Abstract
Reproductive aging of the male is characterized by decreasing fertility; however, factors that protect against reproductive aging in the male are largely unknown. Previous work has demonstrated that both female presence and aging have a dramatic effect on fertility in the male; yet, the effect of female presence on fertility in the aging male mouse is unknown. The objective of this work was to determine the effect of long-term isolation or cohabitation with females on fertility in aged male mice. Male mice were housed with or without females until between 16 and 32 mo of age. Males were subjected to fertility tests at specific ages, after which serum and testes were isolated for radioimmunoassay and histological analysis. We show that male mice continuously housed with females remain fertile longer (∼20% of the reproductive lifespan) than male mice housed alone. Fertility became significantly reduced 6 mo sooner for males housed alone compared with males housed with females; however, the rate of decline was the same for males housed with or without females once fertility began to decrease. Testis weight decreased as the mice aged, and a nearly significant positive effect of female presence was observed. Additionally, histological analysis indicated that abnormal spermatogenesis occurred sooner in isolated males, suggesting that defects in spermatogenesis may play a role in the greater decrease in fertility in isolated males. These results have significant implications for the maintenance of male fertility in wildlife, livestock, and human populations.
Keywords: aging, fertility, male sexual function, spermatogenesis, testis
Cohabitation with females during aging dramatically increases the reproductive lifespan of male mice.
INTRODUCTION
Physiologic aging affects many processes, ranging from maintenance of bone density to neurological function. Aging is also significantly linked to defects in reproduction, particularly in the female. The primary result of reproductive aging in the human female, menopause, is directly related to the limited reserve of oocytes within the ovary. Loss of oocytes not only results in infertility due to lack of gametes, but also in the inability of the ovary to produce steroid hormones. Reproductive deficiencies have also been linked to aging in nonhuman females, including livestock and rodents [1–2].
In contrast to females, reproductive aging in males is not as pronounced or as well studied; however, there is a link between aging and infertility [2, 3]. Elderly human males produce less testosterone [4], have fewer motile sperm in the ejaculate [5], and suffer from a higher incidence of erectile dysfunction [6], all of which could contribute to decreased fertility. Reproductive aging in males is also a concern for endangered species and captive populations of mammals [7].
In rodents, like humans, decreased fertility has been observed in aged males, and is caused by a variety of endocrine, spermatogenic, and environmental factors [8]. Franks and Payne [2] reported that numbers of litters sired by wild-type C57/BL Icrf at mice begins to decline at 24 mo of age, and Bronson and Desjardins [9] reported that this reproductive failure is correlated with a loss of episodic release of luteinizing hormone from the pituitary. Additionally, aged mice have increased incidences of abnormal sperm [10] and a decrease in fertilization capacity [11]. Histological examination of aged rat testes revealed abnormal spermatogenesis [12] and a general degeneration of the seminiferous epithelium with advancing age [13]. We have recently demonstrated that, by 12 mo of age, fertility of ROSA26 mice (stock no. 002073; the Jackson Laboratory) is dramatically reduced, and the spermatogonial stem cells can be maintained for much longer than the normal life span of a mouse, indicating that the stem cell niche plays a critical role in male reproductive aging [14]. Finally, environmental factors can affect aging of the reproductive process, including the presence of novel females [8].
The direct effect of females and social environment on reproduction in male rodents has received considerable investigation. Most of these studies have explored effects of early social deprivation on subsequent behavior and fertility in adult animals. Some reports indicate that social isolation has no negative effect on reproduction [15, 16]. Others have reported that isolated male rats had smaller reproductive organs at 9 mo of age [17], decreased testosterone at 6 mo of age [18], and displayed aberrant copulatory behavior at 16 mo of age [19]; however, no change in overall fertility has been reported. Additionally, isolated male rats that were housed with ovariectomized females had poorer quality sperm [20] and smaller testes [21], indicating that the presence of intact females and/or the act of copulation can influence reproduction. Similar results have been observed in mice [8, 22, 23]; however, these experiments have only examined copulatory behavior in mice aged 22–25 mo, and did not evaluate overall fertility. We have noted apparent decreased fertility in male mice housed individually prior to breeding compared with males that were housed with females immediately after weaning. The objective of this work was to determine the effect of maintaining males in isolation or cohabitation with females on fertility over time. We hypothesized that continued exposure of the male to a female might influence reproductive lifespan.
MATERIALS AND METHODS
Materials and Animals
All reagents were purchased from Sigma, unless otherwise noted (www.sigmaaldrich.com). Animals used in these experiments were B6129SF1/J (stock no. 101 043; the Jackson Laboratory, www.jax.org) F1 hybrid mice. This strain of mice was developed by crossing C57BL/6J (stock no. 000664) females with 129S1/SvImJ (stock no. 002448) males. Mice were maintained with water and feed that were accessible ad libitum. All animal protocols were approved by the Animal Care and Use Committee of the University of Pennsylvania in agreement with the Guide for the Care and Use of Laboratory Animals.
Experimental Design
In order to determine the effect of females on the rate and extent of reproductive aging in male mice, males were maintained in cages by themselves or cohabitated in the constant presence of a female mouse. If females died or ceased breeding regularly, they were replaced with new females. When males reached 16, 18, 20, 22, 24, 26, 28, 30, or 32 mo of age (n = 3–4 per treatment per age; the 30-mo time point only had cohabitated males, because not enough isolated males survived), they were placed with two novel virgin females (2–4 mo of age) for 5 wk. Only males that appeared healthy were examined for fertility. All females that were mated to males less than 26 mo of age were fertile (n = 80), indicating that use of nonproven breeders was not a contributing factor to observation of male fertility. Females were subsequently observed for parturition 3 wk (the gestation period of mice) after exposure to the male for a duration of 2 wk. If females had not given birth by 5 wk after exposure to the male, they were considered not pregnant. This experimental design would limit infertility due to general male inexperience, because males had 2 wk to successfully breed. After the female mice gave birth, the number of pups per litter, number of litters, and total number of pups sired per male were recorded. Males were killed by CO2 asphyxiation and testes and blood were removed. Testes were weighed and fixed in Bouins for histological analysis. Blood was stored overnight at 4°C, and serum was removed from the blood and stored at −20°C for radioimmunoassay.
Radioimmunoassay
After mice were killed, blood was removed from aged males by cardiac puncture. Serum was isolated from the blood and used for radioimmunoassay to determine the concentrations of testosterone in the males. All radioimmunoassays were conducted in duplicate in one assay by the Washington State University Center for Reproductive Biology RIA Core using a commercial kit (DSL-400; Diagnostic Systems Laboratory Inc., www.dslabs.com) as directed. The average within-assay coefficient of variation value was 0.0368.
Histology
After mice were killed, testes were removed, weighed, decapsulated, fixed in Bouins and washed in 75% ethanol. Samples were submitted to the University of Pennsylvania Cell Morphology core or the New Bolton Center Pennsylvania Animal Diagnostic Laboratory, where they were blocked in paraffin, sectioned at 6 μm, and stained with hematoxylin and eosin. At least 6 sections, sectioned 30-μm apart, were viewed for representative testes. At least 1 testis from each male in each treatment at 16, 20, 24, 26, 28, 30, and 32 mo of age was examined. The numbers of normal and abnormal seminiferous tubules were determined from three sections (at least 60-μm apart) of each testis, and the percentage of seminiferous tubules with abnormal spermatogenesis was calculated. Only round or slightly oval seminiferous tubule cross-sections were counted. Histology sections were viewed using a Leitz Dialux 20 microscope, and images were obtained with a SPOT Insight 2MP Firewire Color Mosaic Digital Camera (Diagnostic Instruments Inc., www.diaginc.com).
Statistical Methods
When analyzing data sets, univariate ANOVA was conducted using SPSS 15 (www.SPSS.com) to identify significant effects of time (age), treatment (presence or absence of the female), and the interaction between time and treatment. Datasets were considered significant at P ≤ 0.05. If a significant effect of time was present, datasets were analyzed using the least significant difference post hoc test. When a significant effect of treatment was present, data points for specific time points were analyzed using a t-test. Regression analyses and homogeneity of slopes tests were performed on selected data sets to determine if rates of change over time were significantly different between treatments.
RESULTS
Cohabitation Extends the Reproductive Lifespan of Aged Male Mice
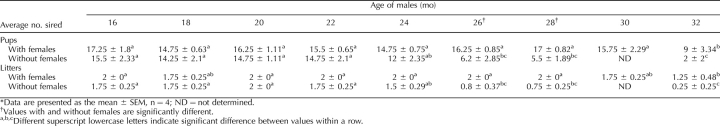
To determine the effect of the presence of the female on fertility in aged male mice, we housed males in the presence and absence of females for 16–32 mo—considerably longer than in previous work. To determine whether the presence of the female affected the fertility of aged males, the males were subjected to breeding trials in which each male was placed with two novel females (2–4 mo of age) at 2-mo intervals beginning at 16 mo of age. Females were observed for 5 wk, and the number of litters and pups sired by each male was recorded. Because pregnancy lasts 3 wk in mice, this procedure tested the ability of the aged male to impregnate 2 females over a 2-wk period. A significant effect of age on both number of pups and litters sired was observed regardless of female presence (Table 1). Additionally, a significant effect of female presence was also observed. Males that were housed alone showed reduced fertility by 26 mo of age (similar to previous reports [2]), whereas males that were housed with females did not show reduced fertility until 32 mo of age. Furthermore, males that were housed alone sired significantly fewer pups and litters at both 26 and 28 mo of age than did males that were housed with females. These results indicate that the presence of a female delayed reproductive aging in the male by 6 mo, which represents approximately 20% of the normal fertile period determined here (6 mo/28.5 mo; 30 mo [greatest age with normal fertility] − 1.5 mo [typical age at puberty] = 28.5 mo).
TABLE 1.
Effect of aging and cohabitation on fertility in males.*
The Rate of Decrease in Fertility in Aged Male Mice Is the Same Regardless of Social Environment
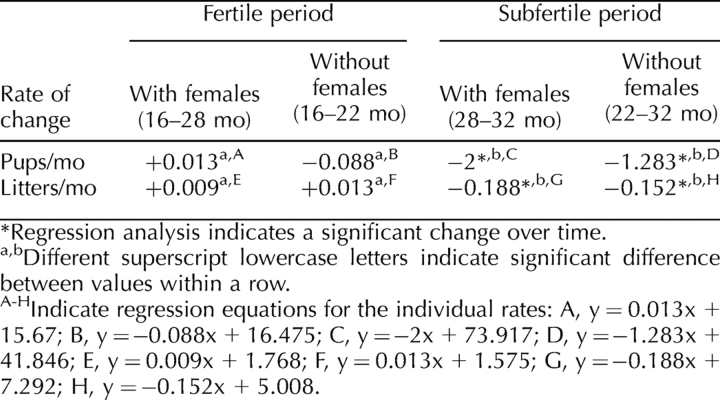
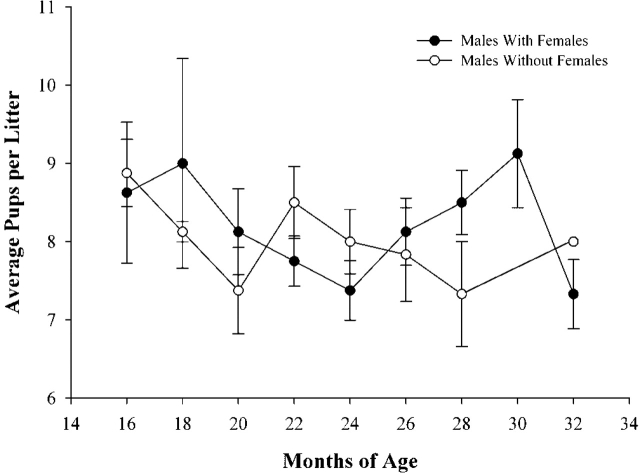
In order to determine if the rate of reproductive aging was similar for isolated and cohabitated males, regression and homogeneity of slope tests were conducted. These analyses indicated that the rate of change in fertility during the fertile periods (16–22 mo in isolated males; 16–28 mo in cohabitated males) was the same for both groups (Fig. 1, A and B; Table 2). Surprisingly, once fertility began to decrease (22–32 mo in isolated males; 28–32 mo in cohabitated males), the rate of decline was also the same for both groups of males (Fig. 1, A and B; Table 2). These results indicate that, once fertility begins to decrease, the rate of decrease is the same, regardless of the presence of the female. The average number of pups sired per litter was not affected by age or cohabitation (Fig. 2), which corroborates experiments of Franks and Payne [2].
FIG. 1.
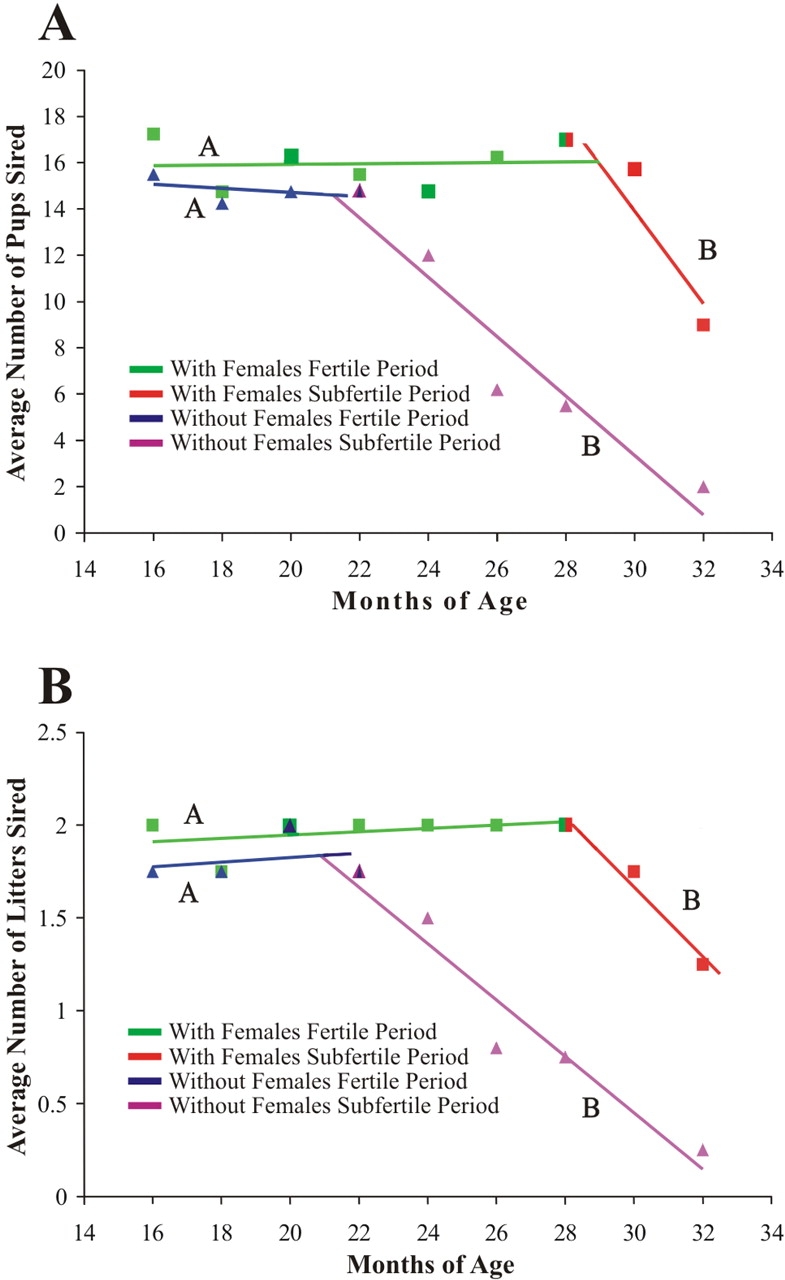
Effects of female presence on the rate of change in fertility in aged male mice. To determine if fertility decreased at a similar rate regardless of female exposure, regression and homogeneity of slope analysis were used to evaluate whether the rate of change (slope of the regression line) for average number of pups sired (A) and average number of litters sired (B) was different in cohabitated (square) or isolated (triangle) males in the fertile and subfertile periods. Regression lines with different letters are significantly different. For both pups and litters sired, fertile periods and subfertile periods had similar rates of change, regardless of the presence of the female (see Table 1 for data and Table 2 for rate changes); n = 4 for each data point.
TABLE 2.
Effect of aging and cohabitation on the rate of change in fertility.
FIG. 2.
Effects of female presence on pups per litter. Neither the age of the male nor the presence of the female had a significant effect on litter size. Error bars are ± SEM.
Testis Weight Decreased as Mice Aged
Testis weight was determined to gain insight into the etiology of decreased fertility in aged males. An effect of cohabitation on testis weight would indicate that abnormal spermatogenesis due to age might be present in isolated males sooner than in cohabitated males. A significant effect of age on testis weight was observed (Fig. 3A), and the rate of decrease in testis weight was the same regardless of treatment (Fig. 3B). Interestingly, the effect of female presence on testis weight was not significant; however, the effect was nearly significant (P = 0.084), suggesting that decreased fertility in aged males housed alone may be due to an early impact of age on spermatogenesis. The seminiferous tubules and endocrine compartments of the testis represent approximately 95% and 5% of testis weight, respectively.
FIG. 3.
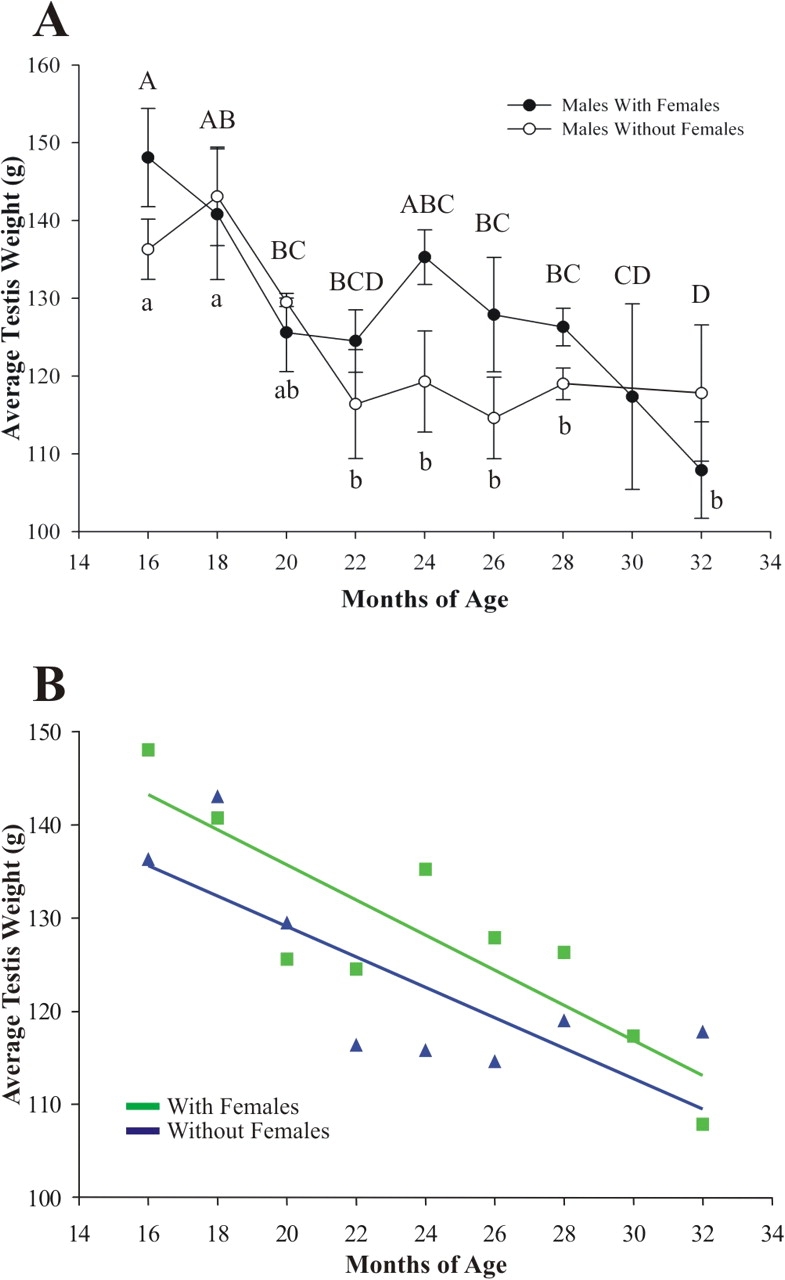
Effects of female presence on testis weight. A) A significant effect of age on testis weight was observed for both cohabitated and isolated males. The effect of the female on testis weight was nearly significant by ANOVA (P = 0.084), indicating that defects in spermatogenesis may account for subfertility. Points with different letters are significantly different within the treatment groups. Capital letters are specific for males with females, and lowercase letters are specific for males without females. Error bars are ± SEM. B) Regression and homogeneity of slope analysis indicated that there was no difference in the rates of decrease (slope of the regression line) in testis weight between cohabitated (square) and isolated (triangle) males (n = 3–4 for each data point). Regression equations: with female, y = −1.885x + 173.428; without female, y = −1.634x + 161.789.
Serum Testosterone Levels Were Not Influenced by the Presence of the Female
To determine if the reduction in fertility in isolated males was due to a decrease in serum testosterone, hormone levels were determined by radioimmunoassay. Testosterone has a profound effect on spermatogenesis [24] and reproductive behavior [25]; however, its secretion is pulsatile, and, therefore, only major changes can be ascertained with individual measurements. No significant difference in the level of testosterone was observed over time or between males housed individually or with females, indicating that the endocrine pathway governing spermatogenesis and reproductive behavior in aged males is essentially intact, regardless of the presence of a female during aging (Fig. 4).
FIG. 4.
Effects of female presence on serum testosterone levels in aged male mice. Serum testosterone concentrations were not affected by age or female presence. Error bars are ± SEM.
Histologically Abnormal Spermatogenesis Appears Sooner in Isolated Males
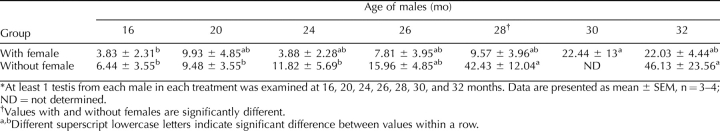
Histological examination of testes was performed to assess the role of female presence on spermatogenesis. Tissues were fixed, sectioned, stained with hematoxylin and eosin, and examined microscopically. Seminiferous tubules appeared normal, and no overt morphology indicating infertility was observed for either housing condition at 16, 20, and 24 mo of age (Fig. 5). Furthermore, seminiferous tubules from 26- and 28-mo-old males housed with females also appeared normal. In contrast, examination of tissues from 26-, 28-, and 32-mo-old males that were housed alone, and 30- and 32-mo-old males housed with females, revealed that some testes had reduced spermatogenesis. This was indicated by abnormal seminiferous tubule morphology, including apparent premature release of germ cells into the seminiferous tubule lumen, vacuolization of seminiferous tubules, and germ cell-deficient (e.g., Sertoli cell only) seminiferous tubules (Fig. 5). Normal testis morphology was sometimes observed in 28-mo-old subfertile and 32-mo-old infertile males. To determine if the observed differences in spermatogenesis were significant, the percentage of seminiferous tubules with abnormal spermatogenesis was evaluated. A significant effect of both age and treatment was observed (Table 3). Isolated males at 28 and 32 mo of age had a significantly higher percentage of seminiferous tubules with abnormal spermatogenesis than 16-, 20-, and 24-mo-old males. However, an effect of age on the percentage of seminiferous tubules with abnormal spermatogenesis in cohabitated males was not observed until 30 mo of age (Table 3). Furthermore, isolated males had significantly more abnormal seminiferous tubules than cohabitated males at 28 mo of age (Table 3). These data indicate that abnormal spermatogenesis appears in some aged males, and that the appearance of abnormal spermatogenesis occurs sooner and is more pronounced in isolated males.
FIG. 5.
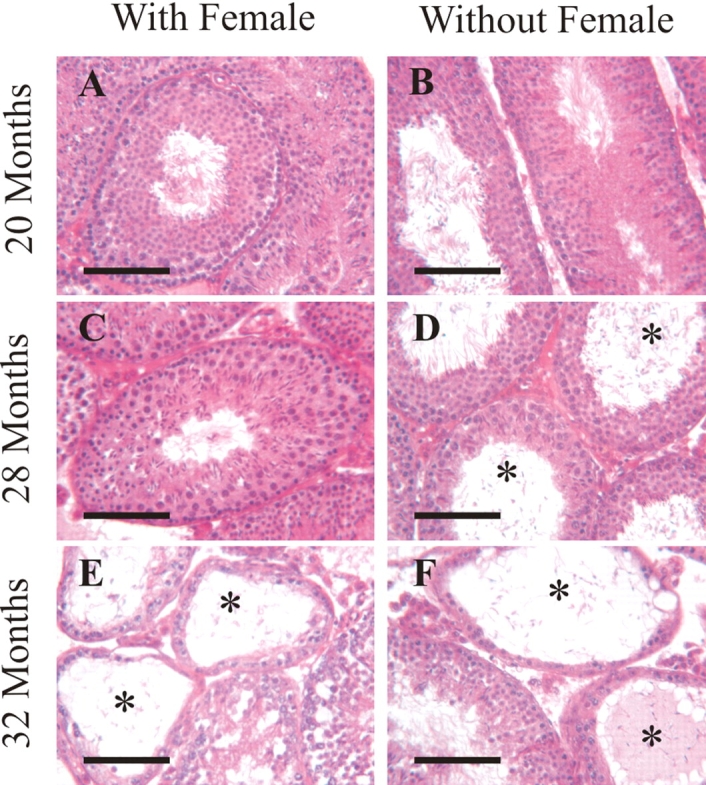
Effects of female presence on testis histology. Testis tissue from 20-mo-old cohabitated males (A) and isolated males (B) appeared normal. Tissue from 28-mo-old cohabitated males appeared normal (C); however, some tissue from 28-mo-old isolated males had abnormal spermatogenesis (D). Some 32-mo-old cohabitated male testis tissue appeared normal; however, some had abnormal spermatogenesis (E). Testis tissue from 32-mo-old isolated males appeared abnormal (F). *Seminiferous tubules with aberrant spermatogenesis; bar = 100 μm.
TABLE 3.
Percentage of seminiferous tubules with abnormal spermatogenesis.*
DISCUSSION
The objective of this work was to determine the effect of the presence of the female on fertility in aged male mice. In order to complete this objective, male mice were aged in the presence or absence of a female for 16–32 mo. At specific ages, males were subjected to breeding trials, after which they were killed and testes and blood were obtained. Testes were weighed and examined histologically, and serum was isolated for radioimmunoassay to determine if either aging or the presence of the female affected spermatogenesis or steroidogenesis. The results indicate that the presence of the female extended the fertile lifespan of the male by 20%, and that the rate of decrease in fertility was the same, regardless of female presence. The observation that isolated males were as fertile as cohabitated males from 16 to 24 mo indicates that the copulatory response is intact in isolated males, and that the effect of cohabitation on reproductive aging is not specifically behavioral. However, we cannot rule out a behavioral change that occurs in isolated males after 24 mo of age.
Analysis of testis weight, testis histology, and serum testosterone indicate that the decrease in fertility is at least in part caused by abnormal spermatogenesis. Testis weight decreased as mice aged, and a nearly significant (P = 0.084) effect of female presence on testis weight was observed. Additionally, abnormal seminiferous tubule histology appeared sooner in testes from isolated males. Radioimmunoassay indicated that production of testosterone was not significantly disrupted; however, this result should be viewed lightly, due to the lack of repeated measures and the extreme variation between individuals. The absence of abnormal spermatogenesis in some subfertile and infertile males suggests that this phenotype was too localized to be observed in the sections, or that the aged phenotype is multifaceted, and abnormal spermatogenesis is only a contributing factor to impaired fertility. Additionally, more subtle problems in aging males, such as physical inability or disease, have been documented [26, 27].
Previous work has demonstrated that cohabitation and previous sexual experience have a positive effect on reproductive behavior [8, 23]; however, a direct evaluation of overall fertility of isolated and cohabitated males has not been conducted. Our results indicate that presence of a female extends the reproductive lifespan of aged male mice by at least 20% (6 mo), which is highly significant. If this phenomenon (20% increase in reproductive lifespan) occurs in other species, changes in management and fertility practices could improve the reproductive lifespan by years in animals such as livestock and endangered wildlife, and even decades in other mammals, such as primates and elephants. Additionally, the fact that the rate of decreasing fertility is the same regardless of female presence suggests that a similar mechanism is responsible for a decrease in fertility, and that presence of the female delays this mechanism. It is possible that the increase in fertile lifespan in cohabitated males would also be present in males that were cohabitated with other males. However, in previous work, when males were cohabitated with either a female or another male, individual males housed with females had larger testes, epididymides, and seminiferous tubule diameters than males housed with males [22], indicating that exposure to females can specifically influence reproductive function. The direct cause of decreased fertility in isolated males still remains to be determined. The facts that breeding trials lasted for 2 wk with 2 females, and that 16- to 22-mo-old isolated males were as fertile as cohabitated males, suggest that aberrant copulatory behavior in isolated males is unlikely. However, it is possible that indirect effects of general aging on overall health and mobility influenced fertility. Loss of libido or the physical ability to mate due to reduced mobility may have contributed to the apparent decreased fertility in aged and isolated males; however, the presence of aberrant spermatogenesis in seminiferous tubules sooner and at a higher rate in isolated males suggests that defects in spermatogenesis also play a role in the decrease in fertility in isolated males.
Many studies have indicated that aging adversely influences reproductive performance in the female, but few studies have examined the effect of age on long-term reproductive performance in the male, especially as it relates to exposure to the female. We were surprised by two aspects of the current study: first, the dramatic extension, 6 mo or 20%, of reproductive lifespan in male mice cohabitated with females compared to male mice housed alone; and, second, that the fertility rate was identical during the fertile period for both sets of males, and then declined at the same rate during the subfertile period. This remarkable similarity suggests that the effect of chronological age on reproductive aging with and without a female is similar once the aging process begins. The mechanisms underlying the process of reproductive aging itself are difficult to determine, yet it is evident that defective spermatogenesis may contribute to the decrease in fertility. Previous studies suggest that spermatogonial stem cells, the foundation of spermatogenesis, are long lived and that the testicular stem cell niche ages more rapidly [14]. Evidence from other studies indicates that blood-borne factors play a critical role in aging of stem cell systems [28]. Thus, we postulate that the female delays aging in one or more systems in the male that support the testicular stem cell niche, which accounts, in part, for extending the reproductive lifespan; however, other systems might also be influenced by female presence. These data also demonstrate that the dramatic influence of the female on the reproductive lifespan of the male could have a significant impact on reproductive management of livestock, endangered species, and humans. Finally, exposure to females may prolong aging of stem cell niches in other tissue systems. Thus, social contexts may have significant impacts on degenerative diseases and aging.
Acknowledgments
We thank C. Freeman and R. Naroznowski for assistance with animal maintenance, the University of Pennsylvania Cell Morphology Core, and the New Bolton Center Pennsylvania Animal Diagnostic Laboratory for histological preparations, the Washington State University Center for Reproductive Biology RIA core for RIA analysis, and the Center for Clinical Epidemiology and Biostatistics for statistical analysis consultation.
Footnotes
1Supported by National Institutes of Health grants HD 052728 and AG 024992, and by the Robert J. Kleberg, Jr., and Helen C. Kleberg Foundation.
REFERENCES
- Erickson BH, Reynolds RA, Murphree RL.Ovarian characteristics and reproductive performance of the aged cow. Biol Reprod 1976; 15: 555–560. [DOI] [PubMed] [Google Scholar]
- Franks LM, Payne J.The influence of age on reproductive capacity in C57BL mice. J Reprod Fertil 1970; 21: 563–565. [DOI] [PubMed] [Google Scholar]
- Kühnert B, Nieschlag E.Reproductive functions of the ageing male. Hum Reprod Update 2004; 10: 327–339. [DOI] [PubMed] [Google Scholar]
- Pirke KM, Sintermann R, Vogt HJ.Testosterone and testosterone precursors in the spermatic vein and in the testicular tissue of old men. Reduced oxygen supply may explain the relative increase of testicular progesterone and 17 alpha-hydroxyprogesterone content and production in old age. Gerontology 1980; 26: 221–230. [DOI] [PubMed] [Google Scholar]
- Ng KK, Donat R, Chan L, Lalak A, Di Pierro I, Handelsman DJ.Sperm output of older men. Hum Reprod 2004; 19: 1811–1815. [DOI] [PubMed] [Google Scholar]
- Lazarou S, Morgentaler A.The effect of aging on spermatogenesis and pregnancy outcomes. Urol Clin North Am 2008; 35: 331–339. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE, Scheuerlein A, Cohen A.Age-related patterns of fertility in captive populations of birds and mammals. Exp Gerontol 2003; 38: 741–745. [DOI] [PubMed] [Google Scholar]
- Bronson FH.Relative effects of exercise, diet, and female stimulation on sexual aging of male mice. J Gerontol 1982; 37: 555–559. [DOI] [PubMed] [Google Scholar]
- Bronson FH, Desjardins C.Reproductive failure in aged CBF1 male mice: interrelationships between pituitary gonadotropic hormones, testicular function, and mating success. Endocrinology 1977; 101: 939–945. [DOI] [PubMed] [Google Scholar]
- Parkening TA, Collins TJ, Au WW.Paternal age and its effects on reproduction in C57BL/6NNia mice. J Gerontol 1988; 43: B79–B84. [DOI] [PubMed] [Google Scholar]
- Parkening TA.Fertilizing ability of spermatozoa from aged C57BL/6NNia mice. J Reprod Fertil 1989; 87: 727–733. [DOI] [PubMed] [Google Scholar]
- Humphreys PN.The histology of the testis in aging and senile rats. Exp Gerontol 1977; 12: 27–37. [DOI] [PubMed] [Google Scholar]
- Wang C, Leung A, Sinha-Hikim P.Reproductive aging in the male brown-Norway rat: a model for the human. Endocrinology 1993; 133: 2773–2781. [DOI] [PubMed] [Google Scholar]
- Ryu B-Y, Orwig KE, Oatley JM, Avarbock MR, Brinster RL.Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells 2006; 24: 1505–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach FA.Comparison of copulatory behavior of male rats raised in isolation, cohabitation and segregation. J Genet Psychol 1942; 60: 121–136. [Google Scholar]
- Beach FA.Normal sexual behavior in male rats isolated at fourteen days of age. J Comp Physiol Psychol 1957; 51: 37–38. [DOI] [PubMed] [Google Scholar]
- Drori D, Floman Y.Effects of cohabitation on the reproductive system, kidneys and body composition of male rats. J Reprod Fertil 1964; 8: 351–359. [DOI] [PubMed] [Google Scholar]
- Dessi-Fulgheri F, Lupo Di Prisco C, Verdarelli P.Effects of two kinds of social deprivation on testosterone and estradiol-17beta plasma levels in the male rat. Experientia 1976; 32: 114–115. [DOI] [PubMed] [Google Scholar]
- Drori D, Floman Y.The sexual behaviour of male rats unmated to 16 months of age. Anim Behav 1967; 15: 20–24. [DOI] [PubMed] [Google Scholar]
- Taylor GT, Weiss J, Frechmann T.Ontogeny of epidydimal sperm reserves during the reproductive lifespan of rats after previous sexual experiences. J Reprod Fertil 1986; 77: 419–423. [DOI] [PubMed] [Google Scholar]
- Taylor GT, Weiss J, Royalty J.Chronic maintenance of rat sperm reserves into old age by previous sexual contact. Experientia 1987; 43: 311–312. [DOI] [PubMed] [Google Scholar]
- Fox KA.Effects of prepubertal habitation conditions on the reproductive physiology of the male house mouse. J Reprod Fertil 1968; 17: 75–85. [DOI] [PubMed] [Google Scholar]
- Huber MHR, Bronson FH.Recovery of sexual activity with experience in aged male mice. Exp Aging Res 1980; 6: 385–391. [DOI] [PubMed] [Google Scholar]
- O'Donnell L, Meachem SJ, Stanton PG, McLachlan RI.Endocrine regulation of spermatogenesis. Neil JD.Knobil and Neill's Physiology of Reproduction, vol. 1, 3rd ed St. Louis:Academic Press;2006: 1071–1148. [Google Scholar]
- Hull EM, Wood RI, McKenna KE.Neurobiology of male sexual behavior. Neil JD.Knobil and Neill's Physiology of Reproduction, vol. 2, 3rd ed St. Louis:Academic Press;2006: 1729–1824. [Google Scholar]
- Bronson FH, Desjardins C.Physical and metabolic correlates of sexual inactivity in aged male mice. Am J Physiol 1986; 250(4 pt 2):R665–R675. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Latham KR, Finch CE.Plasma testosterone levels in C57BL/6J male mice: effects of age and disease. Acta Endocrinol 1975; 80: 744–752. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA.Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005; 433: 760–764. [DOI] [PubMed] [Google Scholar]