219 |
| CGTCGG | 1.10 | 0.78 | 91 | −1.06 | 0.54 | 126 | −4.17 | <.0001 | 219 |
Unadjusted p-values are listed; the highlighted ones are the significant ones AFTER Bonferroni correction (the Bonferroni adjusted significance level is 0.00036, which adjusted for number of haplotypes tested, number of populations tested, and number of outcomes tested, please refer to the test for more detail).
Analyses were restricted to haplotypes with frequencies greater or equal to 0.05; the reference haplotye is GATCTA.
Regression coefficients of haplotype-PAH interactions from the linear regression models.
Haplotype does not exist in Caucasian cohort.
Table 4.
| AA | D | C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Part I: Interaction between PAHs and maternal haplotypes of CYP1B1 gene on MDIs | |||||||||
| Haplotype | βa | P-value | N | βa | P-value | N | βa | P-value | N |
| MDI_12 | MDI_12 | MDI_12 | |||||||
| TTGACC | −4.41 | <.0001 | 165 | −1.26 | 0.044 | 233 | −1.87 | <.0001 | 318 |
| ATGGGT | −2.14 | 0.0025 | 165 | −8.6 | <.0001 | 233 | NA§ | NA | NA |
| ACCGGC | 2.94 | <.0001 | 165 | NA§ | NA | NA | NA§ | NA | NA |
| MDI_24 | MDI_24 | MDI_24 | |||||||
| TTGACC | 1.88 | 1 | 152 | 4.95 | <.0001 | 229 | −4.50 | <.0001 | 303 |
| ACCGGT | −5.06 | <.0001 | 152 | −0.30 | 0.73 | 229 | 0.93 | 0.16 | 303 |
| ATGGGT | −4.018 | 0.0001 | 152 | 3.49 | 0.0001 | 229 | NA§ | NA | NA |
| ACCGGC | 3.10 | 0.0002 | 152 | NA§ | NA | NA | NA§ | NA | NA |
| MDI_36 | MDI_36 | MDI_36 | |||||||
| TTGACC | −5.97 | <.0001 | 149 | 5.69 | <.0001 | 225 | 1.23 | 0.0036 | 289 |
| ACCGCC | NA§ | NA | NA | 6.45 | <.0001 | 225 | −1.06 | 0.017 | 289 |
| ACCGGT | 5.53 | <.0001 | 149 | −1.90 | 0.027 | 225 | 0.96 | 0.077 | 289 |
| ATGGGC | −7.13 | <.0001 | 149 | 4.87 | <.0001 | 225 | NA§ | NA | NA |
| ATGGGT | −6.90 | <.0001 | 149 | −1.05 | 0.21 | 225 | NA§ | NA | NA |
| ACCGGC | 4.90 | <.0001 | 149 | NA§ | NA | NA | NA§ | NA | NA |
| Part II: Interaction between PAHs and newborn haplotypes of gene on MDIs CYP1B1 | |||||||||
| Haplotype | MDI_12 | MDI_12 | MDI_12 | ||||||
| ATGGGT | −5.26 | <.0001 | 106 | 3.95 | 0.0012 | 133 | NA§ | NA | NA |
| MDI_24 | MDI_24 | MDI_24 | |||||||
| ATGGGT | −8.30 | <.0001 | 101 | −8.94 | <.00001 | 131 | NA§ | NA | NA |
| MDI_36 | MDI_36 | MDI_36 | |||||||
| TTGACC | 0.84 | 0.83 | 91 | 3.35 | 0.21 | 126 | 5.56 | <.0001 | 219 |
| ATGGGT | −12.54 | 1 | 91 | −9.26 | <.00001 | 126 | NA§ | NA | NA |
Unadjusted p-values are listed; the highlighted ones are the significant ones AFTER Bonferroni correction (the Bonferroni adjusted significance level is 0.00036, which adjusted for number of haplotypes tested, number of populations tested, and number of outcomes tested, please refer to the test for more detail).
Analyses were restricted to haplotypes with frequencies greater or equal to 0.05; the reference haplotye is ATGGCC.
Regression coefficients of haplotype-PAH interactions from the linear regression models.
Haplotype does not exist in Caucasian cohort or haplotype frequency is less than 0.05 in African American and Dominican cohorts.
To summarize, for the CYP1A1 gene (Table 3), we found that Caucasian newborn haplotypes significantly interact with PAHs on MDI at 2 year and 3 year after Bonferroni correction and in the same direction; Dominican maternal haplotypes significantly interact with PAHs on MDI at 2 year and 3 year after Bonferroni correction but in the opposite direction as the Caucasian newborn haplotype; African American maternal haplotypes and African American newborn haplotypes significantly interact with PAHs after Bonferroni correction and in the same direction but on different years’ MDIs. Note that haplotype GATCTA, which is the most frequent haplotype in Dominican and Caucasian populations but not in African Americans was selected as the reference haplotype for all three populations. Some consistent results of haplotype-PAH interactions were also observed for the CYP1B1 gene (Table 4). One consistent finding is the haplotype-PAH interactions at the gene CYP1B1 in African Americans in all three years, where newborns whose mothers have haplotype ACCGGC of the CYP1B1 gene have higher MDIs than newborns whose mothers have the reference haplotype ATGGCC within the low PAH group and the difference is significantly bigger in high PAH exposure group (with unadjusted p-values <.0001, =.0002, and <.0001 respectively, Figure 1). Note that haplotype ATGGCC, which is the most frequent haplotype in African American and Dominican populations but not in Caucasians was selected as the reference haplotype for all three populations. Although we picked a common reference haplotype for all three populations to easy the interpretation, we would like to point out that some interesting results might be missed in African Americans for the CYP1A1 gene, and some interesting results might be missed in Caucasians for the CYP1B1 gene.
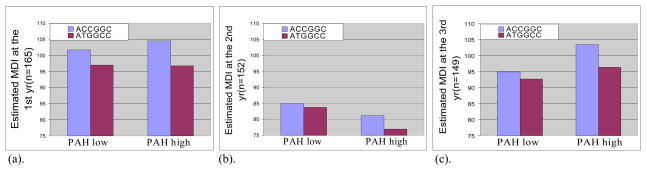
Figure 1.
Interaction effect between maternal haplotype ACCGGC of the CYP1B1 gene and PAHs in African Americans on (a) MDI at 12 months; (b) MDI at 24 months; and (c) MDI at 36 months. More specifically, newborns whose mothers have haplotype ACCGGC have higher MDIs than newborns whose mother have haplotype ATGGCC within the low PAH exposure group and the difference is bigger in high PAH exposure group (interaction β = 2.94, unadjusted-p=<0.0001, n=165 on MDI at 12 months; interaction β = 3.10, unadjusted-p=0.0002, n=152 on MDI at 24 months; and interaction β = 4.90, unadjusted-p=<.0001, n=149 on MDI at 36 months).
Discussion
Our results indicate that MDI at young ages can be modulated by common genetic variants in the key genes CYP1A1, CYP1A2, CYP1B1, GSTT2, and GSTM1. Interaction effects between single genetic markers (ht-SNPs) and PAHs were observed in African American, Dominican, and Caucasian mothers and their newborns before Bonferroni correction for multiple comparisons, with more gene-PAH interaction effects observed in African Americans and Dominicans than in Caucasians. However, little consistency was observed across ethnic groups and across different MDI ages. No single marker-PAH interaction remains significant after Bonferroni correction. The significant interactions between single genetic markers and PAHs in Table 2 are thus only suggestive.
Pairwise LD analysis suggests that the same haplotype block with six SNPs (CYP1A1-78, CYP1A1-109, CYP1A1-06, CYP1A1-15, CYP1A1-14, and CYP1A1-83) was defined in the three populations for the CYP1A1 gene (Supplementary Figure S1), for which similar LD patterns were observed in both mothers and newborns. The LD structure of the CYP1A2 gene and CYP1B1 gene differed substantially in the three populations (Supplementary Figures S2 and S3), which is not surprising because of the differences in population genetic histories and because of the possible selection bias of the chosen SNPs based on the Caucasians in the Breast and Prostate Cancer Cohort Consortium (BPC3) (Hunter et al., 2005). No haplotype block was defined for the gene GSTT2 in the three populations (Supplementary Figure S4). Despite different haplotype blocks defined in the three populations, CYP1A1 and CYP1B1 were considered to have the same haplotype structure in the haplotype-PAH interaction analysis to facilitate interpretation of the results.
We observed more consistent and stronger interaction effects between haplotypes and PAHs than between single markers and PAHs in African American, Dominican, and Caucasian mothers and their newborns. This is consistent with what has been observed by researchers. Clark (2004) in a review paper discussed the role of haplotypes in candidate gene studies and concluded that there were three possible reasons why the phased haplotype should be an improvement over single genetic markers. First, the folding and other properties of the polypeptide chains from the protein products of the candidate genes may depends on particular combination of amino acid. Second, variation in population is inherently structured into haplotypes. Third, the statistical power of association tests with phased haplotypes is likely to be improved because of the reduction in dimension. The consistent findings on interactions between CYP1A1 and CYP1B1 haplotypes and PAHs in all three populations may suggest that CYP1A1 and CYP1B1 are important effect modifiers for MDIs at early ages. Our previous study on DNA adducts (Wang et al, 2008) also suggested that CYP1A1 and CYP1B1 might be important effect modifiers in Caucasians. Savas et al. (1997) have found that CYP1B1 might be an important contributor to activation of PAHs particularly in extra hepatic tissues that are susceptible to cancer where CYP1B1 in contrast to CYP1A1 is constitutively expressed. Notably, we observed that more interactions between maternal genetic markers/haplotypes and PAH had effects on MDIs than did interactions between newborn genetic markers/haplotypes and PAH. At this point, we have no compelling explanation for this difference, since in theory both maternal and fetal genotypes could be important in determining the metabolic fate and toxicity of PAH.
We note that the study examines a modest sample of subjects, which limited the study power in detecting gene-environment interactions. Moreover, the relationships observed for low-income minority women might be different for women of other races or ethnic, cultural, or socioeconomic backgrounds. Furthermore, it is possible that high PAH levels may be associated with living near an exposure source, such as a bus route or garage. This may lead to some confounding socioeconomic factors that were uncontrolled even within our low-income population. A strength of the study is the ability to explore gene-PAH interactions and haplotype-PAH interactions using ht-genetic-markers from genes known to be involved in metabolism and detoxification of PAHs and are relevant to multiple effects of the same pollutants, MDI and environmental monitoring. Another strength of the study is the examination of the possible confounding variables such as dietary PAH and maternal ETS which makes the effect of the prenatal PAHs in the air more accurate. More research efforts are needed to examine these relationships in replication studies. These studies should include fine mapping to identify variables whose causality can be tested by corroborative laboratory analyses.
Supplementary Material
Acknowledgments
Support through grants P01 009600, R01 ES08977, R01 ES111158, R01 ES012468 and ES09089 from the National Institute of Environmental Health Sciences; grants R827027 and R8260901 from the U.S. Environmental Protection Agency; Herbert Irving Cancer Center; Core grant 5P30 CA 13696-23;Gladys and Roland Harriman Foundation; and the New York Community Trust is gratefully acknowledged. All authors declare no conflict of interest on this work.
References
- Abnet CC, Fagundes RB, Strickland PT, Kamangar F, Roth MJ, Taylor PR, Dawsey SM. The influence of genetic polymorphisms in Ahr, CYP1A1, CYP1A2, CYP1B1, GST M1, GST T1 and UGT1A1 on urine 1-hydroxypyrene glucuronide concentrations in healthy subjects from Rio Grande do Sul, Brazil. Carcinogenesis. 2005;28:112–117. doi: 10.1093/carcin/bgl131. [DOI] [PubMed] [Google Scholar]
- Akey J, Xiong M. Haplotypes vs. single marker linkage disequilibrium tests: what do we gain? Eur J Hum Genet. 2001;9:291–300. doi: 10.1038/sj.ejhg.5200619. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant Development. 2. San Antonio: Psychological Corp; 1993. [Google Scholar]
- Bennett WP, Alavanja MCR, Blomeke B, Vahakangas KH, Castrén K, Welsh JA, Bowman ED, Khan MA, Flieder DB, Harris CC. Environmental Tobacco Smoke, Genetic Susceptibility, and Risk of Lung Cancer in Never-Smoking Women. J of the Inter. Cancer Institute. 1999;91:2009–2014. doi: 10.1093/jnci/91.23.2009. [DOI] [PubMed] [Google Scholar]
- Burchinal MR, Roberts JE, Riggins R, Zeisel SA, Neebe E, Bryant D. Relating quality of center-based child care to early cognitive and language development longitudinally. Child Dev. 2000;71:338–357. doi: 10.1111/1467-8624.00149. [DOI] [PubMed] [Google Scholar]
- Camann DE, Harding HJ, Clothier JM, Kuchibhatla RV, Bond AE. Dermal and in-home exposure of the farm family to agricultural pesticides. Measurement of Toxic and Related Air Pollutants: Proceedings of an International Symposium; Research Triangle Park, NC: Air & Waste Management Association; 1995. pp. 548–554. [Google Scholar]
- Chisolm RF. Behavioral and mental health effects of the three mile island accident on nuclear workers: a preliminary report. Ann N Y Acad Sci. 1981;365:134–145. doi: 10.1111/j.1749-6632.1981.tb18127.x. [DOI] [PubMed] [Google Scholar]
- Choi H, Jedrychowski W, Spengler J, Camann DE, Whyatt RM, Rauh V, Tsai WY, Perera FP. International Studies of Prenatal Exposure to Polycyclic Aromatic Hydrocarbons and Fetal Growth. Environ Health Perspect. 2006;114:1744–1750. doi: 10.1289/ehp.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Perera F, Pac A, Wang L, Flak E, Mroz E, Jacek R, Chai-Onn T, Jedrychowski W, Masters E, Camann D, Spengler J. Estimating Individual-Level Exposure to Airborne Polycyclic Aromatic Hydrocarbons throughout the Gestational Period Based on Personal, Indoor, and Outdoor Monitoring. Environ Health Perspect. 2008;116:1509–1518. doi: 10.1289/ehp.10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. The Role of Haplotypes in Candidate Gene Studies. Genet Epidemiol. 2004;27:321–333. doi: 10.1002/gepi.20025. [DOI] [PubMed] [Google Scholar]
- Closas GM, Malats N, Silverman D, Dosemeci M, Kogevinas M, Hein DW, Tardón A, Serra C, Carrato A, Closas RG, Lloreta J, Vinyals GC, Yeager M, Welch R, Chanock S, Chatterjee N, Wacholder S, Samanic C, Torà M, Fernández F, Real FX, Rothman N. NAT2 slow acetylation and GSTM1 null genotypes increase bladder cancer risk: results from the Spanish Bladder Cancer Study and meta-analyses. Lancet. 2005;366:649–659. doi: 10.1016/S0140-6736(05)67137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejmek J, Solansky I, Benes I, Lenicek J, Sram RJ. The impact of polycyclic aromatic hydrocarbons and fine particles on pregnancy outcome. Environ Health Perspect. 2000;108:1159–1164. doi: 10.1289/ehp.001081159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenfeld AE, Pulkkinen A, Palomaki GE, Knight GJ, Haddow JE. Simultaneous fetal and maternal cotinine levels in pregnant women smokers. Am J Obstet Gynecol. 1993;68:781–782. doi: 10.1016/s0002-9378(12)90818-2. [DOI] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Cordero SNL, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Geno PW, Camann DE, Villalobos K, Lewis RG. Analytical methods for assessing the exposure of farmers and their families to pesticides. Measurement of Toxic and Related Air Pollutants: Proceedings of the 1993 U.S. EPA/A&WMA International Specialty Conference; Research Triangle Park, NC: Air & Waste Management Association; 1993. pp. 698–705. [Google Scholar]
- Hanazawa T, Kharitonov SA, Barnes PJ. Increased nitrotyrosine in exhaled breath condensate of patients with asthma. Am J Respir Crit Care Med. 2000;162:1273–6. doi: 10.1164/ajrccm.162.4.9912064. [DOI] [PubMed] [Google Scholar]
- Hunter DJ, Riboli E, Haiman CA, Albanes D, Altshuler D, Chanock SJ, Hayes RB, Henderson BE, Kaaks R, Stram DO, Thomas G, Thun MJ, Blanche H, Buring JE, Burtt NP, Calle EE, Cann H, Canzian F, Chen YC, Colditz GA, Cox DG, Dunning AM, Feigelson HS, Freedman ML, Gaziano JM, Giovannucci E, Hankinson SE, Hirschhorn JN, Hoover RN, Key T, Kolonel LN, Kraft P, Le Marchand L, Liu S, Ma J, Melnick S, Pharaoh P, Pike MC, Rodriguez C, Setiawan VW, Stampfer MJ, Trapido E, Travis R, Virtamo J, Wacholder S, Willett WC. A candidate gene approach to searching for low-penetrance breast and prostate cancer genes. Nat Rev Cancer. 2005;5:977–985. doi: 10.1038/nrc1754. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans. Lyon, France: International Agency for Research on Cancer; 1983. Polynuclear aromatic compounds. Part l. Chemical, environmental, and experimental data; pp. 1–451. [PubMed] [Google Scholar]
- Jedrychowski W, Whyatt RM, Camann DE, Bawle UV, Peki K, Spengler JD, Dumyahn TS, Penar A, Perera FP. Effect of prenatal PAH exposure on birth outcomes and neurocognitive development in a cohort of newborns in Poland. Study design and preliminary ambient data. Int J Occup Med Environ Health. 2003;16:21–29. [PubMed] [Google Scholar]
- Kawajiri K, Nakachi K, Imai K, Hayashi S, Watanabe J. Individual differences in lung cancer susceptibility in relation to polymorphisms of P-450IA1 gene and cigarette dose. Princess Takamatsu Symp. 1990;21:55–61. [PubMed] [Google Scholar]
- Lewtas J. Human exposure to complex mixtures of air pollutants. Toxicol Lett. 1994;72:163–169. doi: 10.1016/0378-4274(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Majumdar TK, Camann DE, Geno PW. Analytical method for the screening of pesticides and polynuclear aromatic hydrocarbons from house dust: Proceedings of the 1993 U.S. EPA/A&WMA International Specialty Conference; Research Triangle Park, NC: Air & Waste Management Association; 1993. pp. 685–690. [Google Scholar]
- Moore LE, Huang WY, Chatterjee N, et al. GSTM1, GSTT1, and GSTP1 polymorphisms and risk of advanced colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2005;14:1823–1827. doi: 10.1158/1055-9965.EPI-05-0037. [DOI] [PubMed] [Google Scholar]
- Morris AP, Whittaker JC. Balding DJ: Little loss of information due to unknown phase for fine scales linkage mapping with single-nucleotide-polymorphism genotype data. Am J Hum Genet. 2004;74:945–953. doi: 10.1086/420773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW. Role of genetics and drug metabolism in human cancer risk. Mutat Res. 1991;247:267–281. doi: 10.1016/0027-5107(91)90022-g. [DOI] [PubMed] [Google Scholar]
- Packer BR, Yeager M, Burdett L, Welch R, Beerman M, Qi LQ, Sicotte H, Staats B, Acharya M, Crenshaw A, Eckert A, Puri V, Gerhard DS, Chanock SJ. SNP500Cancer: a public resource for sequence validation, assay development, and frequency analysis for genetic variation in candidate genes. Nucleic Acids Res. 2006;34:617–621. doi: 10.1093/nar/gkj151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Jedrychowski W, Rauh V, Whyatt RM. Molecular epidemiologic research on the effects of environmental pollutants on the fetus. Environ Health Perspect. 1999;107:451–460. doi: 10.1289/ehp.99107s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Tsai WY, Kinney P, Camann D, Barr D, Bernert T, Garfinkel R, Tu YH, Diaz D, Dietrich J, Whyatt RM. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111:201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Tang D, Tu YT, Cruz LA, Borjas M, Bernert T, Whyatt RM. Biomarkers in Maternal and Newborn Blood Indicate Heightened Fetal Susceptibility to Procarcinogenic DNA Damage. Environ Health Perspect. 2004;112:1133–1136. doi: 10.1289/ehp.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera PR, Rauh V, Whyatt RM, Tsai WY, Tang DL, Diaz D, Hoepner L, Barr D, Tu YH, Camann D, Kinney P. Effect of Prenatal Exposure to Airborne Polycyclic Aromatic Hydrocarbons on Neurodevelopment in the First 3 Years of Life among Inner-City Children. Environ Health Perspect. 2006;114:1287–1292. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Whyatt RM, Garfinkel R, Andrews H, Hoepner L, Reyes A, Diaz D, Camann D, Perera FP. Developmental effects of exposure to environmental tobacco smoke and material hardship among innercitychildren. J Neurotoxicol Teratol. 2004;26:373–385. doi: 10.1016/j.ntt.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savas U, Carstens CP, Jefcoate CR. Biological Oxidations and P450 Reactions Recombinant Mouse CYP1B1 Expressed in Escherichia coli Exhibits Selective Binding by Polycyclic Hydrocarbons and Metabolism Which Parallels C3H10T1/2 Cell Microsomes, but Differs from Human Recombinant CYP1B1. Arch Biochem Biophys. 1997;347:181–192. doi: 10.1006/abbi.1997.0339. [DOI] [PubMed] [Google Scholar]
- Schaid DJ. Evaluating Associations of Haplotypes With Traits. Genet Epidemiol. 2004;27:348–364. doi: 10.1002/gepi.20037. [DOI] [PubMed] [Google Scholar]
- Sternberg RJ, Grigorenko EL, Bandy DA. The predictive value of IQ. Merrill-Palmer Q. 2001;47:1–41. [Google Scholar]
- Stram DO, Haiman CA, Hirschhorn JN, Altshuler D, Kolonel LN, Henderson BE, Pike MC. Choosing haplotype-tagging markers based on unphased genotype data using a preliminary sample of unrelated subjects with an example from the multiethnic cohort study. Hum Hered. 2003;55:27–36. doi: 10.1159/000071807. [DOI] [PubMed] [Google Scholar]
- Thomas WR, Smith W, Hales BJ, Carter MD. Functional effects of polymorphisms of house dust mite allergens. Int Arch Allergy Immunol. 1997;113:96–98. doi: 10.1159/000237516. [DOI] [PubMed] [Google Scholar]
- Wang S, Chanock S, Tang DL, Li ZG, Jedrychowski W, Perera FP. An assessment of interactions between PAH exposure and genetic polymorphisms on PAH-DNA adducts in African American, Dominican, and Caucasian Mothers and Newborns. Cancer Epidem Biomar. 2008;17:405–413. doi: 10.1158/1055-9965.EPI-07-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham GC, Eaton A, Hopkins B. Evidence for an association between environmental tobacco smoke exposure and birth weight: a meta-analysis and new data. Paediatr Perinat Epidemiol. 1999;13:35–57. doi: 10.1046/j.1365-3016.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- Yolton K, Dietrich K, Auinger P, Lanphear BP, Hornung R. Exposure to environmental tobacco smoke and cognitive abilities among U.S. children and adolescents. Environ Health Perspect. 2005;113:98–103. doi: 10.1289/ehp.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.