Abstract
A universal response to cellular stress is the expression of transformation-related protein 53 (TRP53). This transcription factor reduces cell proliferation and/or survival and is classed as a tumour suppressor protein. Several stresses (including culture) cause increased TRP53 expression in blastocysts and their reduced long-term developmental potential. This study shows that culture from the zygote stage (but not the 2-cell stage) reduced the development of C57BL6 inbred (but not hybrid) strain mouse embryos. Reduced viability was TRP53 dependent, being partially reversed by a TRP53 inhibitor (Pifithrin-alpha). However, the presence of culture did not cause an increase in Trp53 mRNA levels (levels were reduced following culture, P < 0.001). Transformed mouse 3T3 cell double minute 2 (MDM2) causes the ubiquitination and degradation of TRP53. MDM2 activation is accompanied by phosphorylation of Ser-166, and this is commonly catalyzed by the phosphatidylinositol-3 kinase and RAC-alpha serine/threonine-protein kinase (AKT) signaling pathway. Paf is an autocrine embryotrophin that activates the phosphatidylinositol-3 kinase/AKT pathway. High levels of TRP53 expression occurred following the culture of zygotes lacking the Paf receptor (Ptafr−/−) and following inhibition of phosphatidylinositol-3 kinase or AKT. Inhibition of MDM2 caused a Trp53-dependent reduction in zygote development. Inbred strain embryos cultured from the zygote stage expressed less phosphorylated MDM2 than similar embryos collected from the uterus. The addition of Paf to the media caused increased phosphorylation of MDM2, and this was blocked by inhibitors of phosphatidylinositol-3 kinase and AKT. The study identifies trophic ligand signaling via the activation of phosphatidylinositol-3 kinase and AKT as a mechanism resulting in the activation of MDM2.
Keywords: AKT, apoptosis, assisted reproductive technology, early development, embryo, MDM2, signal transduction, TRP53, zygote
Trophic signaling in the early embryo induces the activation of MDM2 via the actions of phosphatidylinositol-3-kinase and AKT, and this induces the latency of expression of TRP53.
INTRODUCTION
Early embryos develop in an apparently autonomous manner from the time of fertilization until at least the blastocyst stage of development. This involves several rounds of mitoses and the first stage of cellular differentiation. During this phase of development, embryos seem to be particularly susceptible to a range of exogenous stressors. One example of this is the reduction in the viability of many embryos following their production by fertilization in vitro or when they are subjected to prolonged culture in vitro from the zygote stage. The variable loss of viability of embryos under such conditions is thought to be primarily a response of the embryo to a range of stressors that they may be exposed to in vitro. These stressors may include growth and survival factor deprivation [1, 2], metabolic and substrate imbalances [3, 4], oxidative stress [5], and osmotic and shear stresses [6], and may also involve gross or minor genetic [7] and epigenetic defects [8].
In somatic cells, all such stresses are capable of activating the transformation-related protein 53 (TRP53) stress response pathway [9]. TRP53 is a transcription factor that can either reduce cycle-cell progression by, for example, the induction of cyclin-dependent kinase inhibitor 1A or induce apoptosis by, for example, the synthesis of pro-apoptotic mediators, such as Bcl2-associated X protein (BAX). Increased expression of TRP53 is an important mediator of the loss of embryo viability following culture of zygotes in vitro [10–12]. Zygotes that develop poorly in vitro (e.g., the C57BL/6 strain) show a marked up-regulation and nuclear accumulation of TRP53 in the resulting blastocysts, while this does not occur during development in vivo. Embryos that are null for TRP53 (Trp53−/−) show a marked increase in their developmental potential following culture from the zygote stage, showing that the increased TRP53 expression is responsible for a significant component of the loss of developmental potential of embryos subjected to culture in vitro [10]. Embryos from hybrid mice (e.g., B6CBF1) are relatively resistant to the effects of culture, as assessed by their growth rate in vitro and their viability upon embryo transfer. The amount of TRP53 expressed in hybrid blastocysts is modest following being placed in culture. This differential expression of TRP53 by embryos provides a basis for the well-known strain-dependent differences in the susceptibility of embryos to culture.
Metabolic disturbances can also activate TRP53-mediated early embryopathy. Hyperglycemia, secondary to induced diabetes, causes an increased incidence of cell death in embryos with a consequent reduced rate of development. This phenotype is partially ameliorated by the deletion of the Trp53 gene in the mouse embryo [13, 14]. Inbred zygotes cultured to the blastocyst stage show an accumulation of TRP53 within the nuclei. TRP53 is a transcription factor, and its increased expression and nuclear localization results in a TRP53-dependent accumulation of BAX, indicating that it is transcriptionally active under these conditions [11]. Hyperglycemia also results in increased BAX expression in embryos [14]. A study of human embryos produced by intracytoplasmic sperm injection shows that TRP53 expression occurs at high levels within the nucleus of embryos that are degenerate or retarded in development, but is generally expressed at much lower levels in embryos of apparently normal morphology and growth rates [11].
Transcription of Trp53 is under the regulation of a range of transcription factors [15], including positive regulators, for example, transcriptional enhancer factor (TEF-4; officially known as TEA domain family member 2, TEAD2) and transacting transcription factor 1, and negative transcriptional regulators, for example, paired box protein-1, Y box protein 1, or Kruppel-like factor 4. A range of cell stressors, including genotoxic stress, can induce Trp53 transcription in somatic cells [15]. In human preimplantation embryos produced by in vitro fertilization, a negative association between an embryo's Trp53 mRNA concentration and its morphology and rate of development is observed [16, 17]. Thus, embryos of the best morphological grading have the least Trp53 expression. This may indicate that the stressors of culture act via the induction of Trp53 gene expression.
In many settings, it has been shown that regulation of TRP53 expression occurs primarily posttranslationally [18–20] by the regulation of its half-life. TRP53 is subject to rapid ubiquitin-mediated degradation by the 26S proteosome. A range of stressors can suppress this rapid turnover of TRP53, allowing the TRP53 levels within a cell to rapidly increase and accumulate. Transformed mouse 3T3 cell double minute 2 (MDM2) functions as an ubiquitin ligase E3 toward itself and TRP53. It is an essential mediator of TRP53 ubiquitination and degradation [21]. Absence of MDM2 (Mdm2−/−) in the preimplantation embryo results in their death, while a simultaneous lack of TRP53 (Mdm2−/−Trp53−/− compound mutant) rescues embryos from this lethality [22, 23]. This result infers an essential role for MDM2-mediated degradation of TRP53 in controlling preimplantation embryo survival under normal circumstances. MDM2 is commonly activated through its phosphorylation by RAC-alpha serine/threonine-protein kinase (AKT, also known as protein kinase B) that is in turn activated by binding to phosphatidylinositol (3,4,5)-trisphosphate (PIP3). PIP3 is generated by the actions of phosphatidylinositol-3 kinase (PI3 kinase). Activation of PI3 kinase is commonly coupled to ligand-activated membrane receptors. It has not yet been determined whether this mechanism governs the level of TRP53 expression in the preimplantation embryo.
This study assesses the relative roles of Trp53 transcription and MDM2-mediated regulation of TRP53 expression in the embryo's response to the stresses experienced during culture in vitro. The study finds no evidence for increased transcription of Trp53 under culture conditions that lead to increased TRP53 expression. It did find that activation of MDM2 occurs via a trophic factor/PI3 kinase/AKT-dependent pathway, and this activation is perturbed in susceptible embryos during culture. The study shows that the maintenance of TRP53 latency in culture by the actions of a ligand-induced receptor-dependent PI3 kinase/AKT/MDM2 signaling pathway is one requirement for the normal autonomous development and survival of the preimplantation embryo.
MATERIALS AND METHODS
Animals
The use of animals was in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purpose and was approved by the Institutional Animal Care and Ethics Committee. Mice were inbred (C57BL/6J; B6); hybrid (C57BL/6J × CBA/He; B6CBF1); and Trp53−/− (B6.129S2-Trp53tm1Tyj strain, extensively backcrossed with B6 strain) Ptafr−/− mice were provided by Dr. S. Ishii, Department of Biochemistry and Molecular Biology, University of Tokyo. Females were superovulated with 5 IU eCG (Folligon, Intervet International, Boxmeer, The Netherlands) followed 48 h later by 5 IU hCG (Chorulon, Intervet). All were bred and maintained in the Gore Hill Research Laboratories (Royal North Shore Hospital, St Leonards, North South Wales) under 12L:12D cycle and had access to food and water ad libitum. Females were paired with males of proven fertility following hCG injection. Pregnancy was confirmed by the presence of a copulation plug the following morning (Day 0.5).
Mouse Embryo Collection and Culture
Embryos were flushed from the reproductive tract with Hepes-buffered modified human tubal fluid medium (Hepes-HTF) and cultured in modified HTF medium (mod-HTF) [1]. All components of the media were tissue culture grade (Sigma, St. Louis, MO). Media contained 3 mg BSA/mL unless otherwise stated (CSL Ltd., Melbourne, Australia). Collection times post-hCG were as follows: oocytes or zygotes, 20–21 h; 2-cell embryos, 42 h; 8-cell embryos, 66 h; or blastocysts, 90 h. Zygotes were freed from their surrounding cumulus cells by brief exposure to 300 IU hyaluronidase (Sigma) in Hepes-HTF. Embryos were recovered in minimal volume and assigned to various treatments as required in mod-HTF. Embryos were cultured in 10 μl volumes in 60-well plates (LUX 5260, Nunc, Naperville, IL) overlaid by a depth of approximately 2 mm heavy paraffin oil (Sigma). Culture was at 37°C in 5% CO2 for the periods indicated in individual experiments. The developmental stage and morphology of embryos was assessed by visualizing the embryos with an inverted phase contrasted microscope (Nikon Diaphot, Nikon Corporation, Tokyo, Japan) at 24-h intervals after zygote collection.
Treatments
The following pharmacologically active agents were used in indicated experiments: Paf (equal mixture of 1-o-octadecyl and hexadecyl-2-acetyl-sn-glycero-3-phosphocholine; Sigma); Akt inhibitor (1L-6-hydroxymethyl-chiro-inositol 2-[(R)-2-O-methyl-3-O-octadecylcarbonate], Calbiochem, Alexandria, Australia); deguelin ((7aS,13aS)-13,13a-dihydro-9,10-dimethoxy-3,3-dimethyl-3H-bis[1]benzopyrano[3,4-b:6′,5′-e]pyran-7(7aH)-one; Sigma); LY294002 (2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one, Calbiochem); Nutlin-3 (racemic mix of Nutlin-3a and Nutlin-3b, (+/−)-4-[4,5-bis-(4-chlorophenyl)-2-(2-isopropoxy-4-methoxy-phenyl)-4,5-dihydro-imidazole-1-carbonyl]-piperazin-2-one; Calbiochem); and Pifithrin-α (2-(2-imino-4,5,6,7-tetrahydrobenzothiazol-3-yl)-1-p-tolylethanone; Calbiochem).
Quantitative Reverse Transcriptase PCR
The blastocysts that were analyzed were washed three times in PBS, pH 7.4 (Sigma), transferred individually into 5 μl 1× PCR buffer II (final 1× concentrations: 50 mM KCl, 10 ml Tris-HCl, pH 8.3, Applied Biosystems) supplemented with 4 mg/ml glycogen (Roche) and 1 U/μl RNase inhibitor (Applied Biosystems) and immediately frozen in liquid nitrogen. The samples were lysed by thawing on ice and vortexed for 15 sec. Two more freeze, thaw, and vortex cycles were carried out.
Primer annealing to RNA occurred by the addition of 5 μl of a premixed 2× solution containing random hexamers (Roche), MgCl2, deoxyribonucleotide triphosphates (dNTPs; Applied Biosystems), and PCR buffer per 5 μl of sample such that the resulting solution was 1× PCR buffer II (Applied Biosystems, final 1× concentrations: 50 mM KCl, 10 mM Tris-HCl, pH 8.3), supplemented with 2 mg/ml glycogen (Roche), 0.5 mM each dNTP, 2.4 μM random hexamers, and 4.7 ml MgCl2. The RNA was denatured (65°C, 10 min), and placed on ice. The sample was supplemented with 1.25 U/μl MuLV Reverse Transcriptase (Applied Biosystems) and 0.5 U/μl RNase inhibitor (Applied Biosystems), and incubated at 25°C for 10 min for primer annealing and initial cDNA synthesis, and at 42°C for 40 min for completion of cDNA synthesis. The cDNA samples were stored at −20°C.
Real-time reverse transcriptase PCR analysis was performed in a Rotor Gene 3000 Real Time Thermal Cycler (Corbett Life Science, Sydney, Australia). Gene specific primers and internal Taqman probes were used: Trp53 forward primer 5′ CAG CGT GGT GGT ACC TTA TG 3′, reverse primer 5′ CCC CAT GCA GGA GCT ATT AC 3′, yielding a 91-bp product (lacking a 402-bp intron) with an internal probe of 5′ 6-6-carboxyfluorescein (FAM)-CTC AGA GCC GGC CTC GGG–5-carboxytetramethylrhodamine (TAMRA) 3′; Actb forward primer 5′ CTA AGG CCA ACC GTG AAA AG 3′, reverse primer 5′ GTA CGA CCA GAG GCA TAC AG 3′, yielding a 109 bp product (lacking 454 bp intron) with an internal probe of 5′ hexachlorofluorescein (HEX)-TGA AGG TCT CAA ACA TGA TCT GGG TCA–Black Hole Quencher 1 (BHQ)-1 3′ (all primers from Sigma-Genosys, Castle Hill, Australia).
PCR was performed in 25-μl reactions in PCR buffer II (Applied Biosystems), supplemented with cDNA template (3.3 μl per 25 μl reaction). The PCR cycling conditions were: 95°C for 10 min, then 40 cycles of: 95°C for 15 sec, 60°C for 30 sec, and 72°C for 45 sec; readings were acquired on channels appropriate for FAM and HEX labels.
A threshold was set where the amplification was close to the reaction's maximum rate and where negative controls were not significant. The threshold cycle (Ct) was used to calculate the relative quantity of the gene of interest in the cDNA sample. Samples where the Ct of either Trp53 or Actb was outside the known quantitative range (as indicated by the standard curves) were excluded from further analysis. The delta Ct (i.e., = Ct[Trp53] − Ct[Actb]) is a measure of relative changes in Trp53 mRNA content of the embryo. Data was normalized to the freshly isolated blastocysts Trp53 mRNA relative level by subtracting the delta Ct of each sample. The value of 2−(normalized delta Ct) is a measure of relative Trp53 mRNA expression, shown in Figure 1D.
FIG. 1.
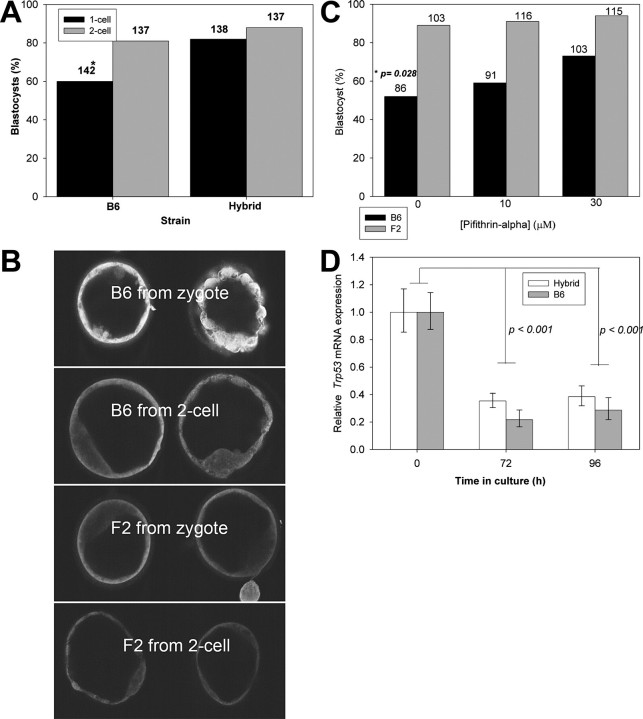
The effect of the stage of embryo development at the initiation of culture on the subsequent expression of TRP53 and rate of embryo development. B6 or hybrid embryos were collected at either the zygote or 2-cell stage and cultured in vitro for 96 or 72 h, respectively. A) The proportion of embryos developing to morphological blastocysts was recorded; the total number of embryos cultured is shown above each column. *P < 0.01. B) The relative intensity of TRP53 staining assessed by immunolocalization and visualization by single equatorial confocal images. The images in each panel are representative of three replicate experiments. Magnification for all images is ×300. F2, B6CBF2 embryos. C) To test that the reduced development of B6 zygotes cultured in vitro compared to hybrid embryos was a consequence of TRP53 expression, embryos were treated to increasing concentration of the TRP53 inhibitor Pifithrin-alpha. The number of embryos in each treatment from three replicates is shown above each bar. Solid bars, B6; shaded bars, hybrid; p, level of significance of the effect of Pifithrin-alpha on B6 zygotes. D) A cohort of embryos from each group, together with a cohort collected fresh from the uterus on Day 3.5 postcoitum, were subjected to quantitative RT-PCR for the relative levels of Trp53/Actb mRNA. The results are the mean and SEM for at least nine determinations for each group.
Western Blot Analysis
Western blot analysis was performed as previously described [24]. Embryos were collected and washed three times in cold PBS and transferred in a maximum volume of 1.5 μl PBS into 1.5 μl of 2× extraction buffer supplemented with protease and phosphatase inhibitors (2× PBS, 2% (v/v) Triton X-100, 24 mM deoxycholic acid, 0.2% (w/v) SDS, 20 mM NaF, 20 mM Na4P2O7, 2 mM PMSF, 3.08 μM aprotinin, 42 μM leupeptin, and 2.91 μM pepstatin A; all from Sigma). The embryos were lysed by three cycles of freezing in liquid nitrogen and thawing (with vortexing). Protein samples were diluted with 1 μl of 5× Laemmli buffer (50 mM Tris-HCl, 5 mM EDTA, pH 8.0, 12.5% (w/v) SDS, 0.05% (w/v) bromophenol blue, and 25% beta-mercaptoethanol) (Sigma), incubated 10 min at 60°C, and size separated using 20% homogenous SDS PAGE (GE Healthcare, Rydalmere, Australia) on a PhastSystem apparatus (GE Healthcare) or using Bio-Rad minigels (Bio-Rad Laboratories, Hercules, CA). Proteins were blotted into polyvinylidene fluoride (PVDF) membranes (Hybond-P, GE Healthcare) in a semidry blotting apparatus overnight using transfer buffer (12 mM Tris, pH 7.0, 96 mM glycine [Sigma], and 20% [v/v] methanol [Merck KGaA, Darmstadt, Germany]). Nonspecific binding was blocked by 5% (w/v) skim milk in PBS supplemented with 0.05% (v/v) Tween-20 (PBST) at room temperature for 1 h. Membranes were probed overnight at 4°C in 5% skim milk in PBST with primary antibody (1:400 rabbit anti-Ser-166 phosphorylated MDM2 (pMDM2) polyclonal IgG; Cell Signaling Technology, Beverly, MA) or with 1:1000 rabbit anti-beta-actin (Sigma). In experiments in which both pMDM2 and actin were assessed, the gel was cut into two pieces along a line corresponding to approximately 60 kDa. The lower molecular weight sections were exposed to the anti-pMDM2, and the higher molecular weight sections to anti-actin. Primary antibodies were detected with a horseradish peroxidase-conjugated secondary antibody applied for 1 h at room temperature. Anti-pMDM2 was developed with 1:2 dilution of Femto Maximum Chemiluminescent Substrates (Pierce, Rockford, IL) for 5 min at room temperature, and anti-actin was detected with 1:3 dilution with SuperSignal West Pico Substrate (Pierce). Membranes were exposed to CL-XPosure x-ray film (Pierce). Analysis was performed on groups of 30 embryos. The relative optical density of blots was measured by the Image-Pro Plus histogram program (Media Cybernetics Inc., Silver Spring, MD). Total density of each blot in three independent replicates was measured.
Immunofluorescence
Embryos were washed three times in PBS with 0.1% (w/v) BSA, 0.1% (v/v) Tween-20, and 0.2% (w/v) sodium azide (washing buffer), fixed with freshly prepared 2% (v/v) paraformaldehyde (Sigma) in PBS (pH 7.4) for 30 min, and then permeabilized with 2% paraformaldehyde with 0.3% Tween-20 (Sigma) at room temperature for 30 min. Embryos were washed three times in washing solution and then were blocked in PBS containing 2% BSA and 30% serum for 3 h. They were stained overnight at 4°C with primary antibodies or an equivalent concentration of isotype control nonimmune immunoglobulin (negative control). Primary antibody was detected by incubation of embryos with secondary antibodies coupled to fluorescein isothiocyanate or Texas Red in PBS, 2% BSA for 1 h at room temperature. In some cases, the nuclei were counterstained with 0.1 μg/ml propidium iodide in PBS. Whole section immunolocalization was performed with mercury lamp UV illumination and epifluorescence on a Nikon Optiphot microscope with Olympus DPlan Apo 40X UV oil objective. Optical sectioning was performed with a Bio-Rad Radiance Confocal microscope, using a Nikon Plan Apo 60X/1.4 oil emersion objective, as previously described [25]. Images were captured using Lasersharp 2000, Version 4.0 (BioRad, Sydney, Australia). Microscope and laser settings were adjusted such that no fluorescence was observed with nonimmune controls. All the test specimens were observed with these same settings. The primary antibodies and their concentrations were: 1:300 anti-TRP53 (Ab-7) polyclonal antibody (Oncogene Research Products, Calbiochem); 1:200 mouse anti-MDM2 (SMP14) monoclonal IgG (Santa Cruz Biotechnology, Santa Cruz, CA); and 1:400 rabbit anti-Ser-166 pMDM2 polyclonal IgG (Cell Signaling Technology). In all the experiments, negative control staining used equivalent concentrations of nonimmune IgG. For all individual experiments, microscope, data-capture, image analysis, and manipulation settings were identical for all treatments, including negative controls.
Statistical Analysis
Statistical analyses were performed on SPSS statistical package (version 11.5, SPSS, Chicago, IL). The proportion of embryos developing to a given developmental landmark following culture in vitro was assessed by binary logistic regression analysis, treating the proportion developing to a given developmental landmark as the dichotomous dependent variable and the treatment and experimental replicate as covariates in the model. The effects of the factors, strain, and treatment on the relative concentration of RNA (dependent variable) was tested by univariate analysis. Difference in the optical density of Western blot analysis was tested by Student t-test.
RESULTS
Mouse embryos cultured from the zygote stage are generally recognized as being more susceptible to the adverse effects of culture than embryos cultured from the 2-cell stage. Inbred strain (B6) and hybrid embryos (B6CBF2) were cultured from either the zygote or 2-cell stage for 96 or 72 h, respectively. Embryos were cultured individually in 10 μl drops of media. B6 embryos cultured from the 2-cell stage had a significantly higher (P < 0.01) rate of development to the blastocyst stage than embryos cultured from the zygote stage for 96h (Fig. 1A). This differential was not observed for B6CBF2 embryos (P > 0.05) (Fig. 1A). B6 blastocysts cultured from the zygote stage showed a pattern of increased TRP53 staining compared with B6 embryos cultured from the 2-cell stage (Fig. 1B). This difference was not obvious for B6CBF2 embryos cultured from either developmental stage (Fig. 1B). The reduction in the rate of development of B6 embryos cultured from the zygote stage was partially reversed (P < 0.05) by the addition of a TRP53 inhibitor (Pifithrin-α) [26] to culture media (Fig. 1C). The inhibitor caused no change in the rate of development of hybrid embryo development compared with controls (P > 0.05) (Fig. 1C), a result consistent with the low level of TRP53 expresssed in these embryos. Thus, under conditions that caused a marked increase in the expression of TRP53 (culture of B6 zygotes), pharmacological inhibition of TRP53 improved the viability of embryos.
There was no effect of mouse strain on the amount of Trp53 mRNA expressed relative to Actb expression (P > 0.05), nor was there a difference in Trp53 expression between blastocysts that had been cultured from the zygote stage or 2-cell stage (P > 0.05) (Fig. 1D). Somewhat surprisingly, the relative levels of Trp53 expression in blastocysts collected directly from the reproductive tract was significantly higher for both strains (P < 0.001) than either of the corresponding cultured groups (Fig. 1D). Thus, conditions that resulted in increased levels of TRP53 expression were not accompanied by increased Trp53 RNA expression.
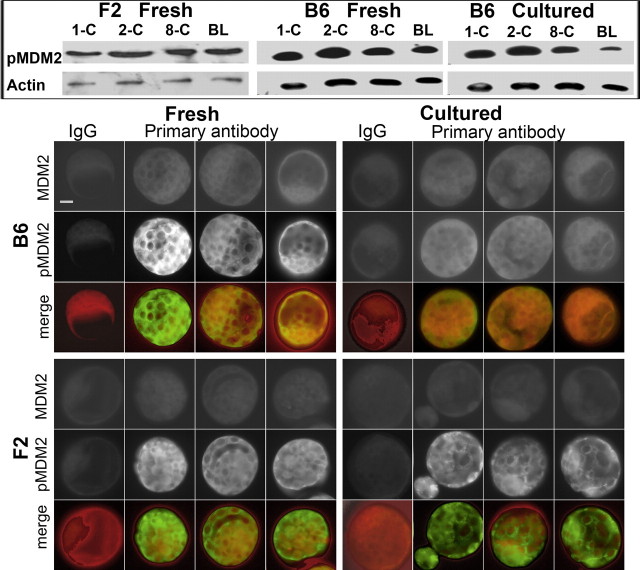
An important determinant of the level of TRP53 expression in cells is its rate of degradation, and this is largely determined by the activity of the protein ligase E3, MDM2. Phosphorylation of MDM2 (at Ser-166 or −188) can result in the activation of its protein ligase activity. Phosphorylated MDM2 was detected by Western blot analysis of embryos throughout the preimplantation phase of development in hybrid embryos and in both fresh and cultured B6 embryos (Fig. 2, inset). Immunolocalization showed that relative to the level of MDM2 staining, pMDM2 staining was lower in B6 blastocysts resulting from zygote culture than in either freshly collected hybrid or B6 blastocysts, or hybrid blastocysts resulting from culture of zygotes (Fig. 2, main). Thus, conditions that resulted in poor embryo viability (i.e., culture of B6 blastocyst) also resulted in a diminished level of MDM2 phosphorylation. It is noteworthy that where the expression of pMDM2 was low (cultured B6), the general pattern of staining was for expression throughout the cells of the embryo. By contrast, treatments that led to high levels of pMDM2 expression showed a general pattern of enhanced cytoplasmic staining in many cells.
FIG. 2.
Effect of culture on the expression of MDM2 and pMDM2 in mouse embryos. Inset: Western blot analysis of pMDM2 and actin in zygotes (1-C), 2-cell embryos (2-C), 8-cell embryos (8-C), and blastocysts (BL) from F2 hybrid embryos collected from the reproductive tract (Fresh) and B6 embryos cultured from zygote stage (Cultured) and also collected from the reproductive tract. Each band represents 30 embryos. Main: Whole section immunofluorescence localization of embryos double stained for MDM2 and pMDM2, and their digitally merged images. Photos show three representative embryos stained for primary antibodies and one example of negative staining with nonimmune IgG control. Embryos were F2 hybrids or B6 inbreds, either collected directly from the reproductive tract (Fresh) or cultured from the zygote stage for 96 h (Cultured). In merged images, MDM2 is red and pMDM2 is green. The results are representative of three independent replicates with at least 10 embryos for each treatment. Bar = 10 μm.
AKT causes Ser-166/Ser-188 phosphorylation of MDM2 [27]. Compared to untreated embryos, treatment of B6CBF2 zygotes with an inhibitor of PI3 kinase (LY294002) or AKT (Akt inhibitor) (Fig. 3A) caused an up-regulation of expression and nuclear localization of TRP53. An autocrine embryotrophin (Paf) induces the formation of PIP3 by activation of PI3 kinase resulting in the downstream activation of AKT [28, 29]. The absence of the receptor for Paf (Ptafr−/−) reduced the rate of development of zygotes in vitro [28] and induced the increased expression and nuclear localization of TRP53 in zygotes cultured for 96 h (Fig. 3A).
FIG. 3.
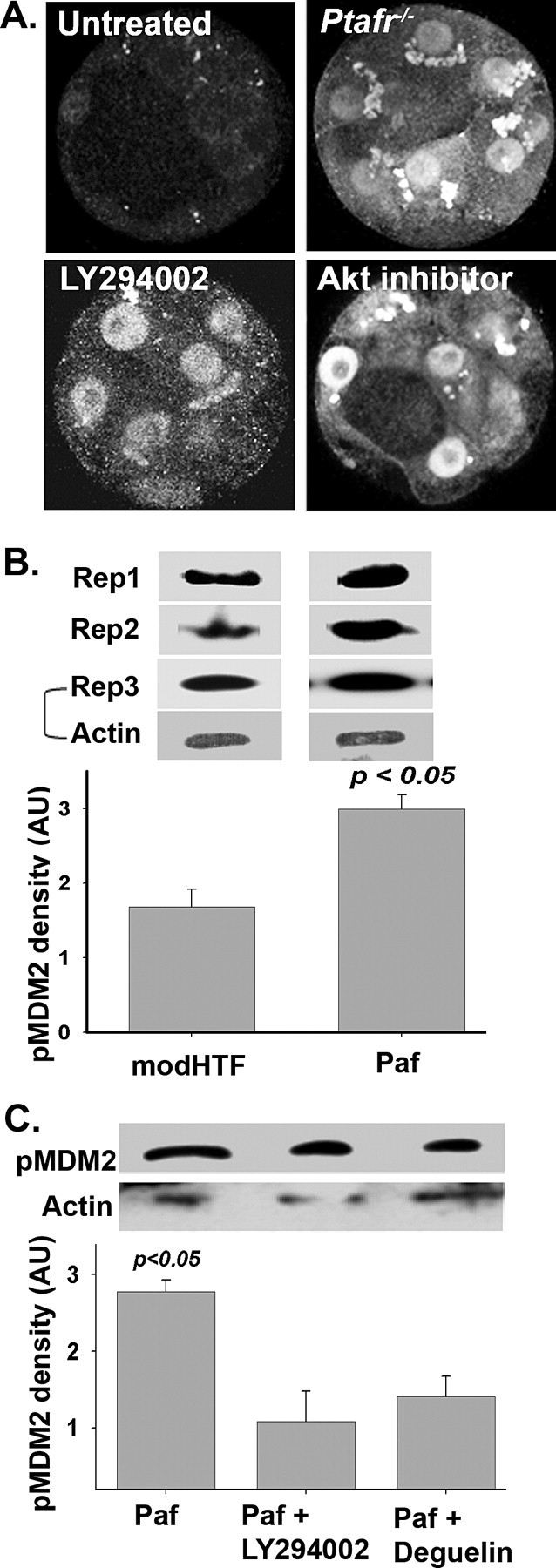
The effect of inhibiting the Paf/PI3 kinase/AKT pathway on TRP53 and pMDM2 expression in zygotes cultured for 90 h in vitro. A) Hybrid zygotes cultured for 96 h without treatment; embryos lacking the Paf-receptor (Ptafr−/−); B6CBF2 zygotes cultured in an inhibitor of PI3 kinase (LY294002, 15 μM); or B6CBF2 zygotes cultured in an inhibitor of AKT (AKT inhibitor 10 μM). Images are single equatorial confocal sections of representative embryos from three replicate experiments. Magnification ×450. B) Mouse zygotes were cultured overnight in media with or without Paf. The total amount of pMDM2 staining in the resulting 2-cell embryos was assessed by Western blot analysis. pMDM2 Western blot analysis from three replicates are shown, and the staining for actin from the same gel is also shown for replicate 3 (Rep3). Densitometric analysis (mean + SEM) of the Western blots are also shown. AU, arbitrary units, modHTF, modified HTF. C) In an experiment of similar design as B, embryos cultured in the presence of Paf were treated with a PI3-kinase inhibitor (LY294002) or an AKT inhibitor (deguelin) and the amount of pMDM2 assessed by Western blot analysis. A representative Western blot is shown and the densitiometric analysis (mean + SEM) of four such blots is presented in the graph.
Paf is known to activate the PI3 kinase/AKT pathway during the zygote to 2-cell stage; thus, it was of interest to note that expression of pMDM2 was highest at the 2-cell stage (Fig. 2, inset). Zygotes were cultured to the 2-cell stage in the presence or absence of Paf, and the expression of pMDM2 was assessed. Western blot analysis showed that Paf induced an increase in the rate of MDM2 phosphorylation (Fig. 3B) without causing any change in the expression of actin. In a further analysis, we showed that inhibitors of PI3 kinase (LY294002) and AKT (deguelin) reduced the staining of pMDM2 induced by treatment of embryos with Paf (Fig. 3C), also without any consistent change in actin. These results indicate that pMDM2 staining in these embryos was at least partially under the regulation of the PI3 kinase and AKT pathway.
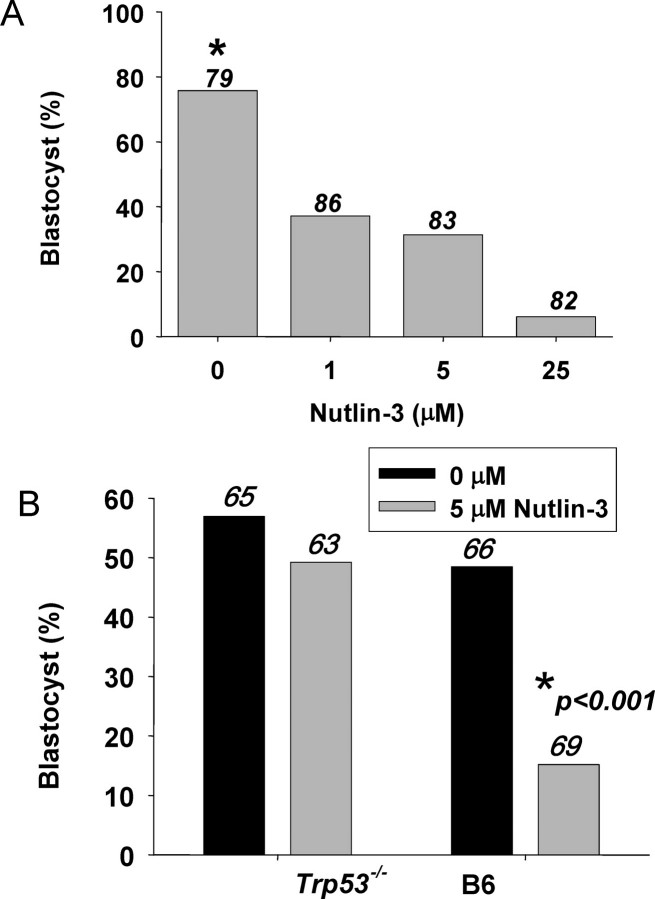
An inhibitor of MDM2 (Nutlin-3) [30] induced a dose-dependent decrease in the proportion of B6CBF2 hybrid zygotes developing to the blastocyst stage in vitro (P < 0.001) (Fig. 4A). Blocking MDM2 activity with Nutlin-3 also exacerbated the culture-induced embryopathy observed in B6 embryos, but this did not occur in B6 background embryos lacking the Trp53 gene (Trp53−/−) (Fig. 4B)..
FIG. 4.
The role of MDM2 in embryo development in vitro. A) The effect of an MDM2 inhibitor (Nutlin-3) on the development of hybrid zygotes in vitro. Embryos were cultured individually in 10 μL of medium. The proportion of zygotes developing to morphologically normal blastocysts after 96 h was recorded. The number of zygotes in each treatment is shown in numbers above the columns. *P < 0.001, effect of Nutlin-3 on blastocyst formation. B) The effect of culture of zygotes in the absence (solid bars) or presence of Nutlin-3 (5 μM, shaded bar) on the development of wild-type (B6) or Trp53−/− zygotes. The number of zygotes in each treatment is shown in numbers above the columns. *P < 0.001, effect of Nutlin-3 on blastocyst formation in B6 embryos.
The study shows that changes in the level of TRP53 expression in embryos that were highly susceptible to the adverse effects of culture occurred independently of an increase in the expression of Trp53 mRNA. Inhibition of the PI3 kinase/AKT/MDM2 pathway mediated by embryotrophic factors resulted in increased TRP53 expression and TRP53-dependent embryopathy. The study indicates that trophic stimulation of the early embryo by this pathway plays an important part in maintaining the latency of TRP53 expression. This latency is required for the normal development and survival of the preimplantation embryo.
DISCUSSION
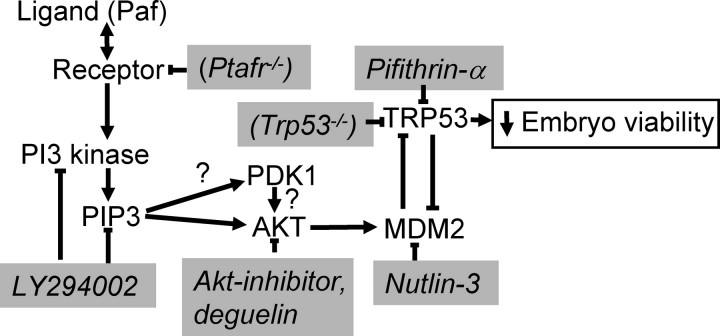
This study shows that the level of TRP53 expression in the blastocyst is primarily regulated by the activity of MDM2, which in turn is regulated by the activity of PI3 kinase and AKT (summarized in Fig. 5). It is generally considered that TRP53 and MDM2 exist as an autoregulatory feedback loop; MDM2 transcription may be induced by TRP53, and MDM2 in turn binds to the N-terminal transactivation domain of TRP53, thereby inactivating TRP53 transcriptional activity [21, 31]. The net effect of the feedback loop is to maintain a low level of TRP53 expression and activity in normal cells. While this negative feedback mechanism is considered a major regulator of TRP53 expression in cells, the rate of transcription of Trp53 can also influence its activity. The current study shows that the level of TRP53 expressed in the mouse blastocyst is not primarily governed by changes in the level of its RNA. While embryo culture induced increased TRP53 expression, this was not associated with increased transcription of the Trp53 gene. Indeed, Trp53 expression was lower in embryos following their culture. This may be a manifestation of the broader perturbation of transcription that apparently occurs in the early embryo as a consequence of culture in vitro [32–35]. The relatively high levels of Trp53 expression in blastocysts collected from the reproductive tract was surprising. TRP53 is known to exert an autoregulatory positive feedback in some cell types [15]. Hence, it might have been expected that transcription would be highest in cells expressing high TRP53 (culture B6). Yet many other transcription factors exert direct positive and negative regulatory actions on Trp53 expression, creating a complex regulatory network. The large effect of all cultures on Trp53 expression indicates that culture influences this regulatory network in an as yet unidentified manner.
FIG. 5.
A summary of the ligand-mediated control of TRP53 expression via the PI3 kinase/AKT/MDM2 pathway. The points of action of the pharmacological inhibitors and genetically modified lines used in this study are shown in italics in shaded boxes. The symbol “?” indicates interactions that have not yet been demonstrated to occur in the early embryo.
Our results do not exclude the possibility that within individual embryos Trp53 is related to the morphology of that embryo, as has been observed in the human [16, 17], however, that question was not addressed in this study. The results do show that on a population basis, the level of Trp53 expression was not a primary determinant of embryo development or TRP53 expression.
The current study confirms the findings of Li et al. [10] that culture of B6 strain zygotes caused the latency of TRP53 expression to be breached and shows that pharmacological inhibition of TRP53 (Pifithrin-α) reduced the adverse effects of culture. The study also showed that increased TRP53 was associated with an increased expression of pMDM2. We show that pharmacological inhibition of MDM2 induced early embryopathy in hybrid embryos, which were normally resistant to the stressors of culture. In B6 background embryos, the reduced embryo development induced by blocking MDM2 with Nutlin-3 did not occur in embryos that lacked the Trp53 gene (Trp53−/− embryos). The essentially complete absence of an effect of Nutlin-3 in Trp53−/− embryos indicates that TRP53 is the major downstream target of MDM2 in this experimental setting. This result indicates that the early lethality of Mdm2−/− embryos (and the reversal of this effect in Mdm2−/− Trp53−/− compound mutant embryos) [22, 23] was a consequence of the regulation of TRP53 expression by MDM2.
The greater sensitivity of the zygote of some strains to the adverse effects of culture has been previously reported and found to be at least partially due to the deprivation of autocrine trophic ligands that occurs when embryos are cultured from this stage of development [1, 2]. The early embryo responds to a range of trophic ligands, including Paf [36–38]. Blockade of trophic signaling reduces normal embryo development, and in this study we show that selective blockade of Paf signaling (Ptafr−/− embryos) results in their increased TRP53 expression. It is known that the culture of zygotes in vitro reduces the amount of autocrine stimulation the embryo receives (and in vitro the embryo is not exposed to the range of paracrine mediators released into the reproductive tract) (for review, see [39]), and this deprivation is exacerbated when embryos are cultured individually as they were in this study. This trophic deprivation is thought to make a significant contribution to the loss of viability that occurs upon the culture of zygotes and also serves as a good model for studying the actions of autocrine trophic ligands. It is noteworthy that mouse embryos cultured from the 2-cell stage are not as sensitive to reduced autocrine stimulation [1, 2], and in this study we show this is associated with the relative maintenance of TRP53 latency.
Several key proofs provided in this report rely of the use of pharmacological inhibitors. This strategy must always be interpreted with caution given the possibility of nonselective actions. The selectively of PI3 kinase inhibitors at the doses used in this study has been established and reviewed [40], and is consistent with the early embryopathy observed in Pik3cb−/− embryos. The embryopathy induced by Nutlin-3 is in the dose range found to be selective for this drug [30] and is consistent with the known embryopathy of Mdm2−/− embryos [22, 23]. The partial rescue of the viability of B6 zygotes following culture by Pifithrin-α is consistent with a beneficial effect of the Trp53−/− genotype following the culture of zygotes [10]. We have used two structurally unrelated inhibitors of AKT to reduce the risk of off-target actions. Akt-inhibitor is a phosphatidylinositol ether analog that potently and selectively inhibits AKT (IC50 of 5 μM), and it acts only as a week inhibitor on PI3 kinase (IC50 of 83 μM). Deguelin by contrast is a naturally occurring rotenoid of the flavonoid family. It is structurally and functionally different from Akt-inhibitor and has an IC50 of 10 nM. It has previously been demonstrated that these two antagonists causes reduced zygote development in these selective dose ranges [29].
Autocrine signals induce receptor-mediated activation of PI3 kinase [28, 29, 41]. PI3 kinase induces the phosphorylation of membrane inositol phospholipids, resulting in the formation of D-3′ phosphoinositides. The most important reaction may be the conversion of phosphatidylinositol (4,5) bisphosphate to phosphatidylinositol (3,4,5)-trisphosphate [42]. PIP3 acts as a docking site for a range of proteins containing the pleckstrin homology (PH) domain [42]. The generation of PIP3 recruits proteins containing PH domains to the membrane, facilitating their activation. AKT is activated as a consequence of PI3 kinase activity. The resulting PIP3 serves as a docking site for AKT where it is phosphorylated by 3-phosphoinositide-dependent kinase 1 [43].
The preimplantation embryo expresses multiple isoforms of PI3 kinase [28] and AKT [44]. Inhibitors of either PI3 kinase or AKT prevent normal preimplantation embryo development in vitro [28, 45, 46], leading to increased rates of cell death and a reduced number of cells populating the embryo. The embryopathy induced by the inhibition of PI3 kinase or AKT can be partially reversed by the addition of exogenous Paf to embryo culture media [28, 44]. Paf induces the activation of PI3 kinase, resulting in the generation of PIP3 and causing the phosphorylation of AKT (Ser-473 phosphoAKT) in the early embryo [29]. In this study, we show that the phosphorylation of MDM2 is regulated in part by the actions of Paf, PI3 kinase, and AKT. Inhibition of each component of the pathway reduces pMDM2 and increases TRP53, and consequent embryopathy. A range of putative embryotrophins activate PI3 kinase in preimplantation embryos. Both insulin [45] and transforming growth factor alpha [47] are reported to act in a PI3 kinase-dependent fashion in the preimplantation embryo. Injection of mRNA coding for a constitutively active myristoylated AKT into mouse zygotes enhances their rate of cell-cycle progression, and conversely, mRNA of kinase-deficient AKT delays the entry of embryos into mitosis. It has also been found that AKT induces the phosphorylation of M-phase inducer phosphatase 2, and hence induces maturation promoting factor activity [48]. Thus, evidence from a range of sources support the presence and actions of the PI3 kinase/AKT pathway during the preimplantation phase of development. It is likely that the sequential and overlapping actions of a range of ligands acting via this pathway throughout the preimplantation stage cooperate. This study shows that maintenance of the latency of TRP53 expression via the action of MDM2 is one target of action of this pathway.
AKT activation results in its translocation to the nucleus [49], allowing MDM2 phosphorylation to occur in the nuclear compartment [50]. MDM2 is a phosphoprotein, and its phosphorylation state determines its concentration and activity. Phosphorylation of MDM2 can protect it from self-ubiquitination, thereby stabilizing the protein, allowing it to accumulate within cells. MDM2 posses a nuclear export sequence that results in the export of the protein from the nucleus [51]. This export function is required for the export and degradation of TRP53, although the two export events may be independent [52]. The interactions between MDM2 and TRP53 are complex, and different modes of regulation may occur, depending upon the stoichiometric relationship between these two autoregulatory partners [53]. In this study, we found that much of the pMDM2 detected in fresh blastocysts was cytoplasmic, indicative of active nuclear export, while the lower expression in cultured B6 blastocysts was not as completely exported from the nucleus, a result consistent with elevated TRP53 expression.
MDM2 is a target for several kinases. Phosphorylation of MDM2 by AKT results in its activation, yet phosphorylation of MDM2 at other sites by a range of other kinases involved in genotoxic stress responses (e.g., by DNA-dependent protein kinase at Ser-17 [54], cyclin A-dependent kinase at Thr-216 [55], casein kinase II at Ser-267 [56], and ataxia telangiectasia mutated protein kinase at Ser-395 [57]) result in the inhibition of MDM2. Furthermore, cyclin-dependent kinase inhibitor 2A acts to sequester MDM2 to the nucleolus, thus diminishing its interaction with TRP53. MDM2 integrates information from many sources to govern the final concentration and activity of TRP53 in the cell. Further studies are required to investigate the role of alternative pathways in the regulation of TRP53 expression in the early embryo.
This study confirms the role of TRP53 in the stress-induced embryopathy that occurs in some embryos following culture. It shows that a mechanism for the control of TRP53 expression occurs via the negative-feedback loop between MDM2 and TRP53, and that a regulator of the feedback loop is the ligand-mediated activation of MDM2 via a PI3 kinase/AKT signaling pathway. Perturbation of this pathway allowed TRP53 to accumulate in embryos and resulted in their loss of viability. The latency of TRP53 expression is required for the normal development of the preimplantation embryo, and the actions of a survival signaling pathway are shown to be a mechanism for achieving this. The results of this study will help to develop an understanding of the causes of loss of embryo viability in mammalian species following the exposure of embryos to a range of exogenous stressors, including those imposed during forms of assisted reproductive technologies.
Supplementary Material
Acknowledgments
We thank Dr. R. Christensen for maintaining animal pedigrees, the staff of the Gore Hill Research Laboratories for the breeding and care of animals, Greg Mulhearn for assistance in preparation of figures, and N. Gunay and A. Cahana for undertaking preliminary experiments not reported here. I also thank Professor G. Lozano, MD Anderson Cancer Centre, for assistance in performing some preliminary analysis of this question.
Footnotes
1Supported by grants from the Australian Health and Medical Research Council. H.D.M. was supported by a NHMRC C J Martin Fellowship, and V.C. was supported by an Australian Postgraduate Award.
REFERENCES
- O'Neill C. Evidence for the requirement of autocrine growth factors for development of mouse preimplantation embryos in vitro. Biol Reprod 1997; 56: 229 237 [DOI] [PubMed] [Google Scholar]
- O'Neill C. Autocrine mediators are required to act on the embryo by the 2-cell stage to promote normal development and survival of mouse preimplantation embryos in vitro. Biol Reprod 1998; 58: 1303 1309 [DOI] [PubMed] [Google Scholar]
- Leese HJ. Quiet please, do not disturb: a hypothesis of embryo metabolism and viability. Bioessays 2002; 24: 845 849 [DOI] [PubMed] [Google Scholar]
- Leese HJ, Donnay I, Thompson JG. Human assisted conception: a cautionary tale. Lessons from domestic animals. Hum Reprod 1998; 13 (Suppl 4): 184 202 [DOI] [PubMed] [Google Scholar]
- Nasr-Esfahani MM, Johnson MH. The origin of reactive oxygen species in mouse embryos cultured in vitro. Development 1991; 113: 551 560 [DOI] [PubMed] [Google Scholar]
- Rappolee DA. Impact of transient stress and stress enzymes on development. Dev Biol 2007; 304: 1 8 [DOI] [PubMed] [Google Scholar]
- Delhanty JD, Harper JC, Ao A, Handyside AH, Winston RM. Multicolour FISH detects frequent chromosomal mosaicism and chaotic division in normal preimplantation embryos from fertile patients. Hum Genet 1997; 99: 755 760 [DOI] [PubMed] [Google Scholar]
- Thompson JG, Kind KL, Roberts CT, Robertson SA, Robinson JS. Epigenetic risks related to assisted reproductive technologies: short- and long-term consequences for the health of children conceived through assisted reproduction technology: more reason for caution? Hum Reprod 2002; 17: 2783 2786 [DOI] [PubMed] [Google Scholar]
- Agarwal M, Taylor WR, Chernov MV, Chernova OB, Stark GR. The p53 network. J Biol Chem 1998; 273: 1 4 [DOI] [PubMed] [Google Scholar]
- Li A, Chandrakanthan V, Chami O, O'Neill C. Culture of zygotes increases p53 expression in B6 mouse embryos which reduces embryo viability. Biol Reprod 2007; 76: 362 367 [DOI] [PubMed] [Google Scholar]
- Chandrakanthan V, Chami O, Stojanov T, O'Neill C. Variable expressivity of the tumour suppressor protein TRP53 in cryopreserved human blastocysts. Reprod Biol Endocrinol 2007; 5: 39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrakanthan V, Li A, Chami O, O'Neill C. Effects of in vitro fertilization and embryo culture on TRP53 and Bax expression in B6 mouse embryos. Reprod Biol Endocrinol 2006; 4: 61 67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moley KH, Chi MM-Y, Knudson CM, Korsmeyer SJ, Mueckler MM. Hyperglycemia induces apoptosis in pre-implantation embryos through cell death effector pathways. Nat Med 1998; 4: 1421 1424 [DOI] [PubMed] [Google Scholar]
- Keim AL, Chi MM-Y, Moley KH. Hyperglycemia-induced apoptotic cell death in the mouse blastocyst is dependent on expression of p53. Mol Reprod Dev 2001; 60: 214 224 [DOI] [PubMed] [Google Scholar]
- Reisman D, Loging WT. Transcriptional regulation of the p53 tumor suppressor gene. Semin Cancer Biol 1997; 8: 317 324 [DOI] [PubMed] [Google Scholar]
- Wells D, Bermudez MG, Steuerwald N, Thornhill AR, Walker DL, Malter H, Delhanty JDA, Cohen J. Expression of genes regulating chromosome segregation, the cell cycle and apoptosis during human preimplantation development. Hum Reprod 2005; 20: 1339 1348 [DOI] [PubMed] [Google Scholar]
- Wells D, Bermudez MG, Steuerwald N, Malter HE, Thornhill AR, Cohen J. Association of abnormal morphology and altered gene expression in human preimplantation embryos. Fertil Steril 2005; 84: 343 355 [DOI] [PubMed] [Google Scholar]
- Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene 2005; 24: 2899 2908 [DOI] [PubMed] [Google Scholar]
- Coutts AS, Thangue NB. The p53 response: emerging levels of co-factor complexity. Biochem Biophys Res Commun 2005; 331: 778 785 [DOI] [PubMed] [Google Scholar]
- Moll UM, Petrenko O. The MDM2-p53 interaction. Mol Cancer Res 2003; 1: 1001 1008 [PubMed] [Google Scholar]
- Fang S, Jensen JP, Ludwig RL, Vousden KH, Weissman AM. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J Biol Chem 2000; 275: 8945 8951 [DOI] [PubMed] [Google Scholar]
- Jones SN, Roe AE, Donehower LA, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 1995; 378: 206 208 [DOI] [PubMed] [Google Scholar]
- Montes de Oca Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 1995; 378: 203 205 [DOI] [PubMed] [Google Scholar]
- Cahana A, Jin XL, Reiner O, Wynshaw-Boris A, O'Neill C. A study of the nature of embryonic lethality in LIS1−/− mice. Mol Reprod Dev 2003; 66: 134 142 [DOI] [PubMed] [Google Scholar]
- Lu DP, Li Y, Bathgate R, Day M, O'Neill C. Ligand-activated signal transduction in the 2-cell embryo. Biol Reprod 2003; 69: 106 116 [DOI] [PubMed] [Google Scholar]
- Schafer T, Scheuer C, Roemer K, Menger MD, Vollmar B. Inhibition of p53 protects liver tissue against endotoxin-induced apoptotic and necrotic cell death. FASEB J 2003; 17: 660 667 [DOI] [PubMed] [Google Scholar]
- Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci U S A 2001; 98: 11598 11603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DP, Chandrakanthan V, Cahana A, Ishii S, O'Neill C. Trophic signals acting via phosphatidylinositol-3 kinase are required for normal pre-implantation mouse embryo development. J Cell Sci 2004; 117: 1567 1576 [DOI] [PubMed] [Google Scholar]
- Li Y, Chandrakanthan V, Day ML, O'Neill C. Direct evidence for the action of phosphatidylinositol (3,4,5)-trisphosphate-mediated signal transduction in the 2-cell mouse embryo. Biol Reprod 2007; 77: 813 821 [DOI] [PubMed] [Google Scholar]
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004; 303: 844 848 [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature 2000; 408: 307 310 [DOI] [PubMed] [Google Scholar]
- Stojanov T, O'Neill C. In vitro fertilization causes epigenetic modifications to the onset of gene expression from the zygotic genome in mice. Biol Reprod 2001; 64: 696 705 [DOI] [PubMed] [Google Scholar]
- Rinaudo P, Schultz RM. Effects of embryo culture on global pattern of gene expression in preimplantation mouse embryos. Reprod 2004; 128: 301 311 [DOI] [PubMed] [Google Scholar]
- Giritharan G, Talb S, Donjacour A, Di Sebastiano F, Dobson A, Rinaudo P. Effect of in vitro fertilization on gene expression and development of mouse preimplantation embryos. Reprod 2007; 134: 63 72 [DOI] [PubMed] [Google Scholar]
- Stojanov T, O'Neill C. Ontogeny of expression of a receptor for platelet-activating factor in mouse preimplantation embryos and the effects of fertilisation and culture in vitro. Biol Reprod 1999; 60: 674 682 [DOI] [PubMed] [Google Scholar]
- O'Neill C. Partial characterisation of the embryo-derived platelet activating factor in mice. J Reprod Fertil 1985; 75: 375 380 [DOI] [PubMed] [Google Scholar]
- Emerson M, Travis AR, Bathgate R, Stojanov T, Cook DI, Harding E, Lu DP, O'Neill C. Characterization and functional significance of calcium transients in the 2-cell mouse embryo induced by an autocrine growth factor. J Biol Chem 2000; 275: 21905 21913 [DOI] [PubMed] [Google Scholar]
- O'Neill C. The role of paf in embryo physiology. Hum Reprod Update 2005; 11: 215 228 [DOI] [PubMed] [Google Scholar]
- O'Neill C. The potential roles for embryotrophic ligands in preimplantation embryo development. Hum Reprod Update 2008; 14: 275 288 [DOI] [PubMed] [Google Scholar]
- O'Neill C. Phosphatidylinositol 3-kinase signaling in mammalian preimplantation embryo development. Reprod 2008. 136: 147 156 [DOI] [PubMed] [Google Scholar]
- Riley JK, Moley KH. Glucose utilization and the PI3-K pathway: mechanisms for cell survival in preimplantation embryos. Reprod 2006; 131: 823 835 [DOI] [PubMed] [Google Scholar]
- Marte BM, Downward J. PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem Sci 1997; 22: 355 358 [DOI] [PubMed] [Google Scholar]
- Wick MJ, Wick KR, Chen H, He H, Dong LQ, Quon MJ, Liu F. Substitution of the autophosphorylation site Thr516 with a negatively charged residue confers constitutive activity to mouse 3-phosphoinositide-dependent protein kinase-1 in cells. J Biol Chem 2002; 277: 16632 16638 [DOI] [PubMed] [Google Scholar]
- Adiga SK, Toyoshima M, Shiraishi K, Shimura T, Takeda J, Taga M, Nagai H, Kumar P, Niwa O. p21 provides stage specific DNA damage control to preimplantation embryos. Oncogene 2007; 26: 6141 6149 [DOI] [PubMed] [Google Scholar]
- Riley JK, Carayannopoulos MO, Wyman AH, Chi M, Ratajczak CK, Moley KH. The PI3K/Akt pathway is present and functional in the preimplantation mouse embryo. Develop Biol 2005; 284: 377 386 [DOI] [PubMed] [Google Scholar]
- Gross V, Hess M, Cooper G. Mouse embryonic stem cells and preimplantation embryos require signaling through the phosphatidylinositol 3-kinase pathway to suppress apoptosis. Mol Reprod Dev 2005; 70: 324 332 [DOI] [PubMed] [Google Scholar]
- Kawamura K, Kawamura N, Kumagai J, Fukuda J, Tanaka T. Tumor necrosis factor regulation of apoptosis in mouse preimplantation embryos and its antagonism by transforming growth factor alpha/phosphatidylionsitol 3-kinase signaling system. Biol Reprod 2007; 76: 611 618 [DOI] [PubMed] [Google Scholar]
- Feng C, Yu A, Liu Y, Zhang J, Zong Z, Su W, Zhang Z, Yu D, Sun Q-Y, Yu B. Involvement of protein kinase B/AKT in early development of mouse fertilized eggs. Biol Reprod 2007; 77: 560 568 [DOI] [PubMed] [Google Scholar]
- Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJC, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA. Role of translocation in the activation and function of protein kinase B. J Biol Chem 1997; 272: 31515 31524 [DOI] [PubMed] [Google Scholar]
- Feng J, Tamaskovic R, Yang Z, Brazil DP, Merlo A, Hess D, Hemmings BA. Stabilization of Mdm2 via decreased ubiquitination is mediated by protein kinase B/Akt-dependent phosphorylation. J Biol Chem 2004; 279: 35510 35517 [DOI] [PubMed] [Google Scholar]
- Roth J, Dobbelstein M, Freedman DA, Shenk T, Levine AJ. Nucleo-cytoplasmic shuttling of the hdm2 oncoprotein regulates the levels of the p53 protein via a pathway used by the human immunodeficiency virus rev protein. EMBO J( 1998) 17: 554 564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe K, Li H, Zhang Y. Nucleocytoplasmic shuttling of p53 is essential for MDM2-mediated cytoplasmic degradation but not ubiquitination. Mol Cell Biol 2003; 23: 6396 6405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Brooks CL, Wu-Baer F, Chen D, Baer R, Gu W. Mono- versus polyubiquitination: differential control of p53 fate by Mdm2. Science 2003; 302: 1972 1975 [DOI] [PubMed] [Google Scholar]
- Mayo LD, Turchi JJ, Berberich SJ. Mdm-2 phosphorylation by DNA-dependent protein kinase prevents interaction with p53. Cancer Res 1997; 57: 5013 5016 [PubMed] [Google Scholar]
- Zhang T, Prives C. Cyclin A-CDK phosphorylation regulates MDM2 protein interactions. J Biol Chem 2001; 276: 29702 29710 [DOI] [PubMed] [Google Scholar]
- Gotz C, Kartarius S, Scholtes P, Nastainczyk W, Montenarh M. Identification of a CK2 phosphorylation site in mdm2. Eur J Biochem 1999; 266: 493 501 [DOI] [PubMed] [Google Scholar]
- Maya R, Balass M, Kim S-T, Shkedy D, Leal J-FM, Shifman O, Moas M, Buschmann T, Ronai Ze, Shiloh Y, Kastan MB, Katzir E, Oren M. ATM-dependent phosphorylation of Mdm2 on serine 395: role in p53 activation by DNA damage. Genes Dev 2001; 15: 1067 1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.