Abstract
The eutopic endometrium in women with endometriosis demonstrates diminished endometrial receptivity and altered gene expression. It is unknown if the endometrium being defective gives rise to a predisposition toward endometriosis and infertility or, alternatively, if endometriosis causes the altered endometrial receptivity. Here we created experimental endometriosis in mice and examined the expression of several markers of endometrial receptivity in the eutopic endometrium. Methylation of Hoxa10 was also evaluated as a potential mechanism responsible for altered gene expression. Expression of each gene was measured using quantitative real-time RT-PCR at 14 wk after induction of endometriosis. Expression of Hoxa10 and Hoxa11, which are necessary for endometrial receptivity, were decreased in the endometriosis group. Insulin-like growth factor binding protein-1 (Igfbp1) mRNA was decreased in the endometriosis group. However, there was no change in Integrin beta3 (Itgb3) mRNA expression. Total progesterone receptor (Pgr-AB) was increased in the endometriosis group and the ratio of Pgr-B to Pgr-AB was increased, indicating a shift from Pgr-A to Pgr-B expression. Basic transcription element-binding protein-1 (Bteb1), official symbol and name Klf9, Kruppel-like factor 9, which functionally interacts with Pgr in endometrium, was also decreased in the endometriosis group. In addition, hypermethylation of Hoxa10 in the endometriosis group was shown by methylation-specific PCR and confirmed by bisulfite sequencing. These findings demonstrate that normal endometrium, when placed in an ectopic location to create experimental endometriosis, led to characteristic changes in gene expression in eutopic endometrium. These data suggest the existence of a signal conduction pathway from endometriosis that alters endometrial gene expression through altered Pgr signaling and epigenetic programming.
Keywords: endometriosis, female reproductive tract, gene regulation, Hoxa10, Hoxa11, implantation, methylation, Pgr, uterus
Normal endometrium, when placed in an ectopic location creating experimental endometriosis, leads to characteristic changes in gene expression in eutopic endometrium.
INTRODUCTION
Endometriosis is found in 20% to 50% of women with infertility, which is a principal manifestation of endometriosis [1–3]. Several theories, such as Sampson's theory [4], have been proposed to explain the etiology of endometriosis, but the pathophysiology of endometriosis and the related infertility remains unclear [5]. There are ethical limitations that limit investigation of endometriosis in humans. It is also difficult to monitor the progress of endometriosis in humans continuously, making investigation difficult in humans. Primates such as rhesus monkeys and baboons may be considered ideal animal models to investigate endometriosis because they menstruate in a cyclic pattern and develop endometriosis spontaneously [6–8]. However, rodents have the advantage of low costs and ease of availability and handling. Rodent models of endometriosis have been developed by utilizing i.p. or subcutaneous transplantation of human endometrium in immunocompromised mice and the surgical autotransplantation of endometrium in immunocompetent animals [9–14].
Defective implantation has been demonstrated as one mechanism responsible for endometriosis-associated infertility [15–18]. Previous studies have reported the aberrant expressions of genetic markers of endometrial receptivity in endometriosis [19–22]. Homeobox (Hox/HOX) genes encode transcription factors that mediate embryonic development [23]. Hoxa10/HOXA10 and Hoxa11/HOXA11 are expressed in the uterus during embryonic development and in the adult [23]. In the human, HOXA10 and HOXA11 are expressed in endometrial glands and stroma throughout the menstrual cycle and show a dramatic increase during the mid-luteal phase at the time of implantation in response to estrogen and progesterone [24, 25]. Hox/HOX genes may affect endometrial development in a way analogous to their role in embryonic development, leading to endometrial growth, differentiation, and receptivity [19, 26]. Mice with a targeted disruption of either the Hoxa10 or Hoxa11 gene are sterile due to the loss of endometrial receptivity, yet still produce viable embryos [27–29]. Embryos from these mice implant normally to the uteri of wild-type mice, but embryos from wild-type mice do not implant in Hoxa10 or Hoxa11 knockout mice [27–29]. Altered endometrial expression of Hoxa10 using either Hoxa10 antisense or a constitutive Hoxa10 expression construct results in either a decrease or increase in litter size, respectively [30]. These experiments demonstrate cyclic expression of Hoxa10/HOXA10 and Hoxa11/HOXA11 in adult endometrium is necessary for endometrial receptivity. Women with endometriosis fail to upregulate expression of HOXA10 and HOXA11 during the window of implantation [19, 31]. In baboons with induced endometriosis, HOXA10 expression in the eutopic endometrium is decreased [32]. These experiments demonstrate endometriosis leads to altered HOXA gene expression and defective endometrial receptivity. Alterations in Hox/HOX genes may be expected to induce additional alterations in the expression of the downstream target genes of these transcription factors involved in the development of endometrial receptivity.
Implantation in early pregnancy is regulated by the steroid hormones estrogen and progesterone. Progesterone receptor (Pgr/PGR), a member of the nuclear receptor superfamily, is necessary for embryo implantation and subsequent decidualization [33]. Progesterone regulates HOXA10 and HOXA11 expression in endometrial glands and stroma [24, 25] through its cognate receptor, PGR [26, 34]. Hoxa10 is necessary for uterine stromal cell responsiveness to progesterone during implantation and decidualization [35]. Two distinct isoforms of the human progesterone receptor (PGR), termed PGR-A and PGR-B, are encoded by a single gene and they differ in that PGR-B contains N-terminal 164 amino acids [36, 37]. PGR-B functions as a dominant transcriptional activator of progesterone-responsive promoters, whereas PGR-A acts as a dominant repressor of PGR-B and other steroid receptors (estrogen, androgen, and glucocorticoid and mineralocorticoid receptors) [36, 37]. Therefore, the relative expression of Pgr-A/PGR-A to Pgr-B/PGR-B determines, in part, the characteristic cellular response to progesterone [36–39].
Klf9, a member of the Sp/Kruppel-like family of transcription factors, functionally interacts with Pgr-A and Pgr-B to mediate progesterone-responsive gene expression in endometrial epithelial cells [40, 41]. In the uterus of early pregnant Klf9 knockout mice, numbers of implantation sites are decreased, expression of Hoxa10 is decreased, and progesterone responsiveness of several uterine genes is decreased [42].
In this study, we developed an experimental mouse model of endometriosis and evaluated expression of Pgr, Hoxa10, Hoxa11, and several downstream target genes related to endometrial receptivity in the eutopic endometrium of mice with experimental endometriosis. We also evaluated the methylation status in the Hoxa10 gene as a possible mechanism for altered Hoxa10 expression.
MATERIALS AND METHODS
Development of a Mouse Model of Endometriosis
Eight-week-old CD1 female mice were obtained from Charles River Laboratories (Wilmington, MA) and kept under controlled conditions (12L:12D and 22°C). Laparotomy was performed by midline incision under i.p. anesthesia with xylazine (Lloyd Laboratories, Quezon, Philippines) and ketamin (Fort Dodge Animal Health, Overland Park, KS). In four donor mice, the whole uterus was removed and divided into two horns after washing in PBS. Each uterine horn was transplanted into the abdominal cavity of one recipient mouse. The lumen of each horn was opened longitudinally. Then, the opened horn was transversely divided into two pieces. One piece from each uterine horn was sutured to the parietal peritoneum of the anterior abdominal wall (Vicryl 4–0, Ethicon), and another piece was placed in the abdominal cavity. Finally, the abdominal wall was sutured closed. Experimental endometriosis was created in eight mice. At the same time, seven control mice were created by performing an identical incision, placing the same amount of suture material in a similar location as performed in the experimental group, and closing the abdominal cavity with the same suture material. After 14 wk the uterus was removed from each recipient mouse and divided into two horns. One horn was snap-frozen in TRIzol Reagent (Invitrogen Technologies, Carlsbad, CA) and another horn was frozen immediately at −80°C for later use. This study was approved by the Institutional Animal Care and Use Committee, Yale University, confirming to the U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training.
Quantitative Real-Time RT-PCR
Total RNA was extracted using TRIzol Reagent with Phase Lock Gel-Heavy (Eppendorf North America, Westbury, NY) according to the manufacturer's instructions and treated using recombinant shrimp DNase (USB, Cleveland, OH) to eliminate DNA contamination. Total RNA (50 ng) was reverse-transcribed using iScript cDNA synthesis kit (Biorad Laboratories, Hercules, CA) for 5 min at 25°C, 30 min at 42°C, and 5 min at 85°C. Real-time PCR was initially performed at 95°C for 3 min and followed by 45 cycles of 95°C for 15 sec, 60.5°C for 20 sec, and 72°C for 25 sec using SYBR Green (BioRad Laboratories) and the MyiQ Single Color Real-Time PCR Detection System (BioRad Laboratories). The specificity of amplified products was confirmed by a melting curve analysis. The oligonucleotide primers are listed in Table 1. Annealing temperature for all primers was 60.5°C. Sterile water was used as a negative control. Messenger RNA expression of Hoxa10, Hoxa11, Insulin-like growth factor binding protein 1 (Igfbp1), Integrin β3 (Itgb3), total Pgr (Pgr-AB), Pgr-B, and Klf9 was quantified and normalized to β-actin (ACTB) as a control. Then, fold value in expression of each gene was calculated using the 2−ΔΔCT Method [43]. Each assay was conducted in duplicate and repeated a minimum of three times.
TABLE 1.
Primers used in quantitative real-time PCR.
Immunohistochemistry
Tissue was embedded in paraffin, cut into 5-μm sections, and mounted onto slides. Immunohistochemistry for PGR was performed on all slides simultaneously. Slides were first deparaffinized and hydrated through a progression of 10-min xylene and ethanol washes. The tissue was then permeabilized in cold 95% ethanol for 10 min. After being rinsed for 5 min in distilled water, the slides were steamed in 0.01 M sodium citrate buffer for 20 min in order to promote antigen presentation. The slides were then allowed to cool for 20 min in the staining jar containing citrate buffer, followed by a 5-min wash in phosphate buffered saline Tween (PBST). Endogenous peroxidase activity was quenched using a 3% hydrogen peroxide solution for 3 min. After another wash in PBST, the slides were incubated for 1 h at room temperature in a solution of 1.5% normal goat serum in PBST in order to block nonspecific antibody binding. The slides were then incubated at 4°C overnight in a 1:500 dilution of primary antibody in PBST containing 1.5% normal goat serum. The primary antibody used was PR H-190 (sc-7208), purchased from Santa Cruz Biotechnology (Santa Cruz, CA). After three 3-min rinses in PBST, slides were incubated in a biotinylated secondary antibody solution for 1 h at room temperature. The secondary antibody used was goat α-rabbit (BA-1000), purchased from Vector Laboratories (Burlingame, CA) and prepared as a 1:435 dilution in PBST containing 1.5% normal goat serum. Slides were then washed three times in PBST and incubated for 15 min in ABC Elite (Vector). After another three washes in PBST, the slides were incubated for 7 min in diaminobenzidine, followed by a rinse in distilled water. To counterstain the specific binding to PR, slides were dipped into hematoxylin for 15 sec and then rinsed with distilled water. The slides were then dehydrated through a series of 3-min ethanol and xylene washes. Coverslips were mounted using Permount.
Sodium Bisulfite DNA Modification and Methylation-Specific PCR
Frozen uterine samples were thawed. Genomic mouse DNA was isolated from each uterine sample using the DNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. Sodium bisulfite modification using the CpGenome DNA Modification Kit (Chemicon, Temecula, CA) was performed using 1 μg of genomic DNA according to the manufacturer's protocol. In this process, unmethylated cytosine residues are converted to thymine, whereas methylated cytosines remain unchanged [44].
The primers used for the methylation-specific PCR (MSP) amplification were designed from published DNA sequences in the 5′ promoter region of Hoxa10 [45] using the MethPrimer software [46]: Methylated sequence: forward, 5′-GGGCGAAAGGGGGCGGGT-3′, and reverse, 5′-GCGCCCGCCCGCCGCCT-3′; Unmethylated sequence: forward, 5′-GGGTGAAAGGGGGCTGGT-3′, and reverse, 5′-ACACCCACCCACCACCT-3′. The 140-bp region amplified with these primers includes eight CpG sites. PCR amplification of 100 ng bisulfite-treated DNA template was performed in a 50-μl reaction containing 1.5 μl forward and reverse primers and 25 μl SYBR Green. Amplification conditions were: 95°C for 15 min, 40 cycles at 95°C for 30 sec, 62°C (methylated) or 55°C (unmethylated) for 30 sec, and 72°C for 30 sec, followed by a final extension at 72°C for 10 min. CpG Methylated NIH 3T3 Mouse Genomic DNA (New England BioLabs, Beverly, MA) and sterile water were used as positive control and negative control, respectively. The PCR products were separated by electrophoresis on 2% agarose gels and visualized by ethidium bromide staining.
Bisulphite Sequencing of Hoxa10 Gene
The primers used for the bisulfite genomic sequencing PCR amplification were designed from mouse DNA sequences that were conserved in the 5′ promoter region of human HOXA10 gene [47] using the MethPrimer software [46]: forward, 5′-TATTTTGAGGTAGTTTTTATAGTTT-3′, and reverse, 5′-ATAACCCTTTCTAACTAACATT-3′. The 271-bp region amplified with these primers includes 20 CpG sites. PCR amplification of 100 ng bisulfite-treated DNA template was performed in a 50-μl reaction containing 1.5 μl forward and reverse primers, 3 μl of 25 mM Mg2+, 5 μl of 10× buffer, 1 μl of 1.25 mmol/L deoxynucleotide triphosphates, and 0.5 μl of HotStarTaq DNA polymerase (Qiagen). Amplification conditions were: starting at 95°C for 15 min, 40 cycles at 95°C for 30 sec, 53°C for 30 sec, and 72°C for 30 sec followed by a final extension at 72°C for 10 min. Direct sequence analyses of PCR products were carried out on Applied Biosystems 3730 capillary instruments (Applied Biosystems, Foster, CA).
Statistical Analyses
Statistical analyses were performed using SigmaStat (Systat Software, Point Richmand, CA). Statistical differences in expression of genes between the endometriosis model group and control group were calculated by a Mann-Whitney Rank Sum Test. Using bisulphite sequencing, Hoxa10 promoter sequences were analyzed for methylation status in the endometriosis group (n = 7) and control group (n = 4). Since there are 20 CpG sites in the 5′ promoter region of Hoxa10, we analyzed 20 × 7 = 140 CpG sites, and 20 × 4 = 80 CpG sites, respectively, in each experimental group. The percentage of methylated CpGs was calculated by the number of methylated CpGs divided by the total number of CpGs analyzed. The difference in methylation frequencies of Hoxa10 gene between different experimental groups was calculated by a chi-square test. P < 0.05 was considered statistically significant.
RESULTS
Altered Gene Expressions in Mice after Experimental Induction of Endometriosis
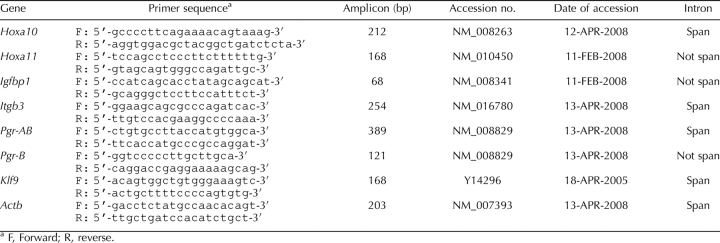
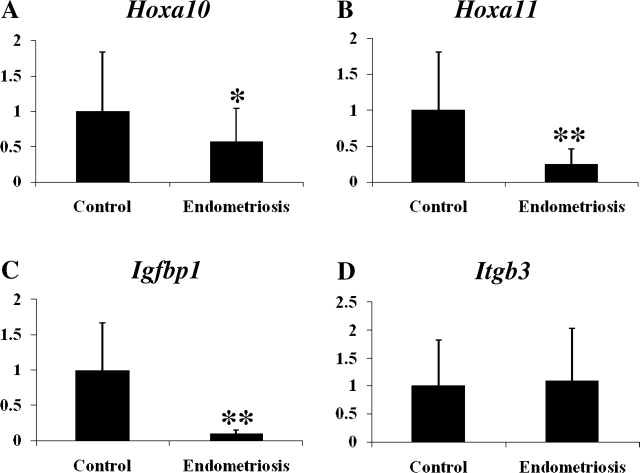
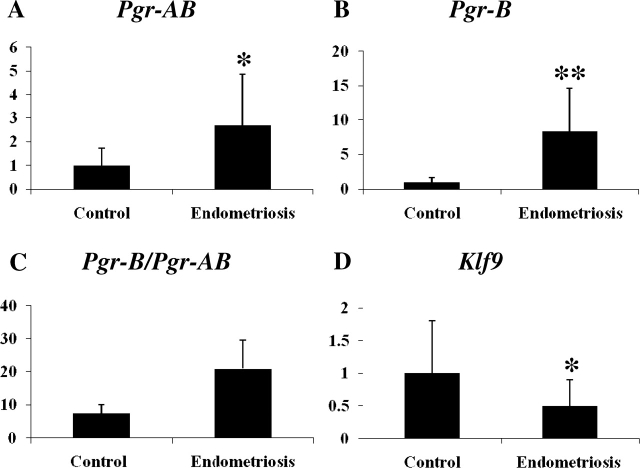
Gene expression in the eutopic endometrium of mice with experimentally induced endometriosis and control mice at 14 wk after the induction of endometriosis were measured by quantitative real-time PCR. Hoxa10 and Hoxa11 mRNA expression decreased 0.57-fold (P = 0.046) and 0.25-fold (P < 0.001) in the endometriosis group compared to the control group, respectively (Fig. 1, A and B). Expression of Igfbp1 and Itgb3, downstream target genes of Hoxa10, were measured. Igfbp1 mRNA, which is positively regulated by Hoxa10 [48, 49], was decreased 0.09-fold (P < 0.001) in the endometriosis group (n = 7) compared to the control group (Fig. 1C). There was no significant change (1.1-fold, P > 0.05; Fig. 1D) in Itgb3 mRNA expression. Total Pgr (Pgr-AB) and Pgr-B mRNA increased 2.7-fold (P = 0.016) and 8.39-fold (P = 0.006) in the endometriosis group compared to the control group, respectively (Fig. 2, A and B). The ratio of Pgr-B to Pgr-AB was increased (7.53 vs. 20.98) in endometriosis. This increase was out of proportion to the increase in Pgr-B, suggesting that Pgr-A expression was decreased (Fig. 2C). Klf9, which functionally interacts with Pgr in endometrium [40–42], was decreased 0.50-fold (P = 0.043) in the endometriosis group compared to the control group (Fig. 2D). The increase in PGR protein expression was confirmed using immunohistochemistry as demonstrated in Figure 3.
FIG. 1.
A–D) Expression of Hoxa10 and its downstream target genes and Hoxa11 in eutopic endometrium of mice with induced endometriosis. The y-axis represents fold change in expression as determined by quantitative real-time PCR and is expressed as mean ± SEM. Asterisk represents statistically significant difference between groups (*P < 0.05, **P < 0.01).
FIG. 2.
A–D) Expression of Pgr and Klf9 in the eutopic endometrium of mice with induced endometriosis. Pgr-AB, Pgr-B, ratio of Pgr-B to Pgr-AB, and Klf9 were expressed as mean ± SEM. The y-axis represents fold change in expression as determined by quantitative real-time PCR. Asterisk represents statistically significant difference between groups (*P < 0.05, **P < 0.01).
FIG. 3.
Expression of PGR protein in the eutopic endometrium of mice with induced endometriosis. Immunohistochemistry was used to demonstrate higher levels of PGR expression in the endometrium of mice with induced endometriosis compared to controls. Original magnification ×600.
Methylation of the Hoxa10 in an Experimental Model of Endometriosis
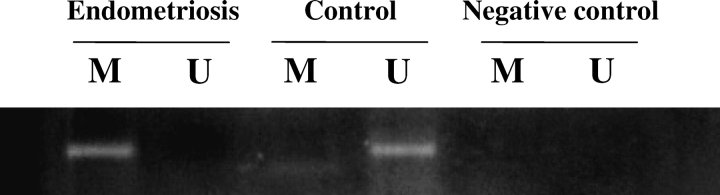
To determine whether hypermethylation of Hoxa10 induced a decrease in Hoxa10 expression in endometriosis, methylation in the 5′ promoter region of Hoxa10 was measured. Several CG-rich regions of the 5′ promoter region of Hoxa10 were measured using both MSP and bisulfite sequencing (accession no. and date of accession: AF246720, 19 July 2002 for MSP; NM_008263, 12 April 2008 for bisulfite sequencing; Fig. 4, A and B). Using MSP, one CpG site showed only methylated bands without any unmethylated bands, suggesting completely methylated CpG in endometriosis. At this same site only unmethylated bands were detected in controls (Fig. 5). A negative control that used non-bisulfite-treated DNA failed to show a band using methylation-specific primers (Fig. 5).
FIG. 4.
The mouse nucleotide sequences subjected to MSP (A) and bisulphate sequencing (B) in the 5′ promoter region of Hoxa10 gene.
FIG. 5.
Methylation status in the 5′ promoter region of Hoxa10 gene by MSP from the endometriosis group and control group. M, Methylated primer; U, unmethylated primer.
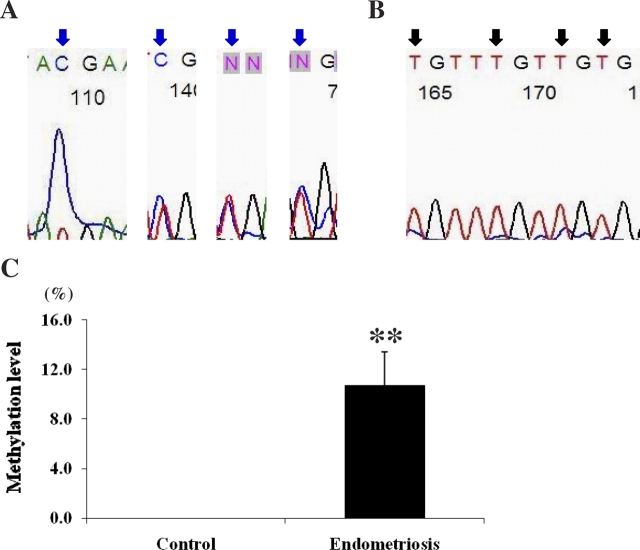
The methylation status of the Hoxa10 gene was further evaluated in the 5′ promoter region by bisulfite sequencing of the PCR product. Twenty CpG sites were analyzed from each sample. CpG was considered as partially methylated when the proportion of C nucleotide signal was less than 90% of the sum of C and T nucleotides at a given position. Partial methylation of 15 CpGs was shown in the endometriosis group (n = 7, a total of 140 CpG sites), and one of the partially methylated CpGs was highly methylated even though we did not consider it as methylated for the purpose of this analysis. In contrast, all of the CpGs were unmethylated in the control group (n = 4, a total of 80 CpG sites). Figure 6, A and B, shows the chromatograms of the 5′ promoter region of Hoxa10 in each group. Percentages of methylated CpGs were calculated in both groups. The endometriosis group demonstrated methylation at a high percentage of CpG sites compared to the control group (0% vs. 10.7%, control vs. endometriosis, P = 0.006; Fig. 6C).
FIG. 6.
The chromatograms from bisulfite sequencing of 5′ promoter region of Hoxa10 in the endometriosis group (A) and the control group (B). Blue arrows indicate partially methylated CpGs, and black arrows indicate unmethylated CpGs. C) Methylation level (mean ± SEM) in 5′ promoter region of Hoxa10 gene by bisulfite sequencing in endometriosis group and control group. Asterisks represent statistically significant difference between groups (**P < 0.01).
DISCUSSION
Experimentally induced endometriosis lowers fecundity in rats, similar to the effect of endometriosis in humans [50]. Hoxa10 expression, a mediator of endometrial receptivity, is decreased in eutopic endometrium in humans with endometriosis, as well as in baboons with induced endometriosis [32]. Here we demonstrate that Hoxa10 and Hoxa11 expression was down-regulated in the eutopic endometrium of mice with induced endometriosis. Altered expression of Hoxa/HOXA genes in animal models of endometriosis is consistent with aberrant expression of the HOXA10 and HOXA11 genes in women with endometriosis [19, 31, 47].
We also demonstrated altered expression of downstream target genes of the Hoxa10 gene in the eutopic endometrium of mice with induced endometriosis. HOXA10 can up-regulate IGFBP1 promoter activity with or without Forkhead transcription factor-1 (FOXO1) in endometrial cells [48, 49], and IGFBP1 secreted by decidualizing endometrial stromal cells is reduced in women with endometriosis [51]. Our data demonstrate that Igfbp1, a marker of decidualization, is down-regulated in the mouse model of endometriosis, consistent with previous reports. However, recently, it has been reported that HOXA10 decreases IGFBP1 expression in decidualizing cells from baboons with endometriosis [32]. This result may reflect the cell culture model as opposed to the in vivo results obtained here and in humans.
ITGB3, which is a marker of endometrial receptivity, is decreased in the endometrium of women with endometriosis [52]. We have previously identified that ITGB3 is directly up-regulated by HOXA10 through a 41-bp 5′-regulatory element in the human endometrium [53]. However, our data demonstrated no change in Itgb3 expression in the eutopic endometrium of mice with induced endometriosis. This result is in contrast to results obtained in baboons with endometriosis, which showed decreased ITGB3 expression [32]. Interestingly, our mouse model and baboon model with induced endometriosis are consistent with regard to Hoxa10 mRNA expression; however, mRNA expression of its downstream target genes differs between models. It is most likely that differences in gene expression are explained based on the duration of endometriosis. We obtained endometrial tissues at 14 wk after the induction of endometriosis in the mouse model, much earlier than in the baboon model (16 mo) and in humans. Here we likely examined an earlier stage of endometriosis compared to reports in baboons and humans.
The relative expression of Pgr-A/PGR-A to Pgr-B/PGR-B modifies progesterone responsiveness in target cells [36–39]. Here we demonstrated an altered ratio of Pgr-B to Pgr-A mRNA and increased PGR protein expression in the eutopic endometrium of mice with induced endometriosis compared to controls. These findings suggest that whereas total Pgr and Pgr-B were increased, Pgr-A was expressed at a lower level in animals with endometriosis. PGR expression is also similarly altered in the eutopic endometrium of baboons with induced endometriosis [54]. A recent microarray-based study in women with moderate to severe endometriosis also reported similarly increased total PR in women with endometriosis compared to controls [55]. We therefore believe that our model most closely reflects moderate to severe disease in women. He we show that despite increased total Pgr, Pgr-A is significantly decreased.
In normal human endometrial epithelium, both PGR-A and PGR-B are increased by estrogen during the proliferative phase but are reduced during the secretory phase under the influence of rising serum progesterone levels [56, 57]. PGR-A predominates throughout the cycle in the stroma, suggesting a function for this isoform in progesterone-mediated stromal decidualization [2, 57]. Pgr-A knockout mice have defective implantation based on loss of progesterone-regulated expression of several genes associated with uterine receptivity [38]. Treatment of ovariectomized Pgr-A knockout mice with estrogen and progesterone induces progesterone-dependent proliferative activity mediated through Pgr-B in uterine epithelium, suggesting that Pgr-A is essential in order to diminish both progesterone (acting via Pgr-B) and estrogen-mediated proliferative responses in uterus [38]. Pgr-B knockout female mice are fertile and sustain a normal pregnancy, suggesting normal uterine responses to progesterone [39]. Therefore, those studies demonstrate that only Pgr-A, but not Pgr-B, is necessary to elicit progesterone-dependent reproductive responses [38, 39]. Progesterone, which inhibits estrogen-mediated mitosis in endometrium [58], mediates its antiproliferative activity through Pgr-A [38, 59]. The antiproliferative effects of progesterone are less pronounced in endometriosis compared with normal endometrium [58]. Therefore, we suggest that the relatively low expression of Pgr-A in the eutopic endometrium of mice with induced endometriosis may result in a defect in proliferation. These data may also explain the decrease in expression of Hoxa10 and Hoxa11, which are progesterone-responsive genes. Taken together, these results suggest increased total PGR expression is accompanied by a decreased expression of the critical PGR-A in endometriosis. The altered PGR expression leads to diminished PGR response and decreased expression of progesterone-responsive genes.
Recently, it has been reported that Klf9 expression in normal murine endometrium during early pregnancy is predominantly localized to stromal cells and temporally coincides with Pgr-A-dependent decidual formation at the time of implantation, suggesting a functional contribution of Klf9 to Pgr-A action in uterine endometrial stromal cells [42]. Here we demonstrated that Klf9 is down-regulated in the eutopic endometrium of mice with induced endometriosis. Therefore, we suggest that decreased Klf9 and Pgr-A mRNA expression may contribute to defective endometrial receptivity in mice with induced endometriosis. In our study, Klf9 is down-regulated in concert with a decrease in Hoxa10 expression in the eutopic endometrium of mice with induced endometriosis, corresponding to a previous report showing decreased Hoxa10 expression in Klf9 knockout mice [42].
DNA methylation is an epigenetic modification of DNA that has an important role in embryonic development, tumorigenesis, aging, and other diseases [60–62]. When promoter CpG islands become methylated, the associated gene typically becomes permanently silenced or repressed due to suppressed transcriptional activity. Recent studies have reported HOXA10 methylation is one possible mechanism by which HOXA10 levels are decreased in endometriosis. HOXA10 in the eutopic endometrium of both women with endometriosis and baboons with induced endometriosis is hypermethylated compared with those without endometriosis [47, 32]. The expression levels of three genes that code for DNA methyltransferase (DNMT1, DNMT3A, and DNMT3B) are overexpressed in the epithelial component of endometriotic implants in humans [63]. Here we demonstrate that 5′ promoter region of Hoxa10 is hypermethylated in the eutopic endometrium of mouse with induced endometriosis. Hoxa10/HOXA10 methylation may, in part, explain mechanism through which Hoxa10/HOXA10 is down-regulated in endometriosis.
The eutopic endometrium in women with endometriosis demonstrates altered endometrial receptivity and altered gene expression. Similarly, we demonstrate significant changes in multiple markers of endometrial receptivity in the eutopic endometrium after induction of endometriosis, suggesting that the mouse model is useful in investigating endometriosis. These findings also suggest that normal endometrium can develop endometrial defects in endometriosis; an abnormal endometrium is not a prerequisite for the development of endometriosis or associated abnormalities. Finally these data also suggest the existence of altered signal conduction pathways resulting from endometriosis that change endometrial gene expression. These pathways include altered progesterone receptor, cofactor, and target gene expression levels as well as epigenetic transcriptional repression.
Acknowledgments
We acknowledge Amy Tetrault for technical assistance.
Footnotes
1Supported by NIH HDO36887, ES010610, and HD052668.
REFERENCES
- Farquhar CM.Extracts from the “clinical evidence”. Endometriosis. BMJ 2000; 320: 1449–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice LC, Kao LC.Endometriosis. Lancet 2004; 364: 1789–1799. [DOI] [PubMed] [Google Scholar]
- Olive DL, Pritts EA.Treatment of endometriosis. N Engl J Med 2001; 345: 266–275. [DOI] [PubMed] [Google Scholar]
- Sampson JA.Ovarian hematomas of endometrial type (perforating hemorrhagic cysts of the ovary) and implantation adenomas of endometrial type. Boston Med Surg J 1922; 186: 445–473. [Google Scholar]
- Sasson IE, Taylor HS.Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci 2008; 1127: 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg BS, Busch C.Endometriosis in rhesus monkeys. Ups J Med Sci 1984; 89: 129–134. [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM, Bambra CS, Cornillie FJ, Isahakia M, Koninckx PR.Prevalence and laparoscopic appearance of spontaneous endometriosis in the baboon (Papio anubis, Papio cynocephalus). Biol Reprod 1991; 45: 411–416. [DOI] [PubMed] [Google Scholar]
- Merrill JA.Spontaneous endometriosis in the Kenya baboon (Papio doguera). Am J Obstet Gynecol 1968; 101: 569–570. [DOI] [PubMed] [Google Scholar]
- Bergqvist A, Jeppsson S, Kullander S, Ljungberg O.Human uterine endometrium and endometriotic tissue transplanted into nude mice. Morphologic effects of various steroid hormones. Am J Pathol 1985; 121: 337–341. [PMC free article] [PubMed] [Google Scholar]
- Eggermont J, Donnez J, Casanas-Roux F, Scholtes H, Van Langendonckt A.Time course of pelvic endometriotic lesion revascularization in a nude mouse model. Fertil Steril 2005; 84: 492–499. [DOI] [PubMed] [Google Scholar]
- Awwad JT, Sayegh RA, Tao XJ, Hassan T, Awwad ST, Isaacson K.The SCID mouse: an experimental model for endometriosis. Hum Reprod 1999; 14: 3107–3111. [DOI] [PubMed] [Google Scholar]
- Bruner-Tran KL, Eisenberg E, Yeaman GR, Anderson TA, McBean J, Osteen KG.Steroid and cytokine regulation of matrix metalloproteinase expression in endometriosis and the establishment of experimental endometriosis in nude mice. J Clin Endocrinol Metab 2002; 87: 4782–4791. [DOI] [PubMed] [Google Scholar]
- Cummings AM, Metcalf JL.Induction of endometriosis in mice: a new model sensitive to estrogen. Reprod Toxicol 1995; 9: 233–238. [DOI] [PubMed] [Google Scholar]
- Somigliana E, Vigano P, Rossi G, Carinelli S, Vignali M, Panina-Bordignon P.Endometrial ability to implant in ectopic sites can be prevented by interleukin-12 in a murine model of endometriosis. Hum Reprod 1999; 14: 2944–2950. [DOI] [PubMed] [Google Scholar]
- Barnhart K, Dunsmoor-Su R, Coutifaris C.Effect of endometriosis on in vitro fertilization. Fertil Steril 2002; 77: 1148–1155. [DOI] [PubMed] [Google Scholar]
- Hahn DW, Carraher RP, Foldesy RG, McGuire JL.Experimental evidence for failure to implant as a mechanism of infertility associated with endometriosis. Am J Obstet Gynecol 1986; 155: 1109–1113. [DOI] [PubMed] [Google Scholar]
- Arici A, Oral E, Bukulmez O, Duleba A, Olive DL, Jones EE.The effect of endometriosis on implantation: results from the Yale University in vitro fertilization and embryo transfer program. Fertil Steril 1996; 65: 603–607. [DOI] [PubMed] [Google Scholar]
- Illera MJ, Juan L, Stewart CL, Cullinan E, Ruman J, Lessey BA.Effect of peritoneal fluid from women with endometriosis on implantation in the mouse model. Fertil Steril 2000; 74: 41–48. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Bagot C, Kardana A, Olive D, Arici A.HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod 1999; 14: 1328–1331. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, Strom BL.Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab 1994; 79: 643–649. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fazleabas AT.Uterine receptivity and implantation: the regulation and action of insulin-like growth factor binding protein-1 (IGFBP-1), HOXA10 and forkhead transcription factor-1 (FOXO-1) in the baboon endometrium. Reprod Biol Endocrinol 2004; 2: 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misao R, Iwagaki S, Fujimoto J, Sun W, Tamaya T.Dominant expression of progesterone receptor form B mRNA in ovarian endometriosis. Horm Res 1999; 52: 30–34. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Vanden Heuvel GB, Igarashi P.A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol Reprod 1997; 57: 1338–1345. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Arici A, Olive D, Igarashi P.HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest 1998; 101: 1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HS, Igarashi P, Olive DL, Arici A.Sex steroids mediate HOXA11 expression in the human peri-implantation endometrium. J Clin Endocrinol Metab 1999; 84: 1129–1135. [DOI] [PubMed] [Google Scholar]
- Vitiello D, Kodaman PH, Taylor HS.HOX genes in implantation. Semin Reprod Med 2007; 25: 431–436. [DOI] [PubMed] [Google Scholar]
- Satokata I, Benson G, Maas R.Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature 1995; 374: 460–463. [DOI] [PubMed] [Google Scholar]
- Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL.Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development 1996; 122: 2687–2696. [DOI] [PubMed] [Google Scholar]
- Hsieh-Li HM, Witte DP, Weinstein M, Branford W, Li H, Small K, Potter SS.Hoxa 11 structure, extensive antisense transcription, and function in male and female fertility. Development 1995; 121: 1373–1385. [DOI] [PubMed] [Google Scholar]
- Bagot CN, Troy PJ, Taylor HS.Alteration of maternal Hoxa10 expression by in vivo gene transfection affects implantation. Gene Ther 2000; 7: 1378–1384. [DOI] [PubMed] [Google Scholar]
- Gui Y, Zhang J, Yuan L, Lessey BA.Regulation of HOXA-10 and its expression in normal and abnormal endometrium. Mol Hum Reprod 1999; 5: 866–873. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Taylor HS, Lu Z, Ladhani O, Hastings JM, Jackson KS, Wu Y, Guo SW, Fazleabas AT.Altered expression of HOXA10 in endometriosis: potential role in decidualization. Mol Hum Reprod 2007; 13: 323–332. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW.Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 1995; 9: 2266–2278. [DOI] [PubMed] [Google Scholar]
- Ma L, Benson GV, Lim H, Dey SK, Maas RL.Abdominal B (AbdB) Hoxa genes: regulation in adult uterus by estrogen and progesterone and repression in müllerian duct by the synthetic estrogen diethylstilbestrol (DES). Dev Biol 1998; 197: 141–154. [DOI] [PubMed] [Google Scholar]
- Lim H, Ma L, Ma WG, Maas RL, Dey SK.Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol Endocrinol 1999; 13: 1005–1017. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP.Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol 1993; 7: 1244–1255. [DOI] [PubMed] [Google Scholar]
- Giangrande PH, McDonnell DP.The A and B isoforms of the human progesterone receptor: two functionally different transcription factors encoded by a single gene. Recent Prog Horm Res 1999; 54: 291–313;.discussion 313–314. [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM.Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 2000; 289: 1751–1754. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM.Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A 2003; 100: 9744–9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhang XL, Michel FJ, Blum JL, Simmen FA, Simmen RC.Direct interaction of the Krüppel-like family (KLF) member, BTEB1, and PR mediates progesterone-responsive gene expression in endometrial epithelial cells. Endocrinology 2002; 143: 62–73. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Zhang D, Michel FJ, Blum JL, Simmen FA, Simmen RC.Selective interactions of Kruppel-like factor 9/basic transcription element-binding protein with progesterone receptor isoforms A and B determine transcriptional activity of progesterone-responsive genes in endometrial epithelial cells. J Biol Chem 2003; 278: 21474–21482. [DOI] [PubMed] [Google Scholar]
- Simmen RC, Eason RR, McQuown JR, Linz AL, Kang TJ, Chatman L, Jr, Till SR, Fujii-Kuriyama Y, Simmen FA, Oh SP.Subfertility, uterine hypoplasia, and partial progesterone resistance in mice lacking the Kruppel-like factor 9/basic transcription element-binding protein-1 (Bteb1) gene. J Biol Chem 2004; 279: 29286–29294. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD.Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Frommer M, McDonald LE, Millar DS, Collis CM, Watt F, Grigg GW, Molloy PL, Paul CL.A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci U S A 1992; 89: 1827–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Ma L, Chiang T, Burow M, Newbold RR, Negishi M, Barrett JC, McLachlan JA.Promoter CpG methylation of Hox-a10 and Hox-a11 in mouse uterus not altered upon neonatal diethylstilbestrol exposure. Mol Carcinog 2001; 32: 213–219. [DOI] [PubMed] [Google Scholar]
- Li LC.Designing PCR primer for DNA methylation mapping. Methods Mol Biol 2007; 402: 371–384. [DOI] [PubMed] [Google Scholar]
- Wu Y, Halverson G, Basir Z, Strawn E, Yan P, Guo SW.Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am J Obstet Gynecol 2005; 193: 371–380. [DOI] [PubMed] [Google Scholar]
- Gao J, Mazella J, Tseng L.Hox proteins activate the IGFBP-1 promoter and suppress the function of hPR in human endometrial cells. DNA Cell Biol 2002; 21: 819–825. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Taylor HS, Akbas GE, Foucher I, Trembleau A, Jaffe RC, Fazleabas AT, Unterman TG.Regulation of insulin-like growth factor binding protein-1 promoter activity by FKHR and HOXA10 in primate endometrial cells. Biol Reprod 2003; 68: 24–30. [DOI] [PubMed] [Google Scholar]
- Grümmer R.Animal models in endometriosis research. Hum Reprod Update 2006; 12: 641–649. [DOI] [PubMed] [Google Scholar]
- Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ.Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril 2006; 85: 564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, Strom BL.Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab 1994; 79: 643–649. [DOI] [PubMed] [Google Scholar]
- Daftary GS, Troy PJ, Bagot CN, Young SL, Taylor HS.Direct regulation of beta3-integrin subunit gene expression by HOXA10 in endometrial cells. Mol Endocrinol 2002; 16: 571–579. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Brudney A, Chai D, Langoi D, Bulun SE.Steroid receptor and aromatase expression in baboon endometriotic lesions. Fertil Steril 2003; 80(suppl 2):820–827. [DOI] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC.Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinol 2007; 148: 3814–3826. [DOI] [PubMed] [Google Scholar]
- Okulicz WC, Savasta AM, Hoberg LM, Longcope C.Immunofluorescent analysis of estrogen induction of progesterone receptor in the rhesus uterus. Endocrinology 1989; 125: 930–934. [DOI] [PubMed] [Google Scholar]
- Mote PA, Balleine RL, McGowan EM, Clarke CL.Colocalization of progesterone receptors A and B by dual immunofluorescent histochemistry in human endometrium during the menstrual cycle. J Clin Endocrinol Metab 1999; 84: 2963–2971. [DOI] [PubMed] [Google Scholar]
- Gurates B, Bulun SE.Endometriosis: the ultimate hormonal disease. Semin Reprod Med 2003; 21: 125–134. [DOI] [PubMed] [Google Scholar]
- McDonnell DP, Goldman ME.RU486 exerts antiestrogenic activities through a novel progesterone receptor A form-mediated mechanism. J Biol Chem 1994; 269: 11945–11949. [PubMed] [Google Scholar]
- Kiefer JC.Epigenetics in development. Dev Dyn 2007; 236: 1144–1156. [DOI] [PubMed] [Google Scholar]
- Robertson KD.DNA methylation and human disease. Nat Rev Genet 2005; 6: 597–610. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Esteller M.Epigenetics and aging: the targets and the marks. Trends Genet 2007; 23: 413–418. [DOI] [PubMed] [Google Scholar]
- Wu Y, Strawn E, Basir Z, Halverson G, Guo SW.Aberrant expression of deoxyribonucleic acid methyltransferases DNMT1, DNMT3A, and DNMT3B in women with endometriosis. Fertil Steril 2007; 87: 24–32. [DOI] [PubMed] [Google Scholar]