Abstract
As a key degrader of fibrillar collagens, matrix metalloproteinase 13 (MMP13), may contribute to the progression of pelvic organ prolapse. Here we aimed to define the regulation of MMP13 by estradiol and progesterone in the vaginal supportive tissues. Fibroblasts cultured from the arcus tendineous fasciae pelvis of three pre- and three postmenopausal women with prolapse were treated with 17-beta-estradiol (E2), progesterone (P4), E2 + P4, or E2 + ICI 182,780 (ICI). Collagenase inhibitor I (CI) and MG-132 were employed to investigate the mechanism of MMP13 degradation into inactive fragments (fragmentation) by hormones. The regulation of MMP13 in vivo was assessed by comparing tissues of ovariectomized (ovx) vs. sham-operated rats. Expression of MMP13 (proenzyme and active and fragment forms) was quantitated by Western immunoblotting, and MMP13 enzymatic activity was measured using a substrate degradation assay. The amount of cellular active MMP13 and MMP13 proteolytic activity decreased in the presence of hormones. The decrease was paralleled by increased proenzyme and fragment forms. MG-132, not CI, suppressed cellular MMP13 fragmentation. Active MMP13 increased in rats following ovx and was suppressed by E2 + P4 supplementation. Active MMP13 is suppressed in vivo and in vitro by estradiol and progesterone, suggesting a protective effect against vaginal supportive tissue deterioration.
Keywords: estradiol, fragmentation, matrix metalloproteinase 13, progesterone, ubiquitin-proteasome
Estrogen and progesterone inhibit active MMP13 in part by accelerating the fragmentation of MMP13 via the ubiquitin-proteasomal degradation pathway.
INTRODUCTION
Pelvic organ prolapse is a common disease negatively affecting the lives of millions of women. Among women 50-yr old and older, more than 50% have some degree of prolapse, with a steady increase with advancing age [1, 2]. Menopause may contribute to the increased prevalence of symptomatic pelvic organ prolapse in older women [1, 2]; however, the precise mechanism remains unknown.
In addition to the levator ani muscle complex, the vagina and its supportive tissues comprise one of the primary means of support to the pelvic organs. Functional failure of this support apparatus results in the descent of the pelvic organs into the vagina [3]. The collagen content in these tissues provides the primary source of tensile strength and directly correlates with their biomechanical behavior [4]. We previously reported that the ratio of collagens I/(III + V), an indicator of tissue tensile strength, was decreased in vaginal supportive tissues of postmenopausal women not on hormone therapy relative to premenopausal women and postmenopausal women with prolapse on hormone therapy. The altered ratio was found to be primarily due to a decrease in collagen I [5]. Such accelerated degradation of collagen is thought to play a key role in the onset and progression of pelvic organ prolapse [6].
The degradation of collagen is precisely regulated by a family of zinc-dependent endopeptidases, the matrix metalloproteinases (MMPs). Collectively, MMPs are capable of degrading all the components of extracellular matrix and are the enzymes known to degrade fibrillar collagen [7–10]. The posttranslational processing of MMPs, the predominant mechanism of regulating activity [11], occurs at multiple levels, including cleavage of proenzyme into active form [11], binding to endogenous inhibitors [12], and degradation into inactive fragments (fragmentation) [13]. In a previous study, we demonstrated that one of the mechanisms by which estrogen and progesterone suppress the amount of active MMP1 in fibroblasts derived from the vaginal supportive tissue is accelerated fragmentation [14]. Although this finding provided an intriguing explanation for the apparent decline of vaginal support with menopause, it was unclear whether fragmentation of active MMP was also a site of regulation by estrogen and progesterone for other collagenases. Moreover, the mechanism by which the hormones regulate fragmentation was not determined in the previous study [14].
In this study, we focus our analysis on MMP13, a key collagenase in the vaginal supportive tissues that not only directly degrades fibrillar collagens but also initiates the degradation cascade by activating other MMPs [15]. In a highly controlled acellular environment, MMP13 spontaneously degrades into two fragments (autocatalysis): a 29-kDa fragment catalytic domain and a 27-kDa fragment C-terminal domain [16]. Whether or not this phenomenon occurs in a cellular environment is not known. In the current study, we aimed to determine the impact of estradiol and progesterone on the complete expression profile of MMP13 (including the proenzyme and active and fragment forms) in cells derived from the vaginal supportive tissues. To take into account the impact of endogenous inhibitors on MMP13 activity, we performed a substrate degradation assay. We explored two possible mechanisms of MMP13 fragmentation: spontaneous fragmentation into inactive fragments via autocatalysis or cellular-mediated degradation via ubiquitin-proteasomal proteolysis (UPP) [17]. Finally, as an in vivo correlate, we induced surgical menopause in rats via ovariectomy and examined the impact on the amount of MMP13 proenzyme and active and fragment forms.
MATERIALS AND METHODS
Human Subjects and Tissue Procurement
Following the approval by the Institutional Review Board of Magee-Womens Hospital and informed consent, biopsies of arcus tendenious fasciae pelvis (ATFP) were obtained from three pre- and three postmenopausal women undergoing surgery for vaginal prolapse. We chose the ATFP because it is an easily identifiable uniform structure in the female pelvis that provides support to the lateral vaginal walls. Premenopausal women had regular menstrual periods over 12 mo prior to surgery and serum FSH <35 IU/L. Postmenopausal women had no menstruation and no use of exogenous sex steroid hormones for the previous 12 mo, serum FSH >35 IU/L, and estradiol <40 pg/ml. For inclusion, premenopausal women had to be aged >24 yr and <55 yr with greater than stage II prolapse and regular periods over the preceding 12 mo. Postmenopausal women were required to be >50 yr with greater than stage II prolapse, absent menses over the preceding 12 mo, and not on hormone therapy for >1 yr. Women were excluded if they had a history of pelvic malignancy or connective tissue disease affecting collagen or elastin remodeling, blood loss during surgery exceeding 500 cc, adhesions or scarring at the biopsy site, surgeon's judgment that a biopsy was contraindicated, history or presence of endometriosis, morbid obesity (body-mas index [BMI] > 35 kg/m2), or inability to provide informed consent. All biopsies were obtained from an intact portion of the arcus tendineous fascia pelvis at the pelvic sidewall, 4 cm toward the pubic symphysis from the most distal point of prolapse. All biopsy sites were repaired with permanent or delayed absorbable suture at a 90° angle to the direction of strain. There were no adverse events associated with procurement of the biopsies. The specimens were immediately passed off to a trained technician, and cells were derived as detailed below.
Subject demographic variables were collected by certified staff, including age, BMI, gravidity and parity, maximal stage of prolapse, and stage of menstrual cycle. A Pelvic Organ Prolapse Quantification Exam as described by the International Continence Society was performed on all subjects to define the stage of prolapse [18].
Tissue Cultures and Hormonal Treatments
The six cell culture conditions and hormonal treatments have been described in detail in a previous publication [14]. The cells were confirmed to be fibroblasts by immunofluorescent labeling with a specific biomarker of prolyl hydrolase beta (Acris Antibodies GmbH, Hiddenhausen, Germany) [14]; the possibility of their being myofibroblasts were further excluded by immunoblotting using a specific biomarker of myocytes: smoothelin (Santa Cruz Biotechnology, Santa Cruz, CA; data not shown). Briefly, after achieving 95% confluence, the ATFP fibroblasts were changed to hormone-free media containing 0.2% (w/v) lactalbumin enzymatic hydrosylate (LAH) for 24 h and then exposed to hormones consisting of 17-β-estradiol (E2, 10−10–10−8 mol/L), progesterone (P4, 10−10–10−8 mol/L), E2 (10−8 mol/L) + P4 (10−10–10−8 mol/L), and E2 (10−8 mol/L) + ICI 182, 780 (ICI, 10−8–10−6 mol/L). Cell layers were harvested 48 h later and stored at −80°C until use. Passages 5 to 7 were used for the experiments. Cells cultured in the identical conditions but without hormonal treatment were employed as control (representing a status of hormone deprivation alone). Cellular proteins were extracted by sonicating the cells in extraction buffer (pH 7.5) consisting of 30 mmol/L Tris, 2.0% SDS, 1.0% Triton X-100, 10 mmol/L EDTA, and protease inhibitor cocktail. Total protein concentration of cellular lysates was determined in duplicate using a DC Protein Assay (Bio-Rad Laboratories, Hercules, CA). Twenty micrograms of total cellular proteins were used for immunoblotting.
In a pilot study, cell viability was investigated by Trypan blue exclusion that indicated >90% viability throughout the experiment. Hormones were directly dissolved in media while ICI required dimethyl sulfoxide (DMSO, Sigma-Aldrich) to completely dissolve. Therefore, DMSO has been tested and has been shown to have no independent effect on MMP13 expression (data not shown).
Inhibitor Treatments
To investigate the possible mechanism of cellular MMP13 fragmentation via autocatalysis vs. UPP, a cell culture was randomly selected from the above mentioned six fibroblastic cultures. Fibroblasts at 95% confluence were incubated with hormone-free media containing 0.2% (w/v) LAH for 24 h and then pretreated with MG-132 (an inhibitor of ubiquitin-proteasomal proteolysis, 5 μmol/L, BostonBiochem, Cambridge, MA) or collagenase inhibitor I (CI, an inhibitor of MMP autocatalysis, 5 μmol/L, EMD Biosciences Inc.) for 1 h followed by the addition of E2 (10−8 mol/L) + P4 (10−10 mol/L). Cell layers were harvested 48 h later and stored at −80°C until use. The cellular protein extraction and determination of total protein concentration were the same as outlined above.
Coimmunoprecipitation
The conjugation of MMP13 protein with ubiquitin was examined by incubating 100 μg of total cellular proteins with a rabbit anti-human ubiquitin agarose conjugate (IgG, 25 μl, Santa Cruz Biotechnology Inc., Santa Cruz, CA) at 4°C overnight. The agarose beads were repeatedly washed with extraction buffer and then boiled with 1× SDS sample buffer consisting of 1.7% SDS, 1.5% dithiothreitol, 0.7% Tris, and 5% glycerol at 100°C for 5 min. After centrifuging, the supernatant was collected and probed for MMP13 expression on Western blot as described below. Rabbit IgG was used as the negative control.
In Vitro Fragmentation Assay
Five nanograms of purified recombinant human MMP13 protein (rhMMP13, predicted molecular weight of 55 kDa, from R&D Systems, Minneapolis, MN) were dissolved in extraction buffer and incubated with MG-132 (5 μmol/L) or CI (5 μmol/L) for 15 min at 37°C. The process was stopped by boiling with 1× SDS sample buffer. Samples were then probed with MMP13 antibody as detailed below.
Animal Experiments
Following approval by the Institutional Animal Care and Use Committee (IACUC #3–003) at Magee-Womens Hospital, 23 virgin rats (Long Evans, Charles River Laboratories Inc., Wilmington, MA) aged 3 mo were anesthetized through the inhalation of isoflurane (2%–4% flow; Abbott Laboratories, North Chicago, IL) and underwent ovariectomy (ovx, N = 9), ovariectomy with subcutaneous implantation of E2 (0.1 mg) + P4 (10 mg) (Innovative Research of America, Sarasota, FL) (ovx + E2P4, N = 6), or sham surgery (N = 8) following the surgical protocols previously described [19]. Torbutrol (10% v/v; Fort Dodge Animal Health, Fort Dodge, Iowa) was administered to all rats following surgery to reduce the pain. Animals were pair fed until 8 wk later and then sacrificed. The decision to sacrifice at Week 8 was based on a time course done previously in which we determined 8 wk to be the amount of time required for a significant biomechanical deterioration (15%–20% decrease in the ultimate load at failure and 25%–30% decrease in stiffness) of the pelvic supportive tissue complex in young rats following ovariectomy [19].
Following sacrifice, the vagina was excised, minced, and sonicated in the extraction buffer (pH 7.5) consisting of 150 mmol/L NaCl, 50 mmol/L Tris, 0.1% Tween-20, 10 mmol/L EDTA, 1 mmol/L phenylmethanesulphonylfluoride, and protease inhibitor cocktail. Total protein concentration of the tissue extracts was determined in duplicate as detailed above. Eighty micrograms of tissue extracts were used for probing MMP13 expression on immunoblot. The procedure was performed as described below.
Western Immunoblotting
The proteins in the human cell or rat vaginal extracts were separated by electrophoresis on a 12% SDS-gel under reducing conditions and then transferred to a polyvinylidene fluoride membrane (Millipore Co, Bedford, MA) in an electrophoretic transfer cell (Bio-Rad). The blots were incubated with the appropriate primary antibody as follows: mouse anti-human MMP13 (clone: VIIIA2, 1:200, recognizing both proenzyme and active forms, EMD Biosciences Inc., San Diego, CA) was for MMP13 expression and mouse anti-human ubiquitin (1:200, Santa Cruz Biotechnology Inc.) for ubiquitin expression in human cells. For rat tissue extracts, the primary antibody was mouse anti-rat MMP13 (clone: VIIIC4, 1:200, recognizing both proenzyme and active forms, EMD Biosciences Inc.). The secondary antibody was goat anti-mouse IgG (H+L) alkaline phosphatase-conjugated (1:5000, Promega, Madison, WI). The anti-human MMP13 antibody was validated with recombinant human MMP13 (R&D Systems) that served as positive control. To control for nonspecific binding of antibody, blots were probed with nonimmune mouse IgG (Sigma-Aldrich) or incubated with the secondary antibody alone. The bands were subsequently visualized by chemiluminesence (CDP-Star kit, Roche, Indianapolis, IN) and imaged with a scanner (ScanJet 5370C, Hewlett-Packard) interfaced to a Macintosh OS X computer (Apple Computer Inc, Cupertino, CA). A research specialist who was blind to the identification of the samples performed the densitometry measurements. Software of UN-SCAN-IT gel (version 4.3, Silk Scientific Co, Orem, Utah) was used to concurrently quantitate the signal intensity of the scanned bands on the same blot to determine the relative quantity of each. The values were expressed as arbitrary units relative to an internal control loaded in duplicate on each gel. The internal control (same cell type at 0 h, 1.0 unit) is the same for all treatments with each cell type. In this way, we were able to compare bands quantitated on different gels within a cell type. Equal loading was ensured by normalizing to the same gel stained with 0.5% Coomassie brilliant blue. The estimation of molecular weight was made relative to Prestained SDS-PAGE Standards (Bio-Rad). For the coimmunoprecipitates, the secondary antibody was rabbit anti-mouse IgG (whole molecule) alkaline phosphatase-conjugated (IgG, 1:15 000, Sigma-Aldrich). All experiments were performed in triplicate.
Endogenous MMP13 Enzyme Activity Assay
To determine the proteolytic activity of active MMP13, a substrate degradation assay was performed. Cellular protein was extracted by sonicating the cell pellet in lysis buffer (pH 7.5) containing 50 mmol/L Tris, 150 mmol/L NaCl, and protease inhibitor cocktail. After centrifuging, the supernatant of the lysates was collected and total protein concentration was determined as described above.
MMP13 enzyme activity per 100 micrograms of total cellular proteins was measured in duplicate using a Fluorokine E enzyme activity assay kit (human active MMP13, R&D System) following the manufacture's protocol. Briefly, a standard of MMP13 and cell lysates were pipetted into the wells of the microplate precoated with monoclonal antibody that captured both proenzyme and active forms of MMP13. After washing away any unbound substances, the MMP activator, p-aminophenylmercuric acetate (APMA), was added to the standards but not the samples. Following a wash, a fluorogenic substrate (cleavage site by active MMP13 is -Gly-Leu-) linked to a quencher molecule was added. In this way, any bound active enzyme with proteolytic activity would cleave the peptide linker between the fluorophore and the quencher molecule, eliminating the distance-dependent resonance energy transfer between the fluorophore and the quencher molecule, thereby emitting a fluorescent signal that was proportional to the amount of enzyme activity in the sample. The fluorescence intensity indicative of enzyme activity was determined at Ex/Em = 320nm/405nm with a SpectraMax M2 system (Molecular Devices, Sunnyvale, CA). Since APMA was not added to the tested samples, the assay determined solely the enzyme activity of endogenous active MMP13 in these samples. In this way, the sum of MMP13 activity measured reflected the balance of active MMP13 and its inhibitors. Enzyme activity was expressed as nanogram/milliliter (ng/mL).
Statistical Analysis
All statistical analyses in this study were performed by a statistician (L.A.M.) who was blind to the study aims. Analysis of variance was used to evaluate the associations among MMP13 subtype expression, activity, and type and dose of hormonal treatments as well as menopausal status. Post hoc pair-wise comparisons were made using the Dunnett multiple comparison procedure. Analysis of variance using the Sidak procedure to adjust for post hoc pair-wise comparisons was used to evaluate differences between the ovx, ovx + E2P4, and sham-operated rats. All statistical tests were evaluated at the 0.05 significance level.
RESULTS
Biopsies of ATFP obtained from three pre- and three postmenopausal women were used to establish six corresponding primary cultures. As shown in Table 1, women in the two groups had a similar gravidity, parity, BMI, and stage of prolapse. All women in the premenopausal group were in the luteal phase of the menstrual cycle at the time of biopsy. Menopausal women were significantly older.
TABLE 1.
Patients' basic clinical demographics.
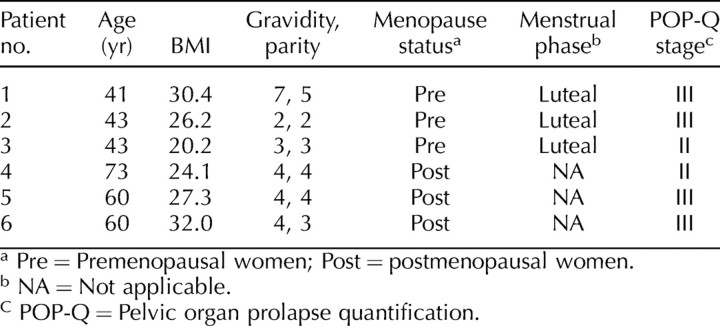
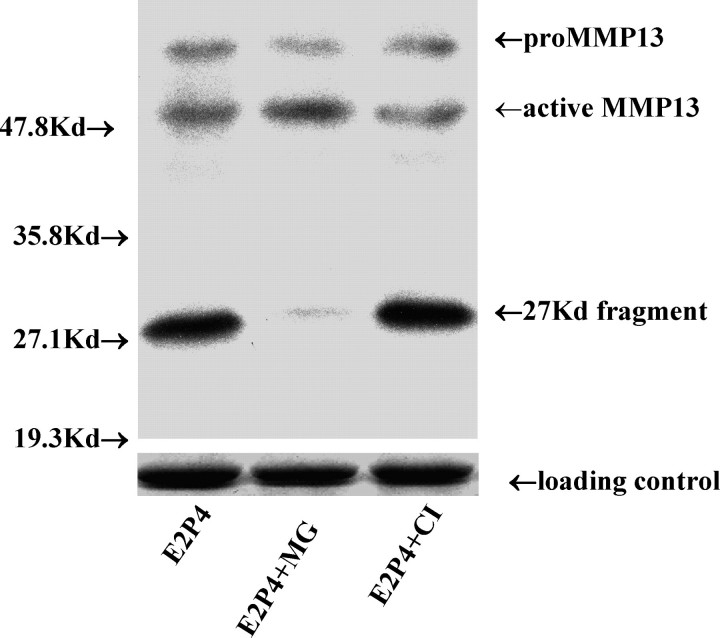
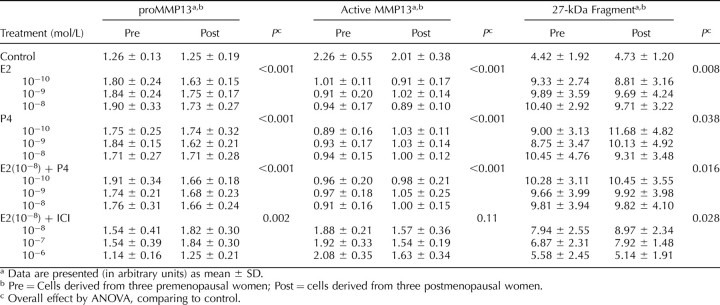
With an antibody recognizing both the proeznyme and active forms of MMP13, we were able to visualize three major MMP13 products in the cell lysates on Western blots (Figs. 1 and 2). In addition to the bands of proenzyme form (60 kDa) and active form (48 kDa), a band corresponding to the fragment form with an estimated size of 27 kDa was identified. As mentioned previously, MMP13 in an acellular environment autocatalyzes into two fragments of 27 and 29 kDa [16]. To confirm that our antibodies recognized the 29-kDa fragment form and that it was truly absent from the cell lysates, we treated recombinant MMP13 in the absence of cell lysate with the same antibodies and demonstrated two fragmentation products of 27 and 29 kDa (Fig. 3). Thus, the 29-kDa fragment was not present in our cells. It is not clear whether the 27-kDa band from cell lysates corresponds to two fragments of a similar size or whether the 29-kDa fragment undergoes further degradation in these cells.
FIG. 1.
A representative blot of proeznyme (proMMP13), active, and fragment forms of MMP13 demonstrating impact of hormone treatment (E2 + P4). The expression of active MMP13 and 27-kDa fragment was significantly higher than that of proMMP13. Therefore, active MMP13 and 27-kDa fragment are shown from the same blot at a lower intensity within the linear range. The proMMP13 is represented at a higher intensity. Control, hormone-free incubation; E2+P4, 17-β-estradiol (10−8 mol/L) plus progesterone (10−10 mol/L); kilodalton (kDa), labeled as Kd.
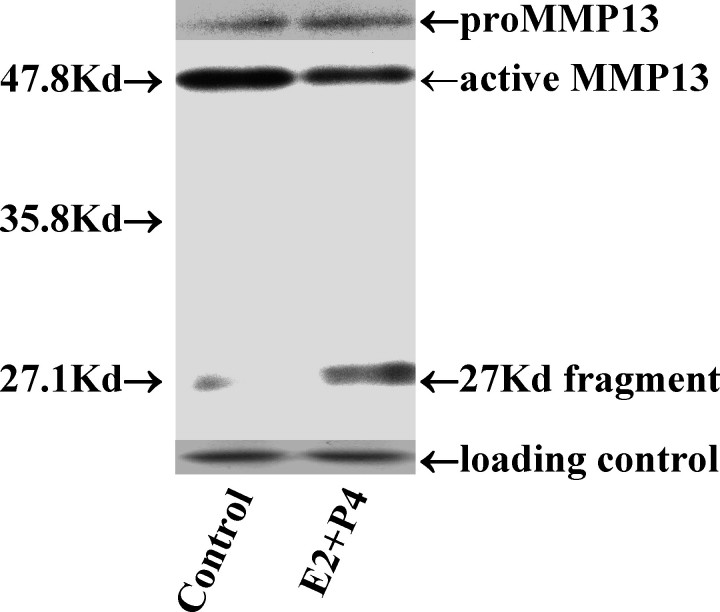
FIG. 2.
A representative blot of the various forms of MMP13 following the treatment of estradiol plus and progesterone in the absence and presence of either proteasome inhibitor (MG-132, 5 μmol/L) or collagenase inhibitor I (CI, 5 μmol/L), demonstrating inhibition of fragmentation by MG-132 but not CI. E2P4, 17-β-estradiol (10−8 mol/L) plus progesterone (10−10 mol/L); kilodalton (kDa), labeled as Kd.
FIG. 3.
The fragmentation of purified rhMMP13 under acellular condition in the presence of proteasome inhibitor (MG-132, 5 μmol/L) or collagenase inhibitor I (CI, 5 μmol/L). In the absence of cell lysate and in the absence of an inhibitor, rhMMP13 spontaneously fragmented into the 29- and 27-kDa peptides and smaller fragments (referred to as autocatalysis [16]). MG-132 did not block the progression while CI inhibited the process, resulting in the accumulation of every form of rhMMP13in the noncellular environment. rhMMP13, recombinant human MMP13 (5 ng; predicted molecular weight, 55 kDa); kilodalton (kDa), labeled as Kd.
Active MMP13 and Its Proteolytic Activity Are Suppressed by Hormones
As shown in Table 2, relative to control (no hormones), the cellular expression of proMMP13 was increased by E2 (up to 51%), P4 (up to 46%), and 10−8 mol/L E2 + P4 (up to 52%) above the control. Further analysis revealed that the increase in proMMP13 was not related to the dose of hormone, hormone type, or the menopausal status of the subject from whom the cells were derived. In the presence of an estrogen receptor antagonist (ICI 182, 780) at a 100-fold excess relative to E2 (10−6 mol/L), the increase in proMMP13 by E2 (10−8 mol/L) was blocked, indicating the involvement of an estrogen receptor-mediated event.
TABLE 2.
Cellular expression of different forms of MMP13 following exposure to hormonal treatments.
Concomitant with the increase in proMMP13, the amount of active MMP13 was decreased in the presence of E2 (up to 60%), P4 (up to 61%), and 10−8 mol/L E2 + P4 (up to 60%) below the control. The decrease in the active form was not affected by hormone dose, hormone type, or the subject's menopausal status. Similar to what was observed with the proenzyme form, the response to E2 (10−8 mol/L) was abrogated by ICI 182, 780 when added at doses of 1–100 fold excess (10−8 to 10−6 mol/L) over E2.
In agreement with the above-mentioned changes in proenzyme and active forms, the expression of the 27-kDa fragment was significantly increased, to a maximum of 147%, by the individual hormones and hormone combinations independent of concentration. ICI 182, 780 completely blocked the increase in the amount of 27-kDa fragment by E2 (10−8 mol/L) at concentrations ranging from 10−6 to 10−7 mol/L, again indicating an estrogen receptor-dependent process.
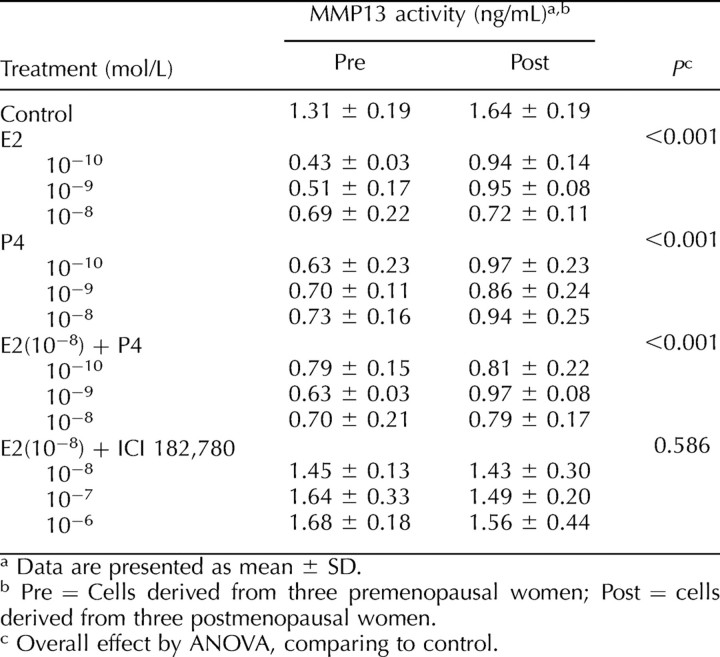
To determine whether the quantitative changes in the expression of active MMP13 on Western blot correlate with the functional endpoint of altered enzyme activity, we measured the endogenous amount of MMP13 proteolytic activity using a collagen degradation assay. In contrast to the Western blot, this assay does not measure endogenous inhibitor bound active MMP13 that lacks proteolytic activity. As shown in Table 3, relative to the control, the enzyme activity of MMP13 was inhibited by E2 (up to 67%), P4 (up to 52%), and 10−8 mol/L E2 + P4 (up to 52%). The inhibition by hormones occurred independent of hormone type, dose, and menopausal status. Similar to what was seen on the Western blots, the inhibition by E2 was blocked by the estrogen receptor antagonist, ICI 182, 780.
TABLE 3.
Cellular enzymatic activity of MMP13 following various treatments.
MMP13 Fragmentation in ATFP Cells Occurs via the Ubiquitin-Proteasome Pathway and Not Autocatalysis
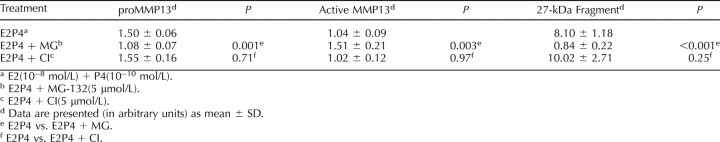
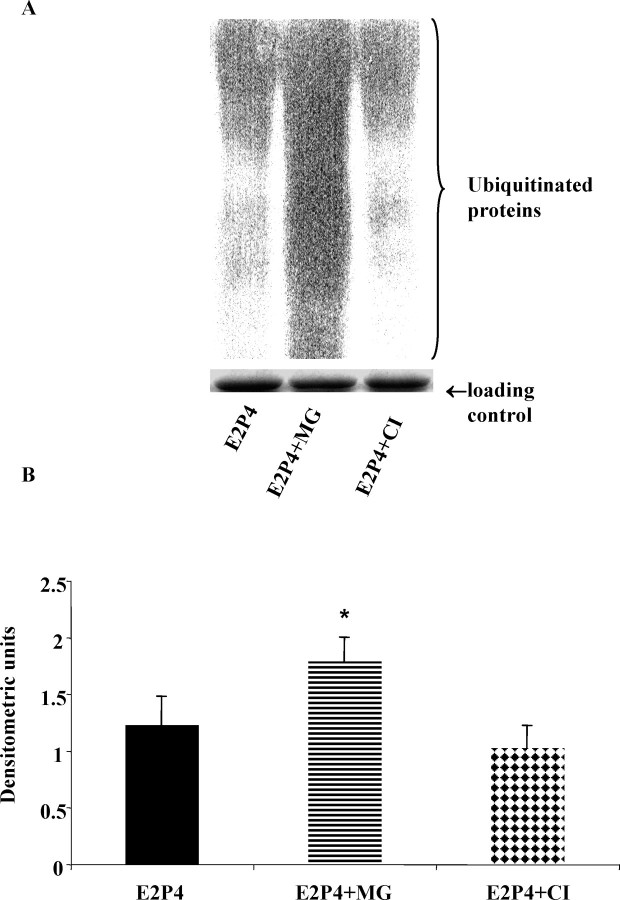
To investigate the mechanism of cellular fragmentation of MMP13 by hormones, cells were preincubated with an inhibitor of UPP—MG-132—versus an inhibitor of MMP autocatalysis—CI—prior to the addition of hormones. As shown in the representative Western blot in Figure 2 and the quantitative data in Table 4, MG-132 inhibited the MMP13 fragmentation induced by E2 + P4, resulting in an increase in active MMP13 and decrease in proMMP13. In contrast, the amount of proenzyme and active and fragment forms was unchanged in the presence of CI, demonstrating an absence of an inhibitory effect. The Western blots were then reprobed with an anti-ubiquitin antibody. By doing this, we were able to demonstrate an increase or accumulation of ubiquitinated proteins (increased density of the smeared lane) following treatment with MG-132 but not with CI (Fig. 4), confirming that the hormone-induced MMP13 fragmentation was UPP-mediated and not autocatalytic.
TABLE 4.
Expression of MMP13 following treatment of E2 + P4 in the presence of MG-132 or CI.
FIG. 4.
A) A representative blot of the expression of ubiquitinated proteins following a treatment with E2 + P4 in the presence of proteasome inhibitor (MG-132, 5 μmol/L) or collagenase inhibitor I (CI, 5 μmol/L). B) Densitometric analysis demonstrated an accumulation of ubiquitinated proteins (higher density of the whole lane) in the presence of MG-132, but not CI, indicating a blockage of the ubiquitin-proteasome pathway. Error bars indicate ± SD. *P < 0.05; E2P4, 17-β-estradiol (10−8 μmol/L) plus progesterone (10−10 μmol/L).
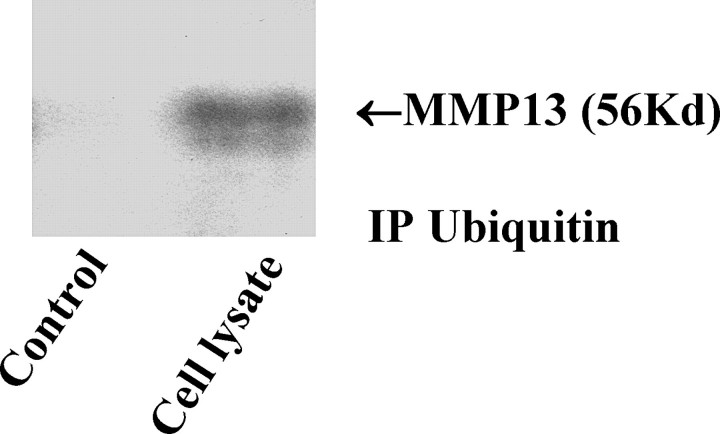
To determine whether MMP13 protein was truly a target of the UPP pathway, we next sought to show that MMP13 binding to ubiquitin occurred prior to fragmentation. To do this, cellular-ubiquitinated proteins were immunoprecipitated out of cell lysates using an anti-ubiquitin antibody. These ubiquitinated proteins were then separated on gels, and the presence of MMP13 protein in these ubiquitinated proteins was identified by Western immunoblotting. As shown in Figure 5, the pooled ubiquitinated proteins contained active MMP13 protein (of 56 kDa), indicating the latter had been previously conjugated with ubiquitin, targeting it for proteasomal proteolysis.
FIG. 5.
A Western blot of MMP13 protein identified in ubiquitin-immunoprecipitated proteins. An anti-ubiquitin antibody was used to isolate the ubiquitinated proteins in cell lysates. MMP13 was then identified in the precipitates on Western blot. Rabbit IgG was used as the negative control. IP Ubiquitin, ubiquitin-immunoprecipitated proteins; kilodalton (kDa), labeled as Kd.
We then sought to exclude the possibility that MG-132 may directly inhibit fragmentation independent of the presence of UPP. As alluded to previously, in the absence of a cell lysate under highly controlled conditions, isolated MMP13 protein spontaneously degrades into the 27- and 29-kDa peptides and smaller fragments (via autocatalysis) [16]. We, therefore, argued that if the MG-132 suppressed fragmentation occurred via inhibition of the UPP pathway, then it should be ineffective in the absence of a cell lysate. To this end, purified recombinant human MMP13 protein was directly incubated with MG-132 in the absence of a cell lysate. As a positive control, rhMMP13 was also incubated with CI, which should inhibit autocatalysis of rhMMP13 directly. As demonstrated in Figure 3, in the absence of a cellular environment, MG-132 failed to block the progression of rhMMP13 autocatalytic fragmentation. Thus, MG-132 indirectly inhibited cellular MMP13 fragmentation via the ubiquitin-proteasomal pathway. On the other hand, CI inhibited the rhMMP13 fragmentation, resulting in accumulation of the various forms of rhMMP13 in the noncellular environment, thereby confirming its role as an inhibitor of MMP autocatalysis.
Active MMP13 Is Increased in Ovariectomized Rats
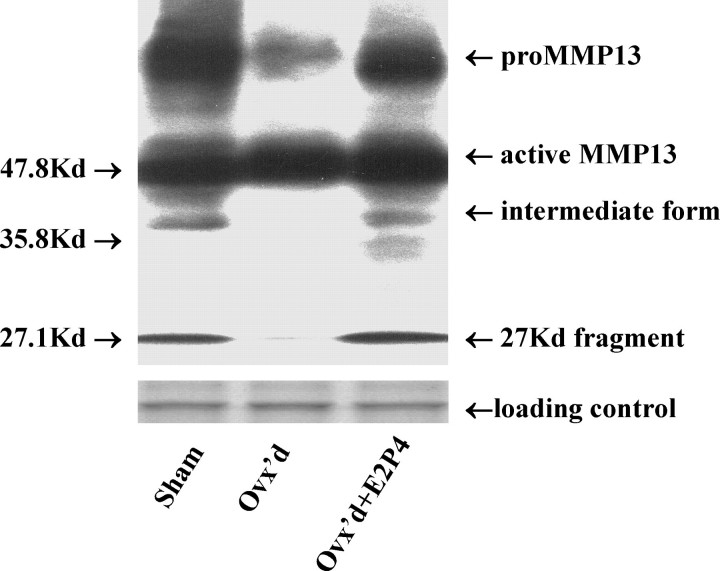
To this point, our data had been obtained from highly controlled in vitro conditions. We, therefore, were interested in determining whether the amount of active MMP13 actually increased in vivo with a surgically induced menopause and decreased with hormone supplementation. To this end, we ovariectomized (ovx) 15 rats and treated 6 with estrogen and progesterone (ovx + E2P4) for 8 wk. We then compared the overall expression profile of MMP13 in the ovx and ovx + E2P4 rats relative to sham-operated controls (N = 8). As shown in Figure 6, on Western immunoblots of the tissue extracts of rat vaginas, in addition to the bands of proenzyme form and active form, a band of an intermediate form with estimated size of 37 kDa and a 27-kDa fragment were detected. Because the 37-kDa form was not observed on the blots of cell lysates, we did not include this form in the subsequent analysis and focused on the 27-kDa fragment.
FIG. 6.
A representative blot of the various forms of MMP13 in the tissue extracts of rat vagina. The intermediate form of 37 kDa was not included in the data analysis because it was not observed on the blots of cell lysates. Sham, sham ovariectomized; Ovx'd, ovariectomized; Ovx'd+E2P4, ovariectomized with subcutaneous implantation of estrogen (0.1 mg) plus progesterone (10 mg); kilodalton (kDa), labeled as Kd.
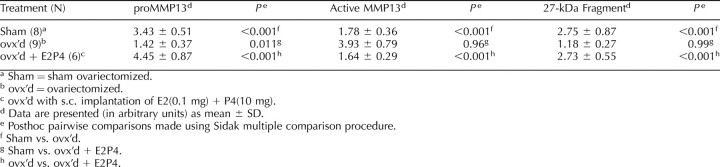
Ovariectomy induced vaginal atrophy as displayed by decreases in midvaginal width compared to sham surgery or ovx supplemented with hormones [19], demonstrating the typical vaginal response to an absence of hormones. As shown in Table 5, ovx induced an increase in the amount of active MMP13 in parallel with a decrease in the amount of proMMP13 and the 27-kDa fragment relative to sham-operated controls while supplementation with hormones inhibited these changes. In this way, the vivo data confirmed an up-regulation of active MMP13 in the absence of hormones and suppression in their presence consistent with the in vitro data (Table 2).
TABLE 5.
Comparison of expression of MMP13 in vaginal tissue of rats following various treatments.
DISCUSSION
Although vaginal childbirth is likely the initial event that predisposes a woman to pelvic organ prolapse, the number of women seeking medical care for prolapse increases substantially after the age of 50, indicating that menopause may play a role [1, 2]. We hypothesize that ovarian hormones of premenopausal vaginally parous women delay the onset and/or progression of pelvic organ prolapse by providing structural support to the vagina and supportive tissues. On the contrary, with the onset of menopause, tissue integrity deteriorates in the absence of hormonal support. Previously, we have shown that menopausal women have decreased collagen ratios due to a decrease in collagen I relative to collagen III [5]. Here we propose that one mechanism for this decline is due to increased collagenase activity.
To this end, we defined the mechanism of regulation of a key collagenase, MMP13, by estradiol and progesterone in cells derived from the arcus tendenious fasciae pelvis. We assessed the expression of MMP13 along with its enzyme activity in the presence of increasing concentrations of E2, P4, and E2 + P4. In addition, we correlated our in vitro findings with an in vivo assay in rats. The most important findings of this paper were that the amount of active MMP13 is decreased in the presence of estradiol and progesterone. The decrease was associated with an increase in the amount of the proenzyme and fragment forms with a net effect of decreased MMP13 proteolytic activity. Another relevant finding was that a key mechanism by which this decrease occurs is via an increase in MMP13 fragmentation, mediated through the cell-associated ubiquitin-proteasome pathway.
The decrease in active MMP13 protein and its activity in the presence of hormones supports our hypothesis that estrogen and progesterone are protective against tissue deterioration in the vaginal supportive tissues. A similar inhibition of active MMP1 has been identified [14]. To date, most studies on the vaginal supportive tissues have focused on mRNA synthesis; however, MMP mRNA is not necessarily correlated with protein expression level [11] and the discrepancy between mRNA level and protein level is more likely a rule rather than an exception [20]. In our current study and previous study [14], we demonstrated that even at the protein level, different MMP forms (proenzyme and active and fragment forms) were not necessarily correlated with each other either. It is thus very important to measure the amount of active MMP and/or endogenous MMP enzyme activity because the latter takes into account the effect of the inhibitors of MMP.
Similar to what we demonstrated for MMP1, here we found that in the presence of estradiol and progesterone, the fragmentation of MMP13 in ATFP cells was accelerated. In addition, the inhibition of MMP13 fragmentation by MG-132 was paralleled by an increase in the expression of active MMP13. The data suggest that the MMP13 fragmentation is a key site of regulation of the amount of active MMP13, which is in agreement with our previous finding that cellular MMP1 fragmentation is a key site regulated by estradiol and progesterone [14].
The increase in proMMP13 with estrogen and progesterone may reflect either an increase in synthesis, decrease in activation, or both. In RUCA-I endometrial tumor cells [21], rabbit synoviocyte cells HIG-82 [22], rat uterus [21], and skin [23], estrogen has been shown to up-regulate MMP13 mRNA by enhancing the MMP13 promoter activity by interacting with the activator protein-1 site [22]. However, our finding that the increase in proMMP13 was paralleled by a decrease in active MMP13 is more likely suggestive of an accumulation of the proenzyme form due to decreased activation. One of the mechanisms by which hormones inhibit proMMp13 activation is via the suppression of its activators such as MMP2, 3, and 14 [24, 25]. Further studies are needed to determine whether an inhibition of activation of proMMP13 is playing a key role in decreasing the amount of active MMP13.
In this study, we determined that the process of induction of proMMP13, suppression of active MMP13, and acceleration in MMP13 fragmentation by estradiol could be mitigated by a pure estrogen receptor antagonist: ICI 182, 780. These cells have been previously shown to express both estrogen and progesterone receptors [26]. Therefore, it is reasonable to speculate that the regulation of MMP13 by estradiol occurs in part via an estrogen receptor-dependent pathway.
We performed an animal experiment as a correlate to the in vitro cell study and found that active MMP13 increased in the absence of hormones (ovariectomized rats). Previously, we performed a biomechanical study of the vagina and supportive tissues and demonstrated inferior biomechanical properties of this tissue complex in ovx animals relative to sham-operated controls. We also found that supplementation with hormones restored the biomechanical properties of this tissue complex in ovx rats [19]. Our data, here, support a biochemical basis for the altered biomechanical properties [19]. Thus, the in vivo data not only substantiates the in vitro hormonal regulation of MMP13, but also further confirms that hormones are important in maintaining the biomechanical integrity of the pelvic supportive tissues.
Ubiquitin-proteasomal proteolysis is a principle mechanism for cytosolic protein degradation in eukaryotic cells. Accordingly, the targeted proteins are first covalently attached by chains of ubiquitin molecules and then catalyzed by the 26S proteasome complex that digests the proteins to small fragments [27]. Recently, it has been shown that UPP is likely involved in tissue remodeling through the regulation of collagen, MMP, and tissue inhibitors of metalloproteinases [17, 28, 29]. In this study, we demonstrated that the proteasome inhibitor, MG-132, blocked the cell-associated MMP13 fragmentation, resulting in increased active MMP13 along with an accumulation of ubiquitinated proteins. The fact that MG-132 could not directly inhibit the fragmentation of purified rhMMP13 in an absence of a cellular environment is further indicative of the involvement of UPP in this process. Moreover, we identified MMP13 protein in the cellular extract ubiquitinated precipitate, thereby demonstrating that MMP13 protein is indeed conjugated with ubiquitin for proteasome-mediated degradation. However, for the analysis of MMP13, we used whole cell extract. Therefore, it is not clear as to whether the MMP13 observed in this study is intracellular, cell-associated, or both. A more detailed investigation into the site and mechanism of association of UPP with MMP is warranted in future studies.
There are limitations to this study. The major shortcoming is that our results are based on fibroblasts originated from vaginal supportive connective tissue of patients with prolapse. Therefore, it may not be appropriate to generalize the conclusions from this paper to all women, especially women without prolapse. Due to ethical issues (i.e., disruption of a key vaginal support structure), ATFP biopsies were not procured from a control group of women without prolapse. It is noteworthy, however, that all ATFP biopsies from women with prolapse were obtained from a site where the ATFP was intact. This was possible because in most women, the ATFP fails at the ischial spine first before progressing toward the pubic symphysis. In this way, there is almost always a portion toward the pubic symphysis that is intact. It is that intact portion of the ATFP that we chose to biopsy in this study. A second limitation of the study is that although we have verified the in vitro pattern of hormonal regulation on cellular MMP13 with an in vivo rat model, further studies on human tissues are currently in progress. Finally, in the present study, we did not investigate the pathway via which progesterone exerts its effect on MMP13 by the use of a progesterone receptor inhibitor. We were surprised by the impact of progesterone on MMP expression and have studies currently in progress investigating the role of progesterone and its interaction with the estrogen receptor. In spite of the limitations, mechanistic studies such as this one are imperative for improving our currently limited understanding of connective tissue remodeling in the female vaginal supportive connective tissues. Indeed, such information is crucial if we are to develop targeted therapies aimed at mitigating the deterioration in pelvic organ support following menopause. Clearly, understanding the mechanisms of regulation of the enzymes that degrade the structural proteins in the pelvic floor is an important first step in this process.
In summary, estradiol and progesterone decrease the amount of active MMP13 by inhibiting the conversion of proMMP13 into active form and accelerating the active MMP13 fragmentation. In addition, ubiquitin-proteasomal proteolysis is critically involved in the fragmentation of MMP13.
Acknowledgments
We would like to thank Augustine Rajakumar, Ph.D., for valuable suggestions with the experiments and kindly providing MG-132 compound and anti-ubiquitin antibody. We also thank Susan C. Street, B.S., for assistance in densitometry, Kristen M Debes, B.S., and Suzan E Stein, B.S., for assistance in the animal experiments, Marcia J. Gallaher, B.S., for technical assistance in the enzyme activity measurement.
Footnotes
1Supported by a grant from the National Institute of Health ( HD 045590) to P.A.M. Part of the cell data was presented at the 2006 SGI Annual Meeting & Scientific Forum, Toronto, Canada, March 22–25, 2006; data on proteasomal inhibition was presented at the 2006 ASMB Biennial National Meeting, Nashville, TN, November 1–4, 2006; rat data was orally presented at the ASRM Annual Meeting, Washington, DC, October 13–17, 2007.
REFERENCES
- Carley ME, Turner RJ, Scott DE, Alexander JM. Obstetric history in women with surgically corrected adult urinary incontinence or pelvic organ prolapse. J Am Assoc Gynecol Laparosc 1999; 6: 85 89 [DOI] [PubMed] [Google Scholar]
- Luber KM, Boero S, Choe JY. The demographics of pelvic floor disorders: current observation and future projections. Am J Obstet Gynecol 2001; 184: 1496 1503 [DOI] [PubMed] [Google Scholar]
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol 1992; 166: 1717 1724 [DOI] [PubMed] [Google Scholar]
- Goh JTW. Biomechanical and biochemical assessment for pelvic organ prolapse. Curr Opin Obstet Gynecol 2003; 15: 391 394 [DOI] [PubMed] [Google Scholar]
- Moalli PA, Talarico LC, Sung VW, Klingensmith WL, Shand SH, Meyn LA, Watkins SC. Impact of menopause on collagen subtypes in the arcus tendineous fasciae pelvis. Am J Obstet Gynecol 2004; 190: 620 627 [DOI] [PubMed] [Google Scholar]
- Jackson SR, Avery NC, Tarlton JF, Eckford SD, Abrams P, Bailey AJ. Changes in metabolism of collagen in genitourinary prolapse. Lancet 1996; 347: 1658 1661 [DOI] [PubMed] [Google Scholar]
- Curran S, Murray GI. Matrix metalloproteinases in tumour invasion and metastasis. J Pathol 1999; 189: 300 308 [DOI] [PubMed] [Google Scholar]
- Curran S, Murray GI. Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer 2000; 36: 1621 1630 [DOI] [PubMed] [Google Scholar]
- Murray GI. Matrix metalloproteinases: a multifunctional group of molecules. J Pathol 2001; 195: 135 137 [DOI] [PubMed] [Google Scholar]
- Nagase H, Visse R. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 2003; 92: 827 839 [DOI] [PubMed] [Google Scholar]
- Kasper G, Glaeser JD, Geissler S, Ode A, Tuischer J, Matziolis G, Perka C, Duda GN. MMP activity is an essential link between mechanical stimulus and mesenchymal stem cell behaviour. Stem Cells 2007; 25: 1985 1994 [PubMed] [Google Scholar]
- Curry TE, Osteen KG. The matrix metalloproteinases system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endoc Rev 2003; 24: 428 465 [DOI] [PubMed] [Google Scholar]
- Kremer EA, Chen Y, Suzuki K, Nagase H, Gorski JP. Hydroxyapatite induces autolytic degradation and inactivation of matrix metalloproteinase-1 and -3. J Bone Miner Res 1998; 13: 1890 1902 [DOI] [PubMed] [Google Scholar]
- Zong W, Zyczynski HM, Meyn LA, Gordy SC, Moalli PA. Regulation of MMP-1 by sex steroid hormones in fibroblasts derived from the female pelvic floor. Am J Obstet Gynecol 2007; 196: 349.e1 349.e11 [DOI] [PubMed] [Google Scholar]
- Leeman MF, Curran S, Murray GI. The structure, regulation, and function of human matrix metalloproteinase-13. Crit Rev Biochem Mol Biol 2002; 37: 149 166 [DOI] [PubMed] [Google Scholar]
- Knauper V, Cowell S, Smith B, Lopez-Otin C, O'Shea M, Morris H, Zardi L, Murphy G. The role of the C-terminal domain of human collagenase-3 (MMP-13) in the activation of procollagenase-3, substrate specificity, and tissue inhibitor of metalloproteinase interaction. J Biol Chem 1997; 272: 7608 7616 [DOI] [PubMed] [Google Scholar]
- Wang HX, Wang HM, Lin HY, Yang Q, Zhang H, Tsang BK, Zhu C. Proteasome subunit lmp2 is required for matrix metalloproteinase-2 and -9 expression and activity in human invasive extravillous trophoblast cell line. J Cell Physiol 2006; 206: 616 623 [DOI] [PubMed] [Google Scholar]
- Bump RC, Mattiasson A, Bø K, Brubaker LP, DeLancey JL, Klarskov P, Shull BL, Smith AB. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 1996; 175: 10 17 [DOI] [PubMed] [Google Scholar]
- Moalli PA, Debes KM, Meyn LA, Howden NS, Abramowitch SD. Hormones restore biomechanical properties of the vagina and supportive tissues after surgical menopause in young rats. Am J Obstet Gynecol 2008; 199: 161.e1 161.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol 1999; 19: 1720 1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tushaus L, Hopert AC, Strunck E, Schubert C, Wunsche W, Vollmer G. Estrogenic and antiestrogenic regulation of MMP-1 and MMP-13 mRNA in RUCA-I endometrial tumor cells in vitro and in vivo. Cancer Lett 2003; 198: 99 106 [DOI] [PubMed] [Google Scholar]
- Lu T, Achari Y, Sciore P, Hart DA. Estrogen receptor alpha regulates matrix metalloproteinase-13 promoter activity primarily through the AP-1 transcriptional regulatory site. Biochim Biophys Acta 2006; 1762: 719 731 [DOI] [PubMed] [Google Scholar]
- Pirila E, Ramamurthy N, Maisi P, McClain S, Kucine A, Wahlgren J, Golub LM, Salo T, Sorsa T. Wound healing in ovariectomized rats: effects of chemically modified tetracycline (CMT-8) and estrogen on matrix metalloproteinases -8, -13 and type I collagen expression. Curr Med Chem 2001; 8: 281 294 [DOI] [PubMed] [Google Scholar]
- Schatz F, Kuczynski E, Kloosterbooer L, Krikun G, Buchwalder LF, Rahman M, Lockwood CJ. Tibolone exerts progestational inhibition of matrix metalloproteinase expression in human endometrial stromal cells. Steroids 2006; 71: 768 775 [DOI] [PubMed] [Google Scholar]
- Zhang J, Hampton AL, Nie G, Salamonsen LA. Progesterone inhibits activation of latent matrix metalloproteinase (MMP)-2 by membrane-type 1 MMP: enzymes coordinately expressed in human endometrium. Biol Reprod 2000; 62: 85 94 [DOI] [PubMed] [Google Scholar]
- Moalli PA, Klingensmith WL, Meyn LA, Zycznski HM. Regulation of matrix metalloproteinase expression by estrogen in fibroblasts that are derived from the pelvic floor. Am J Obstet Gynecol 2002; 187: 72 79 [DOI] [PubMed] [Google Scholar]
- Lecker SH, Solomon V, Mitch WE, Golderg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr 1999; 129: 227S 237S [DOI] [PubMed] [Google Scholar]
- Fineschi S, Reith W, Guerne PA, Dayer JM, Chizzolini C. Proteasome blockade exerts an antifibrotic activity by coordinately down-regulating type I collagen and tissue inhibitor of metalloproteinase-1 and up-regulating metalloproteinase-1 production in human dermal fibroblasts. FASEB J 2006; 20: 562 564 [DOI] [PubMed] [Google Scholar]
- Wang HM, Zhang X, Qian D, Lin HY, Li QL, Liu DL, Liu GY, Zhu C. Effect of ubiquitin-proteasome pathway on mouse blastocyst implantation and expression of matrix metalloproyeinases-2 and -9. Biol Reprod 2004; 70: 481 487 [DOI] [PubMed] [Google Scholar]