Abstract
Whether or not oogenesis continues in the ovaries of mammalian females during postnatal life was heavily debated from the late 1800s through the mid-1900s. However, in 1951 Lord Solomon Zuckerman published what many consider to be a landmark paper summarizing his personal views of data existing at the time for and against the possibility of postnatal oogenesis. In Zuckerman's opinion, none of the evidence he considered was inconsistent with Waldeyer's initial proposal in 1870 that female mammals cease production of oocytes at or shortly after birth. This conclusion rapidly became dogma, and remained essentially unchallenged until just recently, despite the fact that Zuckerman did not offer a single experiment proving that adult female mammals are incapable of oogenesis. Instead, 20 years later he reemphasized that his conclusion was based solely on an absence of data he felt would be inconsistent with the idea of a nonrenewable oocyte pool provided at birth. However, in the immortal words of Carl Sagan, an “absence of evidence is not evidence of absence.” Indeed, building on the efforts of a few scientists who continued to question this dogma after Zuckerman's treatise in 1951, we reported several data sets in 2004 that were very much inconsistent with the widely held belief that germ cell production in female mammals ceases at birth. Perhaps not surprisingly, given the magnitude of the paradigm shift being proposed, this work reignited a vigorous debate that first began more than a century ago. Our purpose here is to review the experimental evidence offered in recent studies arguing support for and against the possibility that adult mammalian females replenish their oocyte reserve.
“Never discourage anyone who continually makes progress, no matter how slow.”—Plato (427–347 BC).
Keywords: atresia, fertility, follicle, germ cell, oocyte, oogenesis, ovary, stem cells
The experimental evidence offered in recent studies claiming support for and against the possibility that mammalian females can and do replenish their oocyte and follicle reserve during postnatal life is discussed in detail.
INTRODUCTION
At a recent workshop held every 2 yr to review the newest concepts in ovarian biology, a scientist asked to lecture on the topic of postnatal oogenesis in mammals polled the audience, asking for a show of hands from those who consider themselves “ovarian optimists” (i.e., those who believe there is some truth behind recent challenges to the dogma that oogenesis in mammals ceases at birth) or “ovarian pessimists” (i.e., those who stand by this dogma). The majority of hands were raised in accord with the speaker as being an ovarian pessimist, despite the fact that considering oneself a pessimist seems at odds with being a research scientist (see http://en.wikipedia.org/wiki/Research and http://en.wikipedia.org/wiki/Scientist). Further, since an ovarian optimist was not invited to participate in this session to provide balanced counterpoints to the issues, some members of the audience were concerned that trainees in attendance would view the take-home message as one cautioning against the pursuit of studies outside of traditional thinking.
This scenario also provides an example of the polarization that has now occurred among scientists engaged in this debate, which probably reflects a complex interplay of both scientific data and personal biases [1]. Indeed, the stakes of challenging, and potentially overturning, one of the most basic doctrines in the field of reproductive biology are very high, which in turn has driven the publication of a tremendous amount of commentary on this topic over the past few years [1–16]. Although a few of these commentaries have been neutral or even supportive of efforts to test the validity of this dogma [1, 2, 13, 16], the vast majority of these publications have voiced varying degrees of skepticism if not outright disbelief of any work portrayed as contradictory to the dogma [3–8, 10, 11, 14] (see also responses in Skaznik-Wikiel et al. [9], Johnson et al. [12], and Tilly and Johnson [15]). One of the more vocal critics against studies challenging the dogma has already characterized this debate “as lingering among physicians, but among basic researchers as a closed case against women being able to regenerate oocytes” [1]. This individual also believes that this debate “has been devastating to the field as a specialty area,” because when future therapies based on postnatal oocyte renewal fail to appear, it will tarnish the image of reproductive biologists [1].
Although the conclusion that the dogma is correct has already been reached by at least some individuals, regardless of a mounting body of evidence arguing otherwise, we respectfully disagree with the view that this debate is a closed case among basic researchers. In fact, as will be discussed in detail below, an increasing number of reports have been published from our laboratories and others involved in basic research that continue to raise questions over the validity of this dogma. And although the scientific process requires some degree of skepticism when a dogma is challenged until the “smoking gun” is uncovered or the accumulated evidence hits a critical mass that becomes impossible to dismiss, critical commentaries rooted in personal opinions do not offer much constructive value to this debate and may discourage others—in particular, younger scientists—from entering into this area of research. Our goal here is to focus on the experimental data offered to date from those on both sides of this debate. Where appropriate, a discussion of the potential limitations of various pieces of evidence tendered for and against the dogma will be provided. In addition, some of the obstacles that have been encountered in efforts to determine whether mammalian females lose the capacity for oocyte renewal during the perinatal period will be highlighted. Finally, in weighing the evidence discussed here, the need to separate work specifically questioning this dogma from related, but apparently equally contentious, work on the possibility that extragonadal stem or progenitor cells of various sources can be coaxed to form oocytes will also be addressed.
STUDIES CONTRADICTING THE DOGMA
Holding to the reasoning used by Zuckerman in 1951 [17] (see also Zuckerman [18]), the dogma, which was first proposed by Waldeyer in 1870 [19], would be invalidated simply by producing evidence inconsistent with the idea that the oocyte pool endowed in the ovaries of female mammals at birth is fixed and nonrenewable. To this end, there now exist at least eight different lines of contemporary experimental investigation, all of which differ in approach but are consistent in their yield of positive results contradicting the basic principles of this dogma. Importantly, independent corroboration, which is one of the fundamental building blocks of the formation of scientific fact, is available for two of these experimental data sets (see discussions below). Moreover, other recent studies have now concurred with initial conclusions [20] that putative stem cells exhibiting germline characteristics are present in adult mouse ovaries [21] and postmenopausal human ovaries [22, 23]. As such, there is no longer a single laboratory reporting observations that do not align with the widely held beliefs that mammalian females lose replicative germ cells and the ability to produce new oocytes at or very shortly after birth, and the work is no longer restricted to rodent models.
The first contemporary argument tendered against the dogma was one based on a mathematical discordance in how much follicle numbers should decline with age if no new follicles are being added to the postnatal ovarian reserve versus what occurs in mouse ovaries if one directly measures and accounts for the incidence of follicle loss via atresia [20]. Importantly, this discordance was not only confirmed by a subsequent independent study of follicle dynamics in juvenile and adult mouse ovaries using meticulously validated and unbiased methods for quantifying oocyte numbers [24] (see also discussions in the following paragraph), but it is also corroborated by a similar study of the nonhuman primate ovary performed five decades ago [25]. This latter investigation, aside from the fact that it was conducted with monkeys (hence, data questioning the dogma are not rodent specific), is especially noteworthy for several reasons. First, like our initial work with mice [20], all three variables required for accurate mathematical modeling of postnatal follicular dynamics over time—namely, starting follicle numbers at a given age, incidence of follicle loss (atresia), and rate of clearance of atretic follicles—were experimentally determined and thus accounted for [25]. Second, the discordance in what is predicted from mathematical modeling versus what is observed is even more dramatic in this species, because female rhesus macaques reach puberty by 3–4 yr of age and then remain fertile for an additional 20 or more years. If one estimates how long the ovaries should function based on the follicle reserve at puberty (roughly 58 000 follicles per ovary), the incidence of atresia at any given time (4.5% of the follicle pool), and the rate of clearance of atretic immature follicles from the ovaries (7–14 days maximum) as reported in Vermande-Van Eck [25], the resulting exponential decay curve predicts that 90% of the follicle reserve present at puberty will be lost within 2 yr. However, given that the ovaries of the rhesus monkey remain functional for at least 20 yr following puberty, it was logically concluded that this discordance could only be explained by failure of the mathematical model to account for a fourth and final key variable: neo-oogenesis and follicle renewal (or, input back into the reserve) [25].
Despite the compelling nature of these arguments tendered from observations of follicular dynamics in both rodents and primates, some critics claimed that the conclusions reached were flawed based on an inaccurate assessment of the clearance rates of atretic immature follicles [8]. Although we believe the conclusions drawn from direct assessments of atretic follicle clearance rates using multiple approaches are sound [20, 25], the second line of experimental investigation that produced data inconsistent with the dogma is free of any dependency on atretic follicle clearance rates for interpretation. These studies are based simply on assessing changes in nonatretic follicle numbers during adolescent and early adult life. Specifically, Kerr et al. [24] showed, in agreement with our earlier findings [20], that the size of the primordial follicle pool in C57BL/6 female mice remains relatively constant (around 2100 follicles per ovary) from Day 12 through Day 100 of postnatal life. Although the incidence of atretic immature follicles at Day 12 is extremely low, by Day 42 more than 1000 atretic immature follicles can be detected per ovary in C57BL/6 females [20]. Even if past estimates of atretic follicle clearance rates are wrong, the primordial follicle reserve should be reduced by at least 50%, if not considerably more, by Day 100—and this is if there is no clearance of dead oocytes from the ovaries at all. Hence, Kerr et al. [24] concluded that their observations in adult female mice “provide qualified support [of conclusions from our earlier study [20]] for an as yet unknown mechanism for follicle renewal.”
Although critics found little, if anything, technically wrong with these experiments, a recent commentary [14] has taken a different approach in questioning the validity of the conclusions drawn from studies based on oocyte quantification [20, 24]. Specifically, it has been claimed that the outcomes observed from the follicle counts are ambiguous in interpretation if one uses confidence intervals rather than standard (P value-based) statistical programs in analyzing these data. These authors go on to state that the overall conclusion reached by both Kerr et al. [24] and us [20] “is an example of a common misunderstanding of the nature of statistical inference” and that “confidence intervals are not used as often as they should be in the analysis of experimental data, while tests of significance are perhaps overused” [14]. As one example of their reasoning, Faddy and Gosden state that the work of Kerr et al. [24] was based on results from an analysis of 6–8 mice per group, whereas “a ten-fold increase in sample size [viz. 60–80 mice per group!] would be necessary to narrow the confidence intervals to widths at which less equivocal inferences might be possible” [14]. However, the standard statistical approaches that Kerr et al. [24] and we [20] employed to analyze these outcomes are no different than those used in literally thousands of published reports from others available on PubMed to infer the conclusions drawn, including those focused on oocyte counts (e.g., [26–33]). Instead of singling out only those studies that question the dogma, the merit of the argument tendered by Faddy and Gosden [14] would have benefited by perhaps also comparatively reevaluating a much larger sampling of studies on ovarian and oocyte biology spanning the past 50 years, including some of their own work (e.g., [34–37]), in which P value-based statistics were employed to analyze the data from experiments routinely using 7–10 mice per group. This is particularly apropos for a very recent study that will be addressed in more detail below, which claims to rule out the possibility that circulating stem or progenitor cells can give rise to oocytes [38]. In addition to the use of P value-based statistics, some of the conclusions reached in this paper were based on data derived from analysis of only two (n = 2) mice per group from which a total of seven or fewer eggs were retrieved for analysis [38].
The third set of experimental observations that does not align with the dogma is based on findings that during each reproductive (estrous) cycle in the adult female mouse, the number of primordial follicles fluctuates significantly. The first study to document this was from Allen [39] in 1923, who reported that 400–500 new oocytes are produced in adult female mice during each estrous cycle, with the highest and lowest ovarian reserves observed during metestrous/diestrous and estrous, respectively. More than 80 years later, our own studies of potential changes in primordial follicle numbers during the estrous cycle in mice fully corroborated this initial report, not just in terms of the magnitude of change but also with respect to the specific stages of the estrous cycle during which the highest and lowest follicle reserves were detected [40]. A key point to bear in mind here is that these estimates of cyclic primordial follicle renewal ultimately predict the formation of thousands of new oocytes during the prime reproductive period in females, perhaps even more than those formed during fetal development. At first glance this may appear to present a credibility issue. However, hundreds of developing oocytes are routinely lost from the adult mouse ovary through follicular atresia on a daily to weekly basis [20]. This offsetting pattern of renewal and loss provides some explanation as to why the primordial follicle reserve in mice remains relatively unchanged from Days 12−100 of postnatal life [20, 24]. In turn, when oocyte renewal no longer counterbalances loss due to atresia, the follicle reserve begins its age-associated decline until exhausted, driving ovarian failure [9, 12].
The fourth line of study that has produced results inconsistent with the idea that germ cell renewal ceases at or shortly after birth in female mammals is one [20] that was conducted based on the established properties of the alkylating agent busulfan to specifically target replicative germ cells in males and females. In studies of male germline stem cell (GSC) function in mice, busulfan is frequently used as a conditioning agent to prepare recipient males for transplantation [41–43]. In fact, the efficacy of GSC transplantation in males is dependent on adequate depletion of the host GSC population in the testes by busulfan pretreatment. A similar specificity of busulfan for targeting replicative, and not postmeiotic, germ cells in females has also been reported from studies of fetal ovarian development [44]. Female rats exposed in utero to busulfan show gametogenic failure only if the chemical is given during the time of fetal ovarian germ cell proliferation. If, however, female rats are exposed to busulfan in utero after oogonial proliferation has ceased and oocytes have been formed, female offspring are born with ovaries that are indistinguishable from the ovaries of control females exposed in utero to the vehicle [44]. Keeping the results of these past studies from others in mind, we subsequently reported that young adult female mice treated with busulfan exhibit a gradual loss of the entire primordial follicle reserve over a 3-wk period without a corresponding cytotoxic effect on primordial follicles [20]. Such an outcome would be expected if busulfan were, as past studies contend [44], selectively eliminating replicative germ cells that support primordial oocyte formation. The net result would be the normal rate of follicle loss via atresia no longer partially offset by de novo follicle formation, leading to accelerated depletion of the follicle reserve without the need for a corresponding increase in the rate of oocyte death.
The fifth line of reasoning also stems from work with a chemotherapeutic drug, but unlike busulfan this drug shows apparent cytotoxic selectivity in the female germ lineage for oocytes and not replicative germ cells. The drug in question is the anthracycline-based chemical doxorubicin, which has been used extensively in past studies with mice as a stimulus for the induction of apoptotic cell death in oocytes (e.g., [45–47]). In a recent investigation that evaluated in adult female mice a more extended time course after doxorubicin exposure in vivo, it was demonstrated, as expected based on past findings, that the primordial follicle reserve was reduced by more than 80% within 24 h of a single injection of doxorubicin [40]. However, once this nadir had been reached, there occurred a spontaneous replenishment of the primordial follicle pool over the next 12–24 h. Moreover, this renewed pool of follicles was stable, because by 2 mo after exposure the follicle reserve in doxorubicin-treated animals was no different than that observed in age-matched control females that had never been exposed to the drug [40].
Although the magnitude of the regenerative response aligns most logically with replenishment of the primordial follicle reserve via neo-oogenesis, additional studies to show a synchronized entry of germ cells into meiosis 24−48 h after doxorubicin exposure would further strengthen this conclusion. In this regard, in as-yet unpublished studies we have observed a dramatic increase in expression of the meiosis-commitment gene, Stimulated by retinoic acid 8 (Stra8), in the ovaries during this time period following doxorubicin treatment (Niikura and Tilly, unpublished data). Given the central importance and specificity of STRA8 to premeiotic DNA synthesis and meiosis commitment in male and female germ cells [48, 49], these findings lend support to the conclusion that the marked increase in primordial oocyte numbers seen in ovaries of doxorubicin-treated mice at 24−48 h after exposure is due to an induction of de novo oogenesis. However, one issue that remains unclear is how the ovaries of adult mice are apparently able to generate new oocytes so quickly. This outcome is observed not only in the doxorubicin insult model discussed above, but also following treatment of adult mice with the class I/II histone deacetylase inhibitor trichostatin-A (TSA) [40]. While unexpected, this rapid generation of new oocytes has been observed in two completely different experimental paradigms, and therefore appears real. Although continuing studies have not yet clarified exactly how adult mouse ovaries are able to generate new oocytes within 24 h, progress has been made in understanding the mechanisms and pathways involved in regulating postnatal oogenesis in mice (see next paragraph). Accordingly, a better understanding of these mechanisms may eventually reveal the inner workings of other aspects of the process, including how the rapid time frame for oocyte formation is achieved.
As compelling as the data are from the doxorubicin insult model discussed above, the sixth line of evidence arguing against the validity of the dogma is probably even stronger, especially in light of new preliminary data further clarifying the mechanisms involved in regulating postnatal oogenesis. In a study published in 2005, it was reported that a single injection of TSA into juvenile, young adult, or aging female mice results in a rapid (within 24 h) and significant increase in the number of primordial follicles, with the greatest response observed in aging (8-mo-old) females on the verge of reproductive failure [40]. Importantly, other aspects of follicular dynamics, including the rates of primordial follicle growth activation and follicular atresia, remained unchanged in TSA-treated females, leaving essentially one other logical explanation for these findings—de novo primordial follicle formation. In subsequent preliminary studies [50], we have found that the ability of TSA to induce oogenesis in adult female mice is linked to epigenetic modification of the retinoic acid signaling pathway, which is critical for early germ cell specification [51, 52], along with induction of the meiosis-commitment gene Stra8 [48, 49]. These data appear to provide a framework for the first mechanistic insights into how postnatal oogenesis in mammals is regulated at the molecular level.
The seventh line of study that has produced results contradicting the dogma was based on results from an analysis of oocyte dynamics in two different lines of genetic null mice. The first mutant mouse line evaluated was deficient in the expression of the enzyme CASPASE6. It was shown that although neonatal Caspase6 mutants are provided with a follicle endowment that is similar to wild-type controls, the mutants show a larger primordial follicle reserve as young adults, despite the fact that primordial follicle growth activation rates and the incidence of immature follicle atresia remain unchanged compared with wild-type controls [9]. In a more in-depth study published last year, Lee et al. [53] demonstrated a similar phenotype in mutant female mice lacking the cell cycle-inhibitory protein CABLES1. Quite strikingly, in this latter study the incidence of immature follicle atresia was much higher in adult mutant females, despite the fact that these animals also possessed significantly more nonatretic immature follicles. Additional experiments provided evidence that the increased oogenesis observed in adult Cables1-null females appeared to be offset by a reduction in oocyte quality, as reflected by increased elimination of developing and mature germ cells via apoptosis [53]. Bristol-Gould and colleagues [54] recently reached a similar conclusion from studies showing that injection of prepubertal mice with activin causes a transient increase in oocyte numbers, but the overall quality of the oocytes subsequently obtained from these mice following superovulation was reduced.
The eighth and final set of studies to be discussed in this section has its foundations in the possibility that GSCs, analogous to those present in the testes of adult males that support spermatogenesis [55, 56], exist in adult females. Although to our knowledge mammalian female GSCs have not yet been purified, initial work on the characteristics of candidate female GSCs in mice [20] has been independently corroborated by Zhang et al. [21]. These authors concluded that their results were “in agreement with the recent studies published by Tilly's group, who examined the expression of respective markers in potential germ stem cell populations in the adult mouse ovary.” Of additional note, very recent studies have reported the isolation and preliminary characterization of a similar population of putative stem cells, which spontaneously generate oocytes and parthenogenetic blastocysts in vitro, from adult human ovarian cortical tissue [22, 23]. Interestingly, ovaries from postmenopausal women and women with premature ovarian failure were used as starting material, excluding the possibility that the oocyte-producing stem cells obtained are simply embryonic stem (ES)-like cells derived from parthenogenetic activation of oocytes. Although further characterization of these cells is needed, this work [22, 23], along with previous observations from others [57–59], represents exciting first steps toward validating the existence of stem cells with germline potential in adult human ovaries.
In lieu of having purified candidate GSCs to transplant and test for their ability to generate new oocytes, ovarian grafting was performed in our earlier study [20]. Briefly, one half of an adult ovary collected from a transgenic mouse line with enhanced green fluorescent protein (EGFP) expression driven by the β-actin promoter was surgically placed into the ovarian bursal sac of an adult wild-type female after removal of one half of its ovary. Within 3–4 wk, chimeric follicles consisting of EGFP-positive oocytes enclosed within EGFP-negative somatic cells were found distributed throughout the wild-type ovary tissue [20]. These findings were offered as evidence for the existence of premeiotic germ cells in the grafted (EGFP-transgenic) ovary tissue that migrated into the adjacent wild-type ovary and transitioned into oocytes. Although supportive of the proposal that female GSCs exist in the ovaries of adult mammals, direct transplantation of purified female GSCs, if and when these cells become available, would be the preferred method to unequivocally establish the presence and function of such cells in adult mammalian females.
STUDIES SUPPORTING THE DOGMA
Although many critical commentaries have been published since 2004 that argue against the possibility of postnatal oogenesis in female mammals (for examples, see [3–8, 10, 11, 14]), we are aware of only two published reports providing experimental data that the authors claim support the validity of the dogma. The first of these two studies, published in 2006, describes mathematical predictions of follicular dynamics in CD1 female mice based on empirical data derived from an assessment of follicle numbers and the maturational status in the ovaries of these animals at 6 days, 10 days, 19 days, 45 days, 4.5 mo, 6 mo, and 12 mo of age [60]. These experimental data were then plotted against outcomes predicted from mathematical equations devised by the authors that they believe reflect the dynamics of a fixed (fixed pool model) or a renewable (stem cell model) pool of primordial follicles during adult life. Bristol-Gould and colleagues [60] contend from this work that “the fixed pool model fit the experimental data, accurately representing the maximum observed primary follicle number reached by 4–6 months of age,” and thus their findings support the dogma. Careful reading of this study, however, reveals several issues with the authors' predictions and the methods employed to arrive at the conclusions drawn that warrant some discussion.
First, follicular dynamics cannot be modeled accurately without knowing and accounting for the incidence of follicle loss via atresia, an endpoint not included as a variable in this study [60]. Although changes in the absolute numbers of nonatretic follicles over time can be plotted, it is unknown if these numbers reflect a progressive decay of a fixed pool of follicles provided at birth or a progressive decay at a rate higher than appreciated and partially offset by a follicle renewal process that becomes compromised with increasing maternal age. Bristol-Gould et al. [60] contend their “experimental observations did not indicate a large number of unhealthy primary and secondary follicles, therefore loss of primary and secondary follicles by atresia was considered negligible.” To the contrary, studies from many laboratories have shown that there are hundreds, if not thousands, of atretic immature follicles in the ovaries of mice just prior to puberty and continuing throughout adult reproductive life (e.g., [20, 53, 61–63]). Although it may be that much of this atresia is observed in secondary and tertiary follicles, such large numbers of atretic immature follicles cannot be considered a negligible factor in the context of accurately modeling follicular dynamics, given that all growing follicles ultimately emanate from the primordial follicle pool.
Of further note is that the experimental data generated by Bristol-Gould et al. [60] for primordial and secondary follicle numbers fit the fixed pool model and the stem cell model equally well through 4–6 mo of age (see Figure 1 in Bristol-Gould et al. [60]). The only instance where the experimental data fit just the fixed pool model is limited to the actual versus predicted changes in primary follicle numbers at 4–6 mo of age. Finally, the mathematical equation devised by Bristol-Gould and colleagues to depict the stem cell model predicts that primordial, primary, and secondary follicle numbers remain unchanged from roughly 100 days of life onward. Such a prediction, which conveys the idea that stem cell support of oogenesis continues unabated throughout adult life, does not fit with our current understanding of ovarian biology, and thus raises questions over the validity of the mathematical equation used for plotting the stem cell model and its subsequent comparison to the experimental data. Indeed, we have emphasized that the recent challenge to the dogma does not change the fact that the ovarian reserve is depleted with age to the point of exhaustion. Rather, we have suggested that age-related ovarian failure is due to a loss of follicles through atresia (traditional thinking) coupled with a loss of input of new follicles as the stem cells responsible for postnatal oogenesis undergo replicative senescence with age [9, 12]. Accordingly, the final conclusion of Bristol-Gould et al. [60], that their “results agree with established dogma that the initial endowment of ovarian follicles is not supplemented by an appreciable number of stem cells,” but “rather, it is sufficient to ensure the fertility needs of the adult mouse,” is in our view not supported by the modeling data presented.
The only other published study we are aware of that offers experimental data claiming to support the dogma is based on series of gene expression (primarily RT-PCR-based) analyses performed with adult human ovarian tissue biopsies [64]. Based on an inability to detect the expression of various markers for germ cell replication or meiotic entry in their tissue samples, Liu et al. [64] concluded, “active meiosis, neo-oogenesis and GSCs are unlikely to exist in normal, adult, human ovaries.” Although bold in its interpretation, the overall conclusion reached by Liu et al. [64] is weakened because of several technical and reasoning problems, all of which have been detailed previously [15]. However, one central point is that the entire basis of the argument put forth by Liu et al. [64] revolves around their claim that markers of replicative germ cells or of meiotic entry could not be detected in adult human ovaries which, according to these authors, is evidence for the absence of oogenesis. However, Liu and colleagues also failed to detect expression of NOBOX in the same tissue samples, which others have shown is abundantly expressed in the oocytes of primordial, transitional, primary, and secondary follicles of the adult human ovary [65]. Using the reasoning of Liu and colleagues, their inability to detect NOBOX would be indicative of an absence of immature oocytes in the adult human ovary samples they analyzed, one of which was collected from a patient 27 yr of age [64]. However, histological analysis of the ovarian tissues used by Liu et al. [64] showed the presence of immature oocytes in these samples. Hence, the insensitivity of the assays employed by Liu and colleagues to detect expression of a low-abundance gene like NOBOX, rather than an absence of immature oocytes, is the explanation for this outcome. In turn, a corresponding inability to detect similarly low-abundance markers of meiotic entry or germ cell replication in the same tissue samples is not, in our view, compelling evidence for an absence of rare germline cells that support postnatal oogenesis [64].
One other recent study, which was not conducted to directly assess the validity of the dogma per se, does offer some discussion of its findings in the context of neo-oogenesis. In this investigation, John et al. [66] reported that inactivation of the Foxo3 gene in mice by conventional gene targeting results in an accelerated onset of ovarian failure due to increased rates of primordial follicle growth activation. From these findings, the authors conclude that “the Foxo3−/− phenotype would appear to be inconsistent with such a model,” referring to the possibility of postnatal follicle renewal. This deduction was based on the argument that primordial follicle renewal should offset the accelerated rates of growth activation, thereby sustaining the follicle reserve in the mutant females. Although John et al. [66] do emphasize that FOXO3 may also be required for the function of putative female GSCs, either intrinsically or indirectly via actions in ovarian somatic cells involved in GSC maintenance, this possibility is then discounted for the following reason. From the perspective of John et al. [66], “it is not clear why such defects [in putative GSC function] would be manifest only in adult life since… Foxo3 is not required for early germline or gonadal development, including the initial colonization of the gonad by germline stem cells.” This conclusion is only valid if embryonic primordial germ cells (PGCs) and GSCs are functionally identical entities, as inferred. This is not the case, however, as past work has shown that embryonic PGCs give rise to GSCs in the postnatal gonad, and that PGCs are unable to recapitulate the ability of GSCs to reconstitute gametogenesis following transplantation into the testis of busulfan-conditioned adult male mice [67]. Although similar, these two types of cells and their regulatory pathways are not identical. As such, the early ovarian failure observed to occur in Foxo3 mutants does not necessarily contradict the possibility of postnatal follicle renewal, but instead may simply reflect a more complex phenotype resulting from accelerated primordial follicle growth activation combined with defective GSC function in adult FOXO3-deficient females.
OOGENESIS FROM EXTRAGONADAL CELLS?
Concurrent with the debate over whether or not adult female mammals produce new oocytes and follicles, another equally contentious area of work has surfaced over the past few years—one that is relevant to discussions of the dogma but needs to remain a separate topic for discussion so that confusion and disinformation are minimized. This line of study pertains to oogenesis as well, but it is rooted in the question of whether oocytes can be generated from stem or progenitor cell sources other than embryonic PGCs. Since the initial publication in 2003 that mouse ES cells could spontaneously form oocytes contained within follicles or follicle-like structures [68], many laboratories have reported similar observations with not just ES cells [69, 70] (reviewed in Nagano [71]), but with putative germline cells in mouse bone marrow (BM) [40, 72], mouse peripheral blood [40], porcine skin [73, 74], and rat pancreas [75] as well. Although it is appreciated that these findings radically depart from traditional thinking that gametogenesis is confined to cells of the gonads, earlier studies reported the presence of what appear to be developing germ cells in human and rat BM samples [76, 77]. Further, male germ cells have similarly been generated from mouse ES cells [70, 78, 79] (reviewed in Nagano [71]) and BM [80, 81] and, very recently, from the BM of men [82].
Even with this mounting body of evidence for cells other than PGCs being capable of generating germ cells, questions have arisen regarding the developmental competency of these cells. For example, Novak et al. [69] and Nayernia et al. [80] showed that oocytes derived from mouse ES cells and spermatogonia generated from mouse BM, respectively, retain an immature phenotype and fail to progress through meiosis appropriately. Likewise, Eggan et al. [38] concluded from studies with female mice using BM transplantation (BMT) or parabiotic blood exchange that mature eggs collected from the oviducts after superovulation are not derived from BM or circulating cells. This latter study is of particular note because some have mistakenly touted the findings reported by Eggan and colleagues as contradicting not only our work on donor-derived immature oocyte generation in the ovaries of chemotherapy-conditioned mice following transplantation of BM- or peripheral blood-derived mononuclear cells [40, 72], but also our earlier studies challenging the dogma that female mammals cannot generate new oocytes [20]. It is imperative to emphasize that Eggan et al. [38] did not provide any data that are germane to discussions of the validity of the dogma, because these investigators focused solely on eggs retrieved from the oviducts following superovulation and did not include the outcome of evaluating the ovaries of their recipient mice for donor-derived immature oocytes.
Given the importance of this latter endpoint to any study of oogenesis, as well as the skepticism over our work on BM- and peripheral blood-derived oocytes that has surfaced as a result of the Eggan et al. study [38], we took it upon ourselves to repeat the parabiosis experiments reported by Eggan and co-workers with a germline-specific, EGFP-expressing transgenic line (ΔPE-Oct4 or TgOG2 [83–85]) for oocyte tracking [40, 72]. After receiving hands-on training from Amy Wagers, the senior author of the Eggan et al. paper [38], to perform parabiosis in mice, we set up parabiotic pairs as they reported and observed that EGFP-positive immature oocytes are easily detected in the ovaries of adult wild-type female partners within 4 wk after joining to adult TgOG2 females (Fig. 1). These results, coupled with data provided in a follow-up study from our group [72], reinforce earlier conclusions that immature oocytes can be produced from putative germline stem or progenitor cells in BM or peripheral blood of adult female mice [40]. It is also worthwhile mentioning here that the other principal conclusion reached by Eggan et al. [38] that the donor-derived (EGFP-positive) immature oocytes detected previously in recipient ovaries following transplantation are CD45+ immune cells mistaken for oocytes has been proven incorrect [72]. Not only are CD45+ cells negative for expression of the germline markers (e.g., MVH and NOBOX) found to be coexpressed in EGFP-positive (donor-derived) oocytes in recipient ovaries, but also CD45+ cells of TgOG2 mice, which were utilized as donors for these studies [40, 72], do not express EGFP [72]. The conclusion drawn by Eggan et al. [38] that the donor-derived cells detected are CD45+ immune cells rather than oocytes emanates from their use of a “ubiquitous” (β-actin promoter-driven), rather than a germline-specific, EGFP-expressing transgenic line for cell tracking. This is why we believe that use of non-cell lineage-specific transgenic reporter lines for studies of germ cell derivation should be discouraged, especially given that β-actin-EGFP females do not show uniform expression of the transgene in all oocytes [72].
FIG. 1.
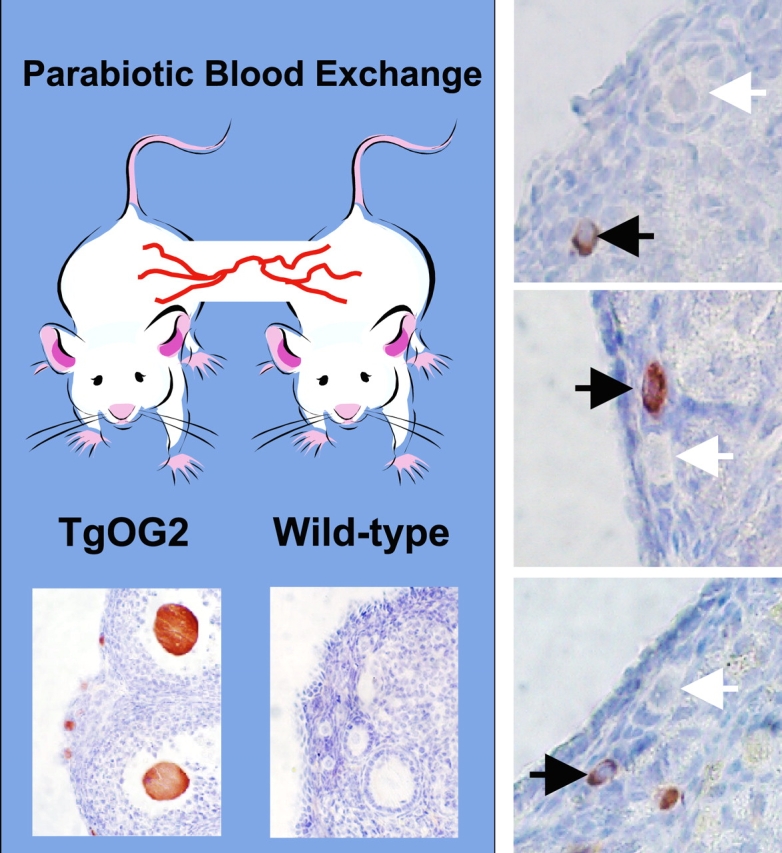
Parabiosis reveals the presence of circulating germline progenitor cells in adult female mice that can form immature (primordial) oocytes. After initiating parabiosis between 2-mo-old wild-type and TgOG2 transgenic females (left; bottom images are from positive and negative controls showing EGFP detection in ovarian sections from TgOG2 and wild-type mice, respectively, prior to parabiosis; see Lee et al. [72] for more details), EGFP-positive immature oocytes enclosed within follicles can be readily detected in the ovaries of wild-type partners within 4 wk of joining (right; data shown are from ovaries of three different mice). Black arrows highlight examples of TgOG2-derived (EGFP-positive; brown) immature oocytes in the ovaries of the wild-type females, whereas white arrows demarcate adjacent wild-type oocytes of approximately the same maturational status. This experiment was reviewed and approved by the institutional animal care and use committee of Massachusetts General Hospital. Original magnification ×40.
This latter point is critical to consider in another more recent study claiming a lack of evidence for immature oocyte generation by circulating cells in adult female mice, which was based on an analysis of wild-type ovarian tissue transplanted into the ovarian bursa or kidney capsule of transgenic female recipients expressing a histone 2B-enhanced EGFP fusion gene under control of a ubiquitous promoter [86]. After scoring 819 oocytes in 30 wild-type ovarian tissue grafts, the authors reported that not a single oocyte scored was EGFP positive. From this, Begum and coworkers [86] concluded that their work “is a further denial of the hypothesis that circulating germ cell progenitors exist in adults” and that “circulating cells do not appear to offer any potential contribution to fertility preservation.” However, several points warrant comment. First, if the objective is to specifically track formation of EGFP-expressing oocytes in wild-type ovary tissue transplanted into transgenic hosts, it remains unclear, at least to us, why investigators interested in this topic continue to use ubiquitous EGFP-expressing transgenic mouse lines as a reporter rather than germline-specific, EGFP-expressing mice (i.e., TgOG2) that are commercially available (strain B6;CBA-Tg(Pou5f1-EGFP)2MnnJ, stock 004654; The Jackson Laboratories, Bar Harbor, ME). The TgOG2 line exhibits high levels of EGFP expression specifically and uniformly in oocytes of adult ovary tissue without background expression in surrounding somatic cells [40, 72] (Fig. 2). This facilitates accurate and rapid identification of TgOG2-derived oocytes in wild-type ovaries after transplantation of BM- or peripheral blood-derived mononuclear cells [40, 72] or parabiosis (Fig. 1). A second issue pertains to the numbers of oocytes scored by Begum et al. [86] on a per-ovary basis. Although the authors emphasize that 819 total oocytes were analyzed and found to be negative for EGFP expression, this number represents the cumulative amount from analysis of 30 grafts. In other words, on average, fewer than 30 oocytes were analyzed in each grafted ovary. This is a key point in view of past studies reporting the formation of EGFP-expressing oocytes following stem cell transplantation, in which only 1.4% of the total oocyte pool per ovary was identified as being donor derived [72]. Accordingly, evaluation of only 30 oocytes per graft does not provide sufficient power to detect this low level of oocyte formation from circulating germline progenitor cells. Finally, it is noteworthy that a parallel analysis of transgenic ovary tissue grafted into transgenic hosts that showed that every oocyte present in the grafted ovary was EGFP positive was not included in this study [86]. Thus, an important positive control is missing, which would allow one to evaluate the success of the grafting procedure in terms of tissue and oocyte viability (both of which could influence transgene expression and/or engraftment efficiency of putative circulating germline cells) as well as the overall technical soundness of the approach used.
FIG. 2.
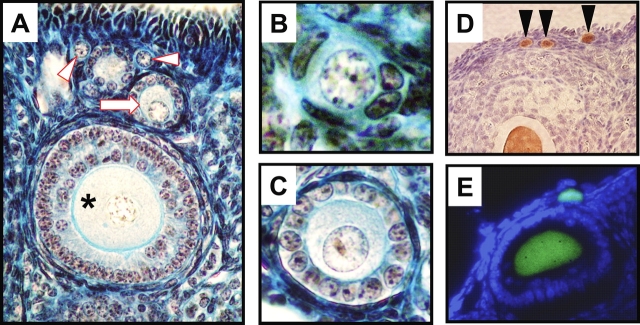
Detection of immature oocytes in ovarian tissue sections of mice by histological approaches and by the use of a transgenic reporter with germline-specific expression of EGFP. A–C) Photomicrographs of oocyte-containing immature follicles in adult mouse ovaries, showing examples of nonatretic primordial (B; arrowheads in A), primary (C; arrow in A), and preantral (asterisk in A) follicles. Original magnifications ×20 (A), ×60 (B), and ×40 (C). D) Use of immunohistochemistry to demonstrate the restricted expression of EGFP (brown immunostaining; see Lee et al. [72] for methodological details) in the oocytes of adult TgOG2 females (arrowheads demarcate primordial oocytes; see also Fig. 1). The ovarian section was counterstained with hematoxylin to visualize tissue architecture. E) Direct fluorescence analysis of EGFP expression (green fluorescence) in ovaries of adult TgOG2 females after counterstaining with a nuclear dye to highlight tissue architecture (blue fluorescence). Two EGFP-positive immature oocytes are shown. Past studies [40] have demonstrated that EGFP-positive oocytes derived from TgOG2 animals coexpress well-accepted markers of the germline (MVH) and of oocytes (NOBOX, GDF-9). This experiment was reviewed and approved by the institutional animal care and use committee of Massachusetts General Hospital. Original magnifications ×20 (D) and ×40 (E).
In any case, oocytes generated from non-PGC sources, although apparently unable to fully complete their own differentiation program under the experimental paradigms that have been tested thus far, can still orchestrate the formation of follicular structures, and these follicles produce classic ovarian-derived hormones (e.g., [68, 69, 73, 74]). Whether or not germ cells produced from these nontraditional sources can be prompted experimentally to yield fertilization-competent eggs (or sperm) remains an important but open issue that is currently under investigation in several laboratories. In the interim, it has been suggested that these germ cells be referred to as oocyte-like cells, reflecting their many similarities to oocytes, including their ability to form hormonally active, gonadotropin-responsive follicles, while at the same time acknowledging their inability to complete meiosis. Finally, very recent preliminary data indicate that ovarian defects arising from inactivation of the follicle-stimulating hormone receptor gene in mice can be rescued by intravenous delivery of BM-derived cells harvested from syngeneic wild-type female donors [87]. These new findings, along with others indicating that transplantation of specifically BM-derived mesenchymal stem cells protects adult rat ovaries from chemotherapy-induced damage [88], suggest that the potential for transplantation-based technologies to benefit female reproductive function could involve reversal of both ovarian germline and somatic cell insufficiency [89].
CONCLUDING REMARKS
More than 4 yr after our initial report questioning the validity of the dogma that oogenesis and folliculogenesis do not persist in the ovaries of adult mammalian females [20], the issue remains unsettled. This is perhaps not surprising, however, because a possible paradigm shift of this magnitude will likely take many years to achieve. In fact, this debate is analogous in many respects to what the neuroscience field experienced in the late 1990s, when claims of neurogenesis in the neocortex of adult primates first surfaced to challenge the century-old assumption that no new neurons are produced in the adult mammalian brain [90–92]. These initial claims were met with skepticism from many neuroscientists (e.g., [93–95]). In addition to offering an array of alternative explanations for these findings—ranging from technical flaws in the cell proliferation assays to questions over the identity of the newly generated brain cells—strong criticisms were voiced about how the work was trumpeted in the popular press as “the most startling discovery” of the decade [96]. In retrospect, many of these same criticisms, particularly those relating to how the work was covered in the lay press, bear an uncanny resemblance to criticisms that our work on postnatal oogenesis has faced since 2004 [20] (see also comments in Powell [1]). However, evidence from subsequent experiments using a variety of models and techniques repeatedly contradicted the underlying principles of this neurobiology dogma. And, with some occasional minor rethinking of the original claims [97–99], widespread acceptance that the adult mammalian brain is, in fact, fully capable of neurogenesis eventually took hold. This, in turn, gave birth to an exciting and ever-growing field in neuroscience—one that is directed at understanding the characteristics and regulation of adult neural stem cells [100, 101], as well as the therapeutic potential of using these cells as new drug targets or in transplantation-based protocols to combat neurodegenerative diseases and even cancer [102, 103]. Although the final outcome of the ongoing challenge to the reproductive biology dogma that adult mammalian females do not make new oocytes remains to be seen, we encourage those interested in this debate to revisit the history of the recent paradigm shift that has occurred regarding neurogenesis in the adult brain as an example of the hurdles faced by those who produce data inconsistent with traditional beliefs of a field.
As one weighs the evidence discussed here, another important issue relevant to discussions of work directed at either challenging the dogma or testing the possibility that cells other than PGCs can be coaxed to form oocytes pertains to the argument that an oocyte is not really an oocyte unless it is shown to produce a viable offspring. Currently, our laboratories and numerous others rely on morphology and gene expression analysis as the two principal endpoints for the study of oocyte biology (Fig. 2). Indeed, the very foundations of the dogma being challenged rest on results of morphometry-based protocols aimed at quantifying oocyte-containing follicle numbers and maturational status (primordial, transitional, primary, preantral) in fixed ovarian tissues analyzed by light microscopy (e.g., [17, 104–107]). Not one of these studies tracked every—or even a single—oocyte scored as an oocyte by standard morphological criteria to ensure that the identified cell was capable of completing maturation to a metaphase II (MII) egg, fertilizing, or producing a live-birth offspring.
Hence, moving forward, a challenge our field will be faced with is to reach a consensus on what parameters constitute the accurate identification of an oocyte and whether said cell has to exhibit full maturational competency (viz. produce an MII egg that can be fertilized to yield viable offspring) to be considered an oocyte. If maturation, fertilization, and embryonic developmental competency are now viewed as basic criteria for identifying oocytes, then the last 100 years of follicle morphometry-based studies—including those that formed the basis of the dogma itself—could be called into question. To us, this would be unwarranted, because the vast majority of oocytes formed in the ovaries never reach the point of being ovulated as an MII egg, much less fertilized [108]. Moreover, one could make a similar argument for the definition of a sperm. Although only a single sperm present in an ejaculate of millions of sperm will fertilize an egg, does this by default make the remainder of the sperm, especially those with obvious morphological or functional defects, not sperm? Although maturation, fertilization, and embryonic developmental competence of oocytes are important aspects of research, by no means should these endpoints now be requested for defining a cell as an oocyte unless, of course, the sole objective of the work is fertility. However, given the central importance of the vast majority of oocytes not destined to fertilize in coordinating so many other aspects of ovarian development and function, it seems shortsighted to restrict the potential utility of postnatally derived oocytes from whatever cell source to only procreation. For example, maintenance or expansion of the oocyte pool in adults for the sole purpose of maintaining an adequate quota of follicles to support cyclic ovarian function may have considerable ramifications for not only promoting continued ovulation of eggs competent for fertilization each cycle but also for improving quality of life as females age [109, 110].
Finally, it is worthwhile mentioning that females of “less evolved” species, such as flies, birds, and fish, can and do generate new oocytes during adult life [111–114]. This begs the question of why the ability of adult females to produce new eggs was lost somewhere during metazoan evolution prior to the emergence of mammals, when the ability of males to produce sperm throughout adulthood was conserved through evolution from flies to man. In other words, why would evolutionary pressure deem it advantageous to endow a fixed complement of oocytes at birth—one that, in humans, would sit and be subjected to years if not decades of aging and insults before being selected for ovulation—rather than to maintain a fresh complement of potential eggs at any given time during adult life to maximize the chances for successful reproduction? Regardless of all of the findings discussed above, it just seems more logical that for both genders, evolution would keep to the course of freshly generated gametes being a better option than stale eggs and sperm to ensure reproductive success.
In conclusion, as support grows—albeit slowly—for the concept that females, like males, have the capacity to renew their germ cell pool during adult life, many implications arise that could not be considered beforehand [89]. For example, the replicative germ cells responsible for maintaining oocyte output during postnatal life could be a new target for novel therapeutics designed to maximize or enhance their activity. The end result of such treatments would be to increase the ovarian follicle reserve when it would be clinically desirable—viz. to postpone age-related ovarian failure and, perhaps, menopause in women of advancing maternal age, and to rescue ovarian function and fertility in female cancer patients after their cytotoxic treatments. Further, female germline or somatic stem cell transplantation-based technologies, similar to those recently reported for BM-derived cells [72, 87, 88], could be developed for the same purposes. Although some have remained steadfast in their opinions that these types of studies will ultimately have no clinical value for fertility preservation or extension of ovarian lifespan [86] (see also comments in Powell [1]), very recent studies with rats have reinforced conclusions drawn in our work with mice [40, 72] that transplantation of BM-derived stem cells conveys significant protection from chemotherapy-induced ovarian damage in adult females [88]. And although this chemoprotective effect yields a return of fertility [72], the small numbers of germ cells formed from donor BM do not appear to generate mature ovulated eggs [38] or directly contribute to the production of offspring [72]. However, periodic reports of a return of ovarian function and fertility following BMT in female cancer patients deemed clinically menopausal due to their prior cytotoxic therapies suggest that the data obtained from transplantation studies in rodents may nonetheless already have clinical relevance (e.g., [115–120]) (reviewed in Oktay and Oktem [121]). Whatever the case, given the controversy that still surrounds this body of work, additional studies are needed to unequivocally establish that premeiotic germ cells capable of producing new oocytes do, in fact, exist in adult female mammals and contribute to normal ovarian function. More work is also required to continue characterization of oocytes or oocyte-like cells derived from non-PGC sources, with the ultimate goal of producing fertilization-competent eggs and viable offspring. Although the challenges of pursuing these types of experiments are considerable, being an ovarian optimist helps one stay focused and motivated when tackling such complex issues.
Acknowledgments
We would like to acknowledge the dedicated efforts of the many postdoctoral trainees who pursued these lines of investigation in the authors' laboratories since 2002. We also thank Dr. Kaisa Selesniemi for technical assistance with figure preparation.
Footnotes
1Supported by the National Institutes of Health grants R01-AG024999 and R37-AG012279 to J.L.T. and R01-CA98333 to B.R.R., the Rubin Shulsky Philanthropic Fund, the Henry and Vivian Rosenberg Philanthropic Fund, the Sea Breeze Foundation, the JM Foundation, and Vincent Memorial Research Funds.
REFERENCES
- Powell K.Going against the grain. PloS Biol 2007; 5: e338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazer FW.Strong science challenges conventional wisdom: new perspectives on ovarian biology. Reprod Biol Endocrinol 2004; 2: 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosden RG.Germline stem cells in the postnatal ovary: is the ovary more like a testis? Hum Reprod Update 2004; 10: 193–195. [DOI] [PubMed] [Google Scholar]
- Greenfeld C, Flaws JA.Renewed debate over postnatal oogenesis in the mammalian ovary. Bioessays 2004; 26: 829–832. [DOI] [PubMed] [Google Scholar]
- Hoyer PB.Can the clock be turned back on ovarian aging? Sci Aging Knowledge Environ 2004; 10: pe11 [DOI] [PubMed] [Google Scholar]
- Telfer EE.Germline stem cells in the postnatal mammalian ovary: a phenomenon of prosimian primates and mice? Reprod Biol Endocrinol 2004; 18: 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertini DF.Micromanagement of the ovarian follicle reserve—do stem cells play into the ledger? Reproduction 2005; 127: 513–514. [DOI] [PubMed] [Google Scholar]
- Byskov AG, Faddy MJ, Lemmen JG, Andersen CY.Eggs forever? Differentiation 2005; 73: 438–446. [DOI] [PubMed] [Google Scholar]
- Skaznik-Wikiel M, Tilly JC, Lee HJ, Niikura Y, Kaneko-Tarui T, Johnson J, Tilly JL.Serious doubts over “Eggs Forever?”. Differentiation 2007; 75: 93–99. [DOI] [PubMed] [Google Scholar]
- Gougeon A.Neo-oogenesis in the postnatal ovary: fantasy or reality? Gynecol Obstet Fertil 2005; 33: 819–823. [DOI] [PubMed] [Google Scholar]
- Telfer EE, Gosden RG, Byskov AG, Spears N, Albertini DF, Anderson CY, Anderson R, Braw-Tal R, Clarke H, Gougeon A, McLaughlin E, McLaren A, et al. On regenerating the ovary and generating controversy. Cell 2005; 122: 821–822. [DOI] [PubMed] [Google Scholar]
- Johnson J, Skaznik-Wikiel M, Lee HJ, Niikura Y, Tilly JC, Tilly JL.Setting the record straight on data supporting postnatal oogenesis in female mammals. Cell Cycle 2005; 4: 1471–1477. [DOI] [PubMed] [Google Scholar]
- Kayisli UA, Seli E.Stem cells and fertility: what does the future hold? Curr Opin Obstet Gynecol 2006; 18: 338–343. [DOI] [PubMed] [Google Scholar]
- Faddy M, Gosden R.Numbers of ovarian follicles and testing germ line renewal in the postnatal ovary. Facts and fallacies. Cell Cycle 2007; 6: 1951–1952. [DOI] [PubMed] [Google Scholar]
- Tilly JL, Johnson J.Recent arguments against germ cell renewal in the adult human ovary. Is an absence of marker gene expression really acceptable evidence of an absence of oogenesis? Cell Cycle 2007; 6: 879–883. [DOI] [PubMed] [Google Scholar]
- Oktem O, Oktay K.Stem cells: a perspective on oocytes. Ann N Y Acad Sci 2008; 1127: 20–26. [DOI] [PubMed] [Google Scholar]
- Zuckerman S.The number of oocytes in the mature ovary. Rec Prog Horm Res 1951; 6: 63–108. [Google Scholar]
- Zuckerman S.Beyond the Ivory Tower. The Frontiers of Public and Private Science New York:Taplinger;1971: 22–34. [Google Scholar]
- Waldeyer W. Eierstock und Ei. Leipzig, Germany:: Engelmann;; 1870. [Google Scholar]
- Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL.Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 2004; 428: 145–150. [DOI] [PubMed] [Google Scholar]
- Zhang D, Fouad H, Zoma WD, Salama SA, Wentz MJ, Al-Hendy A.Expression of stem and germ cell markers within nonfollicle structures in adult mouse ovary. Reprod Sci 2008; 15: 139–146. [DOI] [PubMed] [Google Scholar]
- Virant-Klun I, Zech N, Rožman P, Vogler A, Cvjetičanin B, Klemenc P, Maličev E, Meden-Vrtovec H.Putative stem cells with an embryonic character isolated from the ovarian surface epithelium of women with no naturally present follicles and oocytes. Differentiation 2008; 76: 843–856. [DOI] [PubMed] [Google Scholar]
- Virant-Klun I, Rožman P, Cvjetičanin B, Vrtacnik-Bokal E, Novakovic S, Ruelicke T.Parthenogenetic embryo-like structures in the human ovarian surface epithelium cell culture in postmenopausal women with no naturally occurring follicles and oocytes. Stem Cells Dev 2008; (in press). DOI:10.1089/scd.2007.0238. [DOI] [PubMed]
- Kerr JB, Myers M, Britt KL, Mladenovska T, Findlay JK.Quantification of healthy follicles in the neonatal and adult mouse ovary: evidence for maintenance of primordial follicle supply. Reproduction 2006; 132: 95–109. [DOI] [PubMed] [Google Scholar]
- Vermande-Van Eck G.Neo-ovogenesis in the adult monkey. Anat Rec 1956; 125: 207–224. [DOI] [PubMed] [Google Scholar]
- Flaws JA, Abbud R, Mann RJ, Nilson JH, Hirshfield AN.Chronically elevated luteinizing hormone depletes primordial follicles in the mouse ovary. Biol Reprod 1997; 57: 1233–1237. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Romero C, Hirshfield AN, Ojeda SR.Nerve growth factor is required for early follicular development in the mammalian ovary. Endocrinology 2001; 142: 2078–2086. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Skinner MK.Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol Reprod 2003; 69: 1265–1272. [DOI] [PubMed] [Google Scholar]
- Tomic D, Miller KP, Kenny HA, Woodruff TK, Hoyer P, Flaws JA.Ovarian follicle development requires Smad3. Mol Endocrinol 2004; 18: 2224–2240. [DOI] [PubMed] [Google Scholar]
- Rajkovic A, Pangas SA, Ballow D, Suzmori N, Matzuk MM.NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science 2004; 305: 1157–1159. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA.Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 2004; 301: 215–218. [DOI] [PubMed] [Google Scholar]
- Lohff JC, Christian PJ, Marion SL, Hoyer PB.Effect of duration of dosing on onset of ovarian failure in a chemical-induced mouse model of perimenopause. Menopause 2006; 13: 482–488. [DOI] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, Peng SL, Lan ZJ, Cooney AJ, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 2008; 319: 611–613. [DOI] [PubMed] [Google Scholar]
- Gosden RG.Ovarian support of pregnancy in ageing inbred mice. J Reprod Fertil 1975; 42: 423–430. [DOI] [PubMed] [Google Scholar]
- Gosden RG.Effects of age and parity on the breeding potential of mice with one or two ovaries. J Reprod Fertil 1979; 57: 477–487. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Gosden RG, Felicio LS.Effect of dietary restriction on estrous cyclicity and follicular reserves in aging C57BL/6J mice. Biol Reprod 1985; 32: 515–522. [DOI] [PubMed] [Google Scholar]
- Eichenlaub-Ritter U, Chandley AC, Gosden RG.The CBA mouse as a model for age-related aneuploidy in man: studies of oocyte maturation, spindle formation and chromosome alignment during meiosis. Chromosoma (Berl) 1988; 96: 220–226. [DOI] [PubMed] [Google Scholar]
- Eggan K, Jurga S, Gosden R, Min IM, Wagers AJ.Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature 2006; 441: 1109–1114. [DOI] [PubMed] [Google Scholar]
- Allen E.Ovogenesis during sexual maturity. Am J Anat 1923; 31: 439–482. [Google Scholar]
- Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, Tschudy KS, Tilly JC, Cortes ML, Forkert R, Spitzer T, Iacomini J, et al. Oocyte generation in adult mammalian ovaries by putative germ cells derived from bone marrow and peripheral blood. Cell 2005; 122: 303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci LR, Meistrich ML.Effects of busulfan on murine spermatogenesis: cytotoxicity, sterility, sperm abnormalities, and dominant lethal mutations. Mutat Res 1987; 176: 259–268. [DOI] [PubMed] [Google Scholar]
- Brinster RL, Avarbock MR.Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A 1994; 91: 11303–11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Arechaga JM, Avarbock MR, Brinster RL.Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol 1997; 41: 111–122. [PubMed] [Google Scholar]
- Pelloux MC, Picon R, Gangnerau MN, Darmoul D.Effects of busulphan on ovarian folliculogenesis, steroidogenesis and anti-Müllerian activity of rat neonates. Acta Endocrinol 1988; 118: 218–226. [DOI] [PubMed] [Google Scholar]
- Perez GI, Knudson CM, Leykin L, Korsmeyer SJ, Tilly JL.Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat Med 1997; 3: 1228–1232. [DOI] [PubMed] [Google Scholar]
- Perez GI, Tao XJ, Tilly JL.Fragmentation and death (a.k.a. apoptosis) of ovulated oocytes. Mol Hum Reprod 1999; 5: 414–420. [DOI] [PubMed] [Google Scholar]
- Morita Y, Perez GI, Paris F, Miranda S, Ehleiter D, Haimovitz-Friedman A, Fuks Z, Xie Z, Reed JC, Schuchman EH, Kolesnick RN, Tilly JL.Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med 2000; 6: 1109–1114. [DOI] [PubMed] [Google Scholar]
- Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC.In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet 2006; 38: 1430–1434. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Li Y, Nie R, Friel P, Mitchell D, Evanoff RM, Pouchnik D, Banasik B, McCarrey JR, Small C, Griswold MD.Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol Reprod 2008; 78: 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Tilly JL.Inhibition of histone deacetylase activity amplifies retinoic acid-mediated induction of Stra8 expression and oogenesis in ovaries of adult female mice. Proceedings of the 41st Annual Meeting of the Society for the Study of Reproduction, Kailua-Kona, HI;p132 Abstract 291. [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P.Retinoic acid signaling determines germ cell fate in mice. Science 2006; 312: 596–600. [DOI] [PubMed] [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC.Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A 2006; 103: 2474–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Sakamoto H, Luo H, Skaznik-Wikiel ME, Friel A, Niikura T, Tilly JC, Klein R, Styer A, Zuckerberg L, Tilly JL, Rueda BR.Loss of CABLES1, a cyclin-dependent kinase-interacting protein that inhibits cell cycle progression, results in germline expansion at the expense of oocyte quality in adult female mice. Cell Cycle 2007; 6: 2678–2684. [DOI] [PubMed] [Google Scholar]
- Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Cook RW, Kipp JL, Shea LD, Mayo KE, Woodruff TK.Postnatal regulation of germ cells by activin: the establishment of the initial follicle pool. Dev Biol 2006; 298: 132–148. [DOI] [PubMed] [Google Scholar]
- Lin H.The stem-cell niche theory: lessons from flies. Nat Rev Genet 2002; 3: 931–940. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Ohmura M, Ohbo K.The niche for spermatogonial stem cells in the mammalian testis. Int J Hematol 2005; 82: 381–388. [DOI] [PubMed] [Google Scholar]
- Bukovsky A, Caudle MR, Svetlikova M, Upadhyaya NB.Origin of germ cells and formation of new primary follicles in adult human ovaries. Reprod Biol Endocrinol 2004; 2: 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukovsky A, Svetlikova M, Caudle MR.Oogenesis in cultures derived from adult human ovaries. Reprod Biol Endocrinol 2005; 3: 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukovsky A, Caudle MR, Gupta SK, Svetlikova M, Selleck-White R, Ayala AM, Dominguez R.Mammalian neo-oogenesis and expression of meiosis-specific protein SCP3 in adult human and monkey ovaries. Cell Cycle 2008; 7: 683–686. [DOI] [PubMed] [Google Scholar]
- Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Mayo KE, Shea LD, Woodruff TK.Fate of the initial follicle pool: empirical and mathematical evidence supporting its sufficiency for adult fertility. Dev Biol 2006; 298: 149–154. [DOI] [PubMed] [Google Scholar]
- Peters H.The development of the mouse ovary from birth to maturity. Acta Endocrinol 1969; 62: 98–116. [DOI] [PubMed] [Google Scholar]
- Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM.Molecular characterization of the follicle defects in the growth differentiation factor 9-deficient ovary. Mol Endocrinol 1999; 13: 1018–1034. [DOI] [PubMed] [Google Scholar]
- Myers M, Britt KL, Wreford NGM, Ebling FJP, Kerr JB.Methods for quantifying follicular numbers within the mouse ovary. Reproduction 2004; 127: 569–580. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wu C, Lyu Q, Yang D, Albertini DF, Keefe DL, Liu L.Germline stem cells and neo-oogenesis in the adult human ovary. Dev Biol 2007; 306: 112–120. [DOI] [PubMed] [Google Scholar]
- Huntriss J, Hinkins M, Picton H.cDNA cloning and expression of the human NOBOX gene in oocytes and ovarian follicles. Mol Hum Reprod 2006; 12: 283–289. [DOI] [PubMed] [Google Scholar]
- John GB, Shirley LJ, Gallardo TD, Castrillon DH.Specificity of the requirement for Foxo3 in primordial follicle activation. Reproduction 2007; 133: 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Wakayama T, Nishimune Y.Commitment of fetal male germ cells to spermatogonial stem cells during mouse embryonic development. Biol Reprod 2004; 70: 1286–1291. [DOI] [PubMed] [Google Scholar]
- Hübner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R, Wood J, Strauss JF, III, Boiani M, Schöler HR.Derivation of oocytes from mouse embryonic stem cells. Science 2003; 300: 1251–1256. [DOI] [PubMed] [Google Scholar]
- Novak I, Lightfoot DA, Wang H, Eriksson A, Mahdy E, Höög C.Mouse embryonic stem cells form follicle-like ovarian structures but do not progress through meiosis. Stem Cells 2006; 8: 1931–1936. [DOI] [PubMed] [Google Scholar]
- Kerkis A, Fonseca SA, Serafim RC, Lavagnolli TM, Abdelmassih S, Abdelmassih R, Kerkis R.In vitro differentiation of male mouse embryonic stem cells into both presumptive sperm cells and oocytes. Cloning Stem Cells 2007; 9: 535–548. [DOI] [PubMed] [Google Scholar]
- Nagano MC.In vitro gamete derivation from pluripotent stem cells: progress and perspective. Biol Reprod 2007; 76: 546–551. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Selesniemi K, Niikura Y, Niikura T, Klein R, Dombkowski DM, Tilly JL.Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J Clin Oncol 2007; 25: 3198–3204. [DOI] [PubMed] [Google Scholar]
- Dyce PW, Wen L, Li J.In vitro germline potential of stem cells derived from fetal porcine skin. Nat Cell Biol 2006; 8: 384–390. [DOI] [PubMed] [Google Scholar]
- Dyce PW, Li J.From skin cells to ovarian follicles? Cell Cycle 2006; 5: 1371–1375. [DOI] [PubMed] [Google Scholar]
- Danner S, Kajahn J, Geismann C, Klink E, Kruse C.Derivation of oocyte-like cells from a clonal pancreatic stem cell line. Mol Hum Reprod 2007; 13: 11–20. [DOI] [PubMed] [Google Scholar]
- Logothetou-Rella H.Meiosis in hematological malignancies. In situ cytogenetic morphology. Histol Histopathol 1996; 11: 943–963. [PubMed] [Google Scholar]
- Logothetou-Rella H.Description of primordial germ cells, oogonia, oocytes and embryo-like growth in squash preparations of tissues from hematological malignancies. Histol Histopathol 1996; 11: 965–984. [PubMed] [Google Scholar]
- Toyooka Y, Tsunekawa N, Akasu R, Noce T.Embryonic stem cells can form germ cells in vitro. Proc Natl Acad Sci U S A 2003; 100: 11457–11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijsen N, Horoshak M, Kim K, Gribnau J, Eggan K, Daley GQ.Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature 2004; 427: 148–154. [DOI] [PubMed] [Google Scholar]
- Nayernia K, Lee JH, Drusenheimer N, Nolte J, Wulf G, Dressel R, Gromoll J, Engel W.Derivation of male germ cells from bone marrow stem cells. Lab Invest 2006; 86: 654–663. [DOI] [PubMed] [Google Scholar]
- Lue Y, Erkkila K, Liu PY, Ma K, Wang C, Hikim AS, Swerdloff RS.Fate of bone marrow stem cells transplanted into the testis: implications for men with testicular failure. Am J Pathol 2007; 170: 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusenheimer N, Wulf G, Nolte J, Lee JH, Dev A, Dressel R, Gromoll J, Schmidtke J, Engel W, Nayernia K.Putative human male germ cells from bone marrow stem cells. Soc Reprod Fertil Suppl 2007; 63: 69–76. [PubMed] [Google Scholar]
- Yeom YI, Fuhrmann G, Ovitt CE, Brehm A, Ohbo K, Gross M, Hübner K, Schöler HR.Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development 122: 881–894. [DOI] [PubMed] [Google Scholar]
- Yoshimizu T, Sugiyama N, De Felice M, Yeom YI, Ohbo K, Masuko K, Obinata M, Abe K, Schöler HR, Matsui Y.Germline-specific expression of the Oct-4/green fluorescent protein (GFP) transgene in mice. Dev Growth Differ 1999; 41: 675–684. [DOI] [PubMed] [Google Scholar]
- Szabo PE, Hübner K, Schöler HR, Mann JR.Allele-specific expression of imprinted genes in mouse migratory primordial germ cells. Mech Dev 2002; 115: 157–160. [DOI] [PubMed] [Google Scholar]
- Begum S, Papaioannou VE, Gosden RG.The oocyte population is not renewed in transplanted or irradiated adult ovaries. Hum Reprod 2008; 23: 2326–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadami M, El-Demerdash E, Zhang D, Hassan MH, Nagamani M, Chen X, Chedress J, Sairam MR, Al-Hendy A.Intraveously injected bone marrow cells restore ovarian folliculogenesis and steroid hormones production in female FSHR (−/−) mice. Reprod Sci 2008; 15(suppl):228A Abstract 597. [Google Scholar]
- Fu X, He Y, Xie C, Liu W.Bone marrow mesenchymal stem cell transplantation improves ovarian function and structure in rats with chemotherapy-induced damage. Cytotherapy 2008; 10: 353–363. [DOI] [PubMed] [Google Scholar]
- Tilly JL, Rueda BR.Stem cell contribution to ovarian development, function, and disease. Endocrinology 2008; 149: 4307–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankle WR, Landig BH, Rafii MS, Schiano A, Chen JM, Hara J.Evidence for a postnatal doubling of neuron number in the developing human cerebral cortex between 15 months and 6 years. J Theor Biol 1998; 191: 115–140. [DOI] [PubMed] [Google Scholar]
- Shankle WR, Rafii MS, Landig BH, Fallon JH.Approximate doubling of numbers of neurons in postnatal human cerebral cortex and in 35 specific cytoarchitectural areas from birth to 72 months. Pediatr Dev Pathol 1999; 2: 244–259. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Graziano MS, Gross CG.Neurogenesis in the neocortex of adult primates. Science 1999; 286: 548–552. [DOI] [PubMed] [Google Scholar]
- Korr H, Schmitz C.Facts and fictions regarding post-natal neurogenesis in the developing human cerebral cortex. J Theor Biol 1999; 200: 291–297. [DOI] [PubMed] [Google Scholar]
- Nowakowski RS, Hayes NL.New neurons: extraordinary evidence or extraordinary conclusion? Science 2000; 288: 771 [DOI] [PubMed] [Google Scholar]
- Rakic P.Neurogenesis in the adult primate neocortex: an evaluation of the evidence. Nat Rev Neurosci 2002; 3: 65–71. [DOI] [PubMed] [Google Scholar]
- Blakeslee S.A decade of discovery yields a shock about the brain. New York Times. January4,2000: F1, F4
- Gross CG.Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci 2000; 1: 67–73. [DOI] [PubMed] [Google Scholar]
- Gould E, Vail N, Wagers M, Gould CG.Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc Natl Acad Sci U S A 2001; 98: 10910–10917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Gross CG.Neurogenesis in adult mammals: some progress and problems. J Neurosci 2002; 22: 619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Kozorovitskiy Y, Gross CG, Gould E.Diminished neurogenesis in the marmoset brain precedes old age. Proc Natl Acad Sci U S A 2007; 104: 17169–17173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revishchin AV, Korochkin LI, Okhotin VE, Pavlova GV.Neural stem cells in the mammalian brain. Int Rev Cytol 2008; 265: 55–109. [DOI] [PubMed] [Google Scholar]
- Maurer MH, Kuschinsky W.Screening the brain: molecular fingerprints of neural stem cells. Curr Stem Cell Res Ther 2006; 1: 65–77. [DOI] [PubMed] [Google Scholar]
- Taupin P.Therapeutic potential of adult neural stem cells. Recent Patents CNS Drug Discov 2006; 1: 299–303. [DOI] [PubMed] [Google Scholar]
- Beaumont HM, Mandl AM.A quantitative and cytological study of oogonia and oocytes in the foetal and neonatal rat. Proc R Soc Lond B 1961; 155: 557–579. [Google Scholar]
- Baker TG.A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B 1963; 158: 417–433. [DOI] [PubMed] [Google Scholar]
- Baker TG, Franchi LL.The fine structure of oogonia and oocytes in human ovaries. J Cell Sci 1967; 2: 213–224. [DOI] [PubMed] [Google Scholar]
- Gosden RG.Follicular status at menopause. Hum Reprod 1987; 2: 617–621. [DOI] [PubMed] [Google Scholar]
- Tilly JL.Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol 2001; 2: 838–848. [DOI] [PubMed] [Google Scholar]
- Selesniemi K, Lee HJ, Tilly JL.Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell 2008; 7: 622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez GI, Jurisicova A, Wise L, Lipina T, Kanisek M, Bechard A, Takai Y, Hunt P, Roder J, Grynpas M, Tilly JL.Absence of the pro-apoptotic Bax protein extends fertility and alleviates age-related health complications in female mice. Proc Natl Acad Sci U S A 2007; 104: 5229–5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilly D, Xie T.The Drosophila ovary: an active stem cell community. Cell Res 2007; 17: 15–25. [DOI] [PubMed] [Google Scholar]
- Pearl R, Schoppe WF.Studies on the physiology of reproduction in the domestic fowl. J Exp Zool 1921; 34: 101–118. [Google Scholar]
- Underwood JL, Hestand RS, III, Thompson BZ.Gonad regeneration in grass carp following bilateral gonadectomy. Prog Fish Cult 1986; 48: 54–56. [Google Scholar]
- Draper BW, McCallum CM, Moens CB.nanos1 is required to maintain oocyte production in adult zebrafish. Dev Biol 2007; 305: 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson A, Fuchs T, Simonsson B, Bjorkholm M.Successful pregnancy in a 28-year-old patient autografted for acute lymphoblastic leukemia following myeloablative treatment including total body irradiation. Bone Marrow Transplant 1993; 12: 659–660. [PubMed] [Google Scholar]
- Salooja N, Chatterjee R, McMillan AK, Kelsey SM, Newland AC, Milligan DW, Franklin IM, Hutchinson RM, Linch DC, Goldstone AH.Successful pregnancies in women following single autotransplant for acute myeloid leukemia with a chemotherapy ablation protocol. Bone Marrow Transplant 1994; 13: 431–435. [PubMed] [Google Scholar]
- Sanders JE, Sanders JE, Hawley J, Levy W, Gooley T, Buckner CD, Deeg HJ, Doney K, Storb R, Sullivan K, Witherspoon R, Appelbaum FR.Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood 1996; 87: 3045–3052. [PubMed] [Google Scholar]
- Salooja N, Szydlo RM, Socie G, Rio B, Chatterjee R, Ljungman P, Van Lint MT, Powles R, Jackson G, Hinterberger-Fischer M, Kolb HJ, Apperley JF.Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Pregnancy outcomes after peripheral blood or bone marrow transplantation: a retrospective study. Lancet 2001; 358: 271–276. [DOI] [PubMed] [Google Scholar]
- Hershlag A, Schuster MW.Return of fertility after autologous stem cell transplantation. Fertil Steril 2002; 77: 419–421. [DOI] [PubMed] [Google Scholar]
- Socie G, Salooja N, Cohen A, Rovelli A, Carreras E, Locasciulli A, Korthof E, Weis J, Levy V, Tichelli A.Late Effects Working Party of the European Study Group for Blood and Marrow Transplantation. Nonmalignant late effects after allogeneic stem cell transplantation. Blood 2003; 101: 3373–3385. [DOI] [PubMed] [Google Scholar]
- Oktay K, Oktem O.Regeneration of oocytes after chemotherapy: connecting the evidence from mouse to human. J Clin Oncol 2007; 25: 3185–3187. [DOI] [PubMed] [Google Scholar]