Abstract
Maternal B-vitamin status and homocysteinemia can affect fertility and pregnancy establishment, although the direct effects on ovarian follicle and oocyte development are not known. We report on the effects of restricting the supply of vitamin B12 and methionine from the diet of mature female sheep on ovarian folliculogenesis following follicle-stimulating hormone (FSH) stimulation. The study was split into three batches and involved 76 animals. Surprisingly, the number of growing, estrogen-active antral follicles following FSH treatment was enhanced (P = 0.005) following this dietary intervention. This increase occurred even in the presence of modest live-weight loss (batch 1 only) and depressed plasma insulin concentrations, suggesting a breakdown in the regulation of follicular responsiveness to FSH. This dietary intervention also increased plasma homocysteine concentrations. Physiological concentrations of homocysteine increased granulosa cell proliferation (P < 0.001), estradiol production (P = 0.05), and FSHR transcript expression (P = 0.017) during culture. Transcript levels for growth differentiation factor 9 and bone morphogenetic protein 15 in oocytes from treated ewes were increased (P < 0.05) in the first two batches. Furthermore, regression of BMP receptor 2 (BMPR2) transcript expression and diet on follicle number revealed a significant interaction (P = 0.01); BMPR2 transcript expression was associated with follicle number only in vitamin B12/methionine-restricted animals. Because FSHR transcript expression also was positively (P = 0.007) related to follicle number, the effects of diet may have arisen through enhanced FSH and BMP signaling. Although this remains to be confirmed, the data support an intraovarian impact of vitamin B12/methionine-deficient diets.
Keywords: AMH, follicle, follicle-stimulating hormone, follicle-stimulating hormone receptor, follicular development, granulosa cells, homocysteine, ovarian follicle, vitamin B12
Maternal B-vitamin deficiency, leading to hyper-homocysteinemia, can reduce fertility but, curiously, it can also enhance ovarian response to FSH, possibly by enhanced FSH and Bone Morphogenetic Protein (BMP) signaling via BMP Receptor 2.
INTRODUCTION
There is compelling evidence to indicate that maternal B-vitamin status during the period leading up to and around the time of conception can have a major impact on fertility, pregnancy establishment, and term delivery [1–3]. Mild hyper-homocysteinemia is prevalent in subjects polymorphic for a number of enzymes involved in folate/methionine metabolism, and/or who consume diets deficient in B vitamins, such as vitamin B12 and folate [4]. Total homocysteine concentrations can be measured in follicular fluid, are sensitive to dietary intakes of folic acid [5], and can determine pregnancy outcome in clinical IVF cycles [6, 7]. Furthermore, elevated plasma homocysteine concentrations are frequently detected in women with polycystic ovarian syndrome [8], although the mechanisms underlying this relationship are unclear.
In spite of these reported outcomes, little is known about the effects of B vitamins on ovarian follicle and oocyte development [9]. Although we have shown that transcripts for all major enzymes involved in the linked methionine/folate cycles are present in human embryonic cells [10], it is not clear how these cycles function in the ovary. One enzyme involved in the metabolism of homocysteine is cystathionine β-synthase (CBS; EC 4.2.1.22). Cbs−/− mice are hyperhomocysteinemic, and females are infertile, although fertility can be restored when CBS-deficient ovaries are transplanted into normal ovarectomized females [11]. However, CBS is known to be highly expressed in cumulus cells, particularly following superovulation, and the functional suppression of Cbs by RNA interference results in a significant increase in the number of germinal vesicle-arrested oocytes in mice [12]. Other than this, the specific effects of B vitamins on ovarian function are not known, although clearly they can have an impact on fertility following natural conception and following assisted reproduction, particularly in stimulated cycles.
We recently reported on the extent to which the periconceptional availability of dietary methyl groups (in particular, vitamin B12 and methionine) can perturb homocysteine metabolism in embryo donor ewes, leading to epigenetic modifications to DNA methylation in offspring that became obese, insulin resistant, and hypertensive [13]. As part of ongoing investigations, to further characterize the metabolic status of our embryo donors, we offered the same experimental diets (i.e., control or methyl deficient [MD] diets) to an additional group of 76 ewes in a single experiment that was split into three batches. Curiously, we observed a significant increase in the number of growing antral follicles in MD ewes relative to controls following follicle-stimulating hormone (FSH) treatment, suggesting that the MD diet might affect mechanisms regulating follicular responsiveness to FSH, thereby increasing the proportion of growing antral follicles. The purpose of the current study, therefore, was to identify these mechanisms in an attempt to better understand how B-vitamin/methionine status can determine ovarian responsiveness to gonadotropin treatment.
MATERIALS AND METHODS
Animals and Dietary Treatments
All procedures were reviewed by the Animal Ethics Committee of the University of Nottingham and conducted in accordance with the requirements of the U.K. Home Office Animals (Scientific Procedures) Act 1986. All reagents were purchased from Sigma-Aldrich (Poole Dorset, U.K.) unless otherwise stated.
A single experiment was conducted which was split into three batches, with one batch per year. A total of 76 Scottish Blackface ewes, with an average body weight (±SEM) of 50.3 ± 0.6 kg, were used. Ewes were allocated to one of two dietary groups on the basis of initial body weight (n = 37 control and n = 39 MD). Because it can take several weeks in order to deplete sheep of endogenous reserves of vitamin B12 [14], all ewes were offered a diet of cobalt-deficient barley and hay (<0.04 mg/kg dry matter) over a 4- to 6-wk period prior to the introduction of experimental diets. During this period, ewes were housed singly and offered around 300–400 g of barley and around 600 g of hay daily. Animals were allocated to their experimental diets as soon as mean plasma vitamin B12 concentrations fell below 250 pM, as determined from weekly blood samples collected by jugular venipuncture into ethylenediaminetetraacetic acid-treated tubes.
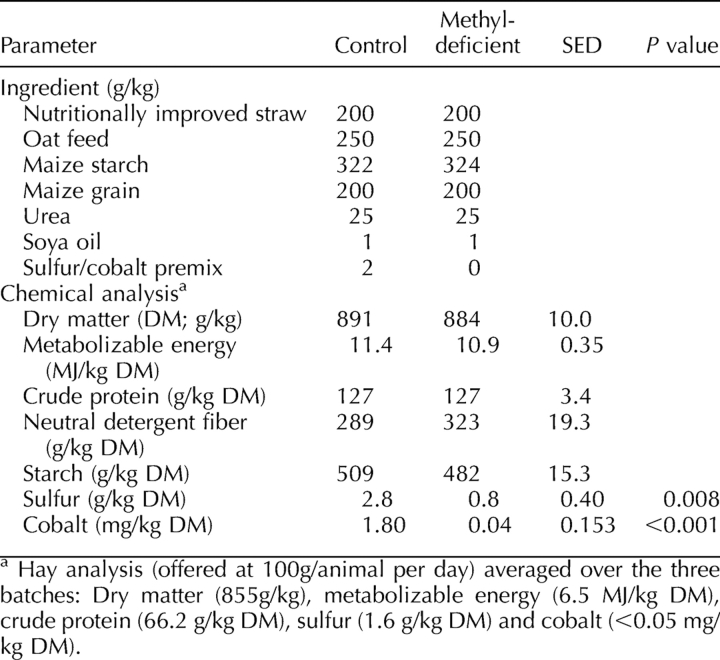
Ewes were offered a complete pelleted diet (Table 1) identical to that offered by Sinclair et al. [13] and calculated to fully meet their energy and protein requirements [15] for a period lasting 7 wk. The two experimental diets were chemically identical except for their content of elemental cobalt and sulfur, which were reduced in the MD diet relative to the control diet in order to diminish the capacity of rumen microorganisms to synthesize sulfur amino acids and vitamin B12, respectively [15]. In addition, control ewes were given a cobalt bolus (containing 3 g cobalt oxide; Rumetrace; Cox Surgical, Surrey, U.K.) at the introduction of experimental diets. In contrast, MD ewes received no such bolus and were placed on the MD diet. The concentrate component of each diet was offered at 900 g/day. Ewes remained on these experimental diets for 49 days, when they were killed and tissues collected.
TABLE 1.
Ingredients and chemical analyses of control and methyl-deficient diets.
Estrous Synchronization, Ovarian Stimulation, and Tissue Collection Postmortem
Full details of the estrous synchronization and ovarian stimulation procedures employed were documented previously [16, 17] and were identical to those employed by Sinclair et al. [13]. Briefly, estrous cycles were synchronized toward the end of the experimental period by the use of progestogen-impregnated intravaginal sponges (30 mg of fluorogestone acetate; Chronogest; Intervet) and prostaglandin F2α (250 μg/ml cloprostenol; Estrumate; Schering-Plough Animal Health), administered intramuscularly on two occasions 7 days apart. Sponges remained in position for 7 days, after which time they were removed and a fresh sponge inserted intravaginally, where it remained for a further 5 days. Ovarian stimulation was induced using a total of 9 mg of ovine FSH (Ovagen; ICP Ltd., Auckland, New Zealand) administered intramuscularly twice daily in equal doses over 4 days beginning on Day 10 of the 12-day progestogen priming period. Intravaginal sponges were withdrawn, on average, 26 h (24–28 h) prior to killing, whereas the final dose of FSH was administered, on average, 17 h (15–19 h) prior to killing.
Following killling by captive bolt and exsanguination, ovaries were recovered, the diameter of individual antral follicles measured using calipers, and follicle number counted. Follicular fluid, oocytes, and granulosa cells were aspirated and pooled from all visible follicles within an animal. Cumulus-oocyte complexes were identified and oocytes denuded by vortexing in 500 μl of PBS containing 0.1% polyvinyl alcohol (PVA). Denuded oocytes were snap frozen in minimal-volume (2–3 μl) PBS/PVA and stored at −80°C until analysis. The remaining aspirants were centrifuged at 500 × g for 10 min, and follicular fluid was stored at −80°C prior to analysis. Granulosa cells were washed in 10 ml of 1× PBS, snap frozen in liquid nitrogen, and stored at −80°C in minimal-volume PBS/PVA until analysis.
Metabolic Analyses
Plasma vitamin B12 and folate were measured in plasma using a commercially available kit (Simultrac Radioassay Kit Vitamin B12 [57Co]/folate [125I]; MP Biomedicals, Strasbourg, France) as described previously [13]. Thiols in plasma were analyzed using an Agilent 1100 HPLC system (Agilent Technologies, Stockport, U.K.), and amino acids (including methionine) in plasma were analyzed using an amino acid analyzer (Pharmacia LKB; Biochrom Ltd., Cambridge, UK) with ninhydrin detection, according to previously published in-house protocols [10]. This method for thiol analysis was modified from the original method of Pfeiffer et al. [18] to reduce the pH of the mobile phase in order to facilitate better separation of cysteinyl glycine and glutathione. For amino acid analysis, the cytosol preparation (490 μl) was mixed with 10 μl of norleucine internal standard and 30 mg of 5 sulphosalicylic acid and was allowed to stand at 4°C for 30 min. After centrifugation at 16 000 × g for 10 min at 4°C, the supernatant was passed through a polyvinylidene fluoride Millipore syringe-driven filter unit with 0.22-μm pore size, and amino acids were measured using Biochrom 20 amino acid analyzer with ninhydrin detection (Pharmacia LKB; Biochrom). Peak integration was performed using EZChrom Elite Software (Scientific Software Inc., San Ramon, CA).
Methylmalonic acid (MMA) determination was based on the method of Shroads et al. [19], with minor modification. The methyl esters were analyzed by Agilent 6890N gas chromatography using an online 5973N mass-selective detector (MSD). The column was a DB-WAX (30 m × 0.25 mm; 0.15-μm film thickness). The injection volume was 1 μl for SCAN mode for qualification and 0.2 μl for selected ion monitoring mode for quantification, both using splitless mode. The injector and detector temperatures were maintained at 250°C and 280°C, respectively. The carrier gas (He) was set at a constant flow rate of 1 ml/min. The chromatograph was programmed for an initial temperature of 35°C for 5 min, raised to 200°C at 8°C min−1, then to 220 at 18°C min−1, and held for 2 min at the final temperature. The MSD was tuned daily and operated in electron impact mode at an ionization energy of 70 eV.
Plasma samples were analyzed for glucose using an Imola Autoanalyser (RX imola; Randox Laboratories Ltd., Antrim, U.K.). The kit for glucose was supplied by Randox Laboratories (Glucose GOD PAP; catalogue no. GL2623).
Primary Granulosa Cell Culture
Ovine granulosa cells from small (2–4 mm) antral follicles were cultured under serum-free conditions based on the method of Campbell et al. [20]. All reagents and media were obtained from Sigma-Aldrich unless otherwise stated. Briefly, ovaries were transported from a local abattoir to the laboratory in warm (37°C) PBS where, on arrival, they were further washed in warm PBS prior to follicular aspiration. Aspirants were transferred to a 60-mm dish, under sterile conditions, containing 0.1% solution of PVA/PBS, and all cumulus-oocyte complexes were recovered. The remaining cells and fluids were centrifuged in 15-ml conical tubes at 300 × g for 5 min, and the granulosa cell pellet was resuspended in 10 ml of 1× PBS prior to a second centrifugation. Granulosa cells were resuspended in culture medium (90% v/v TCM199 and 10% v/v double-distilled water supplemented with 0.1% w/v BSA [fatty acid free], 0.01% w/v l-glutamine, 1 ng/ml FSH [i.e., 0.02 U/ml; catalogue no. F2293; Sigma-Aldrich], 10 ng/ml insulin, 0.5 μg/ml bovine transferrin, 0.5 ng/ml sodium selenite, 50 IU/ml penicillin, and 50 μg/ml streptomycin) and taken through a third wash cycle. Granulosa cells were incubated in 24-well plates at an initial seeding density of 3.5 × 105 viable cells (determined by Trypan blue exclusion) in 500 μl of media for 144 h at 38.8°C in a humidified atmosphere of 5% CO2 in air, with 80% of the medium renewed at 48 h. Spent media collected at 144 h were stored at −20°C until estradiol and progesterone analysis. The number of viable cells at 144 h was determined by Trypan blue exclusion.
To assess the effects of homocysteine and MMA on granulosa cells during culture, these metabolites were added to media at physiological concentrations (homocysteine: 0, 10, 50, and 100 μM [13, 14]; and MMA: 0, 0.85, 8.5, and 85 μM [13, 14]) after the initial 48-h period of culture, and effects were recorded at 144 h. Culture media were replenished every 48 h. For each metabolite, the four doses were applied within a single 24-well plate (i.e., six wells per dose per plate), and there were seven plates (replicates) per metabolite.
Hormone Analysis
Plasma insulin concentrations were determined using a 125I-labeled insulin double-antibody radioimmunoassay (RIA), as described previously [21]. The assay used porcine insulin (I-3505; 24 IU/mg) obtained from Sigma Co. Ltd., as well as guinea pig antiporcine insulin, normal guinea pig serum, and sheep anti-guinea pig immunoglobulin G (IgG) obtained as a gift from the Scottish Antibody Production Unit, Law Hospital (Carluke, Scotland). The [125I] porcine insulin solution was prepared using the chloramine T method. The minimum detection limit of the assay at 80% effective dose (ED80) was 2.6 μIU/ml. Mean (for low, medium, and high controls) intraassay and interassay coefficients of variation were 7.2% and 9.4%, respectively.
Follicular fluid and culture media estradiol (diluted 1:50 and 1:20, respectively) were analyzed without prior extraction using a [125I] estradiol double-antibody RIA [22]. The assay was modified and validated to enable use of a rabbit anti-estradiol first antibody, donkey anti-rabbit IgG, and normal rabbit serum, obtained as gifts from the Scottish Antibody Production Unit. The sensitivity of the assay at ED80 was 44.0 pg/ml. Mean (for low, medium, and high controls) intraassay and interassay coefficients of variation were 7.8% and 13.5%, respectively. Similarly, follicular fluid and culture media progesterone (diluted 1:100) were analyzed without prior extraction using a [125I] progesterone double-antibody RIA [23]. The assay was modified and validated to enable the use of a rabbit anti-progesterone first antibody, donkey anti-rabbit IgG, and normal rabbit serum, obtained as gifts from the Scottish Antibody Production Unit. The sensitivity of the assay at ED80 was 0.54 ng/ml. Mean (for low, medium, and high controls) intraassay and interassay coefficients of variation were 9.1% and 14.5%, respectively.
Transcript Analysis
In support of ongoing studies, it was necessary that granulosa cells from the first two batches were dedicated to amino acid and thiol analyses; some of these data are available as Supplemental Data (all Supplemental Data are available online at www.biolreprod.org). Consequently, although transcript analysis in oocytes was conducted across all three batches, only granulosa cells from the third batch in the series were available for transcript analysis.
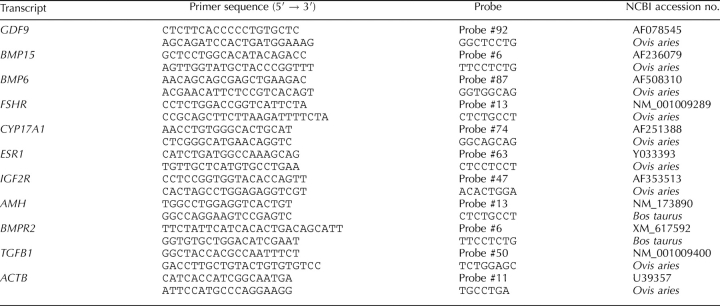
Transcripts for the following genes (Table 2) were selected for analysis by quantitative real-time PCR because of their known stimulatory or inhibitory effects on granulosa cells and ovarian follicles [24, 25]. Growth differentiation factor 9 (GDF9) and bone morphogenetic proteins 15 and 6 (BMP15 and BMP6) are three transforming growth factor superfamily-β members expressed by oocytes. Also included were follicle-stimulating hormone receptor (FSHR), estrogen receptor 1 (ESR1), insulin-like growth factor receptor 2 (IGF2R), anti-Mullerian hormone (AMH), bone morphogenetic protein receptor 2 (BMPR2), and transforming growth factor-β1 (TGFB1). Transcript expression of actin-beta (ACTB) served as a reference.
TABLE 2.
Primers and probes used for real-time quantitative PCR analysis of transcript expression in oocytes and granulosa cells.
Poly A+ RNA was isolated from groups of typically 10 to 40 oocytes (pooled within animal) using Dynabeads Oligo (dT)25 (Dynabeads mRNA DIRECT; Invitrogen Ltd., Paisley, U.K.) according to the method of Kwong et al. [26]. Reverse transcription (RT, Sensiscript Reverse Transcriptase kit; Qiagen) containing 1 μl of random hexamers (36 μg/ml; Promega Ltd.) was followed by PCR amplification using sequence-specific primers and Universal Library Probes (Roche Diagnostics Ltd.; Table 2). The amount of RNA extracted from oocytes was too small to directly quantify. However, working with RNA extracted from groups of oocytes (10 through 40 in duplicate), a preliminary experiment established that the ratio of the transcript of interest to the housekeeping transcript (i.e., ACTB) was unaltered by oocyte number. Polymerase chain reaction was run in duplicate according to the manufacturer's instructions. Because primers for GDF9, BMP15, and BMP6 could not be designed to span two exons, so the following checks were conducted. To ensure the absence of DNA contamination, the remaining poly A+ RNA was used in same reverse transcription reaction, as described above, except that Sensicript enzyme was omitted. A PCR reagent control only also was run at the same time. Agarose gel electrophoresis of PCR products was run in duplicate to confirm further the specificity of primers.
RNA was extracted from granulosa cells using AllPrep DNA/RNA/Protein kit (Qiagen) according to the manufacturer's instructions. RNA yield was quantified using an ND-1000 Nanodrop spectrophometer (Nanodrop, Wilmington, DE) and quality determined using an Agilent Bioanalyzer (Agilent Technologies Ltd.). Only RNA with an integrity number higher than 7 was used. A total of 500 ng of RNA was used for RT, which was conducted using the Transcriptor first-strand cDNA kit (Roche Diagnostics Ltd) according to manufacturer's instructions. Polymerase chain reaction amplification, run in duplicate, used sequence-specific primers and Universal Library Probes (Table 2), as described above. Once again, ACTB served as the reference gene in all reactions, and RT- and PCR-negative reactions and agarose gel electrophoresis were conducted as before to confirm the specificity of primers and absence of genomic DNA.
Statistical Analyses
Body weights were recorded at fortnightly intervals prior to and during the experimental period. These data were regressed against time, and the regression coefficients were analyzed by ANOVA using Genstat release 9.1 [27]. Plasma metabolites, including amino acids, and insulin were measured on at least two occasions within the experiment, and so were analyzed using repeated-measures ANOVA within Genstat. Terms fitted to these regression models were batch (one to three), sample date within batch, dietary treatment, and interactions between these terms. Follicle number was analyzed using a generalized linear model assuming Poisson errors and with log transformations. Terms fitted to this model were batch and dietary treatment. Follicular fluid concentrations of steroid hormones also were analyzed by ANOVA. Estradiol data were not normally distributed and so were log transformed prior to analysis. Data are presented as geometric means. Transcript expression (relative to ACTB) for each of the three genes (GDF9, BMP15, and BMP6) assessed in oocytes for all three batches was analyzed by ANOVA. Residual plots confirmed that the conditions of normality and homogeneity of variance were met. Terms fitted to these models were batch, diet, and interactions between these terms. Follicle number within each of the three batches was regressed against transcript expression using generalized linear models as described before. Terms fitted to these models were diet and transcript expression. Transcript expression (relative to that of ACTB) in granulosa cells from batch 3 was also analyzed by ANOVA. Follicle number was again regressed against transcript expression using models described previously. Terms fitted to these models were diet, transcript expression, and interactions between these terms. Estradiol and progesterone concentrations in spent culture media were log transformed prior to analysis in order to normalize the data and standardize errors. When it came to analyzing the effects of homocysteine and MMA dose on cell proliferation and transcript expression in granulosa cells, the three degrees of freedom associated with the four levels of each of these two metabolites were partitioned into linear and quadratic contrasts, with a residual error term. This permitted the direct assessment of metabolite concentration on gene expression.
RESULTS
Metabolic Effects of Diets
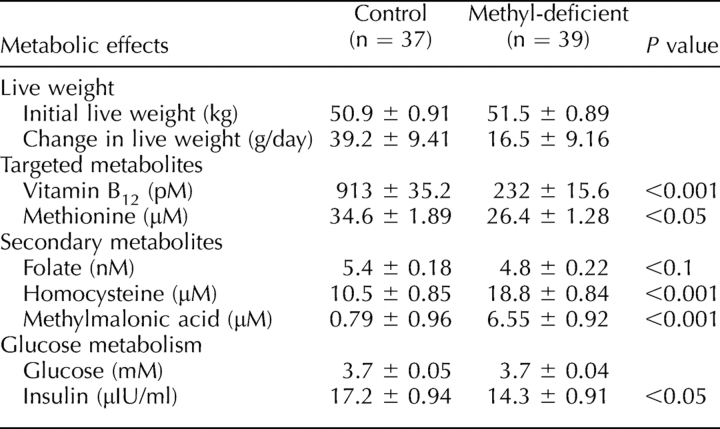
There was no significant difference in body weight between the two experimental groups of animals at the beginning of the experiment (Table 3). Although there was no difference in live-weight gain between the two dietary groups averaged over the three batches, MD ewes in batch 1 actually lost a small amount of weight (50.3 g/day), whereas control ewes gained weight (15.2 g/day; P = 0.05). Plasma vitamin B12 concentrations were reduced (P < 0.001) in MD compared with control ewes over the three batches (Table 3). Plasma methionine concentration also was lower (P < 0.05) in MD than in control ewes. Plasma folate was reduced (P < 0.05) in MD compared with control ewes in batch 1 but not in batches 2 and 3. In contrast, plasma homocysteine and MMA concentrations averaged over the three batches were greater (P < 0.001) in MD compared with control ewes. Whereas plasma glucose concentrations were unaffected by diet, plasma insulin concentrations were lower (P < 0.05) in MD compared with control ewes.
TABLE 3.
Metabolic effects (live-weight change and selected plasma metabolites) of experimental diets.
Ovarian Follicle Development
Averaged across the three batches, mean follicle number was greater (P = 0.005) in MD than in control ewes (39.6 ± 2.1 vs. 29.6 ± 2.3 follicles; Fig. 1A). However, although there was a significant effect of diet on mean follicle number following ovarian stimulation in both batches 1 (P = 0.007) and 2 (P = 0.034), this was not the case in batch 3 (Fig. 1B). Regression of follicle number on diet and granulosa cell homocysteine concentration in batch 1 revealed a significant (P = 0.003) effect of homocysteine, in addition to diet, on follicle number (Supplemental Fig. S1A). However, there was no treatment effect on follicle diameter, which averaged 4.2 ± 0.06 mm. There was, however, a significant increase (P < 0.05) in follicular fluid estradiol concentrations in MD ewes compared with control ewes (geometric means of 30.6 vs. 21.3 ng/ml for MD vs. control ewes). Furthermore, follicle fluid estradiol concentration was positively (P = 0.002) related to granulosa cell homocysteine concentration (Supplemental Fig. S1B). In contrast, there was no significant difference in follicular fluid progesterone concentrations (geometric means of 15.6 vs. 16.2 for control and MD ewes). However, there was an increase (P < 0.05) in the ratio of estradiol to progesterone in follicular fluid for MD relative to control ewes (geometric means of 2.13 vs. 1.46 for MD vs. control ewes).
FIG. 1.
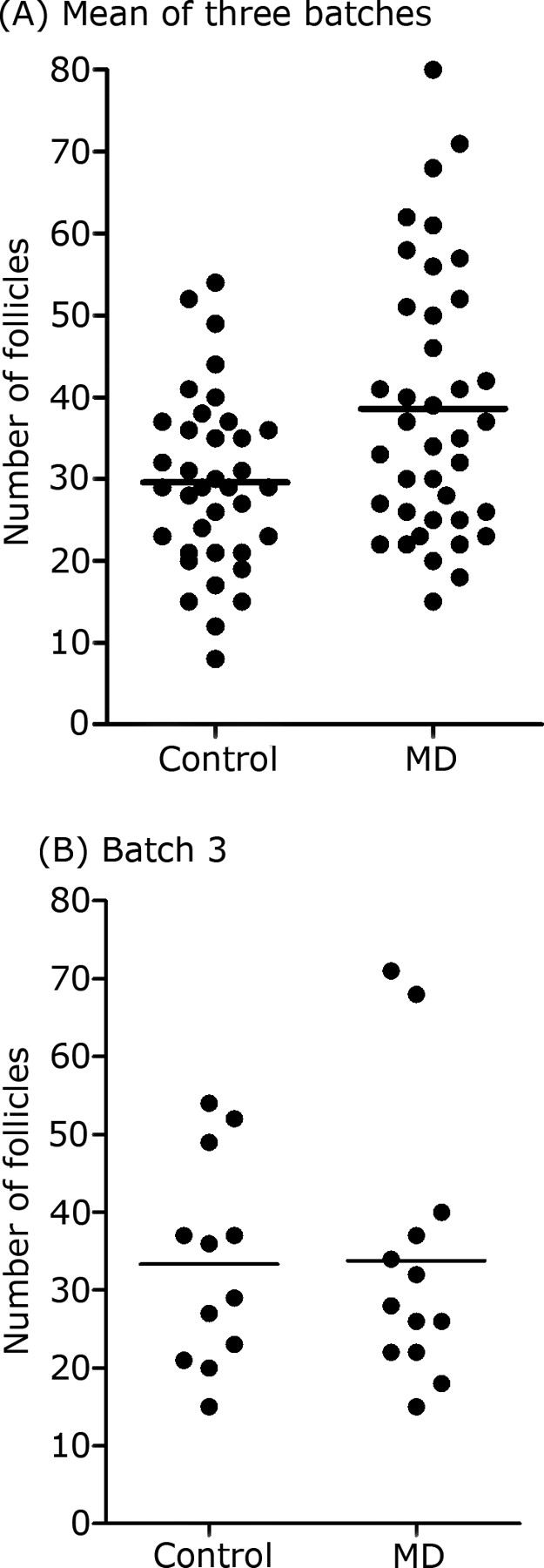
Follicle number (scatter plots with means [horizontal lines]) by treatment for all three batches (A) and for batch 3 (B).
Transcript Expression in Ovarian Follicles
In batches 1 and 2, where there was a significant increase in follicle number due to diet, there was also an increase in expression of GDF9 (batch 1, P = 0.017; batch 2, P = 0.036) and BMP15 mRNA (batch 1, P = 0.045; batch 2, P = 0.028) in oocytes from MD ewes compared with control ewes (Table 4). However, relative transcript abundance was greater in batch 3, where there was no significant treatment difference in expression of transcripts from either of these two genes. For the experiment as a whole, however, GDF9 was significantly correlated (r = 0.80; P < 0.001) with BMP15. BMP6 mRNA expression was not affected by diet in batches 1 and 3, although it differed (P = 0.047) between treatments in batch 2. BMP6 mRNA expression was significantly (P < 0.001) correlated with both GDF9 (r = 0.96) and BMP15 (r = 0.81) mRNA expression.
TABLE 4.
Effect of ewe diet (control vs methyl-deficient) on transcript expression (mean ± sem), relative to ACTB, for GDF9, BMP15, and BMP6 in oocytes recovered in each of the three batches.
In contrast to oocytes, transcript expression analysis in granulosa cells was restricted to batch 3. Here, quantitative real-time PCR analysis failed to detect differences in the expression of any of the selected transcripts between dietary treatments (expression relative to ACTB for control vs. MD treatments: FSHR, 0.72 vs. 0.71, SED = 0.138; AMH, 0.93 vs. 1.25, SED = 0.244; BMP2R, 3.40 vs. 2.62, SED = 0.645; TGFB1, 0.31 vs. 024, SED = 0.113; IGF2R, 0.37 vs. 0.28, SED = 0.118; and ESR1, 0.17 vs. 0.19, SED = 0.035). The granulosa cell phenotype of our cells was confirmed by determining the abundance of a thecal cell marker (i.e., CYP17 [cytochrome P450 steroid 17α-hydroxylase/17,20 lyase] mRNA expression) in these cells and whole-follicle lysates. CYP17 mRNA expression (relative to ACTB) was 0.782 ± 0.28 and 0.006 ± 0.002 for whole-follicle lysates and granulosa cells, respectively (P = 0.028). In contrast, TGFB1 mRNA expression did not differ between these two pools of cells (relative transcript abundance of 0.313 ± 0.048 and 0.270 ± 0.043), confirming expression of TGFB1 transcripts in sheep granulosa cells as well as thecal cells from antral follicles.
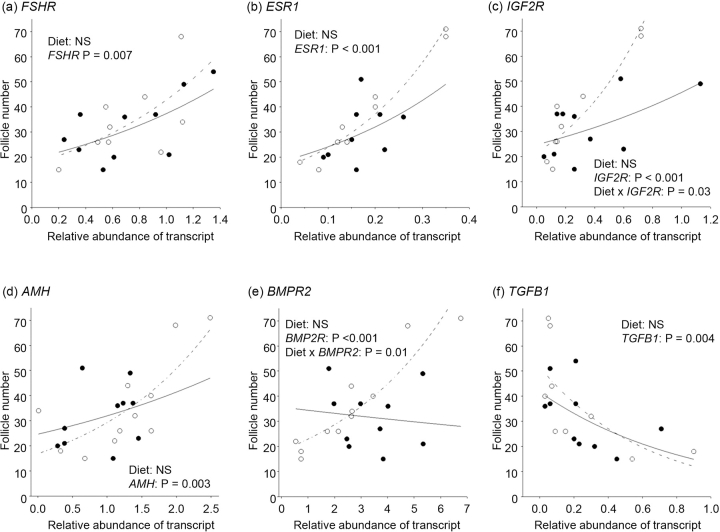
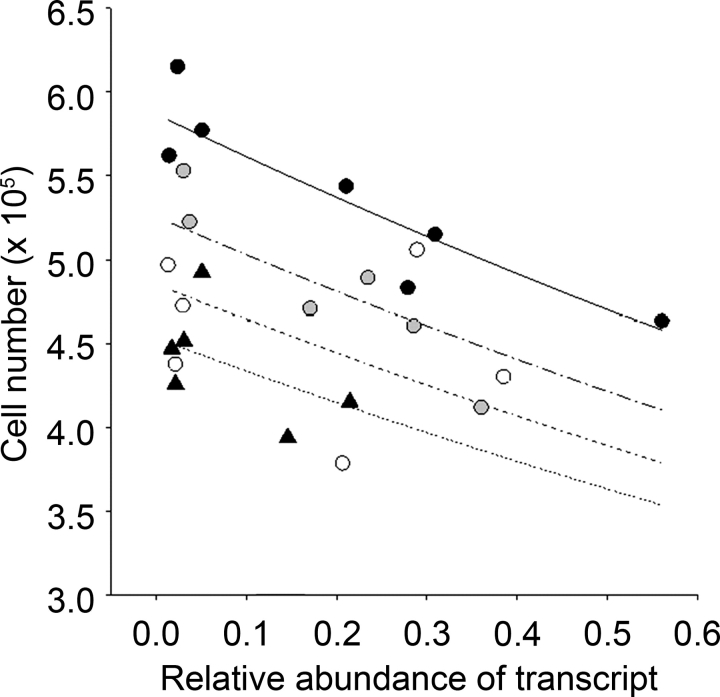
Regression analyses on log-transformed follicle number data using generalized linear models and assuming Poisson errors found that none of the three oocyte-specific transcripts (i.e., GDF9, BMP15, and BMP6) were related to follicle number in any of the three batches once the effects of diet had been accounted for (data not shown). In contrast, the expressions of transcripts for all six granulosa cell-expressed genes were significantly (P ≤ 0.007) related to follicle number (Fig. 2). Although three of these transcripts (i.e., FSHR, ESR1, and AMH) were positively associated with follicle number, TGFB1 was negatively associated with follicle number. Significant (P ≤ 0.03) interactions between diet and transcript expression indicated that both IGF2R and BMPR2 expression were positively associated with follicle number in MD but not control ewes (Fig. 2). Furthermore, FSHR mRNA expression was positively correlated with BMPR2 (r = 0.65; P = 0.009), but was negatively correlated (r = −0.62; P = 0.01) with TGFB1 mRNA expression. AMH mRNA expression was positively correlated with ESR1 mRNA expression (r = 0.67; P = 0.006), but was negatively correlated with TGFB1 mRNA expression (r = −0.66; P = 0.008). Finally, IGF2R mRNA expression also was positively correlated (r = 0.78; P < 0.001) with ESR1 mRNA expression.
FIG. 2.
Regression of follicle number on diet and transcript expression within granulosa cells (batch 3). Terms included in the models were diet (control [closed circles, solid lines] vs. MD [open circles, dashed lines]) and transcript expression and interactions between these terms. NS, not significant.
Effects of Methylmalonic Acid and Homocysteine on Cultured Granulosa Cells
Exposure of granulosa cells for 96 h to physiologically relevant incremental doses of MMA (0, 0.85, 8.5, and 85 μM) had no effect on cell number (5.2 ± 0.3, 5.5 ± 0.3, 5.8 ± 0.4, and 5.5 ± 0.1 × 105 cells, respectively), estradiol (0.93 ± 0.36, 0.81 ± 0.32, 0.66 ± 0.22, and 0.78 ± 0.30 pg/ml, respectively), or progesterone (36.4 ± 5.4, 37.5 ± 6.4, 35.0 ± 5.1, and 35.6 ± 5.7 ng/ml, respectively) concentrations (both steroids adjusted for viable cell number) in spent culture media following 144 h of culture. In contrast, exposure of granulosa cells to physiologically relevant doses of homocysteine for 96 h led to a linear increase (P < 0.001) in cell number (Fig. 3A). This was associated with a significant (P = 0.017) increase in FSHR mRNA expression (Fig. 3B). Homocysteine had no effect on the expression of the other transcripts (i.e., AMH, ESR1, and IGF2R) studied in these cells (data not shown). There was a significant quadratic relationship (P < 0.05) between homocysteine dose and adjusted (for cell number) estradiol concentration in spent culture media at 144 h (0.96 ± 0.28, 1.29 ± 0.32, 1.65 ± 0.30, and 0.80 ± 0.21 pg/ml for 0, 10, 50, and 100 μM homocysteine, respectively). There was no effect of homocysteine on progesterone concentrations in spent media. As for follicle number, each of these four transcripts, together with homocysteine dose, was fitted to generalized linear models of cell number. These analyses revealed a negative (P < 0.001) association between AMH mRNA expression and cell number once the effects of homocysteine dose had been accounted for (Fig. 4). None of the other transcripts had expression significantly associated with cell number in these models.
FIG. 3.
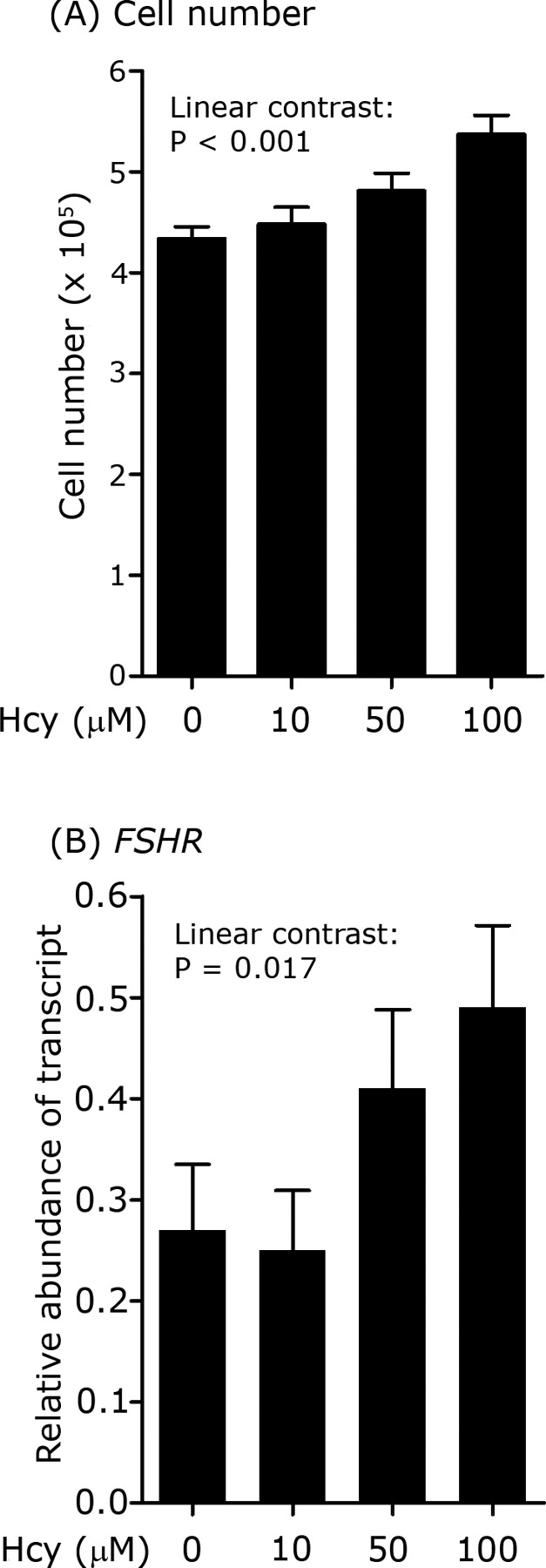
Effect of homocysteine (Hcy) dose on granulosa cell number (A) and the relative abundance of transcripts (B) for FSHR in granulosa cells following 96 h of culture in the presence of homocysteine. Cells were initially plated at density of 3.5 × 105 cells/well.
FIG. 4.
Regression of granulosa cell number on homocysteine dose and the relative abundance of transcripts for AMH following 96 h of culture in the presence of homocysteine. Terms fitted to the model were homocysteine dose (0 μM: solid triangles, dotted line; 10 μM: open circles, fine dashed line; 50 μM: gray circles, dashed line; and 100 μM: black circles, bold dashed line), transcript expression, and interactions between these terms.
DISCUSSION
The novel finding from the current study pertains to the counterintuitive increase in ovarian response, in terms of growing, medium-sized antral follicles, to FSH in animals offered the MD diet. For the most part, this diet led to low plasma vitamin B12, methionine, and folate concentrations and elevated plasma homocysteine and MMA concentrations in ewes (Table 3). The increase in ovarian responsiveness to FSH in MD ewes occurred even in the presence of modest live-weight loss and depressed plasma insulin concentrations (batch 1). A characteristic feature of ovarian follicles in MD ewes was their greater estradiol content. Collectively, these observations suggest a breakdown in the regulation of follicular responsiveness to FSH in MD ewes. However, the expression of transcripts in granulosa cells for some of the key genes known to regulate such responses was unaltered by diet, although these measurements were limited to batch 3, where there was no increase in mean follicle number (Fig. 1). The considerable between-animal variability in follicular response to FSH (Fig. 1) has been noted previously in sheep and other species [28–30], and it may explain the apparent lack of responsiveness in batch 3, which contrasted to the observations made in batches 1 and 2. Nevertheless, although transcript expression was unaffected by diet in batch 3, all transcripts were significantly correlated (either positively or negatively) with follicle number (Fig. 2). Furthermore, transcript levels for GDF9 and BMP15 in oocytes from MD ewes were increased in both batches 1 and 2. Transcripts for these two oocyte-specific genes are normally stably expressed throughout follicle development in sheep [31]. Consequently, their increase further suggests a general breakdown in intrafollicular regulatory mechanisms, the basis of which is not currently understood.
Finally, a characteristic feature of the MD diets was the elevation in plasma homocysteine and MMA concentrations in ewes. Sinclair et al. [13] also reported elevated homocysteine concentrations in granulosa cells recovered from MD ewes, and data from the current study revealed a significant relationship between homocysteine concentration in granulosa cells, follicle number, and follicle fluid estradiol concentration (Supplemental Fig. S1). Consequently, the effects of MMA and homocysteine on cultured granulosa cells were investigated. In contrast to MMA, which had no effect, physiological concentrations of homocysteine increased granulosa cell proliferation, estradiol production, and FSHR transcript expression, and so elevated levels of this methyl cycle metabolite may partly explain the effects of diet on follicle number following FSH treatment.
Follicle Number and Ovulation
Ewes in the current study were subjected to an ovarian stimulation regimen that was identical to that employed previously at our laboratory [13, 16, 17], but animals were killed approximately 24 h prior to ovulation. All four studies used the same genotype of ewe, estrous synchrony regimen, and source of FSH. Ovulation rate was recorded but not reported by Sinclair et al. [13]. Consistent with previous studies in sheep [29], the ovarian response to FSH in that study varied greatly between animals. It averaged 14.0 ± 1.28 corpora lutea (CLs) and did not differ between treatments. This figure is in close agreement to that previously recorded at our laboratory (e.g., 13.2 ± 0.98 CLs [16]) and is markedly lower than the number of growing antral follicles recorded in the current study (Fig. 1), indicating that probably fewer than 50% of the follicles recorded in the current study would go on to ovulate, with a lower proportion of ovulations in MD ewes.
Nature and Timing of Dietary Treatments
By limiting the supply of elemental cobalt and sulfur in the MD diet, the ability of rumen microorganisms to synthesize vitamin B12 and methionine [15] was diminished. Consequently, the MD diet was able to perturb methyl cycle metabolism, both systemically (as evidenced by the increase in plasma concentrations of homocysteine [Table 3] and profile of plasma amino acids, such as glycine, serine, and taurine [Supplemental Table S1]) and locally (as evidenced by the increase in homocysteine concentrations in follicular fluid and granulosa cells, the reduced incorporation of 35S-methionine into S-adenosyl methionine pools by granulosa cells [13], and intracellular concentrations of amino acids, such as sarcosine, glycine and taurine [Supplemental Table S2]). The feeding of such diets to embryo donor ewes during the same timeframe and under almost identical conditions to that of the current study led to widespread epigenetic alterations to DNA methylation determined by restriction landmark genome scanning [13]. Consequently, altered transcript expression in the current study (i.e., for GDF9 and BMP15 in oocytes from batches 1 and 2, and for FSHR in cultured granulosa cells) may have arisen as a result of epigenetic modifications to DNA methylation, although this was not determined. The timing and duration of dietary intervention was designed to embrace the latter stages of preantral follicle development together with the period of antral follicle development, which in the sheep is thought to last around 50 days [32]. Major epigenetic modifications, including global DNA methylation, occur in growing oocytes of between 90 and 100 μm in diameter during the preantral to antral follicle transition in sheep [33], so that this may be a period that is particularly sensitive to nutritional interventions.
Nature and Timing of Dietary Effects
The duration of dietary treatments meant that the observed effects could have been induced at any point during the 7 wk of experimentation, although transcript expression was only determined in oocytes and granulosa cells during the latter stages of antral follicle development in FSH-stimulated ovaries. Given that homocysteine added to cultured granulosa cells was able to increase FSHR mRNA expression (Fig. 3B), it is conceivable that the increased number of growing antral follicles, in response to exogenous FSH in MD ewes (Fig. 1), arose as a consequence of increased FSHR transcript expression in the follicles of responding animals. FSHR mRNA expression in granulosa cells and cAMP responsiveness to FSH have both previously been linked to changes in prolificacy in sheep [34]. There was certainly a significant positive relationship between FSHR transcript expression and follicle number in batch 3 (Fig. 2a), although in this particular batch, there was no significant effect of diet on mean follicle number. In sheep, FSHR mRNA is detectable in granulosa cells from the primary follicle stage onward [35], and there is evidence from studies with ovarian cortical autografts that preantral follicle development is dependent on FSH in this species [36].
In the current study, follicular fluid estradiol concentrations were greater in MD than in control ewes and in spent media from granulosa cells cultured in the presence of 50 μM homocysteine. Once again, this may have arisen, at least in part, from the increased response of granulosa cells to FSH. In vivo and in vitro studies have previously demonstrated that estradiol production by granulosa cells is FSH responsive in this species [37, 38]. Estradiol is known to exert some of its actions in a paracrine manner via one of two receptors (i.e., ESR1 and ESR2), and both isoforms are expressed in sheep granulosa cells during the late preantral and antral stages of follicle development [39], coincident with the period of exposure to dietary treatments in the current study. Although there is an emerging consensus that ESR2 has the more prominent role of the two receptors in mediating the actions of estradiol within the mouse ovary [40], ovarian response to exogenous gonadotropins was significantly reduced in the Esr1 knockout mouse [41], indicating an intraovarian role for this receptor as well. The relative importance of these two isoforms in the sheep ovary is not known; however, in the current study, ESR1 mRNA expression was significantly related to follicle number (Fig. 2b), although a causal relationship remains to be established.
Special mention is reserved for IGF2R and BMPR2, because transcript expression in granulosa cells for these two genes interacted with diet to predict follicle number in the current study (Fig. 2, c and e). Although mean transcript expression for these two genes was unaltered by diet in batch 3, their relationship to follicle number was clearly influenced by diet, and so both are additional putative candidates for the treatment effects on follicle number observed in batches 1 and 2. Furthermore, mean transcript expression for GDF9 and BMP15 was increased in oocytes from MD ewes in batches 1 and 2 (Table 4). In sheep, both GDF9 and BMP15 have known physiological effects in granulosa cells from antral follicles. For example, oGDF9 and oBMP15 acted synergistically to increase 3H-thymidine incorporation, but oGDF9 acted alone to suppress FSH-stimulated progesterone production, in cultured granulosa cells from this species [42]. BMPR2 is the common receptor shared by GDF9 and BMP15 with other BMPs [43], is expressed in ovine granulosa cells at all stages of follicle development [44], and is responsible for mediating the cooperative effects of oGDF9 and oBMP15 on 3H-thymidine incorporation in granulosa cells [45]. However, although follicle number was increased in MD ewes in batches 1 and 2, transcript expression for GDF9 and BMP15 was not related to follicle number once the effects of diet had been accounted for. The reasons for this are not clear. Perhaps the increase in transcript expression for GDF9 and BMP15 (1.6- to 2.5-fold), although statistically significant, was too small and did not translate into a biological effect. Alternatively, as stated earlier, the timing of transcript expression determination (late antral) may not have coincided with the period of effect, which probably preceded FSH treatment.
A degree of uncertainty also surrounds the relationship between diet, IGF2R transcript expression, and follicle number. IGF2R is a multiligand-binding protein that can bind and activate mannose-6-phosphate (M-6-P)-tagged proteins, including TGFB1 [46], to inhibit growth [47]. TGFB1 at levels as low as 0.1 ng/ml was found to reduce cell proliferation (or survival) and progesterone production in cultured ovine granulosa cells [48]. This observation is consistent with an antiproliferative/antidifferentiative role for this TGFB superfamily member, and it supports the negative association found with follicle number (Fig. 2f) and FSHR mRNA expression in the current study. However, the positive interaction between IGF2R mRNA expression and diet, as well as the lack of an association between IGF2R and TGFB1 mRNA expression, casts doubt of a significant biological effect of IGF2R in the current study.
Transcript expression for AMH in the current study was not affected by diet but was positively associated with follicle number (Fig. 2d). This observation is very much in keeping with that of van Rooij et al. [49], who related serum levels of AMH to follicle number, determined by transvaginal ultrasonography, and oocyte retrieval in women undergoing stimulated in vitro fertilization cycles. The emerging consensus is that AMH may be indicative of the size of the ovarian reserve, and hence follicle number at the time of FSH treatment [50]. AMH mRNA expression was also negatively associated with granulosa cell number following 144 h of culture in the current study (Fig. 4). This is significant, because it suggests an inhibitory effect of AMH on proliferation of granulosa cells from antral follicles, at least from FSH-stimulated ovaries in sheep. Previously, AMH was found to decrease FSH- and cAMP-stimulated aromatase activity of postnatal rat and porcine immature granulosa cells, as well as luteinizing hormone receptors in the latter [51]. AMH also is known to inhibit the FSH-stimulated growth of preantral follicles in the mouse [52].
In conclusion, ovarian responsiveness to FSH was enhanced in ewes offered a methyl-deficient (i.e., a vitamin B12- and methionine-deficient) diet, associated with elevated homocysteine concentrations, in the current study. The mechanisms underlying this response are not clear, but they may involve increased expression of FHSR in responsive animals, and possibly enhanced BMP signaling through BMPR2. Transcript expression of this receptor was influenced by diet and linked to follicle number. In keeping with previous studies, the variability in response to FSH was high in the current experiment, so that inconsistencies in response across batches limit interpretation; nevertheless, the data extend the observations of Sinclair et al. [13] by further demonstrating the intraovarian impact of methyl-deficient diets in the period leading up to conception.
Supplementary Material
Acknowledgments
The authors acknowledge the useful advice of R.G. Lea, W.Y. Kwong, and J. Hughes concerning transcript analysis. They also acknowledge the assistance provided by M. Mitchell and N. Saunders with hormone assays and animal care.
Footnotes
1Supported by the National Institute of Child Health and Human Development Cooperative Program on Female Health and the Egg Quality Grant U01-HD044638. R.K. was supported by the ORS award scheme, the British Federation of Woman Graduates, and the University of Nottingham.
REFERENCES
- Reznikoff-Etievant MF, Zittoun J, Vaylet C, Pernet P, Milliez J.Low Vitamin B(12) level as a risk factor for very early recurrent abortion. Eur J Obstet Gynecol Reprod Biol 2002; 104: 156–159. [DOI] [PubMed] [Google Scholar]
- Ronnenberg AG, Venners SA, Xu X, Chen C, Wang L, Guang W, Huang A, Wang X.Preconception B-vitamin and homocysteine status, conception, and early pregnancy loss. Am J Epidemiol 2007; 166: 304–312. [DOI] [PubMed] [Google Scholar]
- Forges T, Monnier-Barbarino P, Alberto JM, Gueant-Rodriguez RM, Daval JL, Gueant JL.Impact of folate and homocysteine metabolism on human reproductive health. Hum Reprod Update 2007; 13: 225–238. [DOI] [PubMed] [Google Scholar]
- Midttun O, Hustad S, Schneede J, Vollset SE, Ueland PM.Plasma vitamin B-6 forms and their relation to transsulfuration metabolites in a large, population-based study. Am J Clin Nutr 2007; 86: 131–138. [DOI] [PubMed] [Google Scholar]
- Boxmeer JC, Brouns RM, Lindemans J, Steegers EA, Martini E, Macklon NS, Steegers-Theunissen RP.Preconception folic acid treatment affects the microenvironment of the maturing oocyte in humans. Fertil Steril 2008; 89: 1766–1770. [DOI] [PubMed] [Google Scholar]
- Haggarty P, McCallum H, McBain H, Andrews K, Duthie S, McNeill G, Templeton A, Haites N, Campbell D, Bhattacharya S.Effect of B vitamins and genetics on success of in-vitro fertilisation: prospective cohort study. Lancet 2006; 367: 1513–1519. [DOI] [PubMed] [Google Scholar]
- Pacchiarotti A, Mohamed MA, Micara G, Linari A, Tranquilli D, Espinola SB, Aragona C.The possible role of hyperhomocysteinemia on IVF outcome. J Assist Reprod Genet 2007; 24: 459–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palep-Singh M, Picton HM, Yates ZR, Barth JH, Balen AH.Plasma homocysteine concentrations and the single nucleotide polymorphisms in the methionine synthase gene (MTR 2756A>G): associations with the polycystic ovary syndrome an observational study. Eur J Obstet Gynecol Reprod Biol 2008; 138: 180–186. [DOI] [PubMed] [Google Scholar]
- Ebisch IM, Thomas CM, Peters WH, Braat DD, Steegers-Theunissen RP.The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum Reprod Update 2007; 13: 163–174. [DOI] [PubMed] [Google Scholar]
- Steele W, Allegrucci C, Singh R, Lucas E, Priddle H, Denning C, Sinclair K, Young L.Human embryonic stem cell methyl cycle enzyme expression: modelling epigenetic programming in assisted reproduction? Reprod Biomed Online 2005; 10: 755–766. [DOI] [PubMed] [Google Scholar]
- Guzman MA, Navarro MA, Carnicer R, Sarria AJ, Acin S, Arnal C, Muniesa P, Surra JC, Arbones-Mainar JM, Maeda N, Osada J.Cystathionine beta-synthase is essential for female reproductive function. Hum Mol Genet 2006; 15: 3168–3176. [DOI] [PubMed] [Google Scholar]
- Liang R, Yu WD, Du JB, Yang LJ, Yang JJ, Xu J, Shang M, Guo JZ.Cystathionine β synthase participates in murine oocyte maturation mediated by homocysteine. Reprod Toxicol 2007; 24: 89–96. [DOI] [PubMed] [Google Scholar]
- Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, Thurston A, Huntley JF, Rees WD, Maloney CA, Lea RG, Craigon J, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci U S A 2007; 104: 19351–19356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DG, Kennedy S, Blanchflower WJ, Scott JM, Weir DG, Molloy AM, Young PB.Cobalt-vitamin B12 deficiency causes accumulation of odd-numbered, branched-chain fatty acids in the tissues of sheep. Br J Nutr 1994; 71: 67–76. [DOI] [PubMed] [Google Scholar]
- Agricultural Research Council. The Nutrient Requirements of Ruminant Livestock. Slough, UK:: Commonwealth Agricultural Bureaux;; 1980. [Google Scholar]
- Sinclair KD, Dunne LD, Maxfield EK, Maltin CA, Young LE, Wilmut I, Robinson JJ, Broadbent PJ.Fetal growth and development following temporary exposure of day 3 ovine embryos to an advanced uterine environment. Reprod Fertil Dev 1998; 10: 263–269. [DOI] [PubMed] [Google Scholar]
- Powell K, Rooke JA, McEvoy TG, Ashworth CJ, Robinson JJ, Wilmut I, Young LE, Sinclair KD.Zygote donor nitrogen metabolism and in vitro embryo culture perturbs in utero development and IGF2R expression in ovine fetal tissues. Theriogenology 2006; 66: 1901–1912. [DOI] [PubMed] [Google Scholar]
- Pfeiffer CM, Huff DL, Gunter EW.Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem 1999; 45: 290–292. [PubMed] [Google Scholar]
- Shroads AL, Henderson GN, Cheung J, James MO, Stacpoole PW.Unified gas chromatographic-mass spectrometric method for quantitating tyrosine metabolites in urine and plasma. J Chromatogr B 2004808: 153–161. [DOI] [PubMed] [Google Scholar]
- Campbell BK, Scaramuzzi RJ, Webb R.Induction and maintenance of oestradiol and immunoreactive inhibin production with FSH by ovine granulosa cells cultured in serum-free media. J Reprod Fertil 1996; 106: 7–16. [DOI] [PubMed] [Google Scholar]
- Sinclair KD, Sinclair LA, Robinson JJ.Nitrogen metabolism and fertility in cattle: I. Adaptive changes in intake and metabolism to diets differing in their rate of energy and nitrogen release in the rumen. J Anim Sci 2000; 78: 2659–2669. [DOI] [PubMed] [Google Scholar]
- Grant SA, Hunter MG, Foxcroft GR.Morphological and biochemical characteristics during ovarian follicular development in the pig. J Reprod Fertil 1989; 86: 171–183. [DOI] [PubMed] [Google Scholar]
- McNeilly AS, Fraser HM.Effect of gonadotrophin-releasing hormone agonist-induced suppression of LH and FSH on follicle growth and corpus luteum function in the ewe. J Endocrinol 1987; 115: 273–282. [DOI] [PubMed] [Google Scholar]
- Knight PG, Glister C.TGF-beta superfamily members and ovarian follicle development. Reproduction 2006; 132: 191–206. [DOI] [PubMed] [Google Scholar]
- Webb R, Campbell BK.Development of the dominant follicle: mechanisms of selection and maintenance of oocyte quality. Soc Reprod Fertil Suppl 2007; 64: 141–163. [DOI] [PubMed] [Google Scholar]
- Kwong WY, Miller DJ, Ursell E, Wild AE, Wilkins AP, Osmond C, Anthony FW, Fleming TP.Imprinted gene expression in the rat embryo-fetal axis is altered in response to periconceptional maternal low protein diet. Reproduction 2006; 132: 265–277. [DOI] [PubMed] [Google Scholar]
- Genstat. Release 9.1 reference manual. Oxford, UK:: Claredon Press, Oxford Science Publications;; 2007. [Google Scholar]
- Driancourt MA.Regulation of ovarian follicular dynamics in farm animals. Implications for manipulation of reproduction. Theriogenology 2001; 55: 1211–1239. [DOI] [PubMed] [Google Scholar]
- González-Bulnes A, Baird DT, Campbell BK, Cocero MJ, Garcia-Garcia RM, Inskeep EK, Lopez-Sebastian A, McNeilly AS, Santiago-Moreno J, Souza CJH, Veiga-Lopez A.Multiple factors affecting the efficiency of multiple ovulation and embryo transfer in sheep and goats. Reprod Fertil Dev 2004; 16: 421–435. [DOI] [PubMed] [Google Scholar]
- Malhi PS, Adams GP, Mapletoft RJ, Singh J.Oocyte developmental competence in a bovine model of reproductive aging. Reproduction 2007; 134: 233–239. [DOI] [PubMed] [Google Scholar]
- Feary ES, Juengel JL, Smith P, French MC, O'Connell AR, Lawrence SB, Galloway SM, Davis GH, McNatty KP.Patterns of expression of messenger RNAs encoding GDF9, BMP15, TGFBR1, BMPR1B, and BMPR2 during follicular development and characterization of ovarian follicular populations in Ewes carrying the Woodlands FecX2(w) mutation. Biol Reprod 2007; 77: 990–998. [DOI] [PubMed] [Google Scholar]
- Lundy T, Smith P, O'Connell A, Hudson NL, McNatty KP.Populations of granulosa cells in small follicles of the sheep ovary. J Reprod Fertil 1999; 115: 251–262. [DOI] [PubMed] [Google Scholar]
- Russo V, Martelli A, Berardinelli P, Di Giacinto O, Bernabo N, Fantasia D, Mattioli M, Barboni B.Modifications in chromatin morphology and organization during sheep oogenesis. Micro Res Tech 2007; 70: 733–744. [DOI] [PubMed] [Google Scholar]
- Abdennebi L, Monget P, Pisselet C, Remy JJ, Salesse R, Monniaux D.Comparative expression of luteinizing hormone and follicle-stimulating hormone receptors in ovarian follicles from high and low prolific sheep breeds. Biol Reprod 1999; 60: 845–854. [DOI] [PubMed] [Google Scholar]
- Tisdall DJ, Watanabe K, Hudson NL, Smith P, McNatty KP.FSH receptor gene-expression during ovarian follicle development in sheep. J Mol Endocrinol 1995; 15: 273–281. [DOI] [PubMed] [Google Scholar]
- Campbell BK, Telfer EE, Webb R, Baird DT.Evidence of a role for follicle-stimulating hormone in controlling the rate of preantral follicle development in sheep. Endocrinology 2004; 145: 1870–1879. [DOI] [PubMed] [Google Scholar]
- Campbell BK, Engelhardt H, McNeilly AS, Harkness LM, Fukuoka M, Baird DT.Direct effects of ovine follicular fluid on ovarian steroid secretion and expression of markers of cellular differentiation in sheep. J Reprod Fertil 1999; 117: 259–269. [DOI] [PubMed] [Google Scholar]
- Campbell BK, Baird DT.Inhibin A is a follicle stimulating hormone-responsive marker of granulosa cell differentiation, which has both autocrine and paracrine actions in sheep. J Endocrinol 2001; 169: 333–345. [DOI] [PubMed] [Google Scholar]
- Juengel JL, Heath DA, Quirke LD, McNatty KP.Oestrogen receptor alpha and beta, androgen receptor and progesterone receptor mRNA and protein localisation withign the developing ovary and in small growing follicles of sheep. Reproduction 2006; 131: 81–92. [DOI] [PubMed] [Google Scholar]
- Woodruff TK, Mayo KE.To beta or not to beta: estrogen receptors and ovarian function. Endocrinology 2005; 146: 3244–3246. [DOI] [PubMed] [Google Scholar]
- Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS.Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science 1999; 286: 2328–2331. [DOI] [PubMed] [Google Scholar]
- McNatty KP, Juengel JL, Reader KL, Lun S, Myllymaa S, Lawrence SB, Western A, Meerasahib MF, Mottershead DG, Groome NP, Ritvos O, Laitinen MPE.Bone morphogenetic protein 15 and growth differentiation factor 9 co-operate to regulate granulosa cell function in ruminants. Reproduction 2005; 129: 481–487. [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Moore RK, Otsuka F, Erickson GF.The bone morphogenetic protein system in mammalian reproduction. Endocr Rev 2004; 25: 72–101. [DOI] [PubMed] [Google Scholar]
- Souza CJH, Campbell BK, McNeilly AS, Baird DT.Effect of bone morphogenetic protein 2 (BMP2) on oestradiol and inhibin A production by sheep granulosa cells, and localization of BMP receptors in the ovary by immunohistochemistry. Reproduction 2002; 123: 363–369. [PubMed] [Google Scholar]
- Edwards SJ, Reader KL, Lun S, Western A, Lawrence S, McNatty KP, Juengel JL.The cooperative effect of growth and differentiation factor-9 and bone morphogenetic protein (BMP)-15 on granulosa cell function is modulated primarily through BMP receptor II. Endocrinology 2008; 149: 1026–1030. [DOI] [PubMed] [Google Scholar]
- Dennis PA, Rifkin DB.Cellular activation of latent transforming growth factor beta requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc Natl Acad Sci U S A 1991; 88: 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JX, Bell J, Leaf A, Beard RL, Chandraratna RA.Retinoic acid alters the intracellular trafficking of the mannose-6-phosphate/insulin-like growth factor II receptor and lysosomal enzymes. Proc Natl Acad Sci U S A 1998; 95: 13687–13691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juengel JL, Bibby AH, Reader KL, Lun S, Quirke LD, Haydon LJ, McNatty KP.The role of transforming growth factor-beta (TGF-beta) during ovarian follicular development in sheep. Reprod Biol Endocrinol 2004; 2: 78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, Themmen AP.Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod 2002; 17: 3065–3071. [DOI] [PubMed] [Google Scholar]
- Visser JA, de Jong FH, Laven JSE, Themmen APN.Anti-Mullerian hormone: a new marker for ovarian function. Reproduction 2006; 131: 1–9. [DOI] [PubMed] [Google Scholar]
- di Clemente N, Wilson C, Faure E, Boussin L, Carmillo P, Tizard R, Picard JY, Vigier B, Josso N, Cate R.Cloning, expression, and alternative splicing of the receptor for anti-Müllerian hormone. Mol Endocrinol 1994; 8: 1006–1020. [DOI] [PubMed] [Google Scholar]
- Durlinger ALL, Gruijters MJG, Kramer P, Karels B, Kumar TR, Matzuk MM, Rose UM, de Jong FH, Uilenbroek JTJ, Grootegoed JA, Themmen APN.Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology 2001; 142: 4891–4899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.