Abstract
Objectives
We investigated the longitudinal association between depressive symptoms and glycemic control (HbA1c) in adults with type 2 diabetes, and the extent to which that association was explained by health behaviors.
Methods
This study assessed data on 998 adults (aged 51 and above) with type 2 diabetes in the US nationally representative Health and Retirement Study and its diabetes-specific mail survey. Participants’ depressive symptoms and baseline health behaviors (exercise, body weight control, and smoking status) were collected in 1998. Follow-up health behaviors and the glycemic control outcome were measured at a 2- and 5-year intervals, respectively.
Results
Nearly one in four of participants (23%) reported moderate or high levels of depressive symptoms at baseline (CES-D score ≥3). Adults with higher levels of depressive symptoms at baseline showed lower scores on baseline and follow-up health behaviors as well as higher HbA1c levels at a 5-year follow-up. Structural equation models (SEM) reveal that health behaviors accounted for 13% of the link between depressive symptoms and glycemic control.
Conclusions
The long-term relationship between depressive symptoms and glycemic control was supported in the present study. Health behaviors, including exercise, body weight control, and smoking status, explained a sizable amount of the association between depressive symptoms and glycemic control. More comprehensive diabetes self-care behaviors should be examined with available data. Other competing explicators for the link, such as endocrinological process and antidepressant effects, also warrant further examination.
Keywords: Depressive symptoms, Health behaviors, Type 2 diabetes, HbA1c, Structural equation models (SEM)
Introduction
The United States is among many industrialized countries facing the threat of a diabetes epidemic. Currently, more than 19.3 million people of all ages and roughly 20% of adults aged 60 and older are living with diabetes in the US [1]. Although the prevention of diabetes is a crucial public health goal, it is also important to help those who already afflicted with the disease.
Depressive symptoms, among many psychological factors, play an important role in diabetes management. High levels of depressive symptoms or depression which afflict 15–40% of individuals with diabetes [2, 3], are associated with poorer glycemic control as well as greater diabetes complications [4–6], leading to increasing research interest in understanding the mechanisms underlying the pathway between depressive symptoms and glycemic outcome. Research on health behaviors has provided preliminary insights, concluding that adults with diabetes and comorbid depression tend to have poorer self-management practices than do those without comorbid depression [7–11], and that poorer adherence to behavioral regimens leads to worsening diabetes outcomes [8, 10, 12–15]. There are, however, many unanswered questions from the extant research due to differing diabetes samples, definition of depressive mood, and measures of health behaviors.
First, the impact of depressive symptoms on health behaviors and the impact of depression-related behaviors on glycemic control have only been examined in isolation in most of the extant studies. To our knowledge, only two studies have explored the depression–behaviors–hyperglycemia link simultaneously [13, 16]. In one study, Lustman et al. [13] examined the potential meditational role of diabetes self-care behaviors (diet, exercise, and glucose testing) in explaining the link between depression symptoms and hyperglycemia, as indexed by HbA1c, in 188 patients with type 1 diabetes. The results of this study showed a weak meditational effect of self-care behaviors in the association. In the second study, Van Tilburg et al. [16] found that self-monitored blood glucose behavior partially mediated the depression–hyperglycemia association in 33 patients with type 1 diabetes. However, both studies focused on type 1 diabetes patients. Given the established knowledge that types 1 and 2 diabetes are distinct diseases (i.e., differing etiologies, ages of onset, risk factors, and treatment regimens) coupled with the skyrocketing prevalence rates of diabetes, especially type 2 diabetes, in middle-aged and older adult populations, the meditational effect of health behaviors on the depressive symptoms–glycemic control link in type 2 diabetes patients deserves further investigation.
Second, the majority of current research in this area has focused on the effects of major depression on diabetes self-care or glycemic control [6, 11, 14, 17–19]. However, recent studies have suggested that depressive symptoms may be more reflective of general emotional and diabetes-specific distress than is clinical depression [20]. For example, Fisher et al. [20, 21] conducted a longitudinal study in 506 type 2 diabetes patients comparing the impact of clinical depression and depressive symptoms on glycemic control, finding that HbA1c was positively related to CES-D, but not to affective and anxiety disorders (e.g., major depressive disorders) over time. Similarly, Gonzalez et al. [7] compared the predictive effect of major depression and the continuous severity of depressive symptoms to diabetes self-care behaviors with 879 type 2 diabetic patients; they found that the severity scores for continuous depressive symptoms were better predictors of non-adherence to diet, exercise, and medications than was categorically defined major depression. The significance of these findings, and the practical value of examining the relationship between depressive symptoms and glycemic control in population-based samples of adults with diabetes have sparked renewed interest in understanding the relationship between depressive symptoms, rather than clinical depression, and glycemic control.
A third important issue in the existing literature is the discrepancy in identifying health behaviors. Variables that have been postulated as highly correlated with depression and poorer glycemic control include smoking [22, 23], weight control [10, 22], and the lack of physical exercise [24–26]. However, these behavioral factors have only been examined in isolation in previous research. The simultaneous effects of such health behaviors in explaining the pathway between depressive symptoms and glycemic control remain unclear. Further, few studies examining the contributing role of depressive symptoms and health behaviors on glycemic control have ruled out the possibility that poor health behaviors cause depressive symptoms [22, 27]. Thus, it is important to control for baseline health behaviors in examining the link between depressive symptoms and diabetes outcomes.
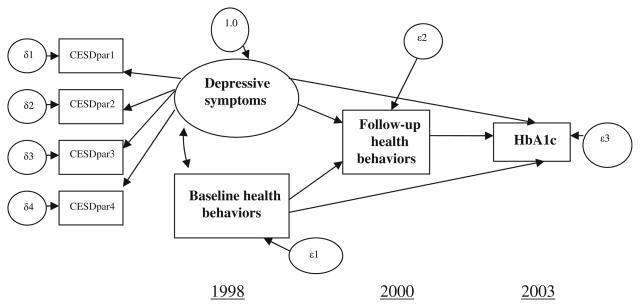
The present study aims to fill the gaps in the current literature by: (1) examining the relationship between depressive symptoms and glycemic control with longitudinal data for a heterogeneous sample of middle-aged and older adults (aged 51 and above) living with type 2 diabetes; and (2) identifying the strength of health behaviors (including physical exercise, weight control, and smoking status) in explaining the link. The structural equation modeling (SEM) approach permit us to develop a measurement model at the latent variable level, thus ameliorating unexplained variances associated with measurement errors. Accordingly, we can examine both the strength of health behaviors in mediating the link between depressive symptoms and glycemic control (indirect effects), as well as the effect of depressive symptoms on glycemic control beyond what health behaviors can explain (direct effects). We hypothesize that depressive symptoms, assessed with the Center for Epidemiological Studies Depression Scale (CES-D), are associated with health behaviors concurrently and longitudinally, and that depressive symptoms-related health behaviors will explain a substantial proportion of the link between depressive symptoms and glycemic control, net of the effects of baseline health behaviors. The hypothesized model is shown in Fig. 1. It should be noted that although the three waves of data in our model permit us to test longitudinal relationships among our factors, the data were not available to control for baseline glycemic control; therefore, tests of the causal relationship of depressive symptoms to glycemic control is beyond the scope of our study.
Fig. 1.
Hypothesized relationships between depressive symptoms, concurrent baseline health behaviors, 2-year follow-up health behaviors, and 5-year follow-up glycemic control. CESDpar1 CESD scale items: feel depressed and restless sleep, CESDpar2 CESD scale items: feel sad and not getting going, CESDpar3 CESD scale items: did not feel happy and did not enjoy life, CESDpar4 CESD scale items: feel lonely and everything is an effort. Source: 1998–2000 Health and Retirement Study (HRS) and 2003 diabetes-specific mail survey
Methods
Participants
The present study included 998 middle-aged and older adults (aged 51 and above at baseline) with self-reported diagnosed type 2 diabetes who: (1) were followed into the fourth and fifth waves (1998 and 2000) of the core Health and Retirement Study (HRS); and (2) had valid HbA1c values in the diabetes-specific mail survey in 2003. Full details regarding the recruitment procedures and characteristics of participants in the HRS core survey and the diabetes-specific mail survey have been described previously [28]. Briefly, the HRS is an ongoing biennial survey initiated in 1992 for tracking the health status and retirement plans of community-dwelling middle-aged and older US adults, with oversampling of Hispanics and African Americans. Beginning in 1998, an older cohort in the Study of Assets and Health Dynamics of the Oldest Old (AHEAD) and two new “age-in” cohorts were added to the HRS, which made the HRS fully representative of US middle-aged and older adults aged 51 and above at that time. The diabetes-specific mail survey, which was fielded in 2003, followed adults who self-reported diagnosed diabetes in one of the previous waves of the core HRS interviews and collected data on a variety of diabetes-specific questions and clinical measures of glycemic control (HbA1c values). 1,901 adults participated in the diabetes-specific mail survey. Of the participating adults, 1,233 (1,074 type 2 diabetes, 159 type 1 diabetes or uncertain) returned valid blood assays. Diabetes type was self-reported by the participants in answer to the follow-up question in the diabetes-specific mail survey: “Which type of diabetes did your doctor say that you have?” Among the 1,074 adults with type 2 diabetes, 40 who did not participate in the 1998 HRS core survey, and 36 who were younger than 51 at baseline were excluded from our analyses, resulting in a sample of 998 adults in the present study.
Measures
Assessment of depressive symptoms
Two instruments were administered in the HRS to measure depressive mood: (1) the CES-D [29] that measures symptoms related to depression and anxiety; and (2) the Short Form Composite International Diagnostic Interview (CIDI-SF) [30, 31] that assesses if respondents have experienced a major depressive disorder (MDD), as described in the Diagnostic and Statistical Manual of Mental Disorders of the American Psychiatric Association, third edition revised (DSM-III-R). In this study, we used the CES-D score to assess participants’ depressive symptoms at baseline in 1998. This instrument was chosen because the CES-D scale has been shown in previous literature to be reliable in older adult respondents, [32] and instead of determining the presence or absence of recognized psychiatric disorders, it measures a continuum of symptoms of depression and anxiety, which has been shown to be more relevant to diabetes distress and diabetes outcomes [7, 20, 21]. An eight-item version, rather than the full-length CES-D, was used in the HRS studies in order to accommodate the time constraints of the interviews. A yes/no response format was used to make the survey easier to be understood and followed by older adults [33]. Reliability coefficients of the eight-item version of the CES-D are excellent, ranging from 0.85 to 0.91; and the factor structures are stable across age [33].
A summary score ranging from 0 to 8 is produced by summing the number of “yes” answers across the eight items: feel depressed, happy, lonely, enjoying life, sad, life being an effort, not getting going, and getting restless sleep (positive items are reverse-scored) in the past week. The composite score was used in the main SEM analysis. Previous literature has suggested that the cutoff of three or more symptoms on the eight-item CES-D scale is sensitive in predicting major depression measured by the CIDI-SF [33]. The descriptive analysis in our data showed that adults who were in the top 10% of those with a depressive mood scored five and above on the CES-D scale; and they were associated with even worse baseline health behaviors than were those who scored between three and four depressive symptoms. For these reasons, we defined depressive symptoms groups to describe the relationship of depressive symptoms, baseline health behaviors, follow-up health behaviors, and follow-up glycemic control. Participants who reported two or fewer depressive symptoms were categorized as low/not-depressed; participants who reported three or four depressive symptoms were categorized as moderately depressed, and participants with five or more depressive symptoms were categorized as highly depressed.
Health behaviors
Among many health behaviors related to diabetes self-care, three health behaviors—physical exercise, body weight control, and current smoking status—were examined in the present study because these behaviors have been demonstrated in the literature to influence glycemic control in adults with type 2 diabetes [34–44]. Respondents were asked the following question to assess their physical exercise behavior: “On an average over the last 12 months have you participated in vigorous activity or exercise three times a week or more? By vigorous physical activity, we mean things like sports, heavy housework, or a job that involves physical labor.” Smoking behavior was assessed by asking respondents: “Do you smoke cigarettes now?” Body weight control was determined by the value of BMI (kg/m2), which was calculated by self-reported weight and height. Participants with a BMI higher than 18.5 and lower than 29.9 were categorized as having good body weight control, whereas those outside of this scope were categorized as not having good body weight control.
In order to estimate the overall meditational effects of health behaviors in the link between depressive symptoms and glycemic control, a composite index score was used—the sum of the three health behaviors (range 0–3). Higher the value of the composite measure, the more positive the health behaviors. The 1998 HRS core interviews assessed baseline health behaviors, and the 2000 HRS core interviews measured those same health behaviors at a 2-year follow up.
Demographic characteristics, clinical characteristics, and glycemic control
Demographic data (age, sex, race/ethnicity) were drawn from the 1998 survey. Clinical characteristics (duration of diabetes, treatment modality) and the study’s outcome variable, the hemoglobin A1c level, were determined by the 2003 diabetes-specific mail survey. The hemoglobin A1c level was determined by blood spot assays returned by diabetes-specific mail survey respondents to Flexsite Diagnostics. In our sample, the hemoglobin A1c ranged from 4.8 to 15.5, with higher values reflecting poorer glycemic control.
Statistical analyses
Two phases of analyses were conducted in the present study. In the first phase, bivariate tests identified relationships among variables. To clarify how subjects with different levels of baseline depressive symptoms exhibited differing health behaviors and HbA1c levels, we used analysis of variance (ANOVA) to compare baseline health behaviors, 2-year follow-up health behaviors, and HbA1c levels in the three groups of subjects. We then examined the inter-correlation of all the numerical latent variables as a prerequisite for proceeding with the structural equation models (SEM). Significant inter correlations between constructs were modeled as paths in the following structural equation model. The ANOVA test and Pearson’s correlations were carried out with SAS 9.1.
In the second phase, structural equation modeling (SEM) using maximum likelihood estimation was applied to evaluate our hypothesized mediation model. We proceeded in two steps in this phase. The first step involved evaluating the measurement and structural models. Assessment of the measurement model was based on the model identification status by which the completely standardized factor loadings on the latent variable, depressive symptoms, were examined. Because the CES-D scale represents multiple dimensions of depressive symptoms, we used a domain representative approach to create “parcels,” which are a combination of items depending on their factor loading in one-factor factor analysis, to be indicators for the latent construct of the depressive symptoms in our analysis. This approach, described in Graham and Tatter-son (2000), helps achieve a better model identification in SEM [45]. Based on the one-factor factor analysis, four parcels were created as indicator variables for the latent variable of depressive symptoms in the SEM: parcel 1 included feeling depressed and restless sleep; parcel 2 feeling sad and not getting going; parcel 3 not feeling happy and not enjoying life; and parcel 4 feeling lonely and everything is an effort. To handle missing data (total missing = 2.61%), assumed to be missing at random in the SEM, we followed the procedure suggested by Graham and Hofer [46] and set the analytic sample sizes to N′= N* (1 – % of total missing) to yield fit estimates. This approach, achieved by using RHO.EXE, is suggested to be much better than using the normal sample size [47]. Goodness of fit for our model was determined by χ2 and three indices of practical fit: non-normed fit index (NNFI/RHO), comparative fit indices (CFI), and root mean square error of approximation (RMSEA). The three indices of practical fit are in wide use and known to be relatively unaffected by sample size [48, 49]. Values less than 0.05 for RMSEA, greater than 0.95 for RHO/NNFI, and greater than 0.96 for CFI are all indications of a good model fit [50, 51].
Our second step in SEM is to identify the total effects of depressive symptoms on glycemic control and to examine the meditational effects of health behaviors by calculating significant relevant path coefficients in the model. The indirect effect from depressive symptoms to glycemic control, by way of health behaviors, was calculated by following the principles described in Baron and Kenny [52]. The total effect of depressive symptoms on glycemic control was the sum of the indirect and (residual) direct effect. The strength of health behaviors in explaining the link between depressive symptoms and HbA1c levels was determined by the proportion of the indirect effect in the total effect (i.e. bindirect/btotal). The SEM were performed in LISREL 8.8. Alpha was set at .05 for all analyses.
Results
Table 1 presents selected demographic and clinical characteristics of the study’s 998 middle-aged and older adults living with type 2 diabetes. On an average, the participants were 65.2 ± 8.1 years, with the duration of diabetes 12.5 ± 10.9 years, and BMI of 29.9 ± 5.7. Females comprises 47.9% of the participants. Respondents self-identified as Caucasian (83.2%), African American (12.8%), and Hispanic and other (4.0%). Their mean level of HbA1c was 7.2% (SD = 1.4), and 17.4% of them reported using the insulin pump.
Table 1.
Demographic and clinical characteristics of the sample (N = 998)
| Characteristic | n (%)a |
|---|---|
| Age (years) | 65.2 ± 8.1 |
| White/Black | 829 (83.2)/128 (12.8) |
| Female | 478 (47.9) |
| Use of insulin pump | 174 (17.4) |
| Duration of diabetes (years) | 12.5 ± 10.9 |
| BMI (kg/m2) | 29.9 ± 5.7 |
| HbA1c (%) | 7.2 ± 1.4 |
| CES-D depression score | 1.6 ± 1.9 |
| Baseline health behaviors index score | 1.9 ± 0.8 |
| 2-year follow-up health behaviors index score | 1.8 ± 0.8 |
The health behaviors index score (range 0–3) was calculated by physical exercise, body weight control, and current smoking behaviors. The healthiest behavior was defined as: physical exercise 3 or more times a week, body weight control resulting in a BMI > 18.8 and <30, and a report of being a current non-smoker. Source: 1998–2000 Health and Retirement Study (HRS) and 2003 diabetes-specific mail survey
HbA1c, glycosylated hemoglobin level; CES-D, Center for Epidemiological Studies Depression Scale (range 0–8)
Continuous variables reported as mean ± SD
As shown in Table 2, nearly one in four of our participants (23%) reported at least three depressive symptoms at baseline. Among them, 13% were moderately depressed (those who self-reported having three or four depressive symptoms), and 10% were highly depressed (those who self-reported having five or more depressive symptoms). Based on the categories of baseline depressive symptoms, highly depressed participants had significantly lower concurrent baseline health behavioral index scores than did participants in low/no and moderately depressed groups (1.5 vs. 1.8 and 1.9, P < 0.05). The moderately and highly depressed groups also showed significantly lower health behavioral index scores than the low/not-depressed group in the 2-year follow-up interview (1.4 and 1.6 vs. 1.9, P < 0.05). Although moderately depressed participants were not different from low/not-depressed participants regarding the 5-year HbA1c levels, highly depressed participants showed significantly higher HbA1c levels than did low/not-depressed participants (7.6 vs. 7.2, P < 0.05). In addition, by comparing baseline and 2-year follow-up health behaviors among the three groups, we found that individuals in the baseline low/not-depressed group did not change their mean health behavioral index score from the baseline to the 2-year follow-up; however, both the baseline moderately and highly depressed groups showed decreases in their health behavioral index scores at the 2-year follow-up.
Table 2.
Analysis of variance test for comparing health behavior index scores and HbA1c levels among three depressive groups
| Depressive groups | N (%) | Baseline health behaviorsa | 2-year follow-up health behaviorsa | 5-year follow-up HbA1ca |
|---|---|---|---|---|
| 1. Highly depressed | 99 (10) | 1.5 ± 0.8 | 1.4 ± 0.8 | 7.6 ± 1.7 |
| 2. Moderately depressed | 130 (13) | 1.8 ± 0.9 | 1.6 ± 0.8 | 7.3 ± 1.1 |
| 3. Low or no depressed | 769 (77) | 1.9 ± 0.8 | 1.9 ± 0.8 | 7.2 ± 1.4 |
| Bonferroni pairwise multiple comparisonb | 2, 3 > 1 | 3 > 2, 1 | 1 > 3 |
The three groups, including highly depressed, moderately depressed, and low/no depressed, were determined by baseline CES-D scores. Participants who reported two or fewer depressive symptoms were categorized as low/no depressed, participants reported three or four depressive symptoms were categorized as moderately depressed group, and participants with five or more depressive symptoms were categorized as highly depressed group. Source: 1998–2000 Health and Retirement Study (HRS) and 2003 diabetes-specific mail survey
Cell values reported as mean ± SD
Significance level was set at 0.05
Inter correlations of all the latent variables in our hypothesized structural equation model are presented in Table 3. As expected, all of the variables were significantly correlated except for baseline health behaviors and the 5-year follow-up HbA1c levels. This path between baseline health behaviors and 5-year follow-up HbA1c levels was therefore excluded from the hypothesized structural equation model.
Table 3.
Standardized inter-correlation among constructs used in the structural equation model (N = 998)
| Baseline depressive symptomsa | Baseline health behaviorsb | 2-year follow-up health behaviorsb | 5-year follow-up HbA1c | |
|---|---|---|---|---|
| Baseline depressive symptomsa | 1.00 | |||
| Baseline health behaviorsb | −0.18 (< 0.0001) | 1.00 | ||
| 2-year follow-up health behaviorsb | −0.19 (< 0.0001) | 0.61 (< 0.0001) | 1.00 | |
| 5-year follow-up HbA1c | 0.08 (0.0098) | −0.04 (0.1847) | −0.11 (0.0004) | 1.00 |
Source: 1998–2000 Health and Retirement Study (HRS) and 2003 diabetes-specific mail survey
Baseline depressive symptoms, as indexed by the CES-D score (range 0–8), include symptoms of feel depressed, happy (score reversed), lonely, enjoying life (score reversed), sad, life being an effort, not getting going, and getting restless sleep
Health behaviors were a composite score of three health-related behaviors: physical exercise, body weight control, and smoking (range 0–3). A higher value represents more positive health behaviors
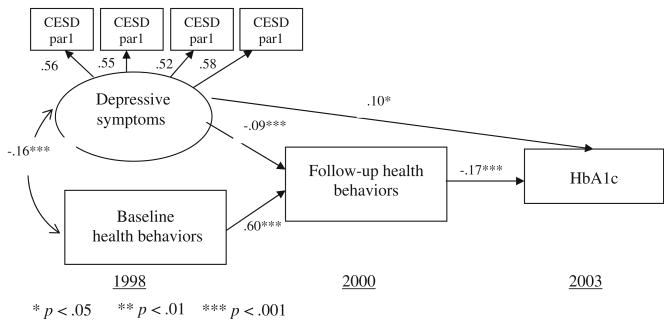
The results from the first step of the SEM in evaluating the measurement and structural models were good. First, completely standardized factor loadings of the four parcels on the depressive symptoms were substantial (0.56, 0.55, 0.52, and 0.58, respectively), suggesting that the identification status in the latent variables was good. Second, the fit of our alternative model, where the path from baseline health behaviors to glycemic control was removed, provided a good fit to our data, meeting all the accepted criteria suggested by Hu and Bentler [51] and Kline [53], with (P = 0.175), NNFI = 0.9948, CFI = 0.9970, and RMSEA = 0.0194. All of the paths in the final model were highly significant. The final model is represented in Fig. 2. Accounting for baseline health behaviors, depressive symptoms were independently associated with worsened 2-year follow-up health behaviors (bdirect = −0.09, t = −3.4). Baseline health behaviors were highly correlated with 2-year follow-up health behaviors (bdirect = 0.60, t = 22.6), independent from baseline depressive symptoms. The 2-year follow-up health behaviors were significantly associated with HbA1c levels at the 5-year follow-up: the higher the health behavior index score, the lower the HbA1c levels (bdirect = −0.17, t = −3.3). There was a significant (residual) direct association beyond what health behaviors explained from baseline depressive symptoms to 5-year follow-up HbA1c levels (bdirect = 0.10, t = 2.0). This effect was controlled for baseline health behaviors.
Fig. 2.
Final model of the relationships between depressive symptoms, baseline health behaviors, follow-up lifestyle behaviors, and HbA1c levels (N = 998). Source: 1998–2000 Health and Retirement Study (HRS) and 2003 diabetes-specific mail survey
In the second step of our results from the SEM, the role of health behaviors in mediating the association between depressive symptoms and HbA1c levels was revealed by calculating both the indirect and total effects of depressive symptoms on glycemic control, which were based on the estimated path coefficients provided in the final model. Following the rules described by Baron and Kenny (1986), the indirect effect of depressive symptoms on HbA1c levels through health behaviors was significant (bindirect = −0.09 × −0.17 = 0.015). The positive value suggests that higher baseline depressive symptoms were associated with higher HbA1c levels (that is, poorer glycemic control) in the 5-year follow-up. The total effect of depressive symptoms on glycemic control was determined by summing the (residual) direct effect and indirect effect (btotal = 0.10 + 0.015 = 0.115). As a result, our model shows that health behaviors accounted for 13% of the association between depressive symptoms and HbA1c levels (bindirect/btotal = 0.015/0.115 = 0.13).
Discussion
The present study examined a potential model for understanding the relationship between depressive symptoms, health behaviors, and glycemic control using a large nationally representative sample of US middle-aged and older adults with type 2 diabetes over a 5-year period. The results of this study suggest that depressive symptoms at baseline were associated with glycemic control 5 years later, and that the association was significantly, but not completely, explained by health behaviors.
Our findings that depressive symptoms were associated with concurrent as well as follow-up general health behaviors support recent clinical findings that even low levels of depressive symptoms are associated with non-adherence to important aspects of diabetes self-care [7]. Our results also add credence to the findings by Lin et al. [11] suggesting that although obesity and smoking are not unique to diabetic patients with depression, high proportions of depressed patients reported infrequent exercise, unhealthful diet and more smoking. We also found that health behaviors were relatively stable in middle-aged and older adults with type 2 diabetes (0.60, P < 0.0001), and were predictive of 3-year follow-up glycemic control (−0.17, P < 0.0001); both support and extend findings by Lustman et al. with cross-sectional data (−0.15, P = 0.04) [13]. It is also important to note that although the effect of depressive symptoms on glycemic control 5 years later is likely to be smaller than the relationship examined within a shorter period of time, we found substantial total effects of depressive symptoms on glycemic control in our data (0.15, P < 0.0001), only somewhat smaller than that found by Lustman et al. (0.23, P < 0.002).
Contrary to other findings regarding type 1 diabetes—that diabetes self-care behavior does not mediate the depression–hyperglycemic link [13]—our investigation concluded that more general health behaviors explained 13% of that link in adults living with type 2 diabetes, and that the mechanism was the same for adults with any level of baseline health behaviors. Further, we found a significant (residual) direct association between depressive symptoms and HbA1c levels, net of baseline and follow-up health behaviors. Thus, although lifestyle behaviors were the logical mechanisms in the link between depressive symptoms and higher HbA1c levels, other underlying effects should be explored in future studies.
Several alternative mechanisms might explain the depression–hyperglycemia relationship. One possibility is that antidepressant therapy may have a direct effect on glycemic control. Research from clinical and animal models has shown that many psychoactive drugs can directly affect glucose metabolism in different ways [54–56]. Other potential mediators of the relationship involve biological mechanisms. For example, the endocrinological process is hypothesized to be an important mechanism underlying the link. It is recognized in the literature that hypercortisolism is a frequent endocrine sign in major depression, and cortisol is a well-known anti-insulinergic hormone [57–60].
Our study has a number of strengths and implications. First, this study includes a longitudinal design over a span of 5 years, which allows us to examine the long-term relationship between depressive symptoms and glycemic control. Second, the use of a structural equation modeling approach enables us to distinguish between indirect and direct relationships among complex psychological, behavioral, and clinical relationships, and to analyze relationships at the latent variable level, which reduces variance due to measurement errors. Third, we used a clinical measure of Hemoglobin A1c, which is not commonly available in such large heterogeneous samples. This increases our confidence in ascertaining the relationship of behavioral and psychological determinants to glycemic control. Further, the relationship between depressive symptoms, rather than major depression, and glycemic control in a non-psychiatric population has been under studied. Our research finding that adults with diabetes who experience moderate or high levels of depressive symptoms have poorer glycemic control highlights the need for clinicians to integrate emotional support systems into diabetes care, which may not only improve adherence to healthy behaviors, but may also directly improve glycemic control. While acknowledging that adherence to one aspect of health behaviors may be unrelated to adherence to others [61], we have focused this study on the general health behaviors that the literature documents as correlates of diabetes (physical exercise, body weight control, and smoking status) and used an index score to investigate the composite effects of these behaviors. This investigation provides a basis for understanding the strength of health behaviors in the link between depressive symptoms and glycemic control, an important issue previously unexplored. Finally, the finding that the link was not completely explained by the three health behaviors signifies that further examination for other diabetes-relevant health behaviors and competing mediators beyond behaviors should be examined.
Three caveats, however, should be acknowledged. First, it is not possible to precisely determine the causal direction between depressive symptoms and glycemic control within the current study. The analyses cast the variables in a temporal order, based on the available literature and using a longitudinal dataset. However, without including baseline glycemic control in the model, the full causal pathways linking depressive symptoms to glycemic control cannot be established.
Second, our finding that health behaviors accounted for 13% of the link between depressive symptoms and glycemic control may represent a conservative assessment of the depressive symptoms–behavior–glycemia relationship for two reasons. On one hand, the time lapse between baseline depressive symptoms and follow-up glycemic control is 5 years, thus the effect of depressive symptoms on glycemic control is likely to be smaller than the effect over a shorter period of time. On the other hand, although we examined three important indicators of health behaviors, other potentially important behavioral factors such as diet, medication taking, blood glucose monitoring, medical appointments, and daily decision making [62] were not available for testing in the current study. The diabetes supplemental study surveyed a broad range of diabetes-specific self-management behaviors, but because they were only measured at a single time point and not prior to our 2003 outcomes, those behaviors were not applicable for the model in the present study. If the HRS fields a follow-up diabetes-specific survey, we may be able to test a further model—including both general and diabetes-specific health behaviors—in a future study. In addition, using BMI to measure body weight control may not closely reflect weight change of our participants. We encourage future researchers to replicate these analyses with different data-sets, measures, and timelines.
Third, the generalization of the results of the present study was limited to middle-aged and older adults with depressive symptoms. As major depression may entail more life demands (e.g., anti-depressant medication taking) and have direct impacts on glycemic control, it may be fruitful for future studies to assess the long-term relationship of major depression, glycemic control and behavioral mechanisms.
In conclusion, given the strengths and limitations of our study, our findings provide support for conceptualizing the long-term association of depressive symptoms and glycemic control in a heterogeneous sample of adults with type 2 diabetes. We identified that general health behaviors—including exercise, body weight control, and smoking status—explained 13% of the association between depressive symptoms and glycemic control. The study’s overall model implies that hemoglobin A1c levels in adults with type 2 diabetes are not simply the result of health behaviors in recent years, but rather follow a pathway through longer-term depressive symptoms and health behaviors to more proximal health behaviors. Our study provides a basis for the further evaluation of other unexamined mediators in the link between depressive symptoms and glycemic control. Additionally, the study results have practical implications in suggesting that effective diabetes care should include attention to not only patients’ external behavioral management, but also their internal psychological health.
Acknowledgments
The authors acknowledge the grant support of National Institute on Diabetes and Digestive and Kidney Disorders. Grant number DK078894 (Linda A. Wray, PI). We acknowledge the valuable statistical consulting help provided by John W. Graham on an earlier version of this paper. The earlier version of this paper was presented at the Society of Behavioral Medicine’s 29th annual meeting on March 26-29, 2008 in San Diego, CA.
References
- 1.Cowie CC, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29(6):1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 2.Gavard JA, Lustman PJ, Clouse RE. Prevalence of depression in adults with diabetes. An epidemiological evaluation. Diabetes Care. 1993;16(8):1167–1178. doi: 10.2337/diacare.16.8.1167. [DOI] [PubMed] [Google Scholar]
- 3.Anderson RJ, et al. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 4.de Groot M, et al. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63(4):619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Lustman PJ, et al. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23(7):934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 6.Lustman PJ, Clouse RE. Depression in diabetic patients: the relationship between mood and glycemic control. J Diabetes Complicat. 2005;19(2):113–122. doi: 10.1016/j.jdiacomp.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez JS, et al. Depression, self-care, and medication adherence in type 2 diabetes. Diabetes Care. 2007;30(9):2222–2227. doi: 10.2337/dc07-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wing RR, Phelan S, Tate D. The role of adherence in mediating the relationship between depression and health outcomes. J Psychosom Res. 2002;53(4):877–881. doi: 10.1016/s0022-3999(02)00315-x. [DOI] [PubMed] [Google Scholar]
- 9.Kilbourne AM, et al. How does depression influence diabetes medication adherence in older patients? Am J Geriatr Psychiatry. 2005;13(3):202–210. doi: 10.1176/appi.ajgp.13.3.202. [DOI] [PubMed] [Google Scholar]
- 10.Sacco WP, et al. Depression in adults with type 2 diabetes: the role of adherence, body mass index, and self-efficacy. Health Psychol. 2005;24(6):630–634. doi: 10.1037/0278-6133.24.6.630. [DOI] [PubMed] [Google Scholar]
- 11.Lin EH, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27(9):2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- 12.Lustman PJ, Griffith LS, Clouse RE. Depression in adults with diabetes Results of 5-yr follow-up study. Diabetes Care. 1988;11(8):605–612. doi: 10.2337/diacare.11.8.605. [DOI] [PubMed] [Google Scholar]
- 13.Lustman PJ, et al. Depression-related hyperglycemia in type 1 diabetes: a mediational approach. Psychosom Med. 2005;67(2):195–199. doi: 10.1097/01.psy.0000155670.88919.ad. [DOI] [PubMed] [Google Scholar]
- 14.Egede LE, Ellis C. The effects of depression on diabetes knowledge, diabetes self-management, and perceived control in indigent patients with type 2 diabetes. Diabetes Technol Ther. 2008;10(3):213–219. doi: 10.1089/dia.2007.0278. [DOI] [PubMed] [Google Scholar]
- 15.Peyrot M, McMurry JF, Jr, Kruger DF. A biopsychosocial model of glycemic control in diabetes: stress, coping and regimen adherence. J Health Soc Behav. 1999;40(2):141–158. [PubMed] [Google Scholar]
- 16.Van Tilburg MA, et al. Depressed mood is a factor in glycemic control in type 1 diabetes. Psychosom Med. 2001;63(4):551–555. doi: 10.1097/00006842-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Ortega AN, et al. Co-occurrence of mental and physical illness in US Latinos. Soc Psychiatry Psychiatr Epidemiol. 2006;41(12):927–934. doi: 10.1007/s00127-006-0121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pouwer F, et al. Serious diabetes-specific emotional problems and depression in a Croatian-Dutch-English Survey from the European Depression in Diabetes [EDID] Research Consortium. Diabetes Res Clin Pract. 2005;70(2):166–173. doi: 10.1016/j.diabres.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 19.Trief PM, et al. Depression and glycemic control in elderly ethnically diverse patients with diabetes: the IDEATel project. Diabetes Care. 2006;29(4):830–835. doi: 10.2337/diacare.29.04.06.dc05-1769. [DOI] [PubMed] [Google Scholar]
- 20.Fisher L, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30(3):542–548. doi: 10.2337/dc06-1614. [DOI] [PubMed] [Google Scholar]
- 21.Fisher L, et al. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with type 2 diabetes. Diabet Med. 2008;25(9):1096–1101. doi: 10.1111/j.1464-5491.2008.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katon W, et al. Behavioral and clinical factors associated with depression among individuals with diabetes. Diabetes Care. 2004;27(4):914–920. doi: 10.2337/diacare.27.4.914. [DOI] [PubMed] [Google Scholar]
- 23.Katon WJ, et al. Cardiac risk factors in patients with diabetes mellitus and major depression. J Gen Intern Med. 2004;19(12):1192–1199. doi: 10.1111/j.1525-1497.2004.30405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vickers KS, et al. Patients with diabetes and depression may need additional support for exercise. Am J Health Behav. 2006;30(4):353–362. doi: 10.5555/ajhb.2006.30.4.353. [DOI] [PubMed] [Google Scholar]
- 25.Ciechanowski PS, et al. The relationship of depressive symptoms to symptom reporting, self-care and glucose control in diabetes. Gen Hosp Psychiatry. 2003;25(4):246–252. doi: 10.1016/s0163-8343(03)00055-0. [DOI] [PubMed] [Google Scholar]
- 26.Lerman I, et al. Psychosocial factors associated with poor diabetes self-care management in a specialized center in Mexico City. Biomed Pharmacother. 2004;58(10):566–570. doi: 10.1016/j.biopha.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clin Psychol Rev. 2001;21(1):33–61. doi: 10.1016/s0272-7358(99)00032-x. [DOI] [PubMed] [Google Scholar]
- 28.Juster FT, Suzman R. An overview of the Health and Retirement Study. J Hum Resour. 1995;30:S7–S56. (special issue on the Health and Retirement Study: data quality and early results) [Google Scholar]
- 29.Radloff L. The CES-D Scale. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 30.Aalto-Setala T, et al. Major depressive episode among young adults: CIDI-SF versus SCAN consensus diagnoses. Psychol Med. 2002;32(7):1309–1314. doi: 10.1017/s0033291702005810. [DOI] [PubMed] [Google Scholar]
- 31.Gigantesco A, Morosini P. Development, reliability and factor analysis of a self-administered questionnaire which originates from the World Health Organization’s Composite International Diagnostic Interview—Short Form (CIDI-SF) for assessing mental disorders. Clin Pract Epidemol Ment Health. 2008;4:8. doi: 10.1186/1745-0179-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hertzog C, et al. Measurement properties of the Center for Epidemiological Studies Depression Scale (CES-D) in older populations. Psychol Assess. 1990;2:64–72. [Google Scholar]
- 33.Steffick DE. HRS/AHEAD Documentation Report. Survey Research Center, Universtiy of Michigan; Ann Arbor, MI: 2000. Documentation of affective functioning measures in the Health and Retirement Study. [Google Scholar]
- 34.Lee S, et al. Exercise without weight loss is an effective strategy for obesity reduction in obese individuals with and without Type 2 diabetes. J Appl Physiol. 2005;99(3):1220–1225. doi: 10.1152/japplphysiol.00053.2005. [DOI] [PubMed] [Google Scholar]
- 35.Barinas-Mitchell E, et al. Effect of weight loss and nutritional intervention on arterial stiffness in type 2 diabetes. Diabetes Care. 2006;29(10):2218–2222. doi: 10.2337/dc06-0665. [DOI] [PubMed] [Google Scholar]
- 36.Agurs-Collins TD, et al. A randomized controlled trial of weight reduction and exercise for diabetes management in older African-American subjects. Diabetes Care. 1997;20(10):1503–1511. doi: 10.2337/diacare.20.10.1503. [DOI] [PubMed] [Google Scholar]
- 37.Bloomgarden ZT. Weight control in individuals with diabetes. Diabetes Care. 2006;29(12):2749–2754. doi: 10.2337/dc06-zb12. [DOI] [PubMed] [Google Scholar]
- 38.Solberg LI, et al. Diabetic patients who smoke: are they different? Ann Fam Med. 2004;2(1):26–32. doi: 10.1370/afm.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen CP, et al. Exploring the relationships between diabetes and smoking: with the development of glucose equivalent concept for diabetes management. Diabetes Res Clin Pract. 2006;73(1):70–76. doi: 10.1016/j.diabres.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Boule NG, Bouchard C, Tremblay A. Physical fitness and the metabolic syndrome in adults from the Quebec Family Study. Can J Appl Physiol. 2005;30(2):140–156. doi: 10.1139/h05-111. [DOI] [PubMed] [Google Scholar]
- 41.Boule NG, et al. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286(10):1218–1227. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 42.Boule NG, et al. Effects of exercise training on glucose homeostasis: the HERITAGE Family Study. Diabetes Care. 2005;28(1):108–114. doi: 10.2337/diacare.28.1.108. [DOI] [PubMed] [Google Scholar]
- 43.Sigal RJ, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(6):357–369. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 44.Ritzwoller DP, et al. Economic analysis of the Mediterranean Lifestyle Program for postmenopausal women with diabetes. Diabetes Educ. 2006;32(5):761–769. doi: 10.1177/0145721706291757. [DOI] [PubMed] [Google Scholar]
- 45.Graham JW, Tatterson JW. Teaching report #00-41. The Methodology Center, Penn State University; USA: 2000. Creating parcels for multidimensional constructs in structural equation modeling. [Google Scholar]
- 46.Graham JW, Hofer SM. Multiple imputation in multivariate research. In: Little TD, Schnabel KU, Baumert J, editors. Modeling longitudinal and multiple-group data: Practical issues, applied approaches, and specific examples. Hillsdale, NJ: Erlbaum; 2000. [Google Scholar]
- 47.Enders CK, Peugh JL. Using an EM covariance matrix to estimate structural equation models with missing data: choosing an adjusted sample size to improve the accuracy of inferences. Struct Equ Model. 2004;11(2):1–19. [Google Scholar]
- 48.Anderson JC, Gerbing DW. The effect of sampling error on convergence, improper solutions, and goodness-of-fit indices for maximum likelihood confirmatiory factor analysis. Psycho-metrika. 1984;49:155–173. [Google Scholar]
- 49.Marsh HW, Bajaj JR, MacDonald RP. Goodness-of-fit indexes in confirmatory factor analysis: the effect of sample size. Psychol Bull. 1988;103:391–410. [Google Scholar]
- 50.Marsh HW, Hau K, Wen Z. In search of golden rules: comment on hypothesis-testing approaches to setting cutoff values for fit indexes and dangers in overgeneralizing Hu and Bentler’s findings. Struct Equ Model. 2004;11:320–341. [Google Scholar]
- 51.Hu L, Bentler PM. Cutoff criteria for fit indices in covariance structure analysis: conventional versus new alternatives. Struct Equ Model. 1999;6:1–55. [Google Scholar]
- 52.Baron RM, Kenny DA. The moderator-mediator variable distinciton in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 53.Kline RB. Principles and practices of structural equation modeling. Guilford; New York: 1998. [Google Scholar]
- 54.Lustman PJ, et al. Effects of nortriptyline on depression and glycemic control in diabetes: results of a double-blind, placebo-controlled trial. Psychosom Med. 1997;59(3):241–250. doi: 10.1097/00006842-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 55.Surwit RS, et al. Alprazolam reduces stress hyperglycemia in ob/ob mice. Psychosom Med. 1986;48(3–4):278–282. doi: 10.1097/00006842-198603000-00013. [DOI] [PubMed] [Google Scholar]
- 56.Lustman PJ, et al. Fluoxetine for depression in diabetes: a randomized double-blind placebo-controlled trial. Diabetes Care. 2000;23(5):618–623. doi: 10.2337/diacare.23.5.618. [DOI] [PubMed] [Google Scholar]
- 57.Weber B, et al. Major depression and impaired glucose tolerance. Exp Clin Endocrinol Diabetes. 2000;108(3):187–190. doi: 10.1055/s-2000-7742. [DOI] [PubMed] [Google Scholar]
- 58.Bjorntorp P, Holm G, Rosmond R. Hypothalamic arousal, insulin resistance and Type 2 diabetes mellitus. Diabet Med. 1999;16(5):373–383. doi: 10.1046/j.1464-5491.1999.00067.x. [DOI] [PubMed] [Google Scholar]
- 59.Drevets WC, et al. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002;71(3):431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- 60.Andrews RC, et al. Abnormal cortisol metabolism and tissue sensitivity to cortisol in patients with glucose intolerance. J Clin Endocrinol Metab. 2002;87(12):5587–5593. doi: 10.1210/jc.2002-020048. [DOI] [PubMed] [Google Scholar]
- 61.Orme CM, Binik YM. Consistency of adherence across regimen demands. Health Psychol. 1989;8(1):27–43. doi: 10.1037//0278-6133.8.1.27. [DOI] [PubMed] [Google Scholar]
- 62.Gonder-Frederick LA, Cox DJ, Ritterband LM. Diabetes and behavioral medicine: the second decade. J Consult Clin Psychol Spec Issue Behav Med Clin Health Psychol. 2002;70(3):611–625. doi: 10.1037//0022-006x.70.3.611. [DOI] [PubMed] [Google Scholar]