Abstract
Marijuana is the most commonly used illicit drug. Although there is some indication that reproductive functions in males are impaired in chronic marijuana users, the genetic evidence and underlying causes remain largely unknown. Herein we show that genetic loss of Faah, which encodes fatty acid amide hydrolase (FAAH), results in elevated levels of anandamide, an endocannabinoid, in the male reproductive system, leading to compromised fertilizing capacity of sperm. This defect is rescued by superimposing deletion of cannabinoid receptor 1 (Cnr1). Retention of Faah−/− sperm on the egg zona pellucida provides evidence that the capacity of sperm to penetrate the zona barrier is hampered by elevated anandamide levels. Collectively, the results show that aberrant endocannabinoid signaling via CNR1 impairs normal sperm function. Besides unveiling a new regulatory mechanism of sperm function, this study has clinical significance in male fertility.
Keywords: anandamide, CNR1, FAAH, male fertility, mouse, sperm, sperm capacitation, sperm motility and transport
Elevated anandamide levels resulting from genetic deletion of fatty acid amide hydrolase (Faah) attenuate sperm's zona-penetrating capacity via CNR1, compromising fertilization.
INTRODUCTION
There is some evidence that male fertility in humans is negatively regulated by long-term exposure to marijuana extracts (reviewed by Wang et al. [1]). The major psychoactive component of marijuana is Δ9-tetrahydrocannabinol (THC). Although in vitro experiments have shown that THC exerts adverse effects on sperm function (reviewed by Rossato et al. [2]), there is no in vivo or genetic evidence that cannabinoids impair male fertility. After THC was identified in 1964 [3], research on cannabinoids exploded with the discovery and cloning of two G protein-coupled cannabinoid receptors, brain-type Cnr1 encoding CNR1 [4, 5] and spleen-type Cnr2 encoding CNR2 [6]. Around the same time, several endogenous lipid molecules targeting CNR1 and CNR2 were identified, collectively called endocannabinoids. Two of the most studied endocannabinoids are N-arachidonoylethanolamide (known as anandamide) and 2-arachidonoylglycerol (2-AG) [7–9]. Anandamide levels are regulated by a balance between the rates of its synthesis and degradation. Anandamide was thought to be produced primarily from N-arachidonoylphosphatidylethanolamine (NAPE) by NAPE-hydrolyzing phospholipase D (NAPEPLD) [10]. However, genetic investigations in NAPEPLD-deficient mice [11] and recent identification of other anandamide synthetic pathways [12, 13] demonstrate that regulation of anandamide synthesis is more complex than previously thought. Anandamide is degraded to ethanolamine and arachidonic acid by a membrane-bound fatty acid amide hydrolase (FAAH) [14, 15]. Although FAAH can hydrolyze other endocannabinoids, including 2-AG [16], investigations in Faah−/− mice show that FAAH has a major role in regulating the magnitude and duration of anandamide signaling [12, 17].
Sperm undergo a long journey to acquire fertilization capacity [18–20]. Through the process of spermatogenesis, spermatogonia differentiate into highly polarized sperm, which then undergo maturation in the epididymis before capacitation, acquiring motility in the female reproductive tract. After traveling through the uterine lumen and reaching ovulated eggs in the oviduct ampulla, capacitated sperm navigate through cumulus cells surrounding the egg to contact the zona pellucida, the outermost membrane of the egg. On binding to the zona, sperm undergo a Ca++-dependent exocytotic event known as the acrosome reaction, which is essential for their zona penetration and homing into the perivitelline space. After a sperm binds to an egg plasma membrane, the two gametes unite, resulting in egg activation, pronuclear formation, and syngamy. Each step in the process is essential for successful fertilization.
There are reports that endocannabinoids and their receptors are present in the testis and sperm of invertebrates and vertebrates, including sea urchins, frogs, rats, mice, boars, and humans [21]. This conserved expression across species suggests that endocannabinoid signaling has important roles in male reproduction. In vitro studies also showed that endocannabinoid signaling inhibits capacitation of boar sperm in a cAMP-dependent pathway and prevents the acrosome reaction [22] and that anandamide reduces human sperm motility by quenching mitochondrial activity [21]. However, there is no in vivo genetic evidence of endocannabinoid signaling affecting male reproductive functions, to our knowledge.
In this study, we used gene-targeted mice for Faah to mimic the conditions of long-term exposure to marijuana. We explored roles of cannabinoid and endocannabinoid signaling in male fertility.
MATERIALS AND METHODS
Mice
Targeted deletion of Faah, Cnr1, or Cnr2 in mice (129/SvJ-C57BL/6J) has been previously described [17, 23, 24]. Double mutants for Faah/Cnr1 or Faah/Cnr2 were generated using appropriate breeding strategies. Adult wild-type (WT), Faah–/–, Faah–/–/Cnr1–/–, and Faah–/–/Cnr2–/– mice were housed at an institutional animal care facility according to National Institutes of Health and institutional guidelines. Experiments were conducted on mice between 3 and 4 mo of age. Testes and epididymis from Faah–/– and WT males were processed for anandamide measurement and in situ hybridization.
Western Blotting
Tissue samples were homogenized in lysis buffer (150 mmol/L of NaCl, 1% nonionic detergent, 0.5% deoxycholate, 0.1% SDS, and 50 mmol/L Tris [pH 8]) containing protease and phosphatase inhibitors. The lysates were centrifuged at 9880 × g for 10 min at 4°C. Supernatants (25 μg) were boiled for 5 min in SDS sample buffer. Samples were run on 10% SDS-PAGE gels under reducing conditions and transferred onto nitrocellulose membranes. Membranes were blocked with 10% carnation milk in Tris-buffered saline with 0.1 Tween-20) and probed with antibodies against mouse FAAH (1:1000; custom made by the laboratory of Cravatt et al. [17], CNR1 (1:2000) [25], CNR2 (1:250; Cayman), and β-actin (1:100; Santa Cruz Biotechnology) overnight at 4°C. After thorough washings, blots were incubated in peroxidase-conjugated donkey/anti-goat IgG (1:2000) or donkey/anti-rabbit IgG (1:2000; Jackson/ImmunoResearch), followed by washings. Protein signals were detected using chemiluminescent reagents (Amersham).
Immunohistochemistry
Immunostaining in Bouin solution-fixed paraffin-embedded sections (6 μm) was performed using antibodies specific to FAAH (1:200) [17], CNR1 (1:200) [25], or CNR2 (1:250; Cayman) following antigen retrieval in citrate buffer (pH 6.0) for 10 min in an autoclave. A Histostain-Plus (DAB) kit (Zymed) was used to visualize the antigen. Reddish brown deposits indicate sites of positive immunostaining.
Immunofluorescence
Sperm were isolated from the epididymis of mature WT males and thoroughly washed in PBS. Sperm were fixed with 1% formaldehyde at room temperature for 15 min. After blocking in 1% BSA/PBS containing 0.05% Tween-20, sperm were incubated with CNR1 antibody (1:200; ∼500 ng/ml of IgG) [25] with or without blocking peptide overnight at 4°C. After thorough washings, secondary antibodies conjugated with Cy3 (Jackson/ImmunoResearch) were used to detect immunofluorescence signaling. SYTO13 green fluorescence dye (Invitrogen) was used for nuclear staining.
Anandamide Assay
Testis and sperm (100 mg) were pooled separately from five WT or Faah−/− mice in each group (n = 3–6) and were assayed for anandamide as previously described [26]. Briefly, the preweighed samples were homogenized in ethyl acetate with 0.5% acetic acid. Immediately before homogenization, 2H8-labeled anandamide was added as an internal standard to a mortar. The homogenate was centrifuged, and the supernatant was dried, reconstituted in chloroform, and purified on a silica-based solid-phase extraction cartridge. The eluent was dried, reconstituted in 1:8 of aqueous silver acetate-methanolic silver acetate, and analyzed by reverse-phase positive-ion electrospray ionization-HPLC-tandem mass spectrometry. Quantification was performed by stable isotope dilution against the octadeuterated internal standard.
In Situ Hybridization
Frozen sections (12 μm) were hybridized with 35S-labeled cRNA probes for mouse Cnr1 or Cnr2 as described previously [27]. Sections hybridized with sense probes served as negative controls and showed no positive signals.
In Vitro Fertilization
In vitro fertilization (IVF) was performed as previously described [28]. Briefly, WT females were superovulated by intraperitoneal injections of 5 IU of eCG (Sigma), followed by injections of 5 IU of hCG (Sigma) 48 h later. Cumulus-oocyte complexes were collected from the oviduct ampulla 12–14 h after hCG injection and placed in 100-μl droplets of human tubal fluid (HTF) medium (Chemicon). In most IVF experiments, zona-intact eggs were used. In some IVF experiments, zona-free eggs were used. Cumulus-oocyte complexes were treated with hyaluronidase (Sigma), and cumulus-free eggs were then exposed to acidic Tyrode solution and passed through a pipette several times until zona pellucidae were dissolved. Eggs were washed three times in HTF medium and incubated longer than 1 h to allow surface proteins to recover [29]. Sperm were collected from the cauda of the epididymis and placed into 400 ml of HTF medium to allow capacitation for 2.5 h in a humidified 5% CO2 incubator at 37°C. Sperm (∼1.2–1.5 × 106 sperm/ml) were then coincubated with eggs to allow fertilization. After 6 h, sperm were removed, and putative zygotes were placed in 200-μl droplets of potassium simplex optimized medium (Chemicon) and incubated in a humidified 5% CO2 incubator at 37°C. The cleavage rate (two-cell stage) after 24 h was used as an index of fertilization. Formation of blastocysts at 120 h indicated developmental potential of fertilized embryos.
Evaluation of Sperm-Zona Binding in IVF
After sperm were incubated with eggs for 2 h in IVF experiments, eggs were removed. Attached sperm were stained with propidium iodide and fluorescein isothiocyanate (FITC)-conjugated antibody specific to Izumo protein, generated in the laboratory of Inoue et al. [30].
Analysis of the Acrosome Reaction by Flow Cytometry
Wild-type and Faah–/– caudal sperm were incubated in HTF medium with anti-Izumo antibody conjugated with FITC to monitor spontaneous acrosome reaction by flow cytometry at 30-min intervals for up to 3 h. Sperm were stained with propidium iodide (10 μg/ml) 2 min before flow cytometry analysis. Viable sperm were selected by propidium iodide staining, while acrosome-reacted sperm were identified by anti-Izumo antibody staining [30].
Evaluation of Sperm Motility
After capacitation for 30 and 90 min, 20 μl of media containing sperm (2 × 106 sperm/ml) was placed on a prewarmed slide under a coverslip. Sperm motility was recorded in 12 frames/sec for 20 sec at a resolution of 640 × 512 pixels. The total travel distance and linear travel distance (linear distance from the starting point to the end point) and the travel time were measured using the Nikon Nis-elements object tracking function. The curvilinear velocity was calculated from the total distance traveled divided by the travel time. The linear velocity was calculated from the linear travel distance divided by the travel time, whereas linearity was calculated from the linear velocity divided by the curvilinear velocity.
RESULTS
Faah−/− Males Have Compromised Fertility
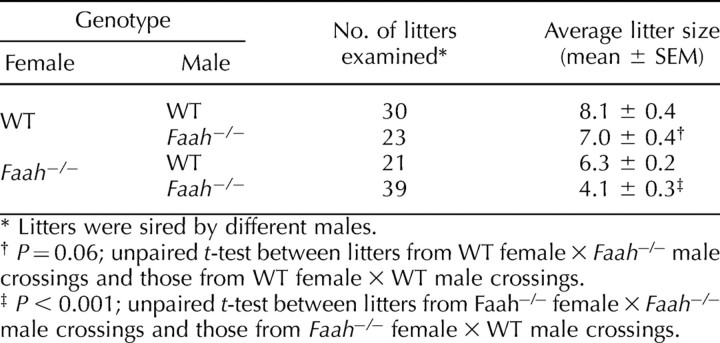
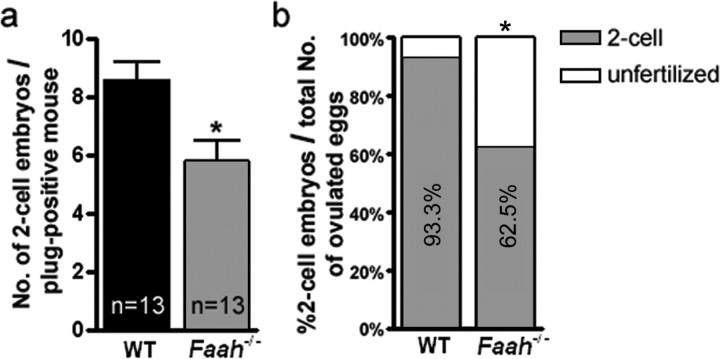
We have previously shown that FAAH is a key metabolic regulator of anandamide levels in mice [17] and that FAAH deficiency results in higher anandamide levels in the female reproductive tract, impairing normal oviductal embryo transport and embryo development [12]. In the course of these studies, analysis of breeding results showed that litter sizes generated by mating WT females with Faah−/− males are 13% smaller than those generated by mating WT females with WT males (Table 1). These results suggested that FAAH deficiency compromises male fertility. This is further evident from our findings of significantly reduced litter sizes generated by mating Faah−/− females with Faah−/− males compared with those generated by mating Faah−/− females with WT males. These breeding results prompted us to further examine fertility of Faah−/− males. We used WT females mated with Faah−/− or WT males. Females were killed on the morning of Day 2 of pregnancy, and oviducts were flushed to record fertilized (two-cell embryos) and unfertilized eggs. We observed that WT females mated with Faah−/− males have significantly fewer fertilized eggs compared with those recovered from WT females mated with WT males. In addition, fewer WT females yielded fertilized egg (Fig. 1). These data corroborate the breeding data that FAAH deficiency impairs male fertility. Collectively, our findings show that Faah−/− sperm underperform even in the WT female reproductive tract and that function of null sperm is further compromised in the Faah−/− female reproductive tract. These observations provide evidence that paternal FAAH deficiency is a cause for compromised fertility.
TABLE 1.
Reproductive performance of Faah−/− males.
FIG. 1.
FAAH deficiency impairs sperm fertility. a) Number of two-cell embryos per plug-positive WT females mated with WT or Faah−/− males. Numbers of plug-positive mice used are shown within the bars (* P < 0.05, unpaired Student t-test). b) Percentage of two-cell embryos and unfertilized eggs retrieved from the same groups. Thirteen mice are used in each group (* P < 0.01, Chi-square test).
Endocannabinoid Signaling Is Present in the Male Reproductive System
The extent and duration of anandamide signaling via CNR1 or CNR2 are mainly regulated by FAAH [17]. Therefore, we examined the expression of CNR1, CNR2, and FAAH in the testis and epididymis to study potential roles of anandamide in regulating male fertility. Western blotting analysis showed that FAAH, CNR1, and CNR2 are present in the testis and epididymis of WT mice (Fig. 2a). We next examined cell-specific localization of FAAH and cannabinoid receptors in the testis and epididymis of WT mice by immunohistochemistry (Fig. 2b). While CNR1 was present in Leydig cells and epididymal epithelial cell surfaces, testicular spermatocytes and spermatids showed modest positive staining. In contrast, CNR2 was localized in spermatocytes and Sertoli cells encircling spermatocytes and spermatids in the testis. In the epididymis, epithelial cell surfaces demonstrated CNR2 immunostaining, whereas signals were undetectable in interstitial cells. FAAH was present in spermatocytes and spermatids, while spermatogonia had little or no positive signal. Sertoli cells and Leydig cells also showed positive staining of FAAH. The localization of FAAH was evident on cell surfaces of the epididymal epithelium. The antibody specificity was confirmed using Faah−/− tissues (Supplemental Figure 1 available online at www.biolreprod.org). The presence of FAAH on the testis and epididymis suggests that endocannabinoid levels are tightly regulated by FAAH in these tissues.
FIG. 2.
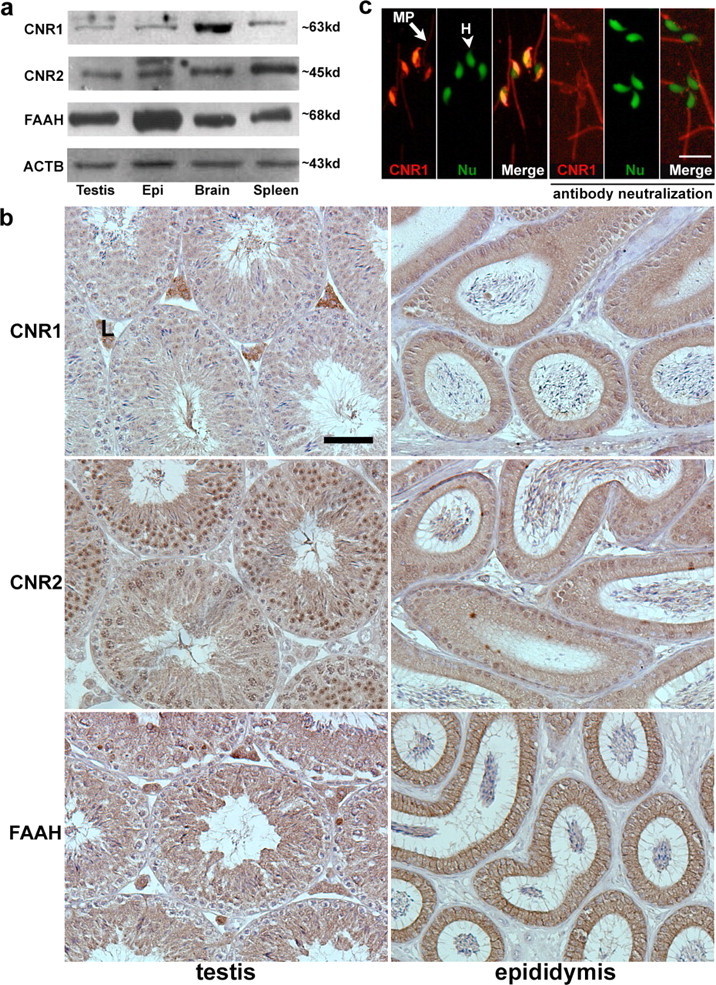
FAAH and cannabinoid receptors are expressed in the male reproductive tract. a) Western blotting of CNR1, CNR2, and FAAH in the WT testis and epididymis. Brain tissue extracts served as positive controls for CNR1 and FAAH, while spleen tissue samples served as positive controls for CNR2. β-Actin (ACTB) is a loading control. Epi, epididymis. b) Immunolocalization of CNR1, CNR2, and FAAH in the testis and epididymis. L, Leydig cells. Bar = 50 μm. c) CNR1 immunostaining (red) in sperm (left three panels) and in sperm exposed to CNR1 antibody preabsorbed with an antigenic peptide (right three panels). In each group, CNR1 staining, nuclear staining, and merged pictures are shown from left to right. Nuclei were counterstained with SYTO13 (green). MP (arrow), sperm midpiece; H (arrowhead), sperm head; Nu, nuclear. Bar = 10 μm.
The presence of CNR1 and CNR2 on sperm was also examined by immunofluorescence. As shown in Figure 2c, CNR1 immunofluorescence is primarily noted in anterior regions of sperm heads, the site of the acrosomal sac, but also in the midpiece. CNR1 is undetectable in the principal piece and endpiece of sperm tails. Sperm incubated with CNR1 antibody preabsorbed with an antigenic peptide showed that, while the signal in the anterior region of sperm heads is specific, the signal is nonspecific in the midpiece. CNR2 was undetectable in sperm (Supplemental Figure 2 available online at www.biolreprod.org). Our findings of the presence of FAAH, CNR1, and CNR2 in the testis and epididymis and the presence of FAAH and CNR1 in sperm suggest that endocannabinoid signaling has a role in spermatogenesis and sperm maturation.
FAAH Deficiency Elevates Anandamide Levels in the Testis and Epididymis
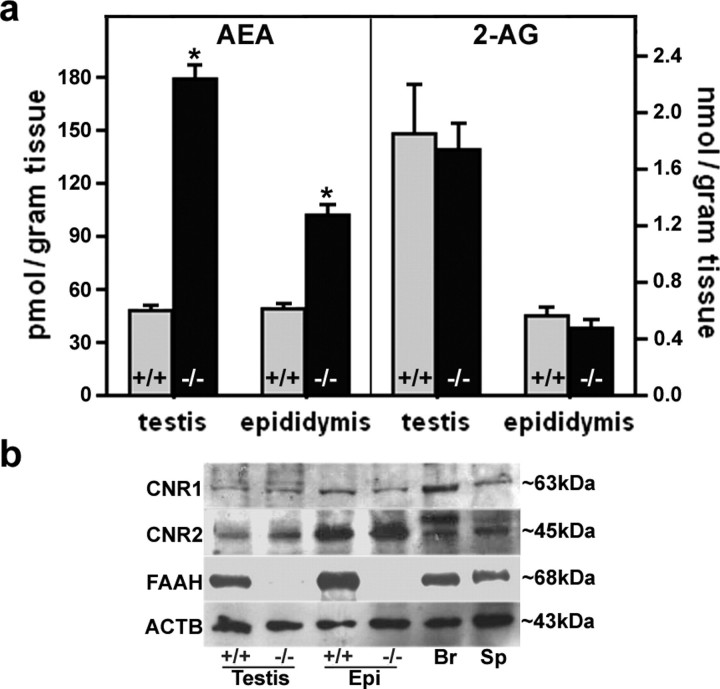
To provide genetic evidence for function of FAAH in the male reproductive system, we measured anandamide and 2-AG levels in the testis and epididymis of WT and Faah−/− mice using reverse-phase HPLC-tandem mass spectrometry. As shown in Figure 3a, testis and epididymis from Faah−/− mice had significantly increased anandamide levels, suggesting that FAAH is a primary enzyme that regulates anandamide turnover in these tissues. Higher testicular anandamide levels in Faah−/− males corroborate our previous observation [31]. However, levels of 2-AG in the testis and epididymis were comparable between Faah−/− and WT males (Fig. 3a). These results are consistent with our previous data in the uterus showing unaltered 2-AG levels in the absence of FAAH [12].
FIG. 3.
FAAH deficiency elevates anandamide levels. a) Anandamide (AEA) levels, but not 2-AG levels, in Faah−/− testis and epididymis were higher than those in WT males (n = 10; * P < 0.05, unpaired Student t-test). b) Western blotting of CNR1, CNR2, and FAAH in the testis and epididymis of WT and Faah−/− males. Brain and spleen samples served as positive controls, while β-actin (ACTB) served as a loading control. Epi, epididymis; Br, brain; Sp, spleen.
Higher anandamide levels in the Faah−/− testis and epididymis prompted us to speculate that reduced fertility in these males is due to persistent or elevated endocannabinoid signaling. However, it is possible that there is a negative feedback loop to downregulate the expression of cannabinoid receptors to counter the consequence of high anandamide levels. To address this possibility, we examined the status of cannabinoid receptors in the testis and epididymis of WT and Faah−/− mice by Western blotting. As shown in Figure 3b, levels of CNR1 and CNR2 protein in these tissues were comparable between Faah−/− and WT males. These results suggest that higher anandamide levels do not appreciably downregulate CNR1 or CNR2 expression. To further confirm that expression of CNR1 and CNR2 is not altered in Faah−/− males, in situ hybridization and immunohistochemistry were performed. Expression patterns of CNR1 and CNR2 were similar in WT and Faah−/− epididymis (data not shown). Collectively, the data suggest that the status of cannabinoid receptors is not altered by higher anandamide levels and that heightened signaling via CNR1 or CNR2 occurs in the presence of increased anandamide levels.
FAAH Deficiency Impairs Sperm Fertilizing Capacity
Our in vivo breeding data led us to speculate that higher anandamide levels in males lacking FAAH results in their reduced fertility. To examine this, we first compared histology of the testis and epididymis, as well as sperm morphology, between Faah−/− and WT males at the age of 3–4 mo. To our surprise, no apparent histological abnormalities were observed in these tissues missing Faah (Supplemental Figure 3 available online at www.biolreprod.org). We next explored whether FAAH deficiency in males impairs the fertilizing capacity of sperm by performing IVF experiments using Faah−/− or WT sperm with WT eggs. Sperm retrieved from the caudal epididymis were subjected to capacitation in vitro for 2 h before placing them with eggs in culture. The fertilization rate was calculated by counting the number of two-cell embryos developed on the second day after IVF. As summarized in Table 2, sperm retrieved from WT males showed a 75% fertilization rate, with 97% of two-cell embryos developing to blastocysts (evaluated on the fifth day of culture). In contrast, Faah−/− sperm showed a remarkably reduced fertilization rate (42%), although development of fertilized eggs into blastocysts was comparable (89%) to that in WT animals (97%). These results suggest that the fertilizing capacity of Faah−/− sperm is compromised because of impairment in the male reproductive tract before ejaculation.
TABLE 2.
Higher anandamide levels impair sperm fertilizing capacity in vitro via CNR1.
Deletion of Cnr1 Reverses Impaired Fertilizing Capacity of Faah−/− Sperm
Sustained higher anandamide levels in the male reproductive tract lacking FAAH are capable of exerting endocannabinoid signaling through CNR1, CNR2, or both. To address this, we generated Faah−/−/Cnr1−/− and Faah−/−/Cnr2−/− double-mutant mice. We again performed IVF using sperm retrieved from Faah−/−/Cnr1−/− or Faah−/−/Cnr2−/− males with eggs isolated from WT females. As summarized in Table 2, sperm isolated from Faah−/−/Cnr1−/− males exhibited a 70% fertilization rate, with 93% of fertilized eggs developing to the blastocyst stage, but sperm isolated from Faah−/−/Cnr2−/− males showed a remarkably low fertilization rate (11%). These data show that, in the absence of CNR1, Faah−/− sperm escape the deleterious effects of higher anandamide levels. The inferior fertilizing capacity of Faah−/−/Cnr2−/− sperm exceeded that of Faah−/− sperm. The results provide genetic evidence that higher anandamide levels work through CNR1 in the Faah−/− male reproductive tract to impair sperm fertilizing capacity.
Faah−/− Sperm Have Poor Zona-Penetrating Ability
Our next objective was to see which step in the fertilization process is impaired in Faah−/− sperm. We first examined whether Faah−/− sperm can adhere to zona pellucidae and, if so, whether they can undergo the acrosome reaction. Izumo, a recently discovered protein, is absent from plasma membranes of acrosome-intact sperm [30]. Following the acrosome reaction, Izumo is exposed and participates in sperm-egg fusion. Therefore, only acrosome-reacted sperm are stained by Izumo antibody.
Wild-type or Faah−/− sperm were incubated with WT eggs for 2 h and then stained with propidium iodide to label cell nuclei. After 2 h of incubation, most WT sperm detached from the zona surface (Fig. 4a), whereas numerous Faah−/− sperm were still attached to the zona. Even after several washings, Faah−/− sperm remained adherent to the zona, indicating good binding of Faah−/− sperm to the zona. These results suggested that most eggs were fertilized by WT sperm but that eggs incubated with Faah−/− sperm were still unfertilized. We then stained the sperm attached to eggs with Izumo antibody. Many Faah−/− sperm remaining on the zona surface showed positive signal by Izumo antibody (Supplemental Figure 4 available online at www.biolreprod.org), indicating that they underwent the acrosome reaction. To further confirm that Faah−/− sperm undergo normal acrosome reaction, we examined the spontaneous acrosome reaction rate of Faah−/− sperm. The acrosome reaction, which occurs during sperm penetration through the zona, can also occur spontaneously without binding to the zona. Analysis of spontaneous acrosome reaction is used to assess the fertilizing ability of human [32] and mouse [33] sperm. We compared the status and time course of spontaneous acrosome reaction of WT and Faah−/− sperm in the fertilization medium by flow cytometry. While viable sperm were selected by propidium iodide staining, acrosome-reacted sperm were identified by Izumo staining. As shown in Figure 4b, the percentage of acrosome-reacted Faah−/− sperm is somewhat lower than that of acrosome-reacted WT sperm, but the difference is not statistically significant. Collectively, these data suggest that Faah−/− sperm can bind to the zona and undergo the acrosome reaction but still have difficulty in fertilizing eggs.
FIG. 4.
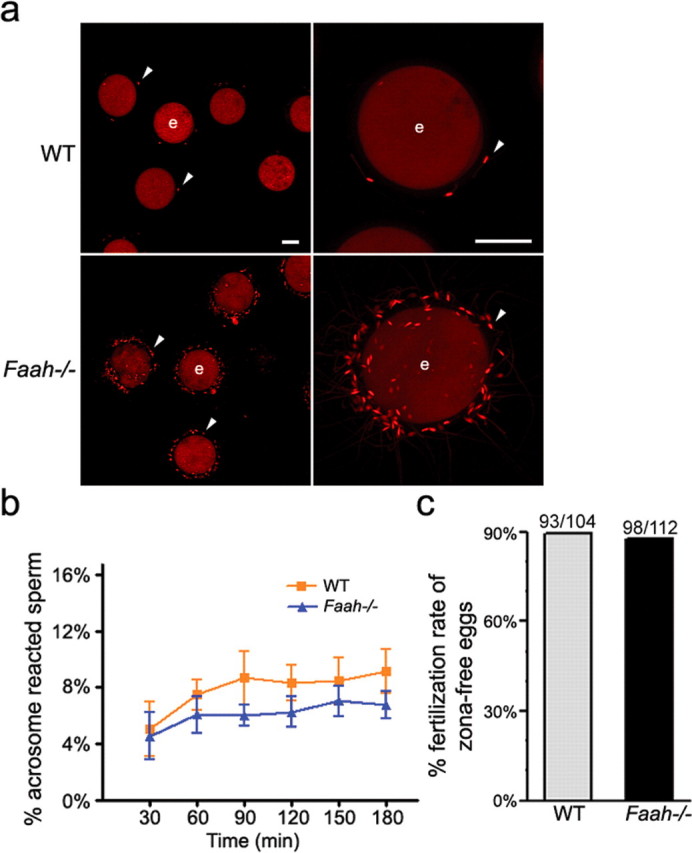
Zona-penetrating capacity of Faah−/− sperm is inferior. a) Sperm-egg interactions using zona-intact WT eggs. After 2 h of incubation with eggs, Faah−/− sperm were still attached to zona pellucidae. Arrowhead, sperm on the zona-intact egg surface; e, egg. Bar = 40 μm. b) Spontaneous acrosome reaction as assessed by flow cytometry. The rate (%) of acrosome-reacted WT and Faah−/− sperm at each time point was analyzed by flow cytometry as described in Materials and Methods, and no statistically significant difference was noted between the two groups as analyzed by Student t-test. c) The IVF rates of zona-free WT eggs fertilized by WT or Faah−/− sperm. Numbers above the bars indicate the number of fertilized eggs/total zona-free eggs used for IVF.
The acrosome reaction is not the only prerequisite for zona penetration. Sperm motility and acrosomal release of proteases are also involved in this process [20]. To examine whether Faah−/− sperm can penetrate the zona successfully, we performed IVF using sperm from Faah−/− or WT mice incubated with zona-free WT eggs. To our surprise, Faah−/− sperm exhibited fertilizing capacity comparable to that of WT sperm (Fig. 4c), indicating that the zona is a major barrier for normal fertilization by Faah−/− sperm.
Sperm Motility Is Attenuated in Faah−/− Males
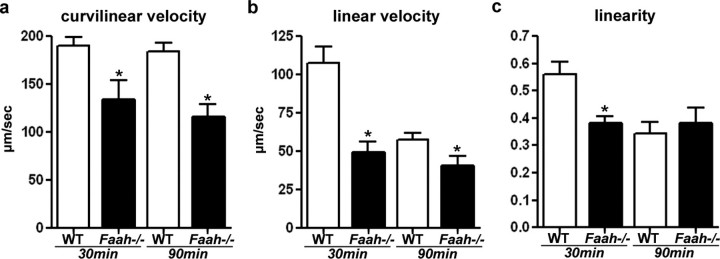
It is generally accepted that robust sperm motility is an important component of normal male fertility [34] and that hyperactivated motility of sperm is correlated with sperm's fertilizing ability of zona-intact eggs [35]. In a low-viscosity medium, motility of hyperactivated sperm is characterized by asymmetrical flagellar bends with large amplitude and curvature, and moving trajectories are irregular and highly curved [36]. We often observed sluggish motility of Faah−/− sperm when they were incubated in the capacitation medium. We speculated that the reduced zona-penetrating ability of Faah−/− sperm could be due to their reduced motility or hyperactivation. Therefore, we assayed motility of WT and Faah−/− sperm after capacitation for 30 and 90 min in vitro. In this measurement, the curvilinear velocity was calculated from the total distance traveled divided by the travel time; this parameter indicates the swimming ability of sperm. The linear velocity was calculated from the distance between the start and end points divided by the travel time. The linearity is the linear velocity-curvilinear velocity ratio; this is an indicator of straightness of sperm movement. The movement of Faah−/− sperm was significantly slower than that of WT sperm at 30 and 90 min of incubation in the capacitation medium (Fig. 5a). The movement of WT sperm was primarily straight at 30 min of capacitation, with symmetrical flagellar beats. After 90 min of capacitation, WT sperm showed hyperactivated movement pattern, resulting in reduced linear velocity (Fig. 5b) and linearity (Fig. 5c); the curvilinear velocity was not significantly changed (Fig. 5a). However, Faah−/− sperm demonstrated irregular movement from 30 min of capacitation, distinguished by decreased linear velocity and linearity (Fig. 5, b and c). Although their moving trajectories were erratic, the seemingly hyperactivated movement of Faah−/− sperm was not the consequence of harder beat of flagellum after capacitation, as the moving speed of Faah−/− sperm stayed at low levels. These results show that heightened anandamide signaling in the male reproductive tract compromises motility of Faah−/− sperm, leading to reduced zona penetration and fertilization.
FIG. 5.
Motility of Faah−/− sperm is inferior. a) Curvilinear velocity of WT and Faah−/− sperm. Curvilinear velocities of Faah−/− sperm were significantly lower than those of WT sperm at 30 and 90 min of capacitation (* P < 0.01, unpaired Student t-test). b) Linear velocity of WT and Faah−/− sperm. Linear velocities of Faah−/− sperm were significantly lower than those of WT sperm (* P < 0.05, unpaired Student t-test). c) Linearity of WT and Faah−/− sperm. Linearity of Faah−/− sperm was significantly lower than that of WT sperm at 30 min of capacitation (* P < 0.05, unpaired Student t-test).
DISCUSSION
Emerging evidence shows that endocannabinoid signaling has critical roles in male reproduction. Endocannabinoid signaling is operative in the oviduct, uterus, and embryo, and aberrant endocannabinoid signaling adversely affects oviductal transport of embryos and their development [1]. Consistent with our present findings, endocannabinoids and their receptors were reported to be present in the testis and sperm of invertebrates and vertebrates [21, 22, 37–40]. However, our findings of the endocannabinoid system in different regions along the male reproductive tract suggest that endocannabinoid signaling has diverse physiological functions. In this respect, Sertoli cells exposed to higher anandamide levels were shown to undergo apoptosis [41], and FAAH activity is regulated by FSH in mouse Sertoli cells [42]. In addition, sperm fertility and the acrosome reaction were reported to be adversely affected if exposed in vitro to high anandamide levels [21, 43].
Our experiments were designed to evaluate in vivo effects of sustained higher anandamide levels in the male reproductive tract on various aspects of sperm function. We used Faah−/− mice with high anandamide levels as a model system to mimic the conditions of long-term exposure to marijuana use to explore the role of cannabinoid and endocannabinoid signaling in male fertility. Results of our IVF experiments with Faah−/− sperm show a resemblance to reduced sperm fertilizing capacity and motility in marijuana users [44–46]. Our findings of compromised fertilizing capacity of Faah−/− sperm in vivo and in vitro, as well as their inability to recover in normal capacitating medium, provide strong evidence that functional impairment of sperm exposed to high anandamide levels in vivo persists for a prolonged period or becomes irreversible. Our results are clinically relevant because long-term in vivo exposure to marijuana is implicated in reduced male fertility [44–46].
The use of zona-free eggs in IVF experiments is an established method to study cellular mechanisms of gamete adhesion and fusion [47]. Using this strategy, we have shown that Faah−/− sperm are capable of adhering to and fusing with zona-free eggs in a manner similar to that of WT sperm. The fact that the fertilization rate of Faah−/− sperm increased from 42% with zona-intact eggs to 90% with zona-free eggs suggests that the zona is a major barrier to Faah−/− sperm, as these null sperm display spontaneous acrosome reaction comparable to that of WT sperm based on Izumo staining and flow cytometry analysis. We speculate that factors other than the acrosome reaction weaken the penetrating capacity of sperm through the zona. Sperm motility and acrosomal enzymes are involved in zona penetration [20]. It is possible that contents released from the acrosomal sac lack appropriate protease activity required for penetration or that Faah−/− sperm cannot acquire hypermotility following capacitation. Our results suggest that reduced motility is a contributing factor for reduced zona-penetrating ability of Faah−/− sperm. However, other factors such as protease activity may contribute to reduced capacity of sperm for zona penetration. Faah−/− sperm show asymmetric flagellar beat at 30 min of capacitation. We do not know whether Faah−/− sperm show straightforward moving trajectory before 30 min of capacitation. Although it would be helpful to know the motility of Faah−/− sperm immediately after they are placed in the capacitation medium, we were unable to obtain this information because of the time necessary for sperm manipulation and counting.
Reversal of the defects of FAAH deficiency in the absence of CNR1 suggests that anandamide signaling exerts its effects on sperm through CNR1. Because CNR1 is expressed in the testis, epididymis, and sperm, it is unclear where and how CNR1-mediated signaling regulates sperm fertility. Because sperm display CNR1, it is possible that higher anandamide levels directly target sperm to alter their function. Alternatively, heightened signaling via CNR1 in the presence of higher anandamide levels in Faah−/− testis and epididymis changes the internal milieu to affect sperm maturation, influencing sperm fertility. Our findings that Faah−/−/Cnr2−/− sperm show much inferior fertilizing capacity than sperm deleted of Faah−/− only suggest that anandamide working via CNR2 is important for normal sperm fertility. Alternatively, in the absence of CNR2, higher levels of anandamide are exclusively available to CNR1, further enhancing its adverse effects on sperm function. The latter speculation seems more probable because homozygous crossings of Cnr2−/− mice have an average litter size of about seven, whereas homozygous crossings of Faah−/−/Cnr2−/− mice produce an average of four pups per litter. Although breeding data are more confounded by maternal factors than IVF results, this observation suggests that CNR2 has limited roles in sperm function under physiological anandamide levels.
The present investigation has physiological significance because sperm in Faah−/− mice and those in chronic marijuana users are subjected to enhanced cannabinoid and endocannabinoid signaling. Beneficial effects of anandamide in neurodegeneration, cancer, pain, and anxiety [48–51] have prompted heightened interest in and effort to develop FAAH inhibitors as novel therapeutic drugs. Therefore, adverse effects of anandamide should be carefully weighed against its beneficial effects. This study provides insights into male fertility regulation by endocannabinoid signaling and may shed light on improving male fertility.
Supplementary Material
Acknowledgments
The authors thank Susanne Tranguch for her critical reading of the manuscript and Jiyoung Hong for her technical help.
Footnotes
1Supported by grants DA06668, HD12304, DA11322, DA21696, and P01-CA-77839 from the National Institutes of Health. S.K. Dey is recipient of Method to Extend Research in Time Awards from the National Institute on Drug Abuse and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
REFERENCES
- Wang H, Dey SK, Maccarrone M. Jekyll and Hyde: two faces of cannabinoid signaling in male and female fertility. Endocr Rev 2006; 27: 427 448 [DOI] [PubMed] [Google Scholar]
- Rossato M, Pagano C, Vettor R. The cannabinoid system and male reproductive functions. J Neuroendocrinol 2008; 20 (suppl 1): 90 93 [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc 1964; 86: 1646 1647 [Google Scholar]
- Devane WA, Dysarz FA, III, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 1988; 34: 605 613 [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990; 346: 561 564 [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993; 365: 61 65 [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992; 258: 1946 1949 [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 1995; 50: 83 90 [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kudo N, Ojima T, Mabuchi-Itoh K, Yamashita A, Waku K. Coenzyme A-dependent cleavage of membrane phospholipids in several rat tissues: ATP-independent acyl-CoA synthesis and the generation of lysophospholipids. Biochim Biophys Acta 1995; 1255: 167 176 [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem 2004; 279: 5298 5305 [DOI] [PubMed] [Google Scholar]
- Leung D, Saghatelian A, Simon GM, Cravatt BF. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry 2006; 45: 4720 4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, Chan AC, Zhou Z, Huang BX, Kim HY, Kunos G. A biosynthetic pathway for anandamide. Proc Natl Acad Sci U S A 2006; 103: 13345 13350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J Biol Chem 2006; 281: 26465 26472 [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996; 384: 83 87 [DOI] [PubMed] [Google Scholar]
- Giang DK, Cravatt BF. Molecular characterization of human and mouse fatty acid amide hydrolases. Proc Natl Acad Sci U S A 1997; 94: 2238 2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney MK, Cravatt BF. Structure and function of fatty acid amide hydrolase. Annu Rev Biochem 2005; 74: 411 432 [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A 2001; 98: 9371 9376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon B, Aitken RJ, McLaughlin EA. New insights into the molecular mechanisms of sperm-egg interaction. Cell Mol Life Sci 2007; 64: 1805 1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe M, Cummins JM. Mechanisms of sperm-egg interactions emerging from gene-manipulated animals. Cell Mol Life Sci 2007; 64: 1945 1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primakoff P, Myles DG. Penetration, adhesion, and fusion in mammalian sperm-egg interaction. Science 2002; 296: 2183 2185 [DOI] [PubMed] [Google Scholar]
- Rossato M, Ion Popa F, Ferigo M, Clari G, Foresta C. Human sperm express cannabinoid receptor Cb1, the activation of which inhibits motility, acrosome reaction, and mitochondrial function. J Clin Endocrinol Metab 2005; 90: 984 991 [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Barboni B, Paradisi A, Bernabo N, Gasperi V, Pistilli MG, Fezza F, Lucidi P, Mattioli M. Characterization of the endocannabinoid system in boar spermatozoa and implications for sperm capacitation and acrosome reaction. J Cell Sci 2005; 118: 4393 4404 [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A 1999; 96: 5780 5785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci U S A 1999; 96: 14136 14141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol 1997; 78: 43 50 [DOI] [PubMed] [Google Scholar]
- Wang H, Guo Y, Wang D, Kingsley PJ, Marnett LJ, Das SK, DuBois RN, Dey SK. Aberrant cannabinoid signaling impairs oviductal transport of embryos. Nat Med 2004; 10: 1074 1080 [DOI] [PubMed] [Google Scholar]
- Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development 1994; 120: 1071 1083 [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Ma W, Smalley W, Trzaskos J, Breyer RM, Dey SK. Diversification of cyclooxygenase-2-derived prostaglandins in ovulation and implantation. Biol Reprod 2001; 64: 1557 1565 [DOI] [PubMed] [Google Scholar]
- Yamagata K, Nakanishi T, Ikawa M, Yamaguchi R, Moss SB, Okabe M. Sperm from the calmegin-deficient mouse have normal abilities for binding and fusion to the egg plasma membrane. Dev Biol 2002; 250: 348 357 [PubMed] [Google Scholar]
- Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 2005; 434: 234 238 [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Saghatelian A, Hawkins EG, Clement AB, Bracey MH, Lichtman AH. Functional disassociation of the central and peripheral fatty acid amide signaling systems. Proc Natl Acad Sci U S A 2004; 101: 10821 10826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K, Saji F, Kato M, Tsutsui T, Tomiyama T, Tanizawa O. Acrobeads test: a new diagnostic test for assessment of the fertilizing capacity of human spermatozoa. Fertil Steril 1995; 63: 625 630 [PubMed] [Google Scholar]
- Inoue N, Ikawa M, Nakanishi T, Matsumoto M, Nomura M, Seya T, Okabe M. Disruption of mouse CD46 causes an accelerated spontaneous acrosome reaction in sperm. Mol Cell Biol 2003; 23: 2614 2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RM. Moving to the beat: a review of mammalian sperm motility regulation. Reprod Fertil Dev 2006; 18: 25 38 [DOI] [PubMed] [Google Scholar]
- Boatman DE, Robbins RS. Bicarbonate: carbon-dioxide regulation of sperm capacitation, hyperactivated motility, and acrosome reactions. Biol Reprod 1991; 44: 806 813 [DOI] [PubMed] [Google Scholar]
- Suarez SS, Katz DF, Owen DH, Andrew JB, Powell RL. Evidence for the function of hyperactivated motility in sperm. Biol Reprod 1991; 44: 375 381 [DOI] [PubMed] [Google Scholar]
- Schuel H, Goldstein E, Mechoulam R, Zimmerman AM, Zimmerman S. Anandamide (arachidonylethanolamide), a brain cannabinoid receptor agonist, reduces sperm fertilizing capacity in sea urchins by inhibiting the acrosome reaction. Proc Natl Acad Sci U S A 1994; 91: 7678 7682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Tonegawa T, Nakane S, Yamashita A, Waku K. Enzymatic synthesis of anandamide, an endogenous cannabinoid receptor ligand, through N-acylphosphatidylethanolamine pathway in testis: involvement of Ca(2+)-dependent transacylase and phosphodiesterase activities. Biochem Biophys Res Commun 1996; 218: 113 117 [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Finazzi-Agro A. The endocannabinoid system, anandamide and the regulation of mammalian cell apoptosis. Cell Death Differ 2003; 10: 946 955 [DOI] [PubMed] [Google Scholar]
- Wenger T, Ledent C, Csernus V, Gerendai I. The central cannabinoid receptor inactivation suppresses endocrine reproductive functions. Biochem Biophys Res Commun 2001; 284: 363 368 [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Cecconi S, Rossi G, Battista N, Pauselli R, Finazzi-Agro A. Anandamide activity and degradation are regulated by early postnatal aging and follicle-stimulating hormone in mouse Sertoli cells. Endocrinology 2003; 144: 20 28 [DOI] [PubMed] [Google Scholar]
- Rossi G, Gasperi V, Paro R, Barsacchi D, Cecconi S, Maccarrone M. Follicle-stimulating hormone activates fatty acid amide hydrolase by protein kinase A and aromatase-dependent pathways in mouse primary Sertoli cells. Endocrinology 2007; 148: 1431 1439 [DOI] [PubMed] [Google Scholar]
- Schuel H, Burkman LJ, Lippes J, Crickard K, Mahony MC, Giuffrida A, Picone RP, Makriyannis A. Evidence that anandamide-signaling regulates human sperm functions required for fertilization. Mol Reprod Dev 2002; 63: 376 387 [DOI] [PubMed] [Google Scholar]
- Kolodny RC, Masters WH, Kolodner RM, Toro G. Depression of plasma testosterone levels after chronic intensive marihuana use. N Engl J Med 1974; 290: 872 874 [DOI] [PubMed] [Google Scholar]
- Hembree WC, Nahas GG, Zeidenberg P, Huang HFS. Changes in human spermatozoa associated with high dose marihuana smoking. Nahas GG, Paton WDM. Marihuana: Biological Effects. New York: Oxford; 1979: 429 439 [DOI] [PubMed] [Google Scholar]
- Nahas GG, Frick HC, Lattimer JK, Latour C, Harvey D. Pharmacokinetics of THC in brain and testis, male gametotoxicity and premature apoptosis of spermatozoa. Hum Psychopharmacol 2002; 17: 103 113 [DOI] [PubMed] [Google Scholar]
- Evans JP. The molecular basis of sperm-oocyte membrane interactions during mammalian fertilization. Hum Reprod Update 2002; 8: 297 311 [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med 2003; 9: 76 81 [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Shelton CC, Advani T, Cravatt BF. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain 2004; 109: 319 327 [DOI] [PubMed] [Google Scholar]
- Bifulco M, Di Marzo V. Targeting the endocannabinoid system in cancer therapy: a call for further research. Nat Med 2002; 8: 547 550 [DOI] [PubMed] [Google Scholar]
- Guzman M. Cannabinoids: potential anticancer agents. Nat Rev Cancer 2003; 3: 745 755 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.