Abstract
Prenatal testosterone excess leads to neuroendocrine and periovulatory disruptions in the offspring culminating in progressive loss of cyclicity. It is unknown whether the mediary of these disruptions is androgen or estrogen, because testosterone can be aromatized to estrogen. Taking a reproductive life span approach of studying control, prenatal testosterone, and dihydrotestosterone-treated offspring, this study tested the hypothesis that disruptions in estradiol-negative but not -positive feedback effects are programmed by androgenic actions of testosterone and that these disruptions in turn will have an impact on the periovulatory hormonal dynamics. The approach was to test estradiol-negative and -positive feedback responses of all three groups of ovary-intact females during prepubertal age and then compare the periovulatory dynamics of luteinizing hormone, follicle-stimulating hormone, estradiol, and progesterone during the first breeding season. The findings show that estradiol-negative but not estradiol-positive feedback disruptions in prenatal testosterone-treated females are programmed by androgenic actions of prenatal testosterone excess and that follicular phase estradiol and gonadotropins surge disruptions during reproductive life are consistent with estrogenic programming. Additional studies carried out testing estradiol-positive feedback response over time found progressive deterioration of estradiol-positive feedback in prenatal testosterone-treated sheep until the time of puberty. Together, these findings provide insight into the mechanisms by which prenatal testosterone disrupts the reproductive axis. The findings may be of translational relevance since daughters of mothers with hyperandrogenism are at risk of increased exposure to androgens.
Keywords: androgens, infertility, neuroendocrinology, ovulation, reproduction
Prenatal testosterone excess reduces responsiveness to estradiol-negative feedback by androgenic actions, while estradiol-positive feedback, follicular phase estradiol, and gonadotropin surge disruptions appear to be programmed by estrogenic actions.
INTRODUCTION
During early development, the hypothalamus is extremely sensitive to negative feedback effects of ovarian estradiol. Puberty in sheep, as in other mammals, is triggered by a reduction in hypothalamic sensitivity to the estradiol (E2)-negative feedback [1, 2]. On achievement of reproductive maturity, reproductive life in female sheep is characterized by recurrent cycles comprised of follicular and luteal phases only to be interrupted by breed- and latitude-specific anestrous periods [3]. The predominant neuroendocrine feedback systems operating during reproductive cyclicity are the E2-negative, E2-positive and progesterone (P4)-negative feedbacks, the first two playing a role during the follicular phase and the third during the luteal phase [4].
Extensive studies carried out using sheep as an animal model have found that prenatal testosterone treatment disrupts all three steroid feedback mechanisms [1, 5–8], the periovulatory sequence of hormonal [9, 10], and follicular dynamics [11–13] and causes progressive loss of cycles [12–15] culminating in anovulatory infertility [10, 12, 16]. Consistent with the progressive loss of cyclicity, our recent studies found that the ovary-intact prenatal testosterone-treated females manifest luteinizing hormone (LH) but not follicle-stimulating hormone (FSH) excess with the magnitude of LH excess increasing from prepubertal to adulthood [17]. These findings suggest that the E2-negative feedback system is also undergoing progressive deterioration. Whether progressive deterioration occurs also at the level of E2-positive feedback is unknown. Furthermore, while considerable information has been gathered relative to programming of neuroendocrine and ovarian disruptions from prenatal testosterone excess, because testosterone can be aromatized to estrogen or reduced to dihydrotestosterone (DHT), a more potent androgen, the relative role of estrogen and androgen in programming the various feedback defects in ovary-intact animals and cyclic hormonal disruptions remains to be determined.
Earlier studies [1, 5] using the ovariectomized and E2-replaced (OVX+E2) sheep model allow some predictions in this regard. The finding that neuroendocrine puberty (timing of increase in tonic LH secretion indicative of escape from E2-negative feedback) is advanced in both prenatal testosterone- and DHT-treated females [1, 18] suggests androgen as the programmer of E2-negative feedback changes. On the contrary, normal E2-positive feedback response in prenatal DHT-treated but its absence in the prenatal testosterone-treated OVX+E2 model [1, 18] suggests that the disruptive effects of prenatal testosterone on E2-positive feedback are not programmed by androgenic actions of testosterone. It is important that predictions from the OVX+E2 model are tested in ovary-intact models, as other ovarian factors may contribute to the maturation of hypothalamic-pituitary-ovarian axis. For instance, E2-positive feedback response is completely ablated in the OVX+E2 model [1, 8], although an LH surge, albeit delayed and severely dampened, is evident in the ovary-intact prenatal testosterone-treated sheep [6, 10].
The present study, taking on a reproductive life span approach, determined which of the neuroendocrine feedbacks and periovulatory hormonal disruptions in the prenatal testosterone-treated sheep are programmed by androgenic actions of testosterone as opposed to its estrogenic actions. As a secondary objective, this study also determined the time course of deterioration of the E2-positive feedback system in prenatal testosterone-treated sheep.
MATERIALS AND METHODS
Prenatal Treatments
All procedures used were approved by the Institutional Animal Care and Use Committee of the University of Michigan and are consistent with National Research Council's Guide for the Care and Use of Laboratory Animals. The study was conducted at the University of Michigan Research Facility (Ann Arbor, MI; 42°18′N). Details of prenatal testosterone and DHT treatments as well as husbandry and nutrition of maternal sheep and newborn lambs have been published [13, 18, 19]. For generating prenatal testosterone- and DHT-treated females, pregnant Suffolk sheep were injected (i.m.) twice weekly from 30 to 90 d of gestation with either 100 mg testosterone propionate (1.2 mg/kg; Sigma Chemical Co., St. Louis, MO) or 100 mg DHT propionate (Steraloids, Inc., Newport, RI) suspended in 2 ml cottonseed oil. Control animals did not receive vehicle since no differences in reproductive characteristics were found between vehicle-treated and nontreated controls in a previous study [10]. Ratio of anogenital to anonavel distance measured 2 days after birth was significantly (P < 0.001) greater in prenatal testosterone- and DHT-treated females (0.84 ± 0.01 and 0.74 ± 0.04, respectively) compared to controls (0.10 ± 0.01). Previous studies found that this mode of testosterone treatment results in adult male and fetal male levels of testosterone in maternal and fetal circulation, respectively [20].
Experimental Design: Objective 1
Androgenic programming of reproductive neuroendocrine and periovulatory dynamics.
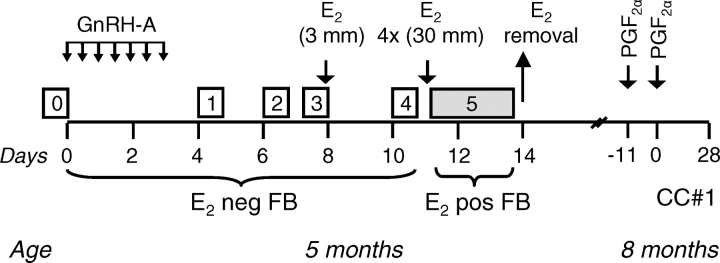
To delineate the role of androgen in programming the progressive deterioration of the reproductive axis, sequential studies across the reproductive life span were undertaken beginning during the prepubertal period and continuing through the first breeding season (Fig. 1). The studies were timed in relation to the natural progression of neuroendocrine events, namely, E2-negative feedback and E2-positive feedback, followed by determination of cyclic function during the first breeding season (Fig. 1).
FIG. 1.
Experimental design of E2-negative and -positive feedbacks at 5 mo of age and cycle characterization study during the first breeding season (8 mo). Boxes numbered 0–4 indicate various periods of frequent blood sampling (every 20 min for 6 h) during the E2-negative feedback test (E2 neg FB). Gray box (#5) indicates collection of blood samples during the E2-positive feedback test (every 2 h for 72 h; E2 pos FB). GnRH-A was administered during E2-negative feedback at 12-h intervals (small arrows, Days 1–3). For initiating cycle characterization study during the first breeding season (CC#1), estrus was synchronized by administering two injections of PGF2α 11 days apart. See Materials and Methods.
E2-negative feedback.
Testing was performed in July during the prepubertal period (∼20 wk of age), when controls are extremely sensitive to E2-negative feedback [21]. The study design (Fig. 1) consisted of a pretreatment phase (0) and four experimental phases [1–4]. During all phases, blood samples were collected for 6 h at 20-min intervals in all females (control: n = 7; prenatal testosterone-treated: n = 6; prenatal DHT-treated: n = 5; only five females were born out of 15 DHT-treated dams). In phase 1, to block GnRH action and reduce endogenous E2 levels in ovary-intact females prior to the E2-negative feedback testing, a GnRH antagonist (GnRH-A), acyline, was administered; 10 μg/kg body weight of acyline given at 12-h intervals for 72 h completely abolishes LH pulsatility [22]. Blood samples were collected for 6 h after the last GnRH-A injection to determine if GnRH-A treatment ablated pulsatile LH release. In phases 2–3, to establish the impact of reducing endogenous E2 with a GnRH-A treatment on subsequent LH frequency, blood samples were collected 48 (phase 2) and 72 (phase 3) h after the GnRH-A treatment. In phase 4, the response to the E2-negative feedback was evaluated by providing a single 3-mm s.c. E2 implant, expected to produce circulating concentrations of <1 pg/ml (luteal-phase levels) [23]. Blood samples were collected beginning 3 d after the E2 implant insertion.
E2-positive feedback.
E2-positive feedback test followed the E2-negative feedback testing (Fig. 1, phase 5) in prepubertal females in the absence of P4 priming. All three groups of females (control: n = 7; prenatal testosterone-treated: n = 6; prenatal DHT-treated: n = 5) were implanted s.c. with four 3-cm E2 implants made up of SILASTIC brand silicon tubing (Dow Corning Corp., Midland, MI) to provide follicular phase levels of E2 [21, 24]. We have used this approach previously [6]. Blood samples were obtained every 2 h for 72 h starting 1 h before placement of E2 implants.
Cycle characterization.
The study was conducted in November during the first breeding season (control: n = 7; prenatal testosterone-treated: n = 7; prenatal DHT-treated: n = 5). Timing of estrus was synchronized with two injections of prostaglandin F2α (PGF2α, 5mg/ml; Lutalyse, Pfizer Animal Health, MI) administered i.m., 11 days apart. Beginning with the second PGF2α injection, blood samples were obtained every 2 h for 120 h (5 days) for the assessment of the periovulatory hormonal changes. Daily blood samples were taken for an additional 23 days. LH and FSH were assayed every 2 h, E2 every 4 h, and P4 in daily samples.
Experimental Design: Objective 2
Developmental progression of the E2-positive feedback disruption.
To complement the reproductive life span study and establish the developmental trajectory of the E2-positive feedback disruptions in prenatal testosterone-treated females, competency of the E2-positive feedback system was tested at different ages using a different cohort of animals. Seven control and seven prenatal testosterone-treated females were inserted with four 3-cm Silastic E2 implants s.c. The testing was done at three different ages: prepubertal (12 wk), pubertal (23 wk), and during the first anestrus season that followed the first breeding season (54 wk). Previous studies have found that E2-positive feedback responses are similar between breeding and anestrous seasons [3].
Hormone Measures
Plasma LH and FSH concentrations were measured in duplicate in all samples using validated assays [25, 26] and expressed in terms of NIH-LH-S12 and NIDDK-ovine FSH-1. The sensitivity of the LH assay was 0.08 ± 0.01 ng/ml (n = 30 assays; mean ± SEM). Mean intra-assay coefficients of variation (CV) based on four quality control pools measuring 3.5 ± 0.1, 7.3 ± 0.1, 13.2 ± 0.3, and 24.1 ± 0.6 ng/ml were 5.9%, 3.8%, 5.4%, and 5.0%, respectively. The corresponding interassay CVs averaged 7.0%, 3.5%, 5.9%, and 6.2%. The sensitivity of the FSH assay was 0.06 ± 0.01 ng/ml (n = 29 assays; mean ± SEM). The intra-assay CVs based on two quality control pools measuring 11.6 ± 0.2 and 25.4 ± 0.7 ng/ml were 6.7% and 4.5%, respectively. The corresponding mean inter-assay CVs were 5.8 and 7.1%. Plasma E2 concentrations were measured in alternate samples for the cycle characterization study using a validated radioimmunoassay first developed by Butcher et al. [27] and modified by Tortonese et al. [28], following extraction of plasma samples with ether. The sensitivity of the E2 assay was 0.07 ± 0.01 pg/ml (n = 18 assays; mean ± SEM). Mean intra-assay coefficients of variation (CV) based on two quality control pools measuring 1.6 ± 0.1 and 4.5 ± 0.3 pg/ml were 12.0 and 10.2%, respectively. The corresponding interassay CVs averaged 22.4% and 14.7%. Plasma P4 concentrations were measured in daily samples in duplicate using a solid-phase radioimmunoassay kit (Coat-A-Count P, Siemens Medical Solutions Diagnostics, Los Angeles, CA) as previously described [29]. The assay sensitivity was 0.02 ± 0.01 ng/ml (n = 8 assays; mean ± SEM) and intra-assay CV, based on the two quality control pools measuring 1.7 ± 0.02 and 14.6 ± 0.4 ng/ml, were 2.9% and 3.3%, respectively. The corresponding interassay sensitivity CVs were 4.1% and 7.9%. For all hormonal assays, to avoid confound from interassay variability, samples were randomly distributed across assays such that each assay included sample sets from control and treated females.
Statistical Analysis
Values below assay sensitivity were assigned the detection limit of the assay. For the E2-negative feedback test, serial LH data from frequent samples were subjected to pulse analysis using the Cluster algorithm, which identifies pulses using criteria that define a pulse such that the peak of the pulse differs significantly from both the preceding and the following nadirs according to two-sample t-tests [30]. All Cluster-identified peaks with LH increase greater than two times assay sensitivity from the preceding nadir were considered as pulses. For the E2-positive feedback tests and cycle characterization studies, the LH surge was defined as circulating LH being elevated three times above baseline for at least 6 h. Onset and end of progestogenic cycles were defined as when daily concentrations of P4 reached above and below 0.5 ng/ml, respectively.
Differences in gonadotropin dynamics during E2-negative feedback (mean LH/FSH, frequency and amplitude of LH pulses) as well as E2-positive feedback (time of gonadotropin surge onset, peak, duration, amplitude, and total LH/FSH released during the gonadotropins surges), changes in E2 and gonadotropin dynamics and its relation to gonadotropin surges (time of E2 peak, E2 peak level, and interval between E2 peak to LH/FSH surge onset and peak), and changes in luteal P4 dynamics (maximal P4 and mean P4 levels during the luteal phase and luteal-phase duration) during cycle characterization were compared using ANOVA after appropriate transformations to account for heterogeneity of variances. For these analyses, only those animals that had LH surges were included. Differences in number of LH pulses, mean LH, and LH pulse amplitude between time points within groups (E2-negative feedback) were determined by repeated-measures ANOVA. Fisher's exact test was used for comparing percentages of females responding to the E2-negative and-positive feedbacks or manifesting gonadotropin surges (cycle characterization study). Developmental progression of the E2-positive feedback (LH surge peak time, amplitude, and duration) was analyzed using repeated-measures ANOVA. In a given developmental time point (prepubertal, pubertal, and anestrus) differences in amplitude, peak time, and surge duration were compared between groups using an independent-sample t-test accounting for equality of variances. All analyses were carried out using SPSS for Windows release 15.0.0. All results are presented as mean ± SEM. Significance was defined as P < 0.05.
RESULTS
Objective 1: Androgenic Programming of Reproductive Neuroendocrine System and Periovulatory Hormonal Dynamics
E2-negative feedback.
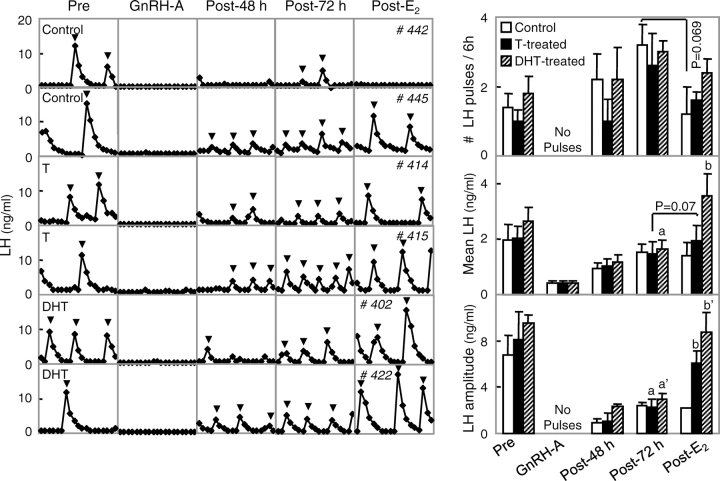
Circulating patterns of LH and summary changes in LH dynamics during the E2-negative feedback test are shown in Figure 2. GnRH-A administration completely ablated LH pulses in all three groups (noted as “no pulses” in Fig. 2). Cessation of GnRH-A treatment resulted in reinitiation of LH pulses. By 72 h post-GnRH-A treatment, all but one prenatal testosterone-treated female had LH pulses. Mean LH, LH pulse frequency, and amplitude increased from 48 to 72 h after cessation of GnRH-A treatment across groups. E2 treatment ablated LH pulses completely in three control females. Consistent with our previous findings [7], mean LH pulse frequency tended to be lower in control (P = 0.069) but not in prenatal testosterone- or DHT-treated females. When the LH pulse frequency data from control and prenatal testosterone-treated females from this study were combined with frequency data from our previous study [7], estradiol significantly suppressed mean LH pulse frequency in control females (P < 0.005) but not prenatal testosterone-treated females. Mean LH pulse amplitude increased after the E2 treatment in both groups of treated females compared to controls. Mean LH increased significantly in prenatal DHT-treated females but tended to do so only in prenatal testosterone-treated females.
FIG. 2.
Results of E2-negative feedback test conducted during the prepubertal period (∼20 wk of age). Left: circulating patterns of LH from two control (two top panels), two prenatal testosterone (T)-treated (two middle panels), and two prenatal DHT-treated (two bottom panels) females during the pretreatment (Pre, phase 0), end of GnRH-A treatment (phase 1), 48 and 72 h after cessation of the GnRH-A treatment (Post-48 h: phase 2; Post-72 h: phase 3), and 3 days postinsertion of E2 implant (Post-E2: phase 4). Inverted triangles represent pulses identified by the Cluster algorithm. Right: mean ± SEM number of pulses (top), mean circulating concentrations (middle), and mean pulse amplitude (bottom) of LH in control (open bar), prenatal testosterone-treated (black bar), and prenatal DHT-treated females (hatched bar) during the different phases of E2-negative feedback test. Different letters (a vs. b and a′ vs. b′) above bars indicate significant differences (P < 0.05). LH pulses were suppressed after GnRH-A treatment in all groups (No Pulses). Two controls and one prenatal testosterone-treated females had no LH pulses throughout the E2-negative feedback test (phases 0–4) and were not included in determination of pulse attributes.
E2-positive feedback.
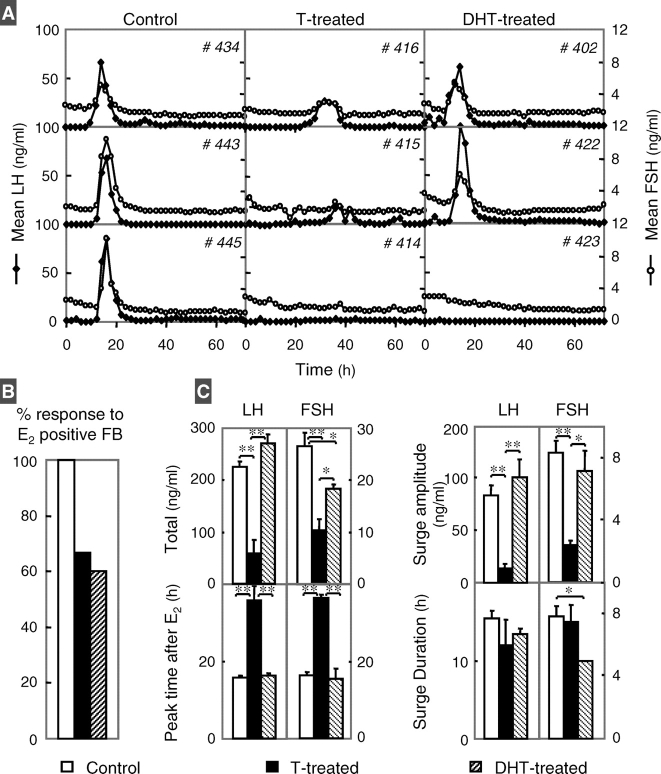
All control females responded to the E2 challenge and had gonadotropin surges, while only four out of six prenatal testosterone-treated and three out of five DHT-treated females did (Fig. 3, A and B). The two prenatal DHT-treated females that did not respond to the E2-positive feedback had elevated P4 levels, which likely blocked the positive feedback response to the E2 challenge [31]. Gonadotropin response to the E2 challenge is summarized in Figure 3C. Total LH released during the E2-induced surge (Fig. 3C, top left) and LH/FSH surge amplitudes (Fig. 3C, top right) were lower in the prenatal testosterone- but not DHT-treated females compared to controls. The timing of LH/FSH surge peaks (Fig. 3C, bottom left) was also delayed in the prenatal testosterone- but not DHT-treated females compared to controls. No differences were found in the LH surge duration (Fig. 3C, bottom right) or interval between the start and peak of LH surge (data not shown). FSH surge duration was shorter in prenatal DHT-treated females compared to controls.
FIG. 3.
Results of E2-positive feedback test conducted during the prepubertal period (∼20 wk of age). A) Two-hour circulating patterns of LH (black diamonds) and FSH (white circles) from three control (left), three prenatal testosterone (T)-treated (middle), and three prenatal DHT-treated females (right). B) Percentage of females that responded to the positive E2 feedback challenge by eliciting an LH surge. C) Mean ± SEM of total LH and FSH released during the surge and amplitude of LH and FSH surges are shown in the top left and right panels, respectively. Timing of gonadotropin surge peak and duration of LH and FSH surges are shown in bottom left and right panels, respectively. Controls are shown as white bars, prenatal testosterone-treated as black bars, and prenatal DHT-treated as hatched bars. *P < 0.05; **P < 0.01. Two prenatal testosterone- and two DHT-treated (had high P4 levels during the test) females did not respond to the E2-positive feedback and are not included in analysis of surge attributes. P4 levels of nonresponder DHT-treated females were 4.6 and 5.5 ng/ml, respectively, thus blocking E2-positive feedback response [31].
Cycle characterization.
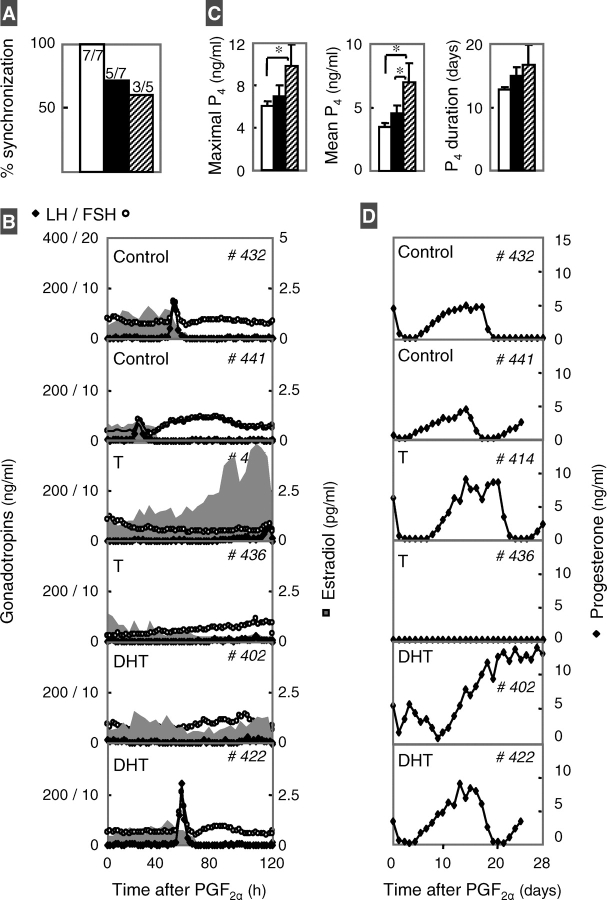
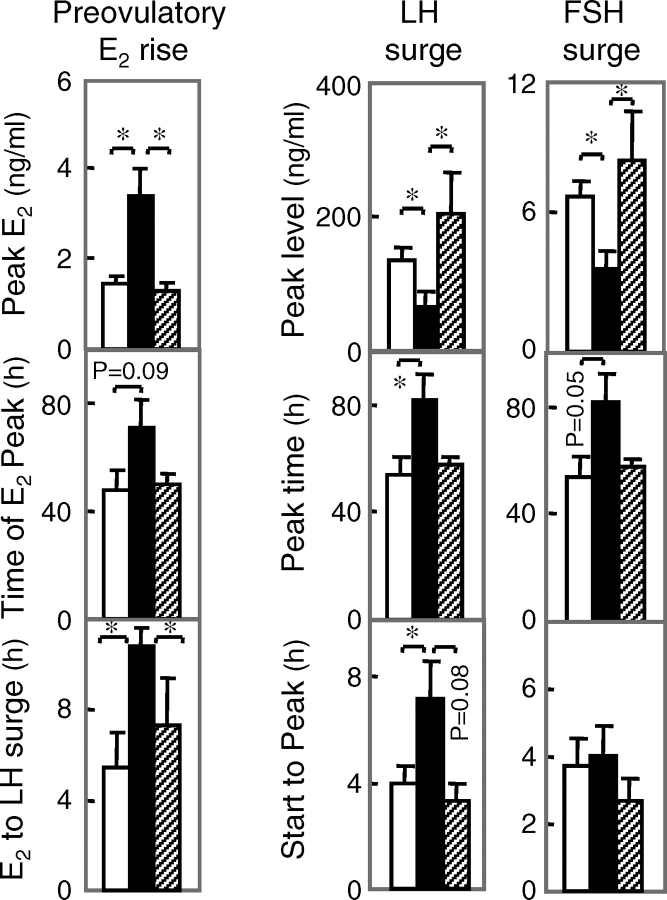
All control, five out of seven prenatal testosterone-treated and three out of five prenatal DHT-treated females responded to PGF2α administration (Fig. 4A). Circulating gonadotropins and preovulatory E2 profiles of two females from each group are presented in Figure 4B (left panels). Summary statistics for preovulatory E2 rise and gonadotropin surges are provided in Figure 5. The preovulatory E2 rise was increased in the prenatal testosterone- but not DHT-treated group compared to controls. The LH/FSH surge peak relative to PGF2α occurred later in the prenatal testosterone- but not DHT-treated females relative to controls. The interval between preovulatory E2 rise and the gonadotropin surge peak was increased in prenatal testosterone- but not DHT-treated females compared to controls. The LH and FSH surge amplitudes were dampened in the prenatal testosterone- but not DHT-treated females compared to controls. The interval between LH surge onset to peak time was longer in prenatal testosterone- but not DHT-treated females compared to controls. Luteal P4 profiles and summary statistics are shown in Figure 4, C and D. Maximal P4 levels were higher and duration tended to be longer (P = 0.08) in prenatal DHT- but not testosterone-treated females.
FIG. 4.
Results from the cycle characterization study. A) Percentage of control (white bar), prenatal testosterone (T)-treated (black), and prenatal DHT-treated (hatched) females that synchronized in response to PGF2α administration. B) Two-hour circulating patterns of LH (black diamonds), FSH (white circles), and 4-h E2 (shaded area) from two control (top panels), two prenatal testosterone-treated (middle panels), and two prenatal DHT-treated (bottom panels) females during the breeding season following estrus synchronization with PGF2α. C) Mean ± SEM of maximal and mean circulating P4 concentrations during the luteal phase and duration of luteal-phase P4 increase from control (white), prenatal testosterone-treated (black), and prenatal DHT-treated (hatched) females during the breeding season following PGF2α synchronization of estrus. Significant differences (P < 0.05). D) Daily circulating patterns of P4 from two controls (top panels), prenatal testosterone-treated (middle panels), and prenatal DHT-treated (bottom panels) females during the breeding season following PGF2α synchronization of estrus.
FIG. 5.
Left: Mean ± SEM of circulating E2, time of E2 rise, and time interval between E2 rise to LH surge peak in control (white), prenatal testosterone-treated (black), and prenatal DHT-treated (hatched) females during the breeding season following estrus synchronization with PGF2α. Asterisks indicate significant differences (P < 0.05). Middle and right: Mean ± SEM of LH and primary FSH surges peak, timing of gonadotropin surge peak, and interval between start and peak level of gonadotropin achieved during the breeding season following PGF2α synchronization of estrus. Controls are shown as white bars, prenatal testosterone-treated as black bars, and prenatal DHT-treated as hatched bars. Asterisks indicate significant differences (P < 0.05). Data from females that did not synchronize were not included in the analysis.
Objective 2: Developmental Progression of the E2-Positive Feedback Disruption
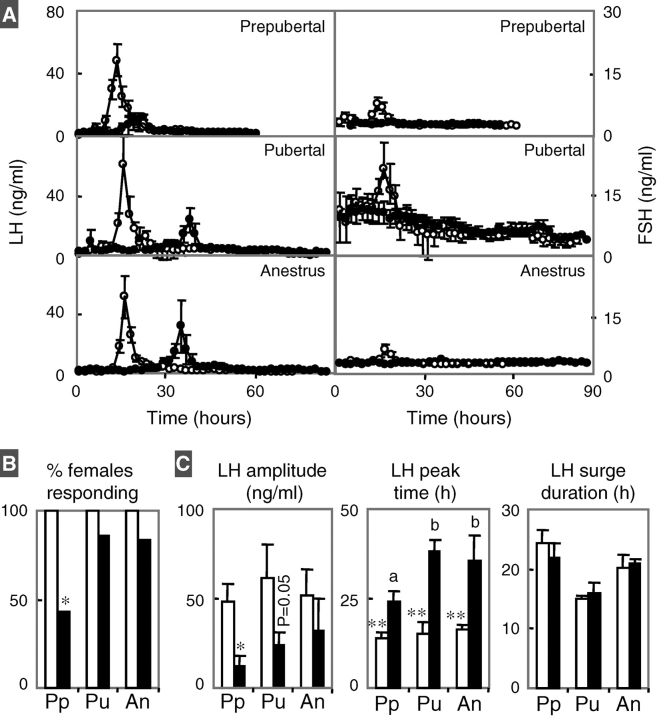
Gonadotropin responses of control and prenatal testosterone-treated females to E2-positive feedback at the three different ages are summarized in Figure 6. While all controls responded to the E2 challenge at all ages, only three out of seven, six out of seven, and five out of six (one testosterone-treated female was not studied because of illness) of the prenatal testosterone-treated females responded at 12, 23, and 54 wk of age, respectively (Fig. 6B). The amplitude of the LH surge was reduced at 12 and 23 wk of age in prenatal testosterone-treated females compared to controls. LH surge peak was delayed at all ages in prenatal testosterone-treated females (Fig. 6C). Repeated-measures ANOVA revealed an age effect relative to timing of LH surge in prenatal testosterone-treated females (P < 0.05). The interval between E2 challenge and LH surge peak was longer during the pubertal and postpubertal anestrus periods relative to the prepubertal period in prenatal testosterone-treated females. There were no differences in the duration of the LH surge in control and prenatal testosterone-treated females. The FSH baseline during the prepubertal period was higher in both groups compared to the prepubertal and anestrus periods. FSH surges paralleled LH surges in control females, but the amplitude of FSH surges in prenatal testosterone-treated females was too small to define surge characteristics.
FIG. 6.
A) Mean ± SEM circulating LH (left) and FSH (right) in response to E2-positive feedback challenge of prepubertal (top), pubertal (middle), and postpubertal (first anestrus, bottom) in control (white circles) and prenatal testosterone-treated (black circles) females. Within control and prenatal testosterone-treated groups, LH surge was aligned to the LH surge peak and then averaged and plotted relative to mean time of surge peak. B) Percentage of control (white) and prenatal testosterone-treated (black) females that responded to E2-positive feedback challenge (top left) during the three time points studied (Pp, prepubertal; Pu, pubertal; An, postpubertal). C) Summary statistics of the LH surge in response to the E2-positive feedback—namely, amplitude, timing of LH surge peak, and duration of LH surge—are shown in left, middle, and right panels, respectively. Asterisks represent significant differences between control and prenatal testosterone-treated groups within a given age (*P < 0.05; **P < 0.01). Different superscripts represent statistical differences within treatment group across time. Two control females had initiated puberty before the positive feedback test at 23 wk and therefore were excluded from the analysis. Data from females that did not synchronize were not included in the analysis.
DISCUSSION
Findings from this study provide evidence that the disruptive effects of prenatal testosterone excess on E2-negative feedback but not E2-positive feedback are likely programmed by androgenic actions of testosterone. Disruptions in 1) E2-negative feedback, which regulates the tonic mode of gonadotropin release, and 2) E2-positive feedback, which triggers the generation of the gonadotropin surge, contribute to the disruptions in periovulatory hormonal dynamics. Presence of follicular phase disruptions (elevated E2 levels achieved, delayed onset of LH/FSH surges relative to E2 rise, and dampened LH and primary FSH surges) in prenatal testosterone- but not DHT-treated females indicates that these follicular phase disruptions are not programmed by androgenic actions of testosterone. In contrast, presence of luteal-phase defects (failure to respond to PGF2α) in both groups of treated females suggest that the luteal defects are likely programmed by androgenic actions of testosterone. These findings as they relate to each developmental milestone are discussed below.
E2-Negative Feedback
The findings from E2-negative feedback tests are consistent with predictions made from the OVX+E2 model [1, 18], where an increase in tonic release of LH, an index of escape from E2-negative feedback suppression, occurred at an earlier age in both groups of treated females. Subsequent studies in ovary-intact sheep provided unequivocal evidence that prenatal testosterone excess reduces sensitivity to E2-negative feedback and validated the use of GnRH antagonist as an approach to study E2-negative feedback in ovary-intact animals [7]. The significant increase in mean LH and LH pulse amplitude in concert with lack of suppression of LH pulse frequency during E2 treatment of DHT-treated relative to controls supports androgenic modulation of E2-negative feedback. The similarity in direction of changes in these variables in prenatal testosterone-treated females (albeit only a trend), the direction of which paralleled findings from our earlier study [7] demonstrating reduced responsiveness of prenatal testosterone-treated animals to E2 feedback, is consistent with androgenic disruption of neural circuitry involved in the control of pulsatile GnRH/LH release. The increase in LH pulse amplitude seen after E2 in both steroid-treated groups is likely the result of increased GnRH pulse amplitude or an increase in pituitary sensitivity to GnRH. Previously, we found increased responsiveness of prenatal testosterone and DHT-treated sheep to GnRH [17].
One caveat in using similarity of responses between prenatal testosterone (aromatizable androgen) and prenatal DHT-treated (nonaromatizable androgen) females as an index to assess androgenic contribution is that DHT can be metabolized into 5α-androstane-3β,17β-diol (3β-diol) [32], which has the ability to act via estradiol receptor beta (ESR2) [32]. However, studies in rodents using knock-out and knock-in approaches point to the classical estradiol receptor alpha (ESR1) but not ESR2 as the mediator of E2-negative feedback [33, 34]. The conclusion that prenatal testosterone effects on E2-negative feedback are programmed by androgenic actions is also corroborated by the recent finding that cotreatment with flutamide, an antiandrogen, prevents early escape from E2 feedback in prenatal testosterone-treated females (OVX+E2 model) [35]. Overall, findings from this study in concert with earlier studies in the OVX+E2-treated model suggest that other ovarian factors are not involved in the organization of neural networks regulating E2-negative feedback and that any postnatal organization by subsequent E2 exposure is independent of the mode of E2 exposure (constant: OVX+E2; changing: ovary intact).
E2-Positive Feedback
The disruption of E2-positive feedback seen in this study and our earlier study [6] in prenatal testosterone-treated but not DHT-treated (this study) females agrees with predictions made from the OVX+E2 model [1, 18] and supports the premise that E2-positive feedback disruptions are not programmed by androgenic actions of testosterone and are more likely facilitated by conversion of testosterone to E2. Absence of LH surge in the two DHT-treated females during the E2-positive feedback test is likely related to the elevated P4 levels seen in these animals (P4 blocks generation of LH surge in sheep [31]), possibly originating from luteinized follicles, as noted in a previous study [13]. These findings differ from the rodent studies where prenatal DHT treatment abolished E2-induced LH surges [36]. These differences may relate to timing of DHT exposure or, alternatively, differences in the role played by P4. In sheep, as in humans [37, 38], P4 blocks the surge [31], while in rodents, P4 plays a facilitatory role [39].
The finding that the LH surge was of normal magnitude in prenatal DHT-treated females, which are also hypergonadotropic like the prenatal testosterone-treated females [17], suggests that depleted pituitary LH store is not the likely cause of dampened LH surge in the prenatal testosterone-treated females. The normalcy of LH surge in prenatal DHT-treated females, however, does not rule out a hypothalamic effect. A reduction in GnRH surge could be missed, as the GnRH requirement for generation of a full LH surge is much lower than the GnRH surge itself [40]. Our conclusion that the positive feedback effect of E2 is likely programmed by estrogenic actions of testosterone appears consistent with rodent studies that show that ESR1 but not ESR2 is mandatory to elicit the surge [34, 41, 42]. Conclusive evidence for this would require documentation that cotreatment with aromatase inhibitor prevents surge disruption in prenatal testosterone-treated sheep.
Developmentally, the disruption of the E2-positive feedback also appears to be progressive, as was the case with the E2-negative feedback [7]. The onset of LH surge in response to E2-positive feedback challenge was much delayed during the pubertal period (38 h) compared to that seen at 12 wk of age (24 h) (Fig. 6). This may reflect continued postnatal organization of the surge system or contribution from ovarian factors. Previous studies using Dorset sheep found that E2-positive feedback response was absent at all time points studied, suggesting that this breed is more sensitive to disruptive effects of prenatal testosterone excess [8]. The absence of differences in the magnitude of LH surge across time points in prenatal testosterone-treated females, albeit reduced compared to controls, suggests that pituitary differentiation is likely completed prenatally. Because prenatal testosterone-treated females are born smaller and show catch-up growth during the first 8–16 wk of life [19] and body weight is highly related to the maturation of the reproductive axis [43], the finding that only 50% of the prenatal testosterone-treated females are able to respond to the E2 challenge at 12 wk suggests that a full maturation of the E2-positive feedback mechanism may not have been completed.
In contrast to definable changes in LH surge dynamics, effect of prenatal testosterone excess on primary FSH surges was hard to discern. Higher presurge levels of FSH in both control and prenatal testosterone-treated sheep at 24 than at 12 wk of age are consistent with the enhanced follicular recruitment that normally occurs around puberty [44–46]. Developmentally, number of antral follicles (>3 mm) reaches its peak at 24 wk of age [45, 46]. Prenatal testosterone-treated females show exacerbated follicular recruitment [47, 48] and enhanced follicular depletion [48]. Absence of differences in FSH between control and prenatal testosterone-treated females in the face of altered follicular dynamics, while consistent with our earlier report [6], suggests that the FSH released may be comprised of a different isoform mix [22, 49] or that ovarian sensitivity to FSH is altered [50, 51].
Periovulatory Hormonal Dynamics
In terms of the follicular phase, the similarity of magnitude of preovulatory E2 rise and magnitude and timing of the primary gonadotropin surges and the interval between E2 rise and LH surge peak in prenatal DHT-treated females and controls suggests that these defects in prenatal testosterone-treated females are not programmed by androgenic actions of testosterone. The selective amplification of E2 rise in prenatal testosterone- but not DHT-treated females is likely related to contributions from the multiple antral follicles that persist in prenatal testosterone- but not DHT-treated females [12, 13].
The magnitude of LH surge during the natural cycle in all groups of females was higher than that achieved during the E2-positive feedback test. This is not a function of age, as previous studies have found that the LH surge magnitude remains the same after ∼15 wk of age [21]. Because P4 induces E2 receptors [52, 53], this difference may relate to the P4 priming that occurs during the natural cycle as opposed to lack of P4 priming during the E2-positive feedback test. A second possibility includes ovarian contributions specifically from the preovulatory follicle. Inhibin has been postulated to play a facilitory role in both sheep [54] and humans [55]. Similarly, the shorter interval between the E2 peak and LH surge peak during the natural follicular phase (5 h) compared to that seen between insertion of E2 and LH surge peak during E2-positive feedback test (18 h) across treatment groups may be a function of the delivery pattern of E2 from the implant compared to what occurs naturally during the surge. A second possibility is that E2 clearance rate is higher in prenatal testosterone-treated females compared to control, requiring a longer time to achieve the threshold level needed for generation of the surge. The longer interval between E2 rise and LH surge in both scenarios in the prenatal testosterone-treated sheep compared to controls suggests that the neural sequence regulating generation of the surge is altered.
Luteal defects seen during the cycle characterization study may stem from altered intrafollicular milieu of preovulatory follicles, leading to a faulty luteinization process and an inability to respond to luteolytic signals. In support of this contention, the percentage of animals responding to the luteolytic agent, PGF2α, was much lower in prenatal testosterone- and DHT-treated females compared to controls (this and previous studies). Luteal persistence seen in the two models may also relate to the hypergonadotropism seen in these females [17] since LH is known to have luteotrophic effects [56]. It is unclear if such defects are programmed by androgenic or estrogenic actions of testosterone. Both prenatal testosterone- and DHT-treated females manifest this defect, which is suggestive of androgenic programming, but, on the other hand, the 3β-diol metabolite of DHT can act via ESR2 [32], which plays a vital role in the ability ovarian follicle to respond to the gonadotropin surge [57].
In summary, the approach of comparing prenatal testosterone- and DHT-treated females provides evidence that the programming of E2-negative feedback is mediated by androgenic actions of prenatal testosterone excess. In contrast, E2-positive feedback and follicular phase E2 and gonadotropins surge disruptions appear to be programmed by estrogenic actions of prenatal testosterone excess.
Acknowledgments
We are grateful to Douglas Doop for his expert animal care, facility management, and help with generation of the experimental lambs and Mohan Manikkam, Carol Herkimer, Jonathan Flak, and Pamela Olton for assistance with prenatal steroid treatment, animal experimentation, or performance of E2/P4 assays.
Footnotes
1Supported by United States Public Health Service grant P01 HD44232 (to V.P.).
REFERENCES
- Wood RI, Foster DL.Sexual differentiation of reproductive neuroendocrine function in sheep. Rev Reprod 1998; 3: 130–140. [DOI] [PubMed] [Google Scholar]
- Huffman LJ, Inskeep EK, Goodman RL.Changes in episodic luteinizing hormone secretion leading to puberty in the lamb. Biol Reprod 1987; 37: 755–761. [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Bittman EL, Foster DL, Goodman RL, Legan SJ, Robinson JE.Neuroendocrine basis of seasonal reproduction. Recent Prog Horm Res 1984; 40: 185–232. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Inskeep EI.Neuroendocrine control of the ovarian cycle of the sheep. Knobil E, Neill JD.The Physiology of Reproduction, 3rd ed Amsterdam:Elsevier Science;2006: 2389–2447. [Google Scholar]
- Robinson JE, Forsdike RA, Taylor JA.In utero exposure of female lambs to testosterone reduces the sensitivity of the gonadotropin-releasing hormone neuronal network to inhibition by progesterone. Endocrinology 1999; 140: 5797–5805. [DOI] [PubMed] [Google Scholar]
- Sharma TP, Herkimer C, West C, Ye W, Birch R, Robinson JE, Foster DL, Padmanabhan V.Fetal programming: prenatal androgen disrupts positive feedback actions of estradiol but does not affect timing of puberty in female sheep. Biol Reprod 2002; 66: 924–933. [DOI] [PubMed] [Google Scholar]
- Sarma HN, Manikkam M, Herkimer C, Dell'Orco J, Welch KB, Foster DL, Padmanabhan V.Fetal programming: excess prenatal testosterone reduces postnatal luteinizing hormone, but not follicle-stimulating hormone responsiveness, to estradiol negative feedback in the female. Endocrinology 2005; 146: 4281–4291. [DOI] [PubMed] [Google Scholar]
- Unsworth WP, Taylor JA, Robinson JE.Prenatal programming of reproductive neuroendocrine function: the effect of prenatal androgens on the development of estrogen positive feedback and ovarian cycles in the ewe. Biol Reprod 2005; 72: 619–927. [DOI] [PubMed] [Google Scholar]
- Savabieasfahani M, Lee JS, Herkimer C, Sharma TP, Foster DL, Padmanabhan V.Fetal programming: testosterone exposure of the female sheep during midgestation disrupts the dynamics of its adult gonadotropin secretion during the periovulatory period. Biol Reprod 2005; 72: 221–229. [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Ye W, Phillips DJ, Herkimer C, Knight PG, Padmanabhan V.Developmental programming: deficits in reproductive hormone dynamics and ovulatory outcomes in prenatal, testosterone-treated sheep. Biol Reprod 2008; 78: 636–647. [DOI] [PubMed] [Google Scholar]
- West C, Foster DL, Evans NP, Robinson J, Padmanabhan V.Intra-follicular activin availability is altered in prenatally-androgenized lambs. Mol Cell Endocrinol 2001; 185: 51–59. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Steckler TL, Welch KB, Inskeep EK, Padmanabhan V.Fetal programming: prenatal testosterone treatment leads to follicular persistence/luteal defects: partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology 2006; 147: 1997–2007. [DOI] [PubMed] [Google Scholar]
- Steckler T, Manikkam M, Inskeep EK, Padmanabhan V.Developmental programming: follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology 2007; 148: 3532–3540. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Scaramuzzi RJ, Short RV.Ovulation in prenatally androgenized ewes. J Endocrinol 1977; 73: 385–389. [DOI] [PubMed] [Google Scholar]
- Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE.Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology 2003; 144: 1426–1434. [DOI] [PubMed] [Google Scholar]
- Steckler TL, Roberts EK, Doop DD, Lee TM, Padmanabhan V.Developmental programming in sheep: administration of testosterone during 60–90 days of pregnancy reduces breeding success and pregnancy outcome. Theriogenology 2007; 67: 459–467. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Thompson RC, Herkimer C, Welch KB, Flak J, Karsch FJ, Padmanabhan V.Developmental programming: impact of prenatal testosterone excess on pre- and postnatal gonadotropin regulation in sheep. Biol Reprod 2008; 78: 648–660. [DOI] [PubMed] [Google Scholar]
- Masek KS, Wood RI, Foster DL.Prenatal dihydrotestosterone differentially masculinizes tonic and surge modes of luteinizing hormone secretion in sheep. Endocrinology 1999; 140: 3459–3466. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V.Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology 2004; 145: 790–798. [DOI] [PubMed] [Google Scholar]
- Wood RI, Ebling FJ, I'Anson H, Bucholtz DC, Yellon SM, Foster DL.Prenatal androgens time neuroendocrine sexual maturation. Endocrinology 1991; 128: 2457–2468. [DOI] [PubMed] [Google Scholar]
- Foster DL, Jackson LM.Puberty in the sheep. Knobil E, Neill JD.The Physiology of Reproduction, 3rd ed New York:Elsevier Science;2006: 2127–2176. [Google Scholar]
- Steckler TL, Lee JS, Ye W, Inskeep EK, Padmanabhan V.Developmental programming: rescue of ovarian function in prenatal testosterone-treated sheep with exogenous gonadotropins. Biol Reprod 2008; 79: 686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza CJ, Campbell BK, Baird DT.Follicular dynamics and ovarian steroid secretion in sheep during the follicular and early luteal phases of the estrous cycle. Biol Reprod 1997; 56: 483–488. [DOI] [PubMed] [Google Scholar]
- Evans NP, Dahl GE, Mauger DT, Padmanabhan V, Thrun LA, Karsch FJ.Does estradiol induce the preovulatory gonadotropin-releasing hormone (GnRH) surge in the ewe by inducing a progressive change in the mode of operation of the GnRH neurosecretory system? Endocrinology 1995; 136: 5511–5519. [DOI] [PubMed] [Google Scholar]
- Niswender GD, Reichert LE, Midgley AR, Nalbandov AV.Radioimmunoassay for bovine and ovine luteinizing hormone. Endocrinology 1969; 84: 1166–1173. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, McFadden K, Mauger DT, Karsch FJ, Midgley AR., Jr.Neuroendocrine control of follicle-stimulating hormone (FSH) secretion: I. Direct evidence for separate episodic and basal components of FSH secretion. Endocrinology 1997; 138: 424–432. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW.Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology 1974; 94: 1704–1708. [DOI] [PubMed] [Google Scholar]
- Tortonese DJ, Lewis PE, Papkoff H, Inskeep EK.Roles of the dominant follicle and the pattern of oestradiol in induction of preovulatory surges of LH and FSH in prepubertal heifers by pulsatile low doses of LH. J Reprod Fertil 1990; 90: 127–135. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Evans NP, Dahl GE, McFadden KL, Mauger DT, Karsch FJ.Evidence for short or ultrashort loop negative feedback of GnRH secretion. Neuroendocrinology 1995; 62: 248–258. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Johnson ML.Cluster analysis: a simple versatile, and robust algorithm for endocrine pulse detection. Am J Physiol 1986; 250: E486–E493. [DOI] [PubMed] [Google Scholar]
- Kasa-Vubu JZ, Dahl GE, Evans NP, Thrun LA, Moenter SM, Padmanabhan V, Karsch FJ.Progesterone blocks the estradiol-induced gonadotropin discharge in the ewe by inhibiting the surge of gonadotropin-releasing hormone. Endocrinology 1992; 131: 208–212. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L.An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta,17beta-diol. Horm Behav 2008; 53: 741–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL.Nonclassical estrogen receptor alpha signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci U S A 2007; 104: 8173–8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Glidewell-Kenney C, Jameson JL, Moenter SM.Classical estrogen receptor {alpha} signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology 2008; 149: 5328–5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LM, Timmer KM, Foster DL.Sexual differentiation of the external genitalia and the timing of puberty in the presence of an antiandrogen in sheep. Endocrinology 2008; 149: 4200–4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foecking EM, Szabo M, Schwartz NB, Levine JE.Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod 2005; 72: 1475–1483. [DOI] [PubMed] [Google Scholar]
- Soules MR, Steiner RA, Clifton DK, Cohen NL, Aksel S, Bremner WJ.Progesterone modulation of pulsatile luteinizing hormone secretion in normal women. J Clin Endocrinol Metab 1984; 58: 378–383. [DOI] [PubMed] [Google Scholar]
- Nippoldt TB, Reame NE, Kelch RP, Marshall JC.The roles of estradiol and progesterone in decreasing luteinizing hormone pulse frequency in the luteal phase of the menstrual cycle. J Clin Endocrinol Metab 1989; 69: 67–77. [DOI] [PubMed] [Google Scholar]
- Micevych P, Soma KK, Sinchak K.Neuroprogesterone: key to estrogen positive feedback? Brain Res Rev 2008; 57: 470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen JM, Dahl GE, Evans NP, Thrun LA, Wang Y, Brown MB, Karsch FJ.Importance of the gonadotropin-releasing hormone (GnRH) surge for induction of the preovulatory luteinizing hormone surge of the ewe: dose-response relationship and excess of GnRH. Endocrinology 1998; 139: 588–595. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, Korach KS, Greiner E, Pérez CA, Schütz G, Herbison AE.Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 2006; 52: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa J, Vigo E, Castellano JM, Gaytan F, Navarro VM, Aguilar E, Dijcks FA, Ederveen AG, Pinilla L, van Noort PI, Tena-Sempere M.Opposite roles of estrogen receptor (ER)-alpha and ERbeta in the modulation of luteinizing hormone responses to kisspeptin in the female rat: implications for the generation of the preovulatory surge. Endocrinology 2008; 149: 1627–1637. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL.The neural basis of puberty and adolescence. Nat Neurosci 2004; 7: 1040–1047. [DOI] [PubMed] [Google Scholar]
- Rawlings NC, Evans AC, Honaramooz A, Bartlewski PM.Antral follicle growth and endocrine changes in prepubertal cattle, sheep and goats. Anim Reprod Sci 2003; 78: 259–270. [DOI] [PubMed] [Google Scholar]
- Bartlewski PM, Beard AP, Cook SJ, Rawlings NC.Ovarian activity during sexual maturation and following introduction of the ram to ewe lambs. Small Ruminant Res 2002; 43: 37–44. [Google Scholar]
- Mahdi D, Khallili K.Relationship between follicle growth and circulating gonadotrophin levels during postnatal development of sheep. Anim Reprod Sci 2008; 106: 100–112. [DOI] [PubMed] [Google Scholar]
- Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V.Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology 2005; 146: 3185–3193. [DOI] [PubMed] [Google Scholar]
- Smith P, Steckler T, Veiga-Lopez A, Padmanabhan V.Developmental programming: differential effects of prenatal testosterone and dihydrotestosterone on follicular recruitment, depletion of follicular reserve, and ovarian morphology. Biol Reprod 2009; 80: 726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Midgley AR, Jr, Beitins IZ, Padmanabhan V.Follicle-stimulating isohormones: characterization and physiological relevance. Endocr Rev 1995; 16: 76–787. [DOI] [PubMed] [Google Scholar]
- Fauser BC, Diedrich K, Devroey P., Evian Annual Reproduction Workshop Group. 2007. Predictors of ovarian response: progress towards individualized treatment in ovulation induction and ovarian stimulation. Hum Reprod Update 2008; 14: 1–14. [DOI] [PubMed] [Google Scholar]
- Loutradis D, Vlismas A, Drakakis P, Antsaklis A.Pharmacogenetics in ovarian stimulation—current concepts. Ann N Y Acad Sci 2008; 1127: 10–19. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Carr AM, Zee MC, Lorang D.Ovarian steroid regulation of estrogen and progesterone receptor messenger ribonucleic acid in the anteroventral periventricular nucleus of the rat. J Neuroendocrinol 1996; 8: 45–56. [DOI] [PubMed] [Google Scholar]
- Blache D, Batailler M, Fabre-Nys C.Oestrogen receptors in the preoptico-hypothalamic continuum: immunohistochemical study of the distribution and cell density during induced oestrous cycle in ovariectomized ewe. J Neuroendocrinol 1994; 6: 329–339. [DOI] [PubMed] [Google Scholar]
- Ghosh BR, Wu JC, Strahl BD, Childs GV, Miller WL.Inhibin and estradiol alter gonadotropes differentially in ovine pituitary cultures: changing gonadotrope numbers and calcium responses to gonadotropin-releasing hormone. Endocrinology 1996; 137: 5144–5154. [DOI] [PubMed] [Google Scholar]
- Hayes FJ, Hall JE, Boepple PA, Crowley WF., JrClinical review 96: Differential control of gonadotropin secretion in the human: endocrine role of inhibin. J Clin Endocrinol Metab 1998; 83: 1835–1841. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Stewart DR, Stouffer RL.Titrating luteinizing hormone replacement to sustain the structure and function of the corpus luteum after gonadotropin-releasing hormone antagonist treatment in rhesus monkeys. J Clin Endocrinol Metab 1999; 84: 342–349. [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Deroo BJ, Korach KS.Estrogen receptor-beta is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology 2005; 146: 3247–3262. [DOI] [PubMed] [Google Scholar]