Abstract
Prenatal testosterone excess programs an array of adult reproductive disorders including luteinizing hormone excess, functional hyperandrogenism, neuroendocrine defects, polycystic ovarian morphology, and corpus luteum dysfunction, culminating in early reproductive failure. Polycystic ovarian morphology originates from enhanced follicular recruitment and follicular persistence. We tested to determine whether prenatal testosterone treatment, by its androgenic actions, enhances follicular recruitment, causes early depletion of follicular reserve, and disrupts the ovarian architecture. Pregnant sheep were given twice-weekly injections of testosterone or dihydrotestosterone (DHT), a nonaromatizable androgen, from Days 30 to 90 of gestation. Ovaries were obtained from Day-90 and Day-140 fetuses, and from 10-mo-old females during a synchronized follicular phase (n = 5–9 per treatment). Stereological techniques were used to quantify changes in ovarian follicle/germ cell populations. Results revealed no differences in numbers of oocytes and follicles between the three groups on Fetal Day 90. Greater numbers of early growing follicles were found in prenatal testosterone- and DHT-treated fetuses on Day 140. Increased numbers of growing follicles and reduced numbers of primordial follicles were found in 10-mo-old, prenatal testosterone-treated females, but not in those treated with DHT. Antral follicles of prenatal testosterone-treated females, but not those treated with DHT, manifested several abnormalities, which included the appearance of hemorrhagic and luteinized follicles and abnormal early antrum formation. Both treatment groups showed morphological differences in the rete ovarii. These findings suggest that increased follicular recruitment and morphologic changes in the rete ovarii of prenatal testosterone-treated females are facilitated by androgenic programming, but that postpubertal follicular growth, antral follicular disruptions, and follicular depletion largely occur through estrogenic programming.
Keywords: developmental biology, follicle, follicular development, folliculogenesis, ovary, PCOS, reproductive aging
Prenatal testosterone excess disrupts follicular dynamics with early disruptions programmed by its androgenic actions and subsequent antral follicular disruptions and follicular depletion largely via estrogenic programming.
INTRODUCTION
Prenatal testosterone-treated sheep manifest reproductive defects similar to those in women with polycystic ovary syndrome (PCOS), the most common cause of anovulatory infertility in women. Effects of prenatal testosterone excess are evidenced as neuroendocrine feedback defects [1–5], ovarian defects [6–10], and insulin resistance [11, 12]. Ovarian defects include polycystic ovarian morphology [10] and follicular persistence [7, 8]. Our recent studies with ovaries from Fetal Day 140 (term = 147 days) provided evidence in support of enhanced follicular recruitment, the transition of nongrowing primordial follicles into the pool of growing follicles, in the Suffolk breed of sheep [9]. Studies using cortical biopsies from ovaries of 8-mo-old Dorset sheep, while not controlled for cycle stage, provided further support in favor of this contention [6]. Although opinion is divided [13–16], evidence has been provided in support of enhanced follicular recruitment in women with PCOS [16]. If the enhanced follicular recruitment, seen as early as Fetal Day 140 in prenatal testosterone-treated sheep [9], persists postnatally, this would result in early depletion of follicular reserve. Such findings would be consistent with progressive loss of cyclicity and early reproductive aging seen in the prenatal testosterone-treated females [7, 8, 17, 18].
In addition, because testosterone can be aromatized to estrogen, the altered ovarian developmental trajectory of prenatal testosterone-treated sheep may be facilitated by estrogenic actions of testosterone. Available information suggests that direct androgen/estrogen action is possible from administered testosterone at the level of the fetal gonad. Conversion of the administered testosterone into estrogens has been demonstrated in early studies with radiolabeled products. For instance, peripheral testosterone is utilized by the ovaries as substrate for estradiol production after Day 12 of pregnancy in rats [19]. Aromatase expression in sheep begins at Day 30 of gestation in the placenta [20], and Days 32–35 in the developing gonad [21, 22]. In addition, androgen and estrogen receptor genes are expressed in the ovary during fetal life, mainly in the surface epithelium (the major source of granulosa cells), those cells surrounding the developing vasculature, and also in the inner cortex and outer medulla [23], facilitating steroid action. Comparison of outcome measures in prenatal testosterone (aromatizable) and dihydrotestosterone (DHT; nonaromatizable androgen)-treated females allows the assessment of whether the effect is mediated by androgenic (if both testosterone and DHT yield similar outcomes) or estrogenic (if testosterone effects differ from DHT) actions. Using this subtractive approach, we found that follicular persistence in prenatal testosterone-treated females is not programmed by androgenic actions of testosterone [8]. Similar information is not available at the level of follicular recruitment.
In addition, because steroids have been hypothesized to play a role in germ cell maturation, the differentiation of germ cells from isolated oogonia to oocytes, and subsequently to follicles, [24, 25], and given the high number of steroidogenic enzymes expressed in the fetal sheep ovary [21, 22], it is unclear whether early effects of prenatal steroid excess also extend to early germ cell differentiation and other ovarian structures. For instance, the mesonepheric remnant is a conglomeration of glomeruli and/or tubules that migrate from regressing mesonephros, through the hilus, to the medulla of the ovary from about Day 55 to Day 75 of gestation, with the timing of appearance and structure of the mesonephric remnant being similar to that described for the connecting rete [26]. While a role for the rete has been suggested in follicle and germ cell development [27–29], little is known about this structure and how it is regulated in late fetal and adult life.
In this study, we tested the hypothesis that prenatal testosterone excess, by its androgenic action, increases early germ cell differentiation, leads to early follicular depletion, and causes disruptions in ovarian morphology.
MATERIALS AND METHODS
Breeding and Prenatal Treatment
The University of Michigan Animal Care and Use Committee approved all procedures involving animals. Two- to three-year-old Suffolk ewes were purchased locally and bred at a nearby University of Michigan Department of Laboratory Animal Medicine-approved farm. Details of husbandry and nutrition of breeder sheep before breeding and during gestation have been published previously [30]. Day of mating was determined by visual confirmation of a paint mark left by an intact ram on the hindquarter of bred ewes. Beginning on Day 30 of gestation, pregnant ewes were injected twice weekly in the musculature of the right shoulder with 100 mg of testosterone propionate (∼1.2 mg/kg; Sigma-Aldrich Corp., St. Louis, MO) or 100 mg DHT propionate (Steraloids Inc., Newport, RI) suspended in cottonseed oil (Sigma-Aldrich Corp.) until Day 90 of gestation. The dose and mode of testosterone and DHT administration were chosen to reflect the large body of data available relative to postnatal reproductive disruptions [1–10]. Levels of testosterone achieved in maternal blood and female fetuses (or umbilical artery, which carries blood from fetuses [31]) following testosterone administration are comparable to that found in adult males and male fetuses [32].
Ovaries from control, prenatal testosterone-, and DHT-treated females were procured on Fetal Day 90 (eight control female fetuses from six dams, nine testosterone-treated fetuses from six dams, and seven DHT-treated fetuses from five dams), Fetal Day 140 (seven control fetuses from five dams, eight testosterone-treated fetuses from seven dams, and seven DHT-treated fetuses from five dams), and at 10 mo of age following the onset of puberty, which occurs around 28 wk [33] of age in sheep (five control animals from five dams, six testosterone-treated animals from six dams, and five DHT-treated animals from five dams). For collection of ovaries from fetuses, dams were euthanized by administration of a barbiturate overdose (Fatal Plus; Vortech Pharmaceuticals, Dearborn, MI) and fetuses removed. As morphology is affected by stage of the estrous cycle, the 10-mo-old females were given two 20-mg injections of Prostaglandin F2α (PGF2α; 5 mg/mL Lutalyse; Pfizer Animal Health, New York, NY) 11 days apart to induce luteolysis and synchronize the initiation of the follicular phase in cycling females. Ewes were euthanized 28 h after the second PGF2α injection, and ovaries collected during the follicular phase. All ovaries were weighed prior to fixation for morphometry. One ovary from each animal was used for ovarian morphometry.
Processing of Ovaries
Fetal ovaries used for morphometry were fixed in Bouins fixative and embedded in plastic (Technovit 7100; Kulzer & Co. GmBH, Werheim, Germany). Whole fetal ovaries were serially sectioned at 20 μm on a rotary microtome (Leica RM2165; Leica Microsystems, Wetzlar, Germany) with every fifth section stained with hematoxylin and eosin. One ovary from each 10-mo-old lamb was fixed in 4% paraformaldehyde in PBS, pH 7.4, embedded in paraffin, and serially sectioned at 5 μm, with every 9th and 10th section stained with hematoxylin and eosin following a standard protocol using Gills Haematoxylin, as described by Bancroft and Cook [34]. The plastic sections were washed in running water for 20 min, stained for 20 min with hematoxylin, 10 min with eosin, dehydrated through alcohol gradient, cleared in xylol, and mounted with Depex. The differing embedding mediums allowed us to employ the most appropriate stereological technique to count follicles: namely, at Days 90 and 140 where small follicles are dense (the optical dissector [35]) and at 10 mo where small follicles are more sparse (the physical dissector [36]).
Germ Cell and Follicular Classification
The zonal pattern of germ cell development in the fetal ovary at the ages studied, combined with their distinctive morphology, allowed easy identification of oocytes without the need for specific markers for oocyte recognition [37, 38]. Isolated germ cells were classified as either oogonia (i.e., large spherical cells with an intact nucleus, low nuclear:cytoplasm ratio, and a pale-staining cytoplasm located in the outer-most regions of the cortex) or oocytes (i.e., large, spherical cells, which had entered meiotic prophase, and were located almost exclusively in the ovigerous cords). Degenerate germ cells contained a dense, small nucleus and/or eosinophillic cytoplasm. Follicles were classified based on the system of Lundy [39]. Primordial follicles (type 1) contained one layer of flattened granulosa cells, transitory follicles (type 1a) contained one layer of mixed flattened and cuboidal granulosa cells, and primary follicles (type 2) contained from one to less than two layers of cuboidal granulosa cells; preantral follicles (secondary) contained two or more layers of granulosa, but no discernible antrum, and, at 10 mo of age, these were further divided into small preantral follicles (type 3), which contained two to four layers of granulosa cells, large preantral follicles contained greater than four layers of granulosa cells and had no discernible antrum. Small antral follicles showed a developing antrum and were ≤1 mm in diameter, and large antral follicles were >1 mm in diameter and had a discernible antrum. Follicle health was assessed in a section containing the oocyte. A follicle was classified as atretic based loosely on the descriptions of Hay et al. (follicles containinig more than three pyknotic granulosa cells, a misshapen oocyte, or a discontinuous membrane) [40]. While some consider type 1a follicles to be the earliest stage of follicle growth, in previous studies we have found that, during fetal life, it is common for some newly formed follicles to resemble type 1a follicles, often with an uneven distribution of pregranulosa cells [21, 41]. The continued appearance of follicles with this characteristic through 10 mo of age, combined with their relatively constant number, suggests that at least some of these follicles may have maintained their original structure. Consequently, in this study, when pooling follicles into growing or nongrowing (primordial), type 1a follicles have not been considered, as they likely represent a mixture of growing and nongrowing follicles. Stereological techniques were employed for counting germ cells and follicles [42]. Due to differences in follicle distribution at different ages, all subclassifications were not possible across all ages. For example, at Day 140, due to the small numbers present, small and large preantral follicles are combined into one class. Similarly, at 10 mo of age, the larger number of antral follicles present allowed further classification into small (<1 mm) and large (>1 mm) follicles.
Ovarian Cortical and Mesonephric Remnant Volume
Ovarian cortical (the outer rim of the ovary containing primordial follicles) and mesonephric remnant volumes were estimated by the Cavalieri principal [43]. Briefly, the area (a) of the tissue was estimated by point counting on every fifth section (Days 90 and 140) or every 50th section (10 mo). Volumes were calculated using the formula Σa × h, with h being the distance between examined sections (100 μm for Days 90 and 140, and 250 μm for 10-mo-old-ovaries). Mesonephric remnant volumes were estimated at Days 90 and 140 of gestation, but not at 10 mo of age, where the remnant represents a much smaller proportion of the ovary, and a far more rigorous sectioning regimen than employed in this study would be required to provide accurate estimates of volume.
Follicle Counts
Germ cells/follicles up to and including type 2 from Day 90 and Day 140 fetuses were counted using the optical dissector [35]. A motorized stepping stage was used to implement a random but uniform sampling strategy. Briefly, a random point remote from the section was selected on the slide, with the stage moving uniformly across and down in a grid pattern, with fields falling on the section used for counting. A high numerical aperture (na) oil immersion objective (Olympus plan apo 100×; na = 1.4; Olympus, Tokyo, Japan) was employed to take optical sections through a known depth (10 μm) of the section. Oocyte nuclei within a counting frame were counted as they came into focus, and classified according to their stage of maturation (i.e., oogonia, oocytes, follicles). Numbers were calculated using the formula N = (ΣQ/(ΣA × H)) × V, where Q is the germ cell nuclei counted within the counting frame, A is the area of the counting frame, H is the depth focused through the section, and V the ovary volume.
For ovaries from 10-mo-old females, primordial and type 1a follicles were counted using the physical dissector and section pairs [36, 38]. In this instance, every 49th and 50th section was used. An unbiased counting frame of area (A) was placed over projected images of the sections, and the numbers of oocyte nuclei (being representative of a follicle) exclusive to one section only (Q) were counted. The follicles represented by these oocytes were classified, and the numbers for the ovary being studied calculated using the formula N = (ΣQ/(ΣA × D)) × V, where D is the distance between sections studied (i.e., 5 μm). For follicles beyond the type 1a stage, all follicles were counted, the oocyte was used as a marker for a follicle, and these were counted in sections, the distance between which was not greater than the diameter of the oocyte (for plastic sections, every fifth section, with the distance between the bottom of section 1 and the top of section 5 being 60 μm; for paraffin, every 10th section, with the distance between the bottom of section 1 and the top of section 10 being 40 μm). The low frequency of oocytes appearing in two consecutive sections studied made these readily identifiable, and these were counted only once. The number of sections examined for both volume estimates and follicle counts averaged 13 at Day 90 and 15 at Day 140 per fetus. At 10 mo of age, an average of 23 sections was examined to determine volumes and small follicle counts, while, for large follicle counts, an average of 107 sections per animal was used.
Mesonephric Remnant Characteristics
Sections were examined throughout the ovary, and the mesonephric-derived cell mass was recorded as either present or absent from the cortex, medulla, or hillium of the ovary. Additionally, the presence or absence of clearly discernible tubules and tubules with a lumen was also recorded.
Statistical Analysis
For Day 90 and Day 140 ovaries, data from twin and triplet fetuses were averaged so that the experimental unit was the dam. One Day 140 DHT-treated fetus was excluded from the analysis, as its morphology was more consistent with that of a Day 120 ovary, suggesting that pregnancy may have been established in this animal one cycle later. All data from all ages were log transformed and subjected to ANOVA, with treatment differences compared by Fisher multiple range test. Significant differences are reported relative to the control group. Data are presented as means ± SEM.
At 10 mo of age, where, for some variables, prenatal DHT-treated animals gave results intermediate between controls and prenatal testosterone-treated animals, a discriminant analysis [44] was performed on standardized, log-transformed data for type 1, type 1a, type 2, preantral, and antral follicles to find the linear combination of these variables (canonical variate scores) that gave the greatest discrimination between the treatment groups. For analyzing the presence or absence of mesonephric remnant in the ovary, the fetus and not the dam was used as the experimental unit. A generalized linear model [45] using a logic function was fitted to the proportions of animals displaying the presence of each attribute within each treatment group on Days 90 and 140 of gestation.
RESULTS
Germs Cell and Follicular Distribution
Fetal Day 90.
Data are summarized in Table 1 and Figures 1 and 2. Prenatal testosterone or DHT treatment had no effect on ovarian weight or volume. The numbers and proportions of germ cells present in each stage of development (i.e., oogonia, oocytes, and follicles) were similar between control, prenatal testosterone-treated, and DHT-treated animals.
TABLE 1.
Ovarian characteristics in control, testosterone (T)-treated, and DHT-treated animals, on Fetal Day 90.*.
FIG. 1.
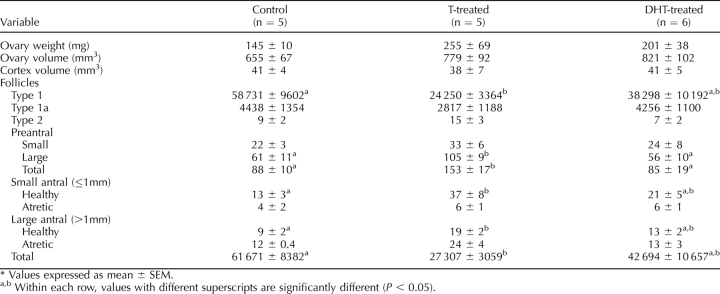
Comparative morphology of ovaries on Day 90 (A–C) and Day 140 of gestation (D–F) and 10 mo of age (G–I) in control (A, D, G), prenatal testosterone-treated (Days 30–90 of gestation; [B, E, H]), and prenatal DHT-treated (Days 30–90 of gestation; [C, F, I]) females. Note the lack of discernible differences between groups on Day 90, increased number of growing follicles in ovaries of prenatal testosterone- and DHT-treated fetuses on Day 140, and increased number of antral follicles in ovaries of 10-mo-old prenatal testosterone-treated females.
FIG. 2.

A) Mean (± SEM) number of primordial follicles (type 1) and growing follicles on Fetal Days 90 and 140 and 10 mo of age in control, testosterone (T)-treated (Days 30–90 of gestation), and DHT-treated (Days 30–90 of gestation) ovaries are shown. Within each age group, different letters indicate significant differences. B) A plot of first and second canonical variate scores (scores 1 and 2) obtained from a discriminant analysis of counts of type 1, type 1a, type 2, preantral, and antral follicles at 10 mo of age, classified by treatment group. Group means for each variable are represented by solid symbols, with 95% confidence circles denoted by labeled lines. The plot illustrates a significant difference between testosterone and both DHT and controls in the first variable ( = 24.75; P < 0.01), while the difference between controls and DHT treatments expressed in the second variable was not significant (
= 24.75; P < 0.01), while the difference between controls and DHT treatments expressed in the second variable was not significant ( = 4.84; P > 0.05). .
= 4.84; P > 0.05). .
Fetal Day 140.
Data are summarized in Table 2 and Figures 1 and 2. Prenatal testosterone and DHT treatment increased ovarian weights (P < 0.05) and volumes (P < 0.05) at this developmental time point. Ovarian cortex volume (the area of the outer ovary containing primordial follicles) from prenatal testosterone-treated animals was significantly greater than in control animals (P < 0.05) (Table 2). Prenatal DHT-treated animals showed a similar tendency (P = 0.07). Numbers of oocytes, nongrowing follicles (type 1 [primordial]), and type 1a follicles did not differ between treatment groups (Fig. 2). The numbers of follicles in the first stage of growth (type 2 [primary]) were significantly higher in both testosterone- and DHT-treated fetuses (P < 0.05) compared to controls. While there was a tendency for both prenatal testosterone and DHT-treated groups to have higher preantral and antral follicle numbers, the high variability between fetuses rendered these nonsignificant. When all growing follicles were considered from type 2 to antral follicles, both prenatal testosterone- and DHT-treated groups had significantly more follicles than the control group (Fig. 2).
TABLE 2.
Ovarian characteristics in control, testosterone (T)-treated, and DHT-treated animals, on Fetal Day 140.*.
Ten-month-old lambs.
Data are summarized in Table 3 and Figures 1 and 2. There were no significant effects of treatment on ovarian weight or ovarian and cortical volumes between controls and either prenatal testosterone- or DHT-treated groups. Total number of follicles in 10-mo-old animals were 65% (control), 28% (prenatal testosterone), and 57% (prenatal DHT) of that seen in Day 140 fetuses, a substantially higher loss in prenatal testosterone-treated females compared with controls and prenatal DHT-treated females. Number of nongrowing (type 1 [primordial]) follicles in prenatal testosterone-treated females were less than half of that found in control animals (P < 0.01). Prenatal DHT-treated animals had primordial follicle numbers intermediate between control and prenatal testosterone-treated females, but did not differ significantly from either group (Fig. 2). The total number of growing follicles (from type 2 to large antral) was significantly higher (P < 0.05) in prenatal testosterone-treated animals when compared with both controls and prenatal DHT-treated females (Fig. 2). Further classification of growing follicles showed that the difference between prenatal testosterone-treated animals and controls was evident at the level of large preantral follicles (P < 0.05), small, healthy antral follicles ≤1 mm (P < 0.05), and healthy antral follicles >1 mm (P < 0.05). A similar trend was present in type 2 follicles and small preantral follicles. There was no significant difference in the number of atretic antral follicles between treatment groups. Prenatal DHT-treated females generally had antral follicle numbers intermediate to the controls and the prenatal testosterone-treated animals, with values being not significantly different from either group.
TABLE 3.
Ovarian characteristics in control, prenatal testosterone (T)-treated, and prenatal DHT-treated animals at 10 mo of age.*.
Discriminant analysis showed a marked contrast in the first dimension ( = 24.75; P < 0.01) between the prenatal testosterone-treated animals and controls and prenatal DHT-treated animals, which had similar scores. This analysis identified a significant difference between prenatal testosterone-treated animals and both the controls and prenatal DHT-treated animals; this difference is related to the ratio of the number of type 1 follicles to the number of follicles that had reached at least the type 2 stage of development. However, it was not possible to differentiate between the controls and prenatal DHT-treated animals. This result reinforces the argument for a marked estrogen-programmed effect at this age, and suggests that any significant androgen-mediated effects are unlikely. .
= 24.75; P < 0.01) between the prenatal testosterone-treated animals and controls and prenatal DHT-treated animals, which had similar scores. This analysis identified a significant difference between prenatal testosterone-treated animals and both the controls and prenatal DHT-treated animals; this difference is related to the ratio of the number of type 1 follicles to the number of follicles that had reached at least the type 2 stage of development. However, it was not possible to differentiate between the controls and prenatal DHT-treated animals. This result reinforces the argument for a marked estrogen-programmed effect at this age, and suggests that any significant androgen-mediated effects are unlikely. .
Ovarian Architecture
Microscopic screening of ovarian sections for morphometry revealed the presence of mesonepheric remnant in all three developmental time points studied. Figures 3 and 4 show the histology of the mesonephric remnant at Fetal Day 90 and 10 mo of age, respectively. The morphology of the mesonephric remnant at Fetal Day 140 of gestation was similar to that at 10 mo of age for all treatment groups (data not shown). Table 4 summarizes the volume and morphological characteristics of the mesonephric remnant in control, prenatal testosterone-treated, and prenatal DHT-treated Day 90 and Day 140 fetuses. Comparison of the mesonephric remnant in Fetal Day 90 ovaries found the volume of mesonephric remnant was reduced in prenatal DHT-treated females compared with controls (Table 4; P < 0.05). Volume of mesonephric remnant in testosterone-treated fetuses was intermediate and did not differ between control and DHT-treated fetuses. The mesonephric remnant was evident in the cortical region of 29% control females, but in none of the prenatal testosterone- or DHT-treated females. Mesonephric remnant was present in the medullary region of all animals of all treatment groups. A lower percentage of prenatal testosterone-treated fetuses had mesonephric remnant in the hillar region of the ovary compared with controls and prenatal DHT-treated females (P < 0.05). The percentage of animals with tubules in the mesonephric remnant was higher in the prenatal DHT-treated group, but not in the testosterone-treated group, relative to controls (P < 0.05). The presence of lumen in the tubules followed a similar trend as the tubules, where at Fetal Day 90 not only did more of the DHT-treated animals have tubules present but more of these tubules contained a lumen compared to similar-age controls and prenatal testosterone-treated females (P < 0.01).
FIG. 3.

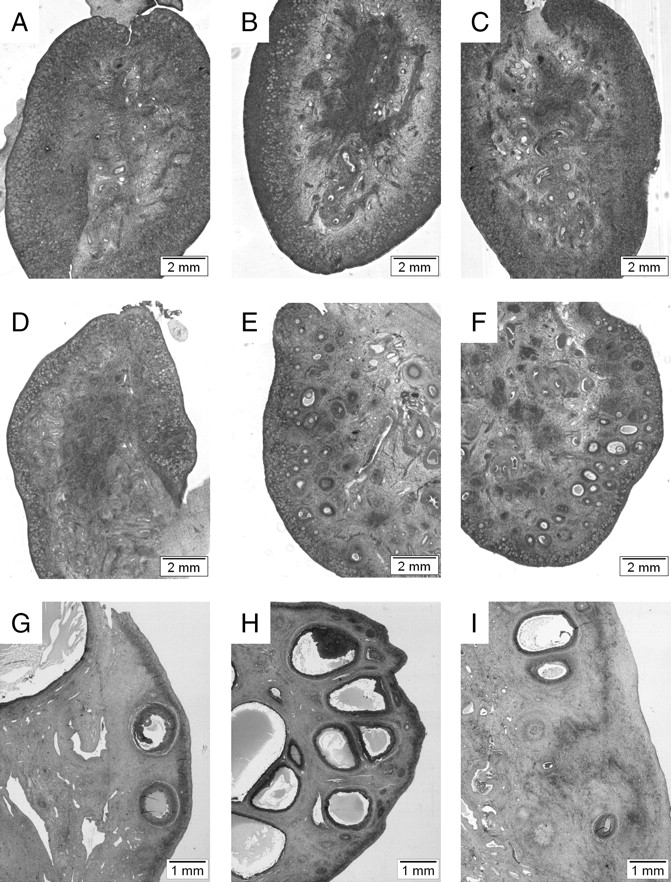
Comparative morphology of mesonephric remnant on Fetal Day 90 of gestation. A and D) control; (B and E) testosterone treated (Days 30–90 of gestation); and (C and F) DHT treated (Days 30–90 of gestation). Note that control and testosterone-treated animals exhibit limited tubule/lumen development within the cell mass, if any, while DHT-treated animals display some tubules with a distinct lumen.
FIG. 4.

Comparative morphology of mesonephric remnant from 10 mo of age. A–F) Connecting rete; (G–I) intraovarian rete. A and D) Connecting rete from the ovary of a control female seen as a discrete, compact structure lined with a columnar epithelium comprising a series of barely distinguishable thick cords lined with a stratified or pseudostratified epithelium interspersed with a small amount of connective tissue. B and E) Connecting rete from the ovary of a prenatal testosterone-treated (Days 30–90 of gestation) female seen as a less discrete, more diffuse structure in which the thick cords appear to be unraveling. Cords appear lined with a mixture of columnar and cuboidal epithelial cells, and show some signs of a lumen. Cords are interspersed with far more connective tissue than controls. C and F) Connecting rete from the ovary of a prenatal DHT-treated (Days 30–90 of gestation) female. The cords, similar to those of the prenatal testosterone-treated female, appear to be unraveling, resulting in a less discrete structure. Cords are lined with a stratified or pseudostratified epithelium comprising a mixture of columnar and cuboidal cells. A distinct lumen is present in many of the cords. G) Intraovarian rete from the ovary of a control female located in the outer medulla and cortex, seen as a system of cell cords, with cells and cords often being indistinguishable from granulosa cells and small follicles. H and I) Intraovarian rete from the ovary of prenatal testosterone-treated (H) and DHT-treated (Days 30–90 of gestation [I]) females, distinguishable from controls by the presence of a lumen.
TABLE 4.
Characteristics of the mesonephric remnant: mesonephric volume, percentage of animals with varying attributes (location of mesonephric remnant, presence of tubules/lumen).
Comparison of Day 140 ovaries found no significant differences in volumes between treatment groups (Table 4). All animals in all treatment groups had mesonephric remnant in the medullary region, but not in the cortical region. Higher percentage of prenatal testosterone- and DHT-treated females also had mesonephric remnant in the hillar region compared with controls (P < 0.05). Mesonephric remnants in 100% of prenatal testosterone and DHT-treated females had associated tubules, while none of the controls had any tubules (P < 0.01). A large percentage of tubules in testosterone- and DHT-treated females also had a lumen (P < 0.05).
Visual comparison show similar direction of changes in the characteristics of the mesonephric remnant when comparing prenatal testosterone-treated, DHT-treated, and control females at 10 mo of age (Fig. 4).
Examination of all follicles beyond the type 2 stage, at 10 mo of age, revealed the appearance of some unique abnormalities in prenatal testosterone-treated animals. Hemorrhagic follicles were present (Fig. 5), typified by a convoluted and disrupted basement membrane, a granulosa cell layer, and antrum containing numerous blood cells. Follicles displaying atypical antrum formation were also noted in prenatal testosterone-treated animals (Fig. 5). In these follicles, the early developing antrum appears lopsided, often with a single layer of granulosa cells separating the antrum from the basement membrane. In these regions, the adjacent theca layer also appeared disorganized. While the incidence of these abnormalities in prenatal testosterone-treated animals was low (<10%), it is of interest that neither abnormality appeared in control or prenatal DHT-treated animals.
FIG. 5.
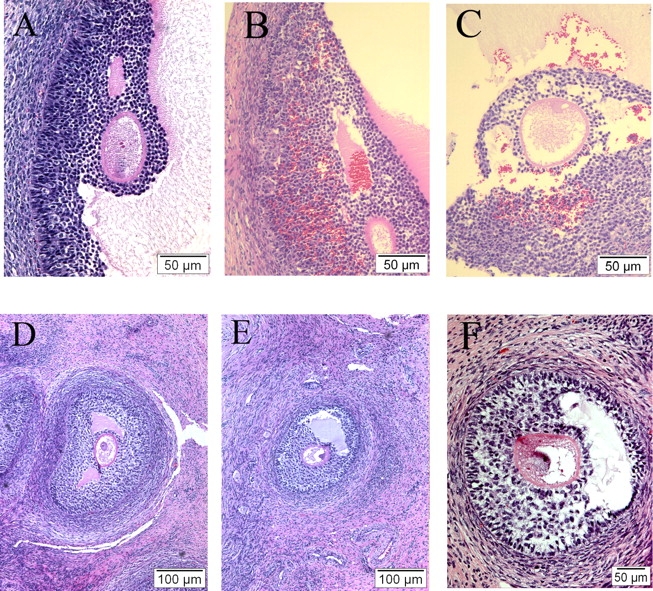
Comparative antral follicle morphology in 10-mo-old control (A, D) and prenatal testosterone-treated (B–C, E–F) ovaries (Days 30–90 of gestation). A) Antral follicle from a control female. Note that blood cells are confined to the theca interna, and the presence of an intact basement membrane. B and C) Hemorrhagic antral follicle from prenatal testosterone-treated female, with discontinuities in the basement membrane and numerous blood cells within the granulosa layer and antrum. D) Small antral follicle from a control female. Note the thick, well-developed theca layer, and multiple concentric layers of granulosa cells. E and F) Abnormal, small antral follicle from prenatal testosterone-treated females, with regions in which a single layer of granulosa separates the developing antrum from the basement membrane. The theca layer appears less developed than in the control, particularly adjacent to the regions lacking multiple layers of granulosa cells. The abnormal antral follicles were only seen in prenatal testosterone-treated females, and constituted less than 10% of antral follicles.
DISCUSSION
The findings from this study, in addition to expanding earlier observation that, during fetal life, prenatal testosterone excess enhances follicular recruitment [9], provide evidence that prenatal testosterone excess: 1) does not affect germ cell development and primordial follicle formation; 2) enhances early follicular recruitment and causes structural changes in mesonephric remnant, likely via androgen-mediated mechanisms; and 3) facilitates follicular growth, depletion, and disruptions in antral follicular morphology, largely via estrogen-mediated mechanisms.
In sheep, Fetal Day 90 represents a period in which a number of developmental events are completed [21, 36, 41], with the majority of the germ cells lost due to atresia between Fetal Days 75 and 90. Although germ cells are still present as oogonia or oocytes undergoing meiosis, the presence of primordial, type 1a, and early growing follicles is also apparent [9, 36]. The lack of significant differences in germ cell/follicle number on Day 90 of gestation in prenatal testosterone- and DHT-treated females relative to controls suggests that prenatal androgen excess does not affect morphologically quantifiable aspects of germ cell maturation. Given the hypothesized role for estradiol in germ cell maturation and follicle formation [46–48], the lack of effect of prenatal testosterone excess, a source of excess estrogen, may relate to the amount of estrogen exposure. Thus far, studies that have documented a role for estrogen in germ cell maturation are based on complete ablation of estrogen. The absence of differences in germ cells between treatments on Fetal Day 90 provides evidence that any difference in ovarian reserve seen at a later time point, fetal or postnatal, are likely the result of increased follicular recruitment or follicle persistence and not differences in initial ovarian reserve.
With advancing development, prenatal testosterone and DHT treatment caused a significant increase in ovarian weight and ovarian volume of 140-day fetuses. This may have resulted from the increased numbers of growing follicles and/or hyperplasia of the stroma and theca, a feature often associated with PCOS [14, 49, 50]. It also reinforces the suggestion of the presence of androgen-mediated effects at Day 140. The significant increase in early growing follicles seen in both prenatal testosterone- and DHT-treated animals at this developmental time point also supports the contention that early differentiation is at least partially under androgenic control. Ongoing developmental studies indicate that prenatal androgen exposure increases expression of androgen receptor in primordial follicles on Days 90 and 140 of gestation, further supporting this contention. That androgens play a role in early follicle differentiation is not a novel concept. Androgens have been shown to promote the differentiation of primordial to primary follicles in the primate [51]. Additionally, in cultures of porcine granulosa cells, androgens have also been shown to augment the mitogenic effects of growth differentiation factor 9 [52], which is known to be expressed in the oocytes of type 1 follicles in the sheep [53].
Postpubertally, at 10 mo of age, there was a 51% decrease in the number of nongrowing (primordial) follicles, with primordial follicles constituting ∼90% of total follicular pool. The reduction in primordial follicles at this age in prenatal testosterone-treated females was not offset by the number of growing follicles, indicating that there is substantial follicular depletion. This loss more likely occurs during early stages of follicular differentiation, as there were no differences in the rate of atresia in the antral follicles. The finding that both prenatal testosterone- and DHT-treated females show increased follicular growth at fetal day 140, while only the prenatal testosterone-treated females show such an increase at 10 mo of age, indicates that this is a transient phenomenon in the prenatal DHT-treated females. Enhanced depletion and increased number of growing follicles in the ovaries of 10-mo-old prenatal testosterone-treated females, but not DHT-treated females, may be reflective of follicular persistence seen in the prenatal testosterone-treated animals [8], and likely influenced by the prevailing steroid milieu [5]. The finding that the prenatal DHT-treated group, at 10 mo of age, were intermediate to the controls and the prenatal testosterone-treated group in number of variables (DHT not different from control and prenatal testosterone treated) may reflect residual effects from the earlier (Day 140) androgen-mediated effects. However, discriminant analysis results, which revealed an overall similarity between controls and prenatal DHT-treated animals, with prenatal testosterone-treated animals being significantly different from both groups, reinforce a marked estrogen-mediated programming in postpubertal females.
While the enhanced follicular differentiation seen at Fetal Day 140 in this study in prenatal testosterone-treated females is consistent with the results of our previous study [9], it does differ markedly in terms of the degree of follicular differentiation. In our previous study, ovaries appeared to contain larger antral follicles than in the present study, suggesting that the ovary was at a more advanced stage of differentiation (i.e., follicles had been growing faster or over a longer time period) [9], and the effect of prenatal testosterone excess was evident not only as significant increase in number of growing follicles, but also as a decrease in the number of nongrowing follicles. A number of fetal studies undertaken in New Zealand over many years have found variations in the degree of ovarian differentiation from year to year [35, 36, 38], perhaps related to differences in the maternal environment. Certainly, nutritional changes could have had an effect on fetal development [54]. Given that the treatment-induced differences appear to stem from enhanced follicular recruitment, it is not surprising that these differences are more pronounced in ovaries at a more advanced stage of development.
The fact that prenatal testosterone, but not DHT treatment, produced a 2-fold increase in the number of growing follicles in tandem with an ∼3-fold decrease in the number of nongrowing follicles in 10-mo-old females suggests a role for estrogenic programming in the growth of follicles, stemming from aromatization of androgen to estrogen. The accelerated rate of follicular recruitment and depletion in prenatal testosterone-treated animals seen at this age suggests involvement of puberty-associated hormonal changes (puberty in sheep occurs at ∼28 wk of age [46], and the onset of puberty does not differ between treatment groups [8]). Morphometric analyses of ovaries from prepubertal animals are necessary to narrow the critical time point at which this accelerated depletion begins to occur in the ovary. The assumption that the reduction in the ovarian reserve seen in 10-mo-old females is a consequence of increased follicle recruitment is logical, considering that there is also an increase in proportion of growing follicles.
Comparing the results of this study with those of an earlier study conducted in prenatal testosterone-treated Dorset sheep aged 8 mo [6], there are both parallels and differences. Both studies support enhanced follicular recruitment. In the current study, 59% fewer primordial follicles and nearly doubling of growing follicles were evident in prenatal testosterone-treated females at 10 mo of age compared with controls, with number of growing follicles accounting for less than 10% of the reduction in primordial follicles. This indicates substantial follicular depletion. Studies with the Dorset breed of sheep found no differences in density of follicles in cortical biopsies of control and prenatal testosetrone-treated sheep [6]. In the earlier study, the distribution of primordial to primary follicles differed between treatment, with 47% of follicles in primordial stage in the prenatal testosterone-treated females as compared with 71% in controls. This was offset by a similar increase in percentage of primary follicles. These findings, while supporting enhanced recruitment, do not support follicular depletion. The differences between these two studies may relate to: 1) breed differences—Suffolk in this study vs. Dorset in the earlier study; 2) differences in approaches used for morphometry—the application of stereological techniques and systematic sampling to the whole ovary in the current study, as opposed to use of core biopsy [55] in the Dorset study; 3) procurement of ovaries after estrus synchronization in this study, as opposed to a mixed cycle stage in the Dorset study; and 4) differences in the classification of follicles (i.e., in the current study, type 1a follicles were not included, while in the Dorset study, these were classified as growing follicles).
Given that less than half the ovarian reserve is left in the prenatal testosterone-treated females by 10 mo of age, it is conceivable that the loss of cyclicity seen in these animals [7, 8, 17, 18] by 2 yr of age is a function of early ovarian aging. Detailed morphometric studies using ovaries at later ages would help address this issue. Ultrasonographic studies carried out with 2-yr-old animals indicate that follicles are not completely depleted at 2 yr of age when most animals are anovulatory [7, 8]. Studies with Dorset females found evidence in support of enhanced follicular recruitment in 8-mo-old, but not in 21-mo-old, Dorset females [6]. If the same holds true for the Suffolk females used in this study, then the findings would suggest a role of cyclic ovarian function in follicular depletion. If so, controls that are continuing to cycle would continue to undergo follicular recruitment/depletion, while the prenatal testosterone-treated females, in which cyclicity is disrupted, will cease to do so, ultimately catching up with each other. The alternate possibility involves postpubertal replenishment of germ cells to the ovarian reserve, as suggested by Johnson et al. [56].
An intriguing finding at the level of ovarian architecture is the effect of prenatal testosterone and DHT treatment on the mesonephric remnant. Despite its presence well into adulthood, the function of this structure remains to be discerned. These structures do show strong expression of genes for steroidogenic enzymes [21, 22] and their receptors [23] at a time when fetuses in these studies were treated with testosterone/DHT (Days 30–90). The mesonephric remnant is a major site of estradiol receptor 2 and androgen receptor gene expression [21, 23], thus making it a likely candidate for effects of prenatal testosterone/DHT treatment. The trend for smaller volumes and more prominent tubules with a distinct lumen may represent a more developed intraovarian rete in prenatal testosterone- and DHT-treated animals, while perhaps the connecting rete, from which the intraovarian rete develops, is in a more advanced state of regression. The prominent lumen in testosterone- and DHT-treated animals is consistent with androgens playing a role in regulation of the rete system. Earlier studies [57, 58] found that secretions into the lumen of the rete system correlated with stages of the estrous cycle and gestation, suggesting that this tissue may be steroid responsive. Given the morphological similarities, the medullary mass is likely to be the connecting rete described by Cassali in the sheep [26] and the tubules that branch from this mass: the intraovarian rete. A role for the rete in follicle formation has been suggested by other investigators [27, 29], but a role in the adult ovary has yet to be determined. The possibility of a link between differences in the development of the rete and increased follicle recruitment (the factors responsible for recruitment of follicles being poorly understood) is a novel concept that remains to be more closely examined in future studies.
The development of ovarian follicles is a process that also involves recurring, regulated angiogenesis [59]. Angiogenesis is also a prominent feature of PCOS, contributing to hyperplasia of the theca and stroma [49]. The appearance of hemorrhagic follicles and follicles with a partially disorganized theca around the time of antrum formation in prenatal testosterone-treated, but not control or prenatal DHT-treated females, suggests possible differences in vascular input to the follicles and a role for angiogenesis in enhanced differentiation. Cells associated with the vasculature appear to express steroid receptor during ovarian development [23]. Interestingly, an association exists between the mesonephric-derived cell streams and the developing vasculature [21, 41]. Angiogenic growth factors, such as vascular endothelial growth factor (VEGF) [60] and endocrine gland-derived VEGF [61] have been implicated in angiogenesis associated with PCOS.
Finally, the relative contribution of neuroendocrine and ovarian reprogramming in prenatal testosterone-/DHT-induced changes in follicle dynamics remains to be elucidated. Clearly, prenatal testosterone excess leads to luteinizing hormone excess in the offspring [3, 62]. Increased number of early growing follicles in prenatal testosterone-treated females, long held to be gonadotrophin-independent events, would suggest direct ovarian programming of enhanced follicular recruitment. Certainly, the abundance of steroidogenic enzyme and receptor expression in the developing ovary throughout fetal life suggests that the machinery is in place to reprogram an ovary by exposure to androgens and estrogens.
In summary, the comparative approach of studying prenatal testosterone- and DHT-treated females revealed that prenatal testosterone treatment, by direct ovarian programming, affects adult follicular dynamics and the morphology of the rete ovarii. The effects on early follicular differentiation during fetal life and mesonephric remnant morphology are mediated by androgenic actions, with subsequent follicular growth, disruptions in antral follicular morphology, and follicular depletion seen in postpubertal females programmed largely by estrogenic actions.
Acknowledgments
We are grateful to Douglas Doop for assistance with breeding and lambing, his expert and conscientious animal care, and sheep facility management. We thank Drs. Mohan Manikkam, Dr. P.S. Mohankumar, Mr. James Lee, and Ms. Carol Herkimer for their help during prenatal steroid treatment and/or tissue procurement. We also thank Dr. Roger Littlejohn of AgResearch Invermay, New Zealand for performance of discriminant analysis, and Dr. Morton Brown for statistical advice.
Footnotes
1Supported by United States Public Health Service grant P01 HD44232 to V.P. and the New Zealand Foundation for Science Research and Technology.
REFERENCES
- Robinson JE, Forsdike RA, Taylor JA.In utero exposure of female lambs to testosterone reduces the sensitivity of the gonadotropin-releasing hormone neuronal network to inhibition by progesterone. Endocrinology 1999; 140: 5797–5805. [DOI] [PubMed] [Google Scholar]
- Sarma HN, Manikkam M, Herkimer C, Dell'Orco J, Welch KB, Foster DL, Padmanabhan V.Fetal programming: excess prenatal testosterone reduces postnatal luteinizing hormone, but not follicle-stimulating hormone responsiveness, to estradiol negative feedback in the female. Endocrinology 2005; 146: 4281–4291. [DOI] [PubMed] [Google Scholar]
- Sharma TP, Herkimer C, West C, Ye W, Birch R, Robinson JE, Foster DL, Padmanabhan V.Fetal programming: prenatal androgen disrupts positive feedback actions of estradiol but does not affect timing of puberty in female sheep. Biol Reprod 2002; 66: 924–933. [DOI] [PubMed] [Google Scholar]
- Wood RI, Foster DL.Sexual differentiation of reproductive neuroendocrine function in sheep. Rev Reprod 1998; 3: 130–140. [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Ye W, Phillips DJ, Herkimer C, Knight PG, Padmanabhan V.Developmental programming: deficits in reproductive hormone dynamics and ovulatory outcomes in prenatal, testosterone-treated sheep. Biol Reprod 2008; 78: 636–647. [DOI] [PubMed] [Google Scholar]
- Forsdike RA, Hardy K, Bull L, Stark J, Webber LJ, Stubbs S, Robinson JE, Franks S.Disordered follicle development in ovaries of prenatally androgenized ewes. J Endocrinol 2007; 192: 421–428. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Steckler TL, Welch KB, Inskeep EK, Padmanabhan V.Fetal programming: prenatal testosterone treatment leads to follicular persistence/luteal defects; partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology 2006; 147: 1997–2007. [DOI] [PubMed] [Google Scholar]
- Steckler T, Manikkam M, Inskeep EK, Padmanabhan V.Developmental programming: follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology 2007; 148: 3532–3540. [DOI] [PubMed] [Google Scholar]
- Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V.Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology 2005; 146: 3185–3193. [DOI] [PubMed] [Google Scholar]
- West C, Foster DL, Evans NP, Robinson J, Padmanabhan V.Intra-follicular activin availability is altered in prenatally-androgenized lambs. Mol Cell Endocrinol 2001; 185: 51–59. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Manikkam M, Recabarren S, Foster D.Prenatal testosterone excess programs reproductive and metabolic dysfunction in the female. Mol Cell Endocrinol 2006; 246: 165–174. [DOI] [PubMed] [Google Scholar]
- Recabarren SE, Padmanabhan V, Codner E, Lobos A, Duran C, Vidal M, Foster DL, Sir-Petermann T.Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. Am J Physiol Endocrinol Metab 2005; 289: E801–E806. [DOI] [PubMed] [Google Scholar]
- Homburg R, Amsterdam A.Polysystic ovary syndrome—loss of the apoptotic mechanism in the ovarian follicles? J Endocrinol Invest 1998; 21: 552–557. [DOI] [PubMed] [Google Scholar]
- Hughesdon PE.Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis”. Obstet Gynecol Surv 1982; 37: 59–77. [DOI] [PubMed] [Google Scholar]
- Maciel GA, Baracat EC, Benda JA, Markham SM, Hensinger K, Chang RJ, Erickson GF.Stockpiling of transitional and classic primary follicles in ovaries of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2004; 89: 5321–5327. [DOI] [PubMed] [Google Scholar]
- Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, Franks S.Formation and early development of follicles in the polycystic ovary. Lancet 2003; 362: 1017–1021. [DOI] [PubMed] [Google Scholar]
- Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE.Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology 2003; 144: 1426–1434. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Scaramuzzi RJ, Short RV.Ovulation in prenatally androgenized ewes. J Endocrinol 1977; 73: 385–389. [DOI] [PubMed] [Google Scholar]
- Bassett SG, Pepe GJ.Utilization of circulating androstenedione and testosterone for estradiol production during gestation in the rat. Biol Reprod 1987; 37: 606–611. [DOI] [PubMed] [Google Scholar]
- Leung ST, Reynolds TS, Wathes DC.Regulation of oxytocin receptor in the placentome capsule throughout pregnancy in the ewe: the possible role of oestradiol receptor, progesterone receptor and aromatase. J Endocrinol 1998; 158: 173–181. [DOI] [PubMed] [Google Scholar]
- Juengel JL, Sawyer HR, Smith PR, Quirke LD, Heath DA, Lun S, Wakefield SJ, McNatty KP.Origins of follicular cells and ontogeny of steroidogenesis in ovine fetal ovaries. Mol Cell Endocrinol 2002; 191: 1–10. [DOI] [PubMed] [Google Scholar]
- Quirke LD, Juengel JL, Tisdall DJ, Lun S, Heath DA, McNatty KP.Ontogeny of steroidogenesis in the fetal sheep gonad. Biol Reprod 2001; 65: 216–228. [DOI] [PubMed] [Google Scholar]
- Juengel JL, Heath DA, Quirke LD, McNatty KP.Oestrogen receptor alpha and beta, androgen receptor and progesterone receptor mRNA and protein localisation within the developing ovary and in small growing follicles of sheep. Reproduction 2006; 131: 81–92. [DOI] [PubMed] [Google Scholar]
- Hillier SG, Ross GT.Effects of exogenous testosterone on ovarian weight, follicular morphology and intraovarian progesterone concentration in estrogen-primed hypophysectomized immature female rats. Biol Reprod 1979; 20: 261–268. [DOI] [PubMed] [Google Scholar]
- Murray AA, Gosden RG, Allison V, Spears N.Effect of androgens on the development of mouse follicles growing in vitro. J Reprod Fertil 1998; 113: 27–33. [DOI] [PubMed] [Google Scholar]
- Cassali GD, Nascimento EF, Cardoso JS, Ferreira DL.Morphological and pathological aspects of the rete ovarii in sheep (Ovis aries). Arq Bras Med Vet Zootec 2000; 52: 47–52. [Google Scholar]
- Byskov AG.The role of the rete ovarii in meiosis and follicle formation in the cat, mink and ferret. J Reprod Fertil 1975; 45: 201–209. [DOI] [PubMed] [Google Scholar]
- Byskov AG, Lintern-Moore S.Follicle formation in the immature mouse ovary: the role of the rete ovarii. J Anat 1973; 116: 207–217. [PMC free article] [PubMed] [Google Scholar]
- Zamboni L, Bezard J, Mauleon P.The role of the mesonephros in the development of the sheep fetal ovary. Ann Biol Anim Bioch Biophys 1979; 19: 1153–1178. [Google Scholar]
- Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V.Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology 2004; 145: 790–798. [DOI] [PubMed] [Google Scholar]
- Whitaker K. Comprehensive Perinatal and Pediatric Respiratory Care. Albany, NY:: Delmar, Thomson Learning;; 2001. [Google Scholar]
- Wood RI, Ebling FJ, I'Anson H, Bucholtz DC, Yellon SM, Foster DL.Prenatal androgens time neuroendocrine sexual maturation. Endocrinology 1991; 128: 2457–2468. [DOI] [PubMed] [Google Scholar]
- Herbosa CG, Wood RI, I'Anson H, Foster DL.Prenatal photoperiod and the timing of puberty in the female lamb. Biol Reprod 1994; 50: 1367–1376. [DOI] [PubMed] [Google Scholar]
- Bancroft JDaC H.C. Manual of Histological Techniques. Edinburgh, Great Britain:: Churchill Livingstone;; 1984. [Google Scholar]
- Smith P, O WS, Corrigan KA, Smith T, Lundy T, Davis GH, McNatty KP.Ovarian morphology and endocrine characteristics of female sheep fetuses that are heterozygous or homozygous for the inverdale prolificacy gene (fecX1). Biol Reprod 1997; 57: 1183–1192. [DOI] [PubMed] [Google Scholar]
- Smith P, O WS, Hudson NL, Shaw L, Heath DA, Condell L, Phillips DJ, McNatty KP.Effects of the Booroola gene (FecB) on body weight, ovarian development and hormone concentrations during fetal life. J Reprod Fertil 1993; 98: 41–54. [DOI] [PubMed] [Google Scholar]
- Da Silva-Buttkus P, van den Hurk R, te Velde ER, Taverne MA.Ovarian development in intrauterine growth-retarded and normally developed piglets originating from the same litter. Reproduction 2003; 126: 249–258. [DOI] [PubMed] [Google Scholar]
- Smith P, Braw-Tal R, Corrigan K, Hudson NL, Heath DA, McNatty KP.Ontogeny of ovarian follicle development in Booroola sheep fetuses that are homozygous carriers or non-carriers of the FecB gene. J Reprod Fertil 1994; 100: 485–490. [DOI] [PubMed] [Google Scholar]
- Lundy T, Smith P, O'Connell A, Hudson NL, McNatty KP.Populations of granulosa cells in small follicles of the sheep ovary. J Reprod Fertil 1999; 115: 251–262. [DOI] [PubMed] [Google Scholar]
- Hay MR, Cran DG, Moor RM.Structural changes occurring during atresia in sheep ovarian follicles. Cell Tissue Res 1976; 169: 515–529. [DOI] [PubMed] [Google Scholar]
- Sawyer HR, Smith P, Heath DA, Juengel JL, Wakefield SJ, McNatty KP.Formation of ovarian follicles during fetal development in sheep. Biol Reprod 2002; 66: 1134–1150. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ.Stereology of arbitrary particles: a review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc 1986; 143: 3–45. [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB.The efficiency of systematic sampling in stereology and its prediction. J Microsc 1987; 147: 229–263. [DOI] [PubMed] [Google Scholar]
- Mardia KV, Kent JT, Bibby JM. Multivariate Analysis. London:: Academic Press;; 1979. [Google Scholar]
- McCullagh P, Nelder JA. Generalized Linear Models. London:: Chapman and Hall;; 1989. [Google Scholar]
- Britt KL, Saunders PK, McPherson SJ, Misso ML, Simpson ER, Findlay JK.Estrogen actions on follicle formation and early follicle development. Biol Reprod 2004; 71: 1712–1723. [DOI] [PubMed] [Google Scholar]
- Pentikainen V, Erkkila K, Suomalainen L, Parvinen M, Dunkel L.Estradiol acts as a germ cell survival factor in the human testis in vitro. J Clin Endocrinol Metab 2000; 85: 2057–2067. [DOI] [PubMed] [Google Scholar]
- Zachos NC, Billiar RB, Albrecht ED, Pepe GJ.Developmental regulation of baboon fetal ovarian maturation by estrogen. Biol Reprod 2002; 67: 1148–1156. [DOI] [PubMed] [Google Scholar]
- Goldzieher JW, Green JA.The polycystic ovary. I. Clinical and histologic features. J Clin Endocrinol Metab 1962; 22: 325–338. [DOI] [PubMed] [Google Scholar]
- Lunde O, Hoel PS, Sandvik L.Ovarian morphology in patients with polycystic ovaries and in an age-matched reference material: a statistical evaluation of 149 cases. Gynecol Obstet Invest 1988; 25: 192–201. [DOI] [PubMed] [Google Scholar]
- Vendola K, Zhou J, Wang J, Famuyiwa OA, Bievre M, Bondy CA.Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod 1999; 61: 353–357. [DOI] [PubMed] [Google Scholar]
- Hickey TE, Marrocco DL, Amato F, Ritter LJ, Norman RJ, Gilchrist RB, Armstrong DT.Androgens augment the mitogenic effects of oocyte-secreted factors and growth differentiation factor 9 on porcine granulosa cells. Biol Reprod 2005; 73: 825–832. [DOI] [PubMed] [Google Scholar]
- McNatty KP, Smith P, Moore LG, Reader K, Lun S, Hanrahan JP, Groome NP, Laitinen M, Ritvos O, Juengel JL.Oocyte-expressed genes affecting ovulation rate. Mol Cell Endocrinol 2005; 234: 57–66. [DOI] [PubMed] [Google Scholar]
- Bloomfield FH, Oliver MH, Hawkins P, Holloway AC, Campbell M, Gluckman PD, Harding JE, Challis JR.Periconceptional undernutrition in sheep accelerates maturation of the fetal hypothalamic-pituitary-adrenal axis in late gestation. Endocrinology 2004; 145: 4278–4285. [DOI] [PubMed] [Google Scholar]
- Lass A.Assessment of ovarian reserve: is there still a role for ovarian biopsy in the light of new data? Hum Reprod 2004; 19: 467–469. [DOI] [PubMed] [Google Scholar]
- Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL.Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 2004; 428: 145–150. [DOI] [PubMed] [Google Scholar]
- Archibald LF, Schultz RH, Fahning ML, Kurtz HJ, Zemjanis R.Rete ovarii in heifers: a preliminary study. J Reprod Fertil 1971; 26: 413–414. [DOI] [PubMed] [Google Scholar]
- Wenzel JG, Odend'hal S, Player EC.Histological and histochemical characterization of the bovine rete ovarii through the estrous cycle and gestation. Anat Histol Embryol 1987; 16: 124–135. [DOI] [PubMed] [Google Scholar]
- Douglas DC, Nakhuda GS, Sauer MV, Zimmermann RC.Angiogenesis and ovarian function. J für Fertilität und Reproduktion 2005; 15: 7–14. [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: a key regulator of physiological angiogenesis. Boston, MA:: Birkhauser;; 2001. [Google Scholar]
- Ferrara N, Frantz G, LeCouter J, Dillard-Telm L, Pham T, Draksharapu A, Giordano T, Peale F.Differential expression of the angiogenic factor genes vascular endothelial growth factor (VEGF) and endocrine gland-derived VEGF in normal and polycystic human ovaries. Am J Pathol 2003; 162: 1881–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Thompson RC, Herkimer C, Welch KB, Flak J, Karsch FJ, Padmanabhan V.Developmental programming: impact of prenatal testosterone excess on pre- and postnatal gonadotropin regulation in sheep. Biol Reprod 2008; 78: 648–660. [DOI] [PubMed] [Google Scholar]