Abstract
Meiotic maturation in oocytes is a prolonged process that is unique because of cell cycle arrests at prophase of meiosis I (MI) and at metaphase of meiosis II (MII). Fluctuations in cyclin-dependent kinase 1 (CDK1/CDC2A) activity govern meiotic progression, yet little is known about how these fluctuations are achieved. CDC14 is a highly conserved dual-specificity phosphatase that counteracts the function of proteins phosphorylated by CDK. Mammals contain two CDC14 homologs, CDC14A and CDC14B. We report that CDC14B localizes with the meiotic spindle in mouse oocytes, and (unlike somatic cells) it does not localize in the nucleolus. Oocytes that overexpress CDC14B are significantly delayed in resuming meiosis and fail to progress to MII, whereas oocytes depleted of CDC14B spontaneously resume meiosis under conditions that normally inhibit meiotic resumption. Depletion of FZR1 (CDH1), a regulatory subunit of the anaphase-promoting complex/cyclosome that targets cyclin B1 (CCNB1) for ubiquitin-mediated proteolysis, partially restores normal timing of meiotic resumption in oocytes with excess CDC14B. These studies also reveal that experimentally altering CDC14B levels generates eggs with abnormal spindles and with chromosome alignment perturbations. Our data indicate that CDC14B is a negative regulator of meiotic resumption and may regulate MI in mouse oocytes.
Keywords: CDC14, CDH1, cell cycle, gamete biology, meiosis, oocyte, phosphatases
CDC14B, a conserved dual-specificity phosphatase, functions through the CDH1 regulatory subunit of the anaphase-promoting complex to prevent meiotic maturation of mouse oocytes.
INTRODUCTION
Meiosis is the two-part cell division that produces haploid gametes from diploid precursors. In female mammals, oocytes arrest at prophase of meiosis I (MI) during fetal development and do not complete meiosis until ovulated. This arrest uses a G protein-coupled receptor, GPR3, which signals to activate adenylyl cyclase (ADCY3) [1, 2]. ADCY3 generates cAMP, which is inhibitory to meiotic resumption [3]. Meiotic resumption is triggered by a preovulatory surge in luteinizing hormone that, through a signaling cascade not completely understood, causes a decrease in intraoocyte cAMP levels [4]. Next, homologous chromosomes are segregated in MI. After MI, the cell cycle arrests again at metaphase of meiosis II (MII), and sister chromatids will not segregate unless the egg is fertilized or activated.
Cyclin-dependent kinase 1 (CDK1/CDC2A) bound to a regulatory cyclin B1 (CCNB1) subunit is the central engine that drives meiosis in the oocyte, and fluctuations in its activity are key for meiotic progression to and arrest at metaphase of MII (Met II). In mitosis, one mechanism that negatively regulates CDC2A is ubiquitin-mediated proteolysis of CCNB1. Proteolysis of CCNB1 is regulated by the anaphase-promoting complex/cyclosome (APC/C), which is activated by binding either the cell division cycle homolog 20 (CDC20) or fizzy/cell division cycle 20 related 1 (FZR1; hereafter called CDH1, which is the more commonly used term) in two sequential steps. In mitotic cells from humans, several core APC/C units are phosphorylated in a CDC2-dependent manner, which triggers recruitment of CDC20 [5]. Concurrently, CDC2-mediated phosphorylation of CDH1 prevents its binding to the APC/C. Next, in late mitosis CDH1 is dephosphorylated and then binds and activates the APC/C [6]. These activating proteins also provide substrate specificity for the APC/C. Oocytes must keep CDC2A activity low to maintain the prophase I arrest. One mechanism that ensures low CDC2A activity is CDH1 activation of the APC/C to maintain low levels of CCNB1 [7]. When meiosis resumes (analogous to a mitotic G2/M transition), CDH1 is phosphorylated and inactivated by CDC2A, thus generating a positive feedback loop that elevates CCNB1 levels and CDC2A activity.
Cell division cycle homolog 14 (CDC14) is a conserved dual-specificity phosphatase that counteracts CDK activity in mitotic cells, and it was initially described as a positive regulator of mitotic exit in budding yeast and of cytokinesis in fission yeast [8–10]. Mammals contain two CDC14 homologs, CDC14A and CDC14B. In human cell lines, CDC14A is critical for genomic stability because it regulates the centrosome cycle, mitosis, and cytokinesis [11, 12]. The function of human CDC14B is less clear. One study [13] that knocked out the CDC14B coding region found that CDC14B is dispensable for mitosis. In contrast, other studies that reduced CDC14B mRNA by RNA interference (RNAi) found that CDC14B is required for a variety of cellular processes. For example, depletion of CDC14B led to centriole amplification [14] and abnormal nuclear morphology and chromosome segregation in cell lines that overexpressed Polo kinase (PLK1) [15]. Furthermore, CDC14B is a pivotal inducer of the mitotic G2 DNA damage checkpoint, where it activates degradation of PLK1 by activating APC/C-CDH1 and thereby causing cell cycle arrest [16].
In yeast, Cdc14 mutants fail to form viable gametes (spores) because of an uncoupling of the spindle and chromosome segregation cycles [17, 18]. These meiotic cells fail to disassemble the MI spindle but attempt to segregate sister chromatids. It is unknown, however, if either CDC14 homolog functions in mammalian meiosis. Because CDC14 counteracts CDC2A activity and because fluctuation in CDC2A activity is critical for meiotic progression, it is likely that at least one of the conserved CDC14 homologs would be required for meiotic maturation in oocytes.
In this study, we demonstrate that CDC14B functions to prevent meiotic resumption by antagonizing CDC2A activity by activating degradation of CCNB1 in mouse oocytes. Perturbing the amount of CDC14B generates eggs with chromosome misalignment and meiotic spindle defects, suggesting that CDC14B acts later to promote the transition from MI to MII. To our knowledge, our data are the first to describe a role for CDC14B phosphatase in mammalian meiosis and indicate that CDC14B functions upstream of the CDH1 subunit of the APC/C to negatively regulate CDC2A activity in oocytes.
MATERIALS AND METHODS
Oocyte Collection, Culture, and Microinjection
Fully grown germinal vesicle (GV)-intact oocytes from equine chorionic gonadotropin-primed (44–48 h before collection) 6-wk-old female CF-1 mice (Harlan) were obtained as previously described [4]. Meiotic resumption was inhibited by the addition of 2.5 μM milrinone to the collection, culture, or injection medium. Oocytes were cultured in Chatot, Ziomek, and Bavister (CZB) medium [19] in an atmosphere of 5% CO2 in air at 37°C and were microinjected in bicarbonate-free Whitten medium supplemented with 10 mM Hepes (pH 7.3) and 0.01% polyvinyl alcohol [20]. Oocytes were injected with 7 pl of mRNA at 0.5 μg/μl (Cdc14b and Gfp) and 0.15 μg/μl (Δ90) of double-stranded RNA (dsRNA) at 106 copies/μl as previously described [21]. Morpholinos (Gene Tools) were used at 1.5 μM, and the sequences of them are the same as previously described [7]. Messenger RNA-injected oocytes were held in CZB plus milrinone for 4 h, for 24–48 h when dsRNA was injected, or for 20 h when morpholinos were injected. For maturation experiments, oocytes were washed and cultured in milrinone-free CZB medium. All animal experiments were approved by the institutional animal use and care committee and were consistent with National Institutes of Health (NIH) guidelines.
NIH 3T3 Cell Culture
NIH 3T3 cells were grown on glass coverslips. They remained at 37°C in Dulbecco modified Eagle medium plus 10% fetal bovine serum (Invitrogen) in an atmosphere of 5% CO2 in air until ∼70% confluency.
Cloning
Full-length cDNA encoding Cdc14b (IMAGE 6835123) was acquired from the IMAGE collection (Invitrogen), cloned into the plasmid in vitro transcription (pIVT) expression vector [22], and verified by sequencing. An amino (N)-terminal 360-bp region of Cdc14b, which does not contain more than 11 bp of sequence identity to Cdc14a, was amplified from the IMAGE clone, TA cloned into pCRII (Invitrogen), sequenced, and used to generate dsRNA. Site-directed mutagenesis of the pIVT construct was performed to generate the catalytically inactive mutant (Quik Change; Stratagene). C314 was changed to S (TGC to TCC) and confirmed by sequencing. CCNB1-Δ90 was generated by amplifying Ccnb1 lacking the first 270 bp from pRN3 (gift from M. Levasseur). The PCR product was cloned into pRN3 and verified by sequencing.
In Vitro Synthesis of mRNA and dsRNA
Plasmids containing Cdc14b, Egfp, and Δ90 sequences were linearized and in vitro transcribed using an mMessage mMachine kit (Ambion), and RNA was purified using RNeasy Mini Kit (Qiagen). A pCRII vector containing 360 bp of Cdc14b was linearized using BamHI (T7) and NotI (SP6) and was in vitro transcribed as described previously [23].
Immunocytochemistry
Oocytes and eggs were fixed in 3.7% paraformaldehyde in PBS for 1 h at room temperature, permeabilized in PBS containing 0.1% Triton X-100 plus 0.3% bovine serum albumin (BSA) for 15 min at room temperature, and rinsed through three drops of blocking solution (0.3% BSA plus 0.01% Tween-20 in PBS) before blocking for 15 min. In a humidified chamber, oocytes were incubated in blocking solution containing primary antibody for 1 h at room temperature. The following dilutions were used: CDC14B (ab26194, 1:100; Abcam), β-tubulin (TUBB) (T4024, 1:500; Sigma), and γ-tubulin (TUBG1) (T6557, 1:100; Sigma). After washing, secondary antibodies were applied for 1 h. Secondary antibodies (Jackson) were Cy5-conjugated anti-chicken and fluorescein isothiocyanate-conjugated anti-mouse IgG (Southern Biotech). DNA was detected by mounting the cells in VectaShield (Vector Laboratories) containing 3 μg/ml of propidium iodide. To detect kinetochore microtubules, in vitro-matured oocytes were incubated in ice-cold maturation medium for 10 min before fixation in 4% paraformaldehyde in 100 mM K-PIPES, 10 mM ethyleneglycoltetracetic acid, 1 mM MgCl2, 0.2% Triton X-100, pH 6.9, for 30 min at 37°C. Somatic NIH 3T3 cells were fixed for 20 min in cold methanol and stained with the CDC14B antibody at 1:500. Fluorescence was detected on a Leica TCS SP laser scanning confocal microscope with Leica confocal software, and images were processed using Photoshop software (Adobe Systems, Inc.). Images were viewed with a 40× oil immersion objective (N.A., 1.25). Figure 1, A and E, were viewed with a 63× oil immersion objective (N.A., 1.32).
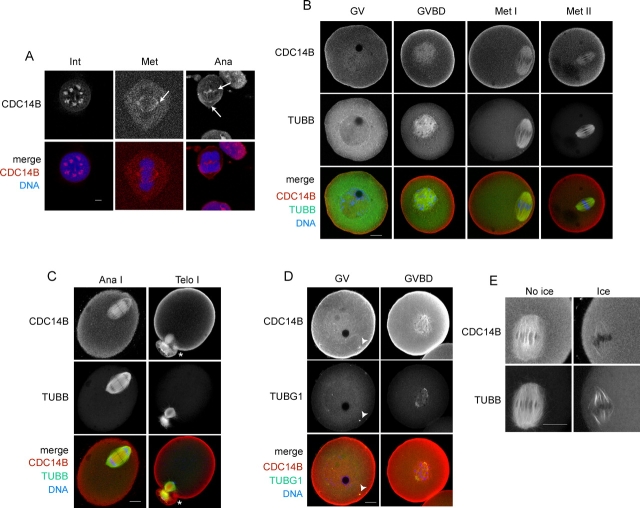
FIG. 1.
Immunocytochemical detection of CDC14B in mouse somatic cells and oocytes. A) The NIH 3T3 cells were fixed in cold methanol for 20 min before immunological detection. The arrows indicate localization at the mitotic centrosome in metaphase (Met) and at the central spindle (upper arrow) and centrosome (lower arrow) in anaphase (Ana). In the merged images, CDC14B is red, and DNA is blue. These experiments were conducted twice, and 50 somatic cells were analyzed by confocal microscopy. Int, interphase; bar = 5 μm. B and C) The GV-intact oocytes were collected and matured in vitro for 0 h (GV), 3 h (GVBD), 7 h (Met I), 9 h (Ana I/telophase [Telo] I), and 16 h (Met II) before fixation. In merged images, CDC14B is red, the spindle (TUBB) is green, and DNA is blue. The asterisk indicates a polar body. D) CDC14B (red) colocalizes with γ-tubulin (TUBG1; arrowheads, green) in oocytes. E) In vitro-matured oocytes were placed in ice-cold medium for 10 min before fixation in a 4% formaldehyde fixative that preserves kinetochore fibers. These experiments were conducted three times, with 15–20 oocytes per stage. Bar = 20 μm.
Immunoblotting
Samples stored at −80°C were thawed on ice, diluted in 2× Laemmli sample buffer [24], and loaded on 8% polyacrylamide gels. Samples were electrophoresed at 15 mA, transferred to polyvinylidene fluoride membrane (Millipore), and either blocked overnight in PBS (pH 7.5) plus 0.1% (v/v) Tween-20 and 2% blocking agent (ECL Advance; GE Healthcare) at 4°C (CDC14B and TUBB) or for 2 h at room temperature (CCNB1 and CDH1). Detection of CDC14B and TUBB was achieved by probing the membrane with anti-CDC14B (ab26194; Abcam) or anti-β-tubulin (T4026; Sigma) at 1:5000 for 1 h at room temperature. After washing, the membrane was incubated with secondary anti-chicken (CDC14B) or anti-mouse (TUBB) antibodies (1:200 000; Amersham) for 1 h at room temperature. To detect CCNB1 and CDH1, membranes were incubated overnight at 4°C with anti-CCNB1 (ab72; Abcam) at 1:500 or with CDH1 (ab3242; Abcam) at 1:100. After washing, the membranes were incubated for 1 h at room temperature with a secondary anti-mouse horseradish peroxidase (1:200 000; Amersham) antibody. Secondary antibodies were detected with chemiluminescence (ECL Advance; Amersham). To quantify changes in CDC14B and CCNB1, the signals were first normalized to TUBB in GV controls, and intensities were measured using ImageJ software (NIH).
Kinase Assays
CDC2A and MAPK1 activities, as assessed by their ability to phosphorylate histone H1 and myelin basic protein, respectively, were assayed in single oocytes as previously described (29). Images were detected using a Typhoon 9410 PhosphorImager (GE Healthcare) and were quantified with ImageJ software.
Statistical Analysis
Two-way ANOVA or Student t-test, as indicated in the figure legends, was used to evaluate the differences between groups using Prism software (GraphPad Software). P < 0.05 was considered significant.
RESULTS
CDC14B Colocalizes with the Meiotic Spindle in Mouse Oocytes
In all organisms studied to date, CDC14 activity is regulated by cell cycle-dependent changes in its localization [25]. In somatic cells, CDC14B is in the nucleolus during interphase and colocalizes with the centrosome during mitosis [11, 12, 15, 26]. Mouse CDC14B is highly similar in sequence to its human homolog. We found that CDC14B had the same subcellular localization in mouse somatic cells (NIH 3T3) as it reportedly has in human tissue culture lines, as we observed that CDC14B localizes in the nucleolus during interphase and is at the spindle poles during mitosis (Fig. 1A). To determine where CDC14B localizes during meiotic maturation in mouse oocytes, we matured prophase-arrested (GV) oocytes in vitro and fixed them at specific times during meiosis. We found that CDC14B localized in the cortex and colocalized with the entire length of the meiotic spindle at all stages (Fig. 1, B and C); no signal was observed when the primary antibody was omitted (Supplemental Fig. S1, B and C; all supplemental figures are available online at www.biolreprod.org). Furthermore, CDC14B colocalized with γ-tubulin (TUBG1) at microtubule organizing centers (MTOCs) and with the cytoplasmic microtubule network in GV oocytes (Fig. 1D). Furthermore, unlike somatic cells in interphase (Fig. 1A), we never observed CDC14B in the nucleolus of GV oocytes, even when oocytes were fixed with methanol, a harsh fixative that allows better antibody penetration into the cell (data not shown). Therefore, the distribution of CDC14B on the meiotic spindle, localization on the cytoplasmic network during prophase I, and lack of nucleolar staining indicate that the localization of CDC14B in oocytes differs somewhat from its somatic cell localization.
The spindle is composed of two types of microtubules, namely, unstable pole-pole microtubules that cross in the middle of the spindle and highly stable microtubules that contact the kinetochore complex at chromosome centromeres. Because mouse CDC14B localized to the meiotic spindle and human CDC14B bundles and stabilizes microtubules in vitro [26], we investigated which type of microtubule associated with CDC14B. We briefly exposed oocytes that were at metaphase of MI (Met I) to ice-cold medium to induce depolymerization of pole-pole microtubules [27] and found that most CDC14B associated with the unstable microtubules. In oocytes incubated on ice, most of the spindle immunostaining was lost except for a faint signal that colocalizes with the remaining kinetochore microtubules (Fig. 1E). This dramatic loss of CDC14B staining suggests that it is less likely to regulate kinetochore microtubules and more likely has a dominant role in regulating meiotic spindle dynamics in mouse oocytes.
Depletion of CDC14B Causes Premature Meiotic Resumption by Increasing CDC2A Activity
To determine if CDC14B is required for mammalian female meiosis, we used an RNAi approach in which we microinjected dsRNA that specifically targeted Cdc14b mRNA in GV oocytes [28]. Compared with oocytes injected with control Gfp dsRNA, we found that Cdc14b mRNA was reduced more than 80% in Cdc14b dsRNA-injected oocytes by 48 h after injection (Supplemental Fig. S2). The RNAi targeting was specific because Cdc14a mRNA levels were not reduced. Compared with CDC14B levels in control oocytes, the amounts of CDC14B protein were reduced by 29% and 60% in Cdc14b dsRNA-injected oocytes held for 24 h and 48 h, respectively, when normalized to TUBB protein levels (Fig. 2A). Furthermore, we stained dsCdc14b-injected oocytes with an antibody against CDC14B and found little to no immunoreactivity on the meiotic spindle (Fig. 2B). These data demonstrate that CDC14B is efficiently turned over in dsCdc14b-injected oocytes and further support our evidence that endogenous CDC14B is colocalized with the meiotic spindle (Fig. 1B).
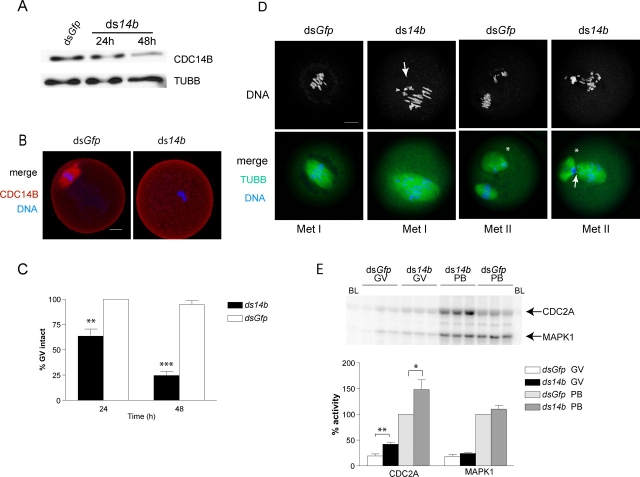
FIG. 2.
Reduction of CDC14B induces meiotic resumption. A) Western blot of 30 oocytes injected either with dsGfp and held for 48 h or with dsCdc14b and held for the indicated times before loading on an 8% polyacrylamide gel. The blot was stripped and reprobed with an anti-β-tubulin (TUBB) antibody for loading standard. B) Oocytes injected with dsCdc14b were held in medium containing milrinone for 48 h before fixation and immunocytochemistry with an anti-CDC14B antibody (red; spindle staining). DNA was detected by staining with propidium iodide (blue). Control dsGfp oocytes were held in medium containing milrinone for 24 h and matured to Met II for 16 h before fixation and processing with the same antibody. Bar = 20 μm. C) Injected oocytes were held in medium containing milrinone for 24 h and 48 h, and the number of oocytes with intact GVs was counted. The experiment was repeated four times, with a total of 65 oocytes. D) Oocytes injected with dsCdc14b were fixed after being held in medium containing milrinone for 24 h (Met I) and 48 h (Met II). Oocytes injected with dsGfp were held in medium containing milrinone for 41 h before maturing to Met I or for 36 h before maturing to Met II and fixation. Spindles were visualized with an anti-β-tubulin antibody, and DNA was stained with propidium iodide. The arrow in Met I points to several misaligned chromosomes, and the arrow in Met II points to a region of the Telo I spindle that has not properly disassembled. The asterisks indicate polar bodies. Bar = 20 μm. E) Injected oocytes were held in milrinone-containing medium for 48 h before freezing single oocytes. For a control (dsGfp polar body [PB]), dsGfp-injected cells were held in medium containing milrinone for 24 h and matured to MII in medium lacking milrinone for 24 h before collection. CDC2A and MAPK1 activity in the dsGfp PB control was used as the reference level. The graph represents quantification of three independent experiments, with nine oocytes per group. C and E) Two-way ANOVA was used to analyze the data, and the data are presented as the mean ± SEM. BL, blank; GV, GV intact. *P < 0.05, **P < 0.01, ***P < 0.0001.
After 24 h of culture in medium containing milrinone (a PDE3A inhibitor that sustains an inhibitory concentration of oocyte cAMP [29]), control dsGfp-injected oocytes maintained the prophase arrest, as monitored by the presence of an intact GV (Fig. 2C). We found that only 64% of oocytes injected with Cdc14b dsRNA remained arrested in prophase when held in culture for 24 h. After 48 h, this phenotype was more pronounced, as only 25% of dsCdc14b-injected oocytes were arrested in prophase (Fig. 2C). Those oocytes that underwent GV breakdown (GVBD) within 24 h did not yet have polar bodies, but by 48 h most CDC14B-depleted oocytes had extruded polar bodies.
We assessed chromosome configuration by DNA staining and meiotic spindles by immunostaining of TUBB in oocytes that spontaneously resumed meiosis. Nonarrested oocytes were fixed 24 h after injection and were compared with control oocytes at Met I. Those oocytes injected with Cdc14b dsRNA were in MI and contained many elongated and stretched chromosomes that seemed as if they were under abnormal tension and contained spindles that were larger and misshapen compared with control Met I oocytes (Fig. 2D). Oocytes injected with Cdc14b dsRNA that resumed meiosis between 24 h and 48 h were fixed 48 h after injection and were compared with control oocytes arrested at Met II. Sixty-five percent of oocytes with reduced CDC14B contained chromosomes improperly aligned on the Met II spindle. Furthermore, we often observed oocytes that were in late telophase I with apparent defects in cytokinesis, as some of their chromosomes were in close proximity to the cleavage furrow rather than at the opposite end of the spindle (Fig. 2D). These data indicate that CDC14B is required to prevent meiotic resumption in mouse oocytes, and the subsequent spindle and chromosome defects suggest that it also acts later to regulate meiotic M phase.
Because CDC2A activity increases shortly after initiation of meiotic resumption and because CDC14 is a negative regulator of CDC2A activity in other organisms [25, 30], we determined whether CDC2A activity was altered in CDC14B-reduced oocytes. We observed a 2-fold increase in CDC2A activity, as assayed by phosphorylation of histone H1, relative to controls, and CDC2A activity remained elevated by 1.5-fold in Met II-arrested eggs (Fig. 2E). In contrast, MAPK1 activity was not significantly altered at either developmental stage. These data suggest that CDC14B promotes meiotic arrest by negatively regulating CDC2A activity.
Overexpression of CDC14B Delays Meiotic Resumption
Overexpression of human CDC14B in interphase-arrested somatic cells causes aberrant microtubule bundling and stabilization [11, 12, 26]. We assessed the consequences of overexpressing mouse CDC14B in the prophase-arrested oocytes by microinjecting GV-intact oocytes with Cdc14b mRNA. As a control, we injected oocytes with catalytically inactive Cdc14b-C314S or Gfp mRNAs. Quantification of the fluorescent signals indicated that microinjection of the Cdc14b mRNA modestly expanded the endogenous pool 2-fold compared with Gfp mRNA-injected controls (Fig. 3A). Consistent with published data demonstrating that some of the localizations of human CDC14B during mitosis do not require catalytic activity [12], the catalytically inactive form of CDC14B was able to localize to the meiotic spindle and did not differ from its wild-type form in oocytes undergoing meiosis (Supplemental Fig. S3). Moreover, we observed the increased CDC14B signal specifically on the meiotic spindle, further confirming our immunocytochemistry results (Fig. 1). Because depletion of CDC14B induced meiotic resumption in medium containing milrinone (Fig. 2C), we first examined the kinetics of meiotic resumption by monitoring the timing of GVBD in oocytes with excess CDC14B. Oocytes microinjected with control mRNAs completed GVBD within 2 h after transfer to milrinone-free medium, whereas oocytes that overexpressed CDC14B had significantly delayed GVBD kinetics (Fig. 3B). By 5 h after transfer to milrinone-free medium, only 40% of oocytes overexpressing CDC14B had resumed meiosis (Fig. 3B). This delay depended on the catalytic activity of CDC14B, as oocytes overexpressing similar amounts of CDC14B-C314S (both were ∼2-fold in excess as determined by quantifying the immunoblot [Fig. 3C]) displayed normal GVBD kinetics (Fig. 3B). These data indicate that CDC14B negatively regulates meiotic resumption. Moreover, the observed phenotype (i.e., delay in maturation) is the opposite and expected phenotype compared with the phenotype observed following RNAi-mediated knockdown of CDC14 (i.e., induction of maturation in the presence of milrinone).
FIG. 3.
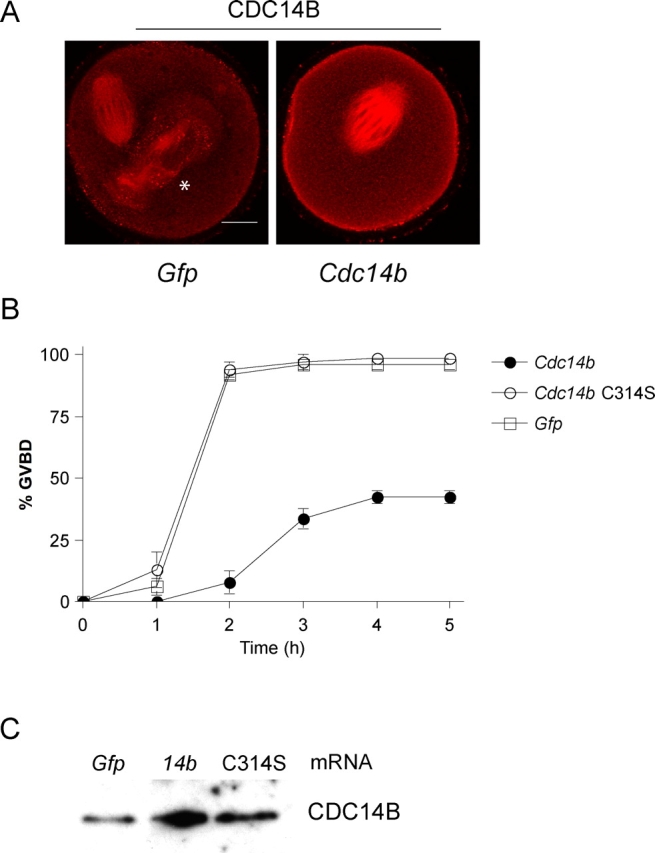
Overexpression of CDC14B delays meiotic resumption in oocytes. A and C) Oocytes were microinjected with the indicated mRNA and either (A) were matured for 16 h before fixation or (C) were held for 16 h before freezing and immunodetection of CDC14B. A) To estimate the level of overexpression, the average intensities of the overexpressing cells (n = 15) were compared with the average intensities in Gfp-injected cells using ImageJ software. The asterisk indicates a polar body. Bar = 20 μm. B) Oocytes were injected with indicated mRNAs and held in medium containing milrinone for 4 h. The oocytes were washed out of the inhibitor, and the absence of a GV (GVBD) was verified every hour by light microscopy. These experiments were repeated three times, with n = 60 for each group. The error bars indicate SEM.
Overexpression of CDC14B Reduces CDC2A Activity and CCNB1 Protein Levels
Next, we sought to determine the molecular mechanism by which CDC14B prevents meiotic resumption in the oocyte. Oocytes that are competent to resume meiosis must have adequate CDC2A activity [31]. We measured the level of CDC2A activity by performing in vitro kinase assays with single GV oocytes injected with Gfp or Cdc14b mRNA. We found that GV-arrested oocytes overexpressing CDC14B have reduced CDC2A activity (∼60% reduction) compared with controls (Fig. 4A), suggesting that CDC14B is a negative regulator of CDC2A in oocytes.
FIG. 4.
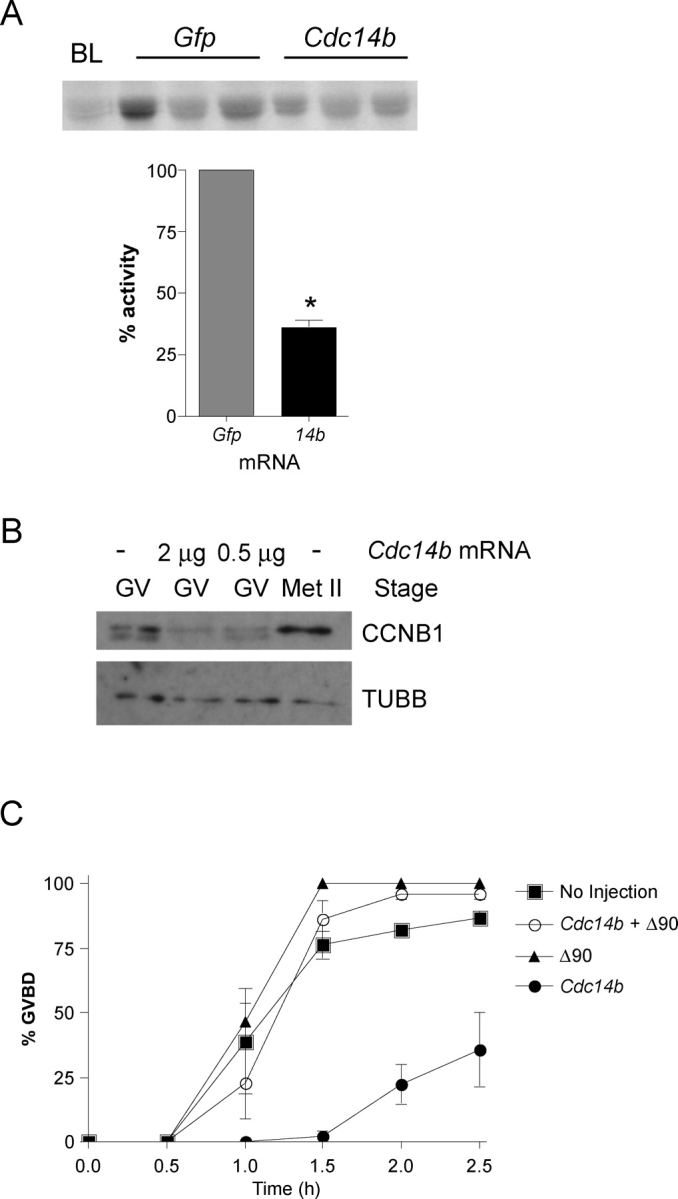
Overexpression of CDC14B reduces CDC2A activity and CCNB1 levels. A) Oocytes were injected with the indicated mRNA and held in medium containing milrinone for 16 h before snap freezing single GV oocytes for CDC2A in vitro kinase assays. The graph represents quantification of three independent experiments, with a total of nine oocytes per group. Student t-test was used to analyze the data, which are presented as the mean ± SEM. BL, blank. *P < 0.05. B) Lysates from oocytes either not injected or injected with the indicated amount of Cdc14b mRNA were electrophoresed in an 8% acrylamide gel and probed with an anti-CCNB1 antibody after transfer to a membrane. The blot was stripped and reprobed with anti-β-tubulin (TUBB) for a protein-loading standard. Each lane contains 50 oocytes. This experiment was conducted twice, and similar results were obtained for each experiment. C) Oocytes were injected with the indicated materials and held for 4 h before maturation. The absence of a GV (GVBD) was verified every 30 min by light microscopy. This experiment was repeated three times, with n = 60 for each group. The data are presented as the mean ± SEM.
To be active, CDC2A must be bound to a regulatory cyclin subunit. In oocytes, the amount of CCNB1 is kept low by the APC/C, and as a consequence, CDC2A activity is low [32]. We asked whether CCNB1 levels were altered in GV oocytes containing excess CDC14B and found by Western blot analysis that GV oocytes overexpressing CDC14B contained ∼50% of the amount of CCNB1 compared with noninjected controls (Fig. 4B). These data indicate that, in oocytes with excess CDC14B, CDC2A activity is reduced likely because steady-state levels of CCNB1 are reduced.
CCNB1 contains a destruction box sequence located in its N-terminus that is recognized by the APC/C [33]. Expressing a nondegradable form of CCNB1 (Δ90) that lacks this sequence should rescue the observed delay in GVBD in CDC14B-overexpressing oocytes. Expression of Δ90 alone slightly advanced the time course for GVBD compared with noninjected controls, as previously reported (Fig. 4C) [34]. Most important, coexpression of Δ90 with CDC14B alleviated the delay in GVBD caused by CDC14B overexpression, as these oocytes underwent GVBD with the same kinetics as the noninjected control (Fig. 4C). These data suggest that CDC14B regulates the stability of CCNB1 to prevent resumption of meiosis in oocytes.
CDC14B Regulates CCNB1 Turnover Through Regulating the CDH1 Subunit of the APC/C
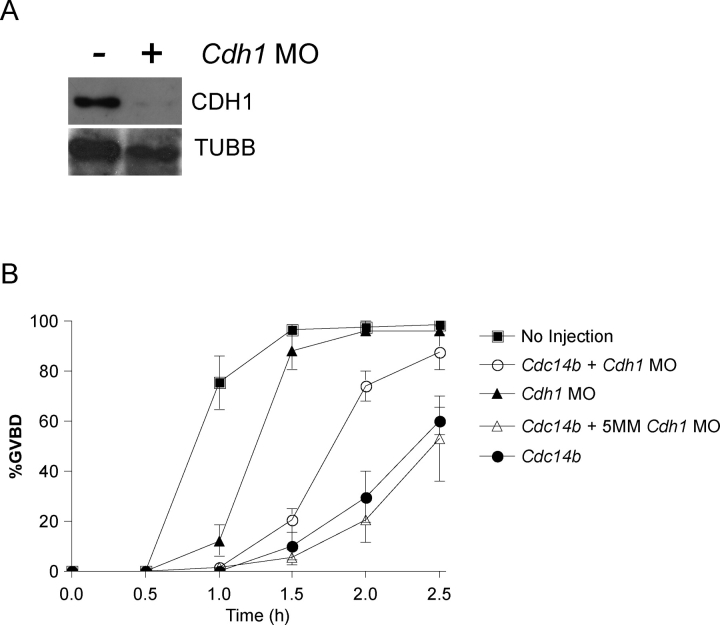
In GV-intact oocytes, degradation of CCNB1 is regulated by CDH1 binding to and activating the APC/C [7]. In human cell lines, CDC14B triggers the G2 DNA damage checkpoint by dephosphorylating CDH1, thus promoting CDH1 binding to the APC/C [16, 35, 36]. Therefore, we hypothesized that overexpression of CDC14B reduces CDC2A activity by promoting increased CCNB1 turnover through positively regulating CDH1. To address this question, we asked whether reducing the amount of CDH1 via morpholino microinjection could rescue the GVBD delay in oocytes containing excess CDC14B. As previously reported, CDH1 is efficiently depleted by ∼90% 20 h after morpholino injection when the samples were normalized to TUBB protein levels [7] (Fig. 5A). Compared with control oocytes either co-injected with a mismatch Cdh1 morpholino (5MM) and Cdc14b or injected with Cdc14b alone, depletion of CDH1 partially suppressed the GVBD delay in CDC14B-overexpressing oocytes (Fig. 5B). These data suggest that CDC14B functions upstream of CDH1 and positively regulates its activity to promote CCNB1 turnover and to prevent meiotic resumption.
FIG. 5.
Depletion of CDH1 partially suppresses the GVBD delay in oocytes containing excess CDC14B. Oocytes were injected with the indicated materials and held for 20 h before freezing (A) or maturation (B). A) Lysates from 30 oocytes either not injected or injected with a Cdh1 morpholino were electrophoresed in a 10% acrylamide gel and probed with an anti-CDH1 antibody after transfer to a membrane. The blot was stripped and reprobed with anti-β-tubulin (TUBB) for a protein-loading standard. B) The absence of a GV (GVBD) was monitored every 30 min by light microscopy. This experiment was repeated three times, with n = 60 for each group. The data are presented as the mean ± SEM. MO, morpholino; 5MM, mismatch morpholino.
Oocytes with Excess CDC14B Block Meiosis Before Met II
Because CDC14B localizes on the meiotic spindle and because depletion of CDC14B caused chromosome alignment defects and abnormally sized spindles, we assessed the effect of overexpression that CDC14B had on chromosome and spindle dynamics during meiotic maturation. We microinjected Cdc14b mRNA into GV-intact oocytes and then matured them in vitro. We found that, when matured for 16 h (a time in which almost all control oocytes have reached Met II), ∼75% of oocytes overexpressing CDC14B failed to reach Met II and that this effect required catalytically active CDC14B (Fig. 6A).
FIG. 6.
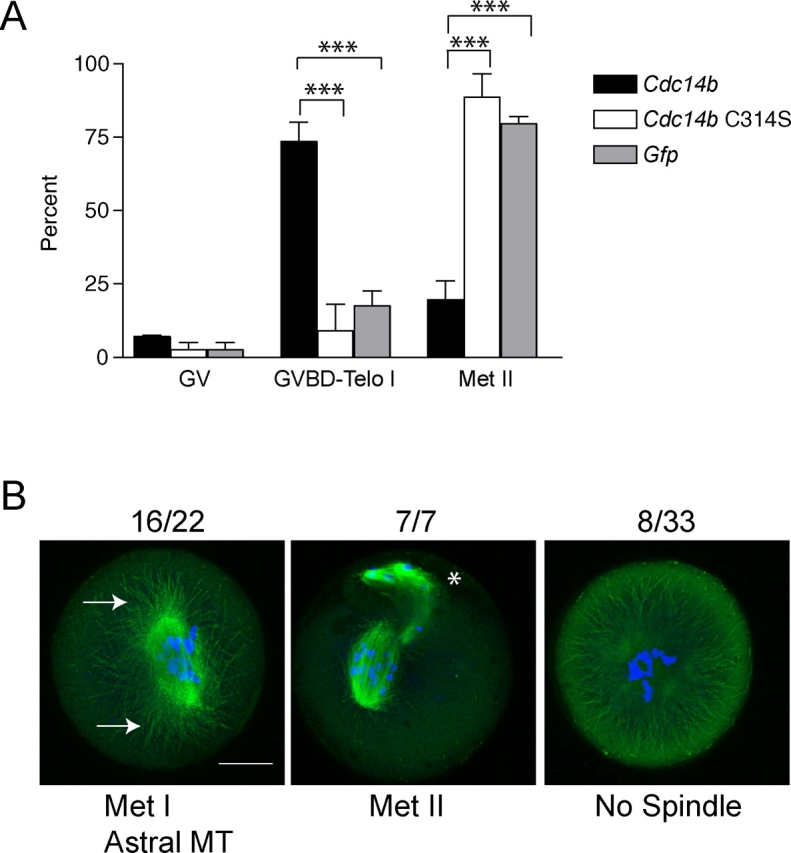
Overexpression of CDC14B prevents completion of meiotic maturation. A) Oocytes were injected with the indicated mRNAs, held in milrinone for 4 h, and matured in the absence of milrinone for 16 h. Cells were fixed and stained with anti-β-tubulin and propidium iodide to determine the meiotic stage. These experiments were conducted three times, with n = 45 for each group. Two-way ANOVA was used to analyze the data, which are presented as the mean ± SEM. Telo I, telophase I. ***P < 0.0001. B) Representative images of oocytes overexpressing CDC14B; DNA is blue, and the spindle (β-tubulin) is green. The asterisk indicates polar bodies. Arrows point to regions of astral microtubule (MT) projections. Bar = 20 μm.
When CDC14B was overexpressed in oocytes, we observed a variety of DNA and spindle abnormalities. Oocytes overexpressing CDC14B had severe chromosome alignment defects regardless of whether they were arrested at Met I (72% of Met I oocytes) or Met II (100% of Met II eggs) (Fig. 6B). Furthermore, many oocytes overexpressing CDC14B contained no spindle (24% of all oocytes) or had persistent astral microtubule projections (40% of all oocytes) (Fig. 6B). These data, together with the spindle localization of CDC14B (Fig. 1, B, C, and E), suggest that CDC14B is a critical regulator of meiotic spindle dynamics in oocytes.
DISCUSSION
We demonstrate herein for the first time (to our knowledge) a role for CDC14B in maturation of mouse oocytes. Meiotic progression is unique in females, as oocytes enter a prolonged prophase (G2 like) arrest during fetal development and do not resume meiosis until sexual maturity is reached. The CDH1 regulator of the APC/C is required for this arrest [7], and herein we find that CDC14B functions upstream of CDH1 to prevent premature meiotic resumption (Fig. 7). In budding yeast, Cdc14 is required for the exit from MI [17, 18]. Our data demonstrate that alteration of CDC14B levels in oocytes causes meiotic spindle and chromosome alignment perturbations, suggesting that it has a role in regulating the exit from MI in female mammalian meiosis. However, although CDC14B localizes on the central spindle during anaphase of MI (Ana I) (Fig. 1E), we were unable to assess a role for CDC14B during the MI/MII transition because of the asynchrony by which oocytes with reduced CDC14B resume meiosis. Future studies are aimed at determining the requirement for CDC14B during meiotic M phase.
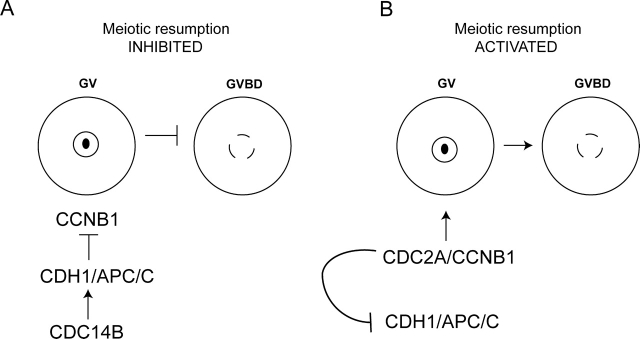
FIG. 7.
A schematic model depicting the role of CDC14B in regulating meiotic resumption in mouse oocytes. A) During the meiotic prophase arrest, CDC2A activity is low. One mechanism used by the oocyte to ensure low CDC2A activity is ubiquitin-mediated proteolysis of its cyclin subunit CCNB1. Our data demonstrate that CDC14B functions upstream of CDH1 to maintain low steady-state levels of CCNB1, thereby maintaining the prophase arrest. It is likely that CDC14B dephosphorylates substrates in addition to CDH1, but these possibilities have not been explored in this study. B) Upon GVBD, CDC2A phosphorylates CDH1, causing its disassociation from the APC/C and subsequent stabilization of CCNB1. It is unknown how CDC14B activity for CDH1 is turned off to promote this transition.
Localization is one mechanism that regulates CDC14 function in many organisms [25]. In budding yeast, Cdc14 is held in the nucleolus by CfiI/NetI, and its cell cycle-dependent release is controlled by two signaling cascades called FEAR (for Cdcfourteen early anaphase release) and MEN (mitotic exit network) [37–39]. In higher eukaryotes, CDC14A and CDC14B contain motifs that govern their localizations in somatic cells. For example, CDC14B contains a nuclear localization sequence in its N-terminus that is required for its nucleolar localization during interphase [11]. Our data demonstrate that the localization of CDC14B in oocytes is somewhat different from its localization in mitotic cells (Fig. 1). We never observe CDC14B in the nucleolus and find that it localizes to the entire length of the meiotic MI and MII spindles (Fig. 1, B, C, and E). Similar to somatic cells, we find CDC14B at MTOCs (Fig. 1D). Human CDC14B activates the G2 DNA damage checkpoint through CDH1 [16]. We find that CDC14B negatively regulates oocyte maturation by regulating CDH1-mediated turnover of CCNB1 (Figs. 4, 5, and 7). Therefore, it is likely that CDH1 is a direct target of CDC14B in mouse oocytes. Understanding how CDC14B activity on CDH1 is regulated will be key to understanding how meiotic resumption is prevented in meiotically competent oocytes.
Oocytes that overexpress CDC14B undergo GVBD with slower kinetics (Fig. 3C) and have reduced CCNB1 levels (Fig. 4B). Moreover, depletion of CDH1 partially rescues the GVBD delay (Fig. 5B). In the morpholino rescue experiments, oocytes were held in medium containing milrinone for 20 h. Although most CDH1 protein is turned over, there are trace amounts of CDH1 in the oocytes (Fig. 5A) that could account for the partial rescue phenotype. Alternatively, CDC14B may signal through substrates in addition to CDH1 to control premature meiotic resumption. For example, Clp1 (the Cdc14 homolog in fission yeast) dephosphorylates and inactivates Cdc25, a phosphatase that activates CDC2A [40]. In oocytes, both CDC25A and CDC25B are required to promote meiotic resumption and are therefore potential substrates [41, 42].
Although we do not know if CDC14B functions later in oocyte maturation, oocytes either containing excess CDC14B or those depleted of CDC14B have abnormal spindles, and CDC14B localizes to the center part of the Ana I spindle, suggesting a role for CDC14B during MI (Figs. 1C, 2E, and 6B). The spindle midzone, the electron dense and centermost region of the anaphase spindle, provides the forces necessary for spindle elongation. In budding yeast, Cdc14 is required for forming the spindle midzone through dephosphorylation of a microtubule bundling factor, Ase1, and for directing the midzone localization of the separase-Slk19 complex [43]. Higher eukaryotes contain an Ase1 homolog called protein regulator of cytokinesis (PRC1), which also localizes to the mitotic spindle midzone when dephosphorylated [44]. Microarray data indicate that Prc1 is present in mouse oocytes and therefore may be a candidate to pursue [45]. Deciphering the role of CDC14B during MI should reveal additional insight as to how MI is regulated in mammals and how it differs from mitosis.
The reported mitotic functions of human CDC14B are conflicting. Our data clearly demonstrate that CDC14B is a critical regulator of the meiotic cell cycle. Meiotic spindle assembly is unique in mammalian oocytes because it occurs in an acentrosomal fashion via de novo MTOC synthesis and chromosome congression [46]. Because CDC14B colocalizes with MTOCs and the meiotic spindle (Fig. 1, B–D), it is tempting to speculate that the requirement for CDC14B during oocyte maturation in mammals is different from that in mitosis because of its unique spindle assembly mechanism. Overexpression of CDC14B delays meiotic events after GVBD, suggesting that it likely functions later in meiosis (Fig. 6B). These oocytes contain abnormal spindles with persistent astral microtubules that emanate from the spindle poles, consistent with data demonstrating that CDC14B bundles and stabilizes microtubules in vitro [26]. Further analysis of the microtubule-related function of CDC14B will provide insight into meiotic spindle assembly in female gametes.
Supplementary Material
Acknowledgments
The authors would like to thank Francesca Duncan, Paula Stein, Mariano Buffone, and Mike Lampson for technical support and helpful advice. The authors thank Jun Ma for culturing the NIH 3T3 cells used in Figure 1A.
Footnotes
1Supported by a grant from the National Institutes of Health (HD22681) to R.M.S. K.S. was supported by HD055822 from the NIH.
REFERENCES
- Mehlmann LM, Jones TL, Jaffe LA.Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science 2002; 297: 1343–1345. [DOI] [PubMed] [Google Scholar]
- Horner K, Livera G, Hinckley M, Trinh K, Storm D, Conti M.Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev Biol 2003; 258: 385–396. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Downs SM.Chemical signals that regulate mammalian oocyte maturation. Biol Reprod 1984; 30: 1–11. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Montgomery RR, Belanoff JR.Regulation of mouse oocyte meiotic maturation: implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol 1983; 97: 264–273. [DOI] [PubMed] [Google Scholar]
- Kraft C, Herzog F, Gieffers C, Mechtler K, Hagting A, Pines J, Peters JM.Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J 2003; 22: 6598–6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer ER, Scheuringer N, Podtelejnikov AV, Mann M, Peters JM.Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol Biol Cell 2000; 11: 1555–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis A, Chang HY, Levasseur M, Jones KT.APCcdh1 activity in mouse oocytes prevents entry into the first meiotic division. Nat Cell Biol 2006; 8: 539–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspersen SL, Charles JF, Tinker-Kulberg RL, Morgan DO.A late mitotic regulatory network controlling cyclin destruction in Saccharomyces cerevisiae. Mol Biol Cell 1998; 9: 2803–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visintin R, Craig K, Hwang ES, Prinz S, Tyers M, Amon A.The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol Cell 1998; 2: 709–718. [DOI] [PubMed] [Google Scholar]
- Trautmann S, Wolfe BA, Jorgensen P, Tyers M, Gould KL, McCollum D.Fission yeast Clp1p phosphatase regulates G2/M transition and coordination of cytokinesis with cell cycle progression. Curr Biol 2001; 11: 931–940. [DOI] [PubMed] [Google Scholar]
- Kaiser BK, Zimmerman ZA, Charbonneau H, Jackson PK.Disruption of centrosome structure, chromosome segregation, and cytokinesis by misexpression of human Cdc14A phosphatase. Mol Biol Cell 2002; 13: 2289–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailand N, Lukas C, Kaiser BK, Jackson PK, Bartek J, Lukas J.Deregulated human Cdc14A phosphatase disrupts centrosome separation and chromosome segregation. Nat Cell Biol 2002; 4: 317–322. [DOI] [PubMed] [Google Scholar]
- Berdougo E, Nachury MV, Jackson PK, Jallepalli PV.The nucleolar phosphatase Cdc14B is dispensable for chromosome segregation and mitotic exit in human cells. Cell Cycle 2008; 7(9):1184–1190. [DOI] [PubMed] [Google Scholar]
- Wu J, Cho HP, Rhee DB, Johnson DK, Dunlap J, Liu Y, Wang Y.Cdc14B depletion leads to centriole amplification, and its overexpression prevents unscheduled centriole duplication. J Cell Biol 2008; 181: 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalepa G, Harper JW.Visualization of a highly organized intranuclear network of filaments in living mammalian cells. Cell Motil Cytoskeleton 2004; 59: 94–108. [DOI] [PubMed] [Google Scholar]
- Bassermann F, Frescas D, Guardavaccaro D, Busino L, Peschiaroli A, Pagano M.The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell 2008; 134: 256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomo SB, Rabitsch KP, Fuchs J, Gruber S, Sullivan M, Uhlmann F, Petronczki M, Toth A, Nasmyth K.Division of the nucleolus and its release of CDC14 during anaphase of meiosis I depends on separase, SPO12, and SLK19. Dev Cell 2003; 4: 727–739. [DOI] [PubMed] [Google Scholar]
- Marston AL, Lee BH, Amon A.The Cdc14 phosphatase and the FEAR network control meiotic spindle disassembly and chromosome segregation. Dev Cell 2003; 4: 711–726. [DOI] [PubMed] [Google Scholar]
- Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I.An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil 1989; 86: 679–688. [DOI] [PubMed] [Google Scholar]
- Whitten W.Nutrient requirements for the culture of preimplantation mouse embryo in vitro. Adv Biosci 1971; 6: 129–139. [Google Scholar]
- Kurasawa S, Schultz RM, Kopf GS.Egg-induced modifications of the zona pellucida of mouse eggs: effects of microinjected inositol 1,4,5-trisphosphate. Dev Biol 1989; 133: 295–304. [DOI] [PubMed] [Google Scholar]
- Igarashi H, Knott JG, Schultz RM, Williams CJ.Alterations of PLCbeta1 in mouse eggs change calcium oscillatory behavior following fertilization. Dev Biol 2007; 312: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda P, Stein P, Schultz RM.RNAi in mouse oocytes and preimplantation embryos: effectiveness of hairpin dsRNA. Biochem Biophys Res Commun 2001; 287: 1099–1104. [DOI] [PubMed] [Google Scholar]
- Laemmli UK, Quittner SF.Maturation of the head of bacteriophage T4, IV: the proteins of the core of the tubular polyheads and in vitro cleavage of the head proteins. Virology 1974; 62: 483–499. [DOI] [PubMed] [Google Scholar]
- Stegmeier F, Amon A.Closing mitosis: the functions of the Cdc14 phosphatase and its regulation. Annu Rev Genet 2004; 38: 203–232. [DOI] [PubMed] [Google Scholar]
- Cho HP, Liu Y, Gomez M, Dunlap J, Tyers M, Wang Y.The dual-specificity phosphatase CDC14B bundles and stabilizes microtubules. Mol Cell Biol 2005; 25: 4541–4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley BR, Cartwright J., JrCold-labile and cold-stable microtubules in the mitotic spindle of mammalian cells. Ann N Y Acad Sci 1975; 253: 428–439. [DOI] [PubMed] [Google Scholar]
- Svoboda P, Stein P, Hayashi H, Schultz RM.Selective reduction of dormant maternal mRNAs in mouse oocytes by RNA interference. Development 2000; 127: 4147–4156. [DOI] [PubMed] [Google Scholar]
- Tsafriri A, Chun SY, Zhang R, Hsueh AJ, Conti M.Oocyte maturation involves compartmentalization and opposing changes of cAMP levels in follicular somatic and germ cells: studies using selective phosphodiesterase inhibitors. Dev Biol 1996; 178: 393–402. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Kishimoto T.Cell cycle dynamics of maturation-promoting factor during mouse oocyte maturation. Tokai J Exp Clin Med 1986; 11: 471–477. [PubMed] [Google Scholar]
- Masui Y, Clarke HJ.Oocyte maturation. Int Rev Cytol 1979; 57: 185–282. [DOI] [PubMed] [Google Scholar]
- de Vantery C, Stutz A, Vassalli JD, Schorderet-Slatkine S.Acquisition of meiotic competence in growing mouse oocytes is controlled at both translational and posttranslational levels. Dev Biol 1997; 187: 43–54. [DOI] [PubMed] [Google Scholar]
- Madgwick S, Nixon VL, Chang HY, Herbert M, Levasseur M, Jones KT.Maintenance of sister chromatid attachment in mouse eggs through maturation-promoting factor activity. Dev Biol 2004; 275: 68–81. [DOI] [PubMed] [Google Scholar]
- Reis A, Madgwick S, Chang HY, Nabti I, Levasseur M, Jones KT.Prometaphase APCcdh1 activity prevents non-disjunction in mammalian oocytes. Nat Cell Biol 2007; 9: 1192–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bembenek J, Yu H.Regulation of the anaphase-promoting complex by the dual specificity phosphatase human Cdc14a. J Biol Chem 2001; 276: 48237–48242. [DOI] [PubMed] [Google Scholar]
- Jaspersen SL, Charles JF, Morgan DO.Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14. Curr Biol 1999; 9: 227–236. [DOI] [PubMed] [Google Scholar]
- Stegmeier F, Visintin R, Amon A.Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell 2002; 108: 207–220. [DOI] [PubMed] [Google Scholar]
- Shou W, Seol JH, Shevchenko A, Baskerville C, Moazed D, Chen ZW, Jang J, Shevchenko A, Charbonneau H, Deshaies RJ.Exit from mitosis is triggered by Tem1-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell 1999; 97: 233–244. [DOI] [PubMed] [Google Scholar]
- Visintin R, Hwang ES, Amon A.Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature 1999; 398: 818–823. [DOI] [PubMed] [Google Scholar]
- Wolfe BA, Gould KL.Fission yeast Clp1p phosphatase affects G2/M transition and mitotic exit through Cdc25p inactivation. EMBO J 2004; 23: 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln AJ, Wickramasinghe D, Stein P, Schultz RM, Palko ME, De Miguel MP, Tessarollo L, Donovan PJ.Cdc25b phosphatase is required for resumption of meiosis during oocyte maturation. Nat Genet 2002; 30: 446–449. [DOI] [PubMed] [Google Scholar]
- Solc P, Saskova A, Baran V, Kubelka M, Schultz RM, Motlik J.CDC25A phosphatase controls meiosis I progression in mouse oocytes. Dev Biol 2008; 317: 260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khmelinskii A, Lawrence C, Roostalu J, Schiebel E.Cdc14-regulated midzone assembly controls anaphase B. J Cell Biol 2007; 177: 981–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Lau E, Schwarzenbacher R, Bossy-Wetzel E, Jiang W.Spatiotemporal control of spindle midzone formation by PRC1 in human cells. Proc Natl Acad Sci U S A 2006; 103: 6196–6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, O'Brien MJ, Wigglesworth K, Eppig JJ, Schultz RM.Transcript profiling during mouse oocyte development and the effect of gonadotropin priming and development in vitro. Dev Biol 2005; 286: 493–506. [DOI] [PubMed] [Google Scholar]
- Schuh M, Ellenberg J.Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell 2007; 130: 484–498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.