Abstract
We show here high levels of expression and secretion of the chemokine CXCL5 in the macrophage fraction of white adipose tissue (WAT). Moreover, we find that CXCL5 is dramatically increased in serum of human obese compared to lean subjects. Conversely, CXCL5 concentration is decreased in obese subjects after a weight reduction program, or in obese non-insulin resistant, compared to insulin resistant obese subjects. Most importantly we demonstrate that treatment with recombinant CXCL5 blocks insulin-stimulated glucose uptake in muscle in mice. CXCL5 blocks insulin signaling by activating the Jak2/STAT5/SOCS2 pathway. Finally, by treating obese, insulin resistant mice with either anti-CXCL5 neutralizing antibodies or antagonists of CXCR2, which is the CXCL5 receptor we demonstrate that CXCL5 mediates insulin resistance. Furthermore CXCR2−/− mice are protected against obesity-induced insulin resistance. Taken together, these results show that secretion of CXCL5 by WAT resident macrophages represents a link between obesity, inflammation, and insulin resistance.
Introduction
White adipose tissue (WAT) is primarily involved in energy storage in the form of triglycerides, and energy release in the form of free fatty acids. However, WAT is no longer considered only as a fat storage organ. Instead, WAT secrete numerous factors that play a role in several physiological and pathological processes including immunological response, angiogenesis, growth and differentiation, atherosclerosis, hypertension, glucose homeostasis, appetite regulation, and cancer (reviewed in (Ahima and Flier, 2000; Trujillo and Scherer, 2006)). External factors such as nutritional status, stress, physical exercise, infection or trauma, will affect WAT biology through various signaling pathways including growth factors and fatty acids. In turn, WAT will then signal other organs in an endocrine manner, thereby transmitting a metabolic, proliferative, growth, or differentiation response. Adipose tissue derived factors are secreted by the different cell compartments of WAT, such as adipocytes or macrophages, and were initially characterized as regulators of metabolic processes, such as regulation of food intake, energy homeostasis, adipocyte differentiation, or insulin sensitivity. Subsequently, it was found that adipose tissue derived factors could modulate inflammatory processes. These factors include WAT-specific factors, such as leptin, adiponectin, and well-known cytokines secreted by several cell types, such as TNFα, IL-6, IL-8, IL-1, or monocyte chemoattractant protein-1 (MCP-1). Increased WAT mass, such as observed in obese subjects is correlated with chronic systemic inflammatory response, demonstrated by increased infiltration of macrophages in WAT (Weisberg et al., 2003; Xu et al., 2003). This inflammatory state results in the development of obesity-associated pathologies including atherosclerosis, hypertension, non alcoholic steatohepatitis, and insulin resistance. These obesity-associated pathologies are the result, at least in part, of changes in the secretion of adipose tissue derived factors, and illustrates the link between inflammatory state and metabolic response (Hotamisligil, 2006).
Chemokines are proinflammatory cytokines that stimulate leukocyte chemoattraction and are produced in response to infectious and other inflammatory stimuli by a number of different cell types. To date more than 50 proinflammatory cytokines or chemokines have been identified and have been classified into four groups according to the location of the conserved cysteine residues: CXCL, CCL, CL, and CX3CL (Zlotnik et al., 2006). These chemokines specifically recruit different cell types to an inflammatory site. CXCL5 or epithelial neutrophil activating peptide (ENA-78) is a cytokine belonging to the family of chemokines that is mainly implicated in the chemotaxis of inflammatory cells through the generation of local concentration gradients (Walz et al., 1991; Walz et al., 1997). It has been shown, to be a recruiter of neutrophils and involved in their activation, through interaction with the CXCR2 receptor. This C–X–C chemokine has been implicated in pulmonary disease, lung cancer, arthritis, and other pathological states (Walz et al., 1991; Walz et al., 1993; Wislez et al., 2004). In the present study we show that CXCL5 is expressed at high levels in WAT, in particular in the macrophage fraction. We further show that circulating levels of CXCL5 are associated with human obesity and insulin resistance, and demonstrate inhibitory effects of the cytokine in insulin-induced glucose transport in muscle cells. Consistent with a role in insulin resistance, we show that treatment of obese, insulin resistant mice with either neutralizing anti-CXCL5 antibodies or CXCR2 antagonists results in an overall decrease in fasting glycemia, and improved insulin sensitivity. Finally, we show that CXCR2 (−/−) mice are resistant to diet-induced insulin resistance and diabetes.
Results
CXCL5 is expressed by WAT resident macrophages
Global analysis of cytokine expression in insulin-sensitive tissues highlighted the presence of high levels of CXCL5 protein expression in WAT in mice, compared to liver or muscle (fig. 1A), suggesting that CXCL5 could be an adipose tissue derived factor. Consistent with this hypothesis, CXCL5 was found to be secreted by WAT explants in culture (fig. 1B). Furthermore, fractionation of human subcutaneous WAT by collagenase digestion showed that the stromal vascular fraction (SVF) expressed higher levels of CXCL5 than did mature adipocytes, as assessed by real-time PCR analysis (fig. 1C). A more detailed analysis by immunoselective isolation demonstrated that the major contribution to CXCL5 concentration was from macrophages from the SVF of human WAT (fig.1C). Isolation of specific cell types was validated with specific markers of each fraction (suppl.table 1). The observed high levels of expression in WAT could point to the participation of CXCL5 in adipogenesis. To address the role of CXCL5 in adipocyte differentiation, we first analyzed CXCL5 mRNA expression during this process. CXCL5 mRNA was highly expressed in mouse and human preadipocytes whereas its expression progressively decreased during adipocyte differentiation (fig. 1D and 1E). PPARγ mRNA level is showed as specific marker of adipocyte differentiation (suppl. Fig.1). This result suggested that CXCL5 was not critical for adipogenesis. To demonstrate this hypothesis the effects of recombinant CXCL5 on 3T3-L1 preadipocyte differentiation were evaluated. 3T3-L1 cells differentiated into adipocytes, as measured by the expression of the adipocyte marker aP2, regardless of the absence or the presence of CXCL5 (fig.1F). Altogether these results suggested that CXCL5 is an adipose tissue derived factor secreted by WAT macrophages.
Figure 1. CXCL5 is expressed by WAT resident macrophages.
A. CXCL5 level was measured by ELISA assay in total protein extract of WAT, liver and muscle from 10-weeks-old C57Bl/6 mice (n=4 mice). * p<0.05, **, p<0,01, ***, p<0,001 here and in following figures or otherwise stated.
B. WAT from 10-weeks-old C57Bl/6 mice (n=4 mice) were incubated in KRBH buffer for 1h, 3h, or 22h. At each time point, secreted CXCL5 in medium was analyzed by ELISA and normalized by DNA content in tissue.
C. CXCL5 mRNA quantification by QPCR in different fractions of isolated human subcutaneous WAT as indicated. SVF: stromal vascular fraction. Quantification of mRNA was normalized by the expression level of rS9, here and in the following figures.
D-E. Quantification of relative CXCL5 mRNA levels at the indicated times of 3T3-L1 (D) or human preadipocyte (E) differentiation in three independents experiments.
F. aP2 mRNA expression was analyzed by quantitative RT-PCR on 3T3L1 adipocytes differentiated with or without recombinant mouse CXCL5 (50ng/ml). Values in (C), (D), (E) and (F), represent means of three independents experiments.
CXCL5 expression and secretion is increased in obesity
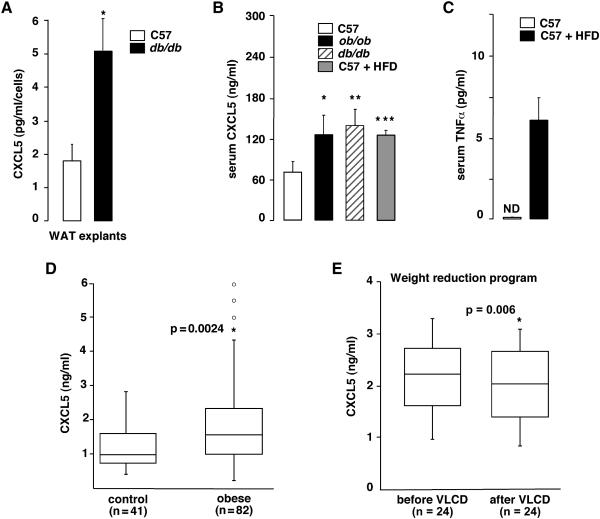
The inflammatory state associated with obesity includes macrophage infiltration in WAT, and increased cytokine secretion by these macrophages. Since it is expressed in WAT macrophages (fig. 1C), we hypothesized that CXCL5 expression would be increased in obesity. Consistent with this hypothesis CXCL5 secretion in WAT explants from obese db/db mice was markedly increased compared to non-obese control mice (2.5-fold, fig. 2A). Most interestingly, circulating serum CXCL5 concentration was increased 2-fold in obese ob/ob and db/db and high fat diet-fed mice compared to lean mice (fig. 2B). Interestingly TNFα levels were also increased in high fat diet fed mice (fig. 2C). These results prompted us to analyze CXCL5 concentration in human obese subjects. Similar to the results observed in mice, CXCL5 serum concentration was significantly increased in an obese French population, compared to lean control subjects (median:1545 [295-25900] versus 970 [395-2790] pg/ml, p=0,025) (fig. 2D). Control subjects were age and gender matched, and the observed differences were independent of age and gender as calculated by Wilcoxon-Mann-Whitney test (p=0.19). As expected serum leptin levels were also increased in the obese population (from 12..37 ±5.83 in lean to 119.87 ±22.93 in obese) Similar results were observed when an English cohort was analyzed (suppl. Fig. 2). To further demonstrate a positive correlation between body weight and CXCL5 circulating levels, calorie restriction experiments were performed. Obese women were subjected to very low calorie diet (VLCD, 800kcal/d) for a period of 4 weeks (Vitkova et al., 2007). An average decrease of 7% in body weight was observed during VLCD. Strikingly, the weight loss is associated with a decrease of 10% in serum CXCL5 concentration (median: 2230 versus 2040 pg/ml, p=0,006), suggesting that CXCL5 circulating concentration is controlled by WAT (fig. 2E).
Figure 2. Obesity increases CXCL5 release from WAT and CXCL5 systemic level.
A. Quantification by ELISA methods of CXCL5 secretion by WAT explants from C57Bl/6 and db/db mice (n=3) after 6h in incubation media. Results were normalized by DNA content in tissue.
B. Circulating CXCL5 levels were measured by ELISA methods in serum from male control C57Bl/6, db/db, ob/ob, and mice fed with a high fat diet (n=5).
C. Circulating TNFα levels were measured by ELISA methods in serum from control C57Bl/6 mice and mice fed with a high fat diet (HFD) for 12 weeks (n=4).
D. Quantification of CXCL5 concentration in serum, as measured by ELISA between normal lean (n=41) and obese patients (n=82).
E. Quantification of CXCL5 concentration in serum, as measured by ELISA in response to body weight loss during a 4-week very low calorie diet (VLCD) in 24 obese patients.
CXCL5 mediates insulin resistance
Some of the insulin desensitizing effects observed during obesity development are mediated by adipokines. The positive association of CXCL5 concentration with obesity suggested that this chemokine could be a mediator of insulin resistance. The biological effects of CXCL5 are mediated through interaction with the chemokine receptor CXCR2. To investigate the role of CXCL5 in insulin resistance, we first aimed to identify tissues that are potentially responsive to CXCL5. Expression analysis in insulin-sensitive tissues showed that CXCR2 mRNA was expressed at higher levels in muscle than in liver or WAT (fig. 3A). The physiological effects of CXCL5 in muscle were next analyzed. Incubation of isolated soleus muscles with recombinant CXCL5 resulted in a diminution of insulin-stimulated glucose transport in this tissue (fig. 3B). This suggested a role of CXCL5 in mediating insulin resistance. Strikingly, CXCL5 treatment inhibited insulin-induced Akt phosphorylation (fig. 3C, from 10- to 6-fold), which was consistent with the observed decrease in glucose transport in CXCL5-treated muscles (fig. 3B). Similarly, CXCL5 inhibited Akt phosphorylation also in primary cultures of mouse embryonic fibroblasts (MEFs) (fig.3C). A more in detail analysis suggested that CXCL5 was inhibiting, insulin signaling through activation of the JAK/STAT pathway, and consequently through increasing the expression of SOCS2, which is a known inhibitor of the insulin receptor, and increasing the activity of Stat5 signaling up to 2.5-fold (fig.3D-E). Strikingly, SOCS 2 regulation by CXCL5 is blunted by a Jak2 inhibitor, AG490 and by CXCR2 antagonist treatments (fig. 3D). From these results we concluded that CXCL5 could block insulin signaling in muscle through activation of the Jak/STAT/SOCS pathway through the CXCR2 receptor. We attempted next to demonstrate a positive correlation between insulin resistance and serum CXCL5 concentration in mice. Obese db/db mice were treated with the insulin-sensitizing drug rosiglitazone. This resulted, as expected in a decrease in glycemia (suppl. Fig.3). Decreased circulating glucose concentration was significantly associated with a 30% decrease in serum CXCL5 concentration in rosiglitazone-treated mice (fig.3F).
Figure 3. CXCL5 is involved in insulin resistance.
A. Comparative expression of CXCR2 mRNA in WAT, liver and muscle from 6-month-old C57Bl/6 mice (n=3).
B. 2-deoxy-glucose uptake in isolated soleus muscles from mice (n=3). Muscle strips were incubated with or without recombinant CXCL5 (100ng/ml) for 1h before stimulation with insulin (10−7M).
C. Protein expression of phospho-Akt, and total Akt in whole cells lysates from isolated muscles (upper panel) described in (B) and from primary MEFs (lower panel) as analyzed by Western blotting with anti-phospho-Ser473-Akt and anti-Akt antibodies. Blot are representative of 3 independents experiments.
D. Relative mRNA level of SOCS2 in primary MEFs treated or not with CXCL5 (300ng/ml) and cotreated with or without Jak2 inhibitor (AG490 50μM) or CXCR2 inhibitor (SB225002 10-6M).
E. Protein expression of phospho-STAT5a/b and total STAT5 in whole cells lysates from primary MEFs treated with or without insulin (10-7M) and/ or CXCL5 (300ng/ml) as analyzed by Western blotting with anti-phospho-Y694/Y699-STAT5a/b and anti-STAT5 antibodies. Blot are representative of 2 independents experiments
F. Relative quantification of circulating CXCL5 concentration (fold) in mice (n=10 per group) after 12 days treatment with 20mg/Kg rosiglitazone (rosi) compared to non-treated mice (control).
G. CXCL5 serum concentration is increased in obese patients with insulin resistance (n=30), compared to obese non-insulin resistant subjects (n=22). Insulin resistance status was assessed by the HOMA index (non IR: HOMA≤2.6; IR: HOMA≥2.6).
H. Quantification of CXCL5 concentration in serum, as measured by ELISA between normal lean (n=9), lipodystrophic patients (n=12) and obese patients (n=11).
We next examined the relationship between CXCL5 and insulin sensitivity in a population of obese subjects. The subjects (n=52) were stratified into two groups according to HOMA values, as a measure of insulin resistance index. Clinical parameters of the subjects are summarized in supplemental table II. We found that serum CXCL5 concentration was higher in insulin-resistant compared to non insulin-resistant obese subjects, suggesting that CXCL5 circulating levels are causative of insulin resistance in these subjects (fig. 3G; IR: 3960pg/ml versus non IR: 3110pg/ml, p=0,021). Furthermore, we have analyzed CXCL5 serum levels in a human model of non-obese insulin resistance. We show that lipodystrophic patients, who are insulin resistant do not have increased levels of CXCL5 (fig. 3H). We can conclude from this data that it is CXCL5 that induces insulin resistance, and not the opposite. Taken together these results suggested that CXCL5 is involved in the development of insulin resistance during obesity.
Inhibition of CXCL5 signaling in insulin resistant mice improves insulin sensitivity
To unequivocally demonstrate the participation of CXCL5 in obesity-induced insulin resistance we analyzed the effects of CXCL5 inhibition in two mice model of insulin resistance. C57BL/6 mice rendered obese by feeding high fat diet for 12 weeks, and obese db/db mice were treated every other day with intraperitoneal injection of neutralizing anti-CXCL5 antibody or control IgG. After one week of treatment, a significant decrease in fasting glucose was observed in mice injected with anti-CXCL5 compared to mice injected with control IgG (figure 4A and 4B). Anti-CXCL5-treated obese mice were more sensitive to insulin as measured by fasted insulin level (figure 4C and 4D) and by insulin tolerance test (figure 4E and 4F). Moreover, treatment with anti-CXCL5 improved glucose tolerance during an intraperitoneal glucose tolerance test in both mouse models of obesity (figure 4G and 4H).
Figure 4. Inhibition of CXCL5 action in obese mice improves insulin sensitivity.
A-D. Fasting glucose (A-B) and insulin (C-D) in 18-week-old obese HFD fed mice (A-C) and 9-weeks-old db/db mice (B-D) after 7 days of treatment with neutralizing antibody against CXCL5 (anti-CXCL5) or control IgG (cont IgG) (n=10 mice per group).
E-H. Glucose clearance after intraperitoneal injection of insulin (0.75U/kg) (E-F)) or glucose (G-H) in animals treated with anti-CXCL5 or control IgG. For the graph E, F and G, glucose values are relative to initial glucose levels. Area under the curve was analyzed (AUC).
Since CXCL5 signaling is mediated by the CXCR2 receptor, we next studied the impact of a selective antagonist of CXCR2, SB225002, on insulin sensitivity. Obese C57BL/6 mice were treated daily with intraperitoneal injection of SB225002 (100mg/kg) for 10 days. Similar to the effect of neutralizing antibody, SB225002 treatment also decreased fasting glucose and insulin level (figure 5A and 5B), and increased insulin sensitivity and glucose homeostasis as demonstrated by insulin and glucose tolerance tests (fig. 5C and 5D). No differences in body weight or food intake were observed as a result of either SB225002 or anti-CXCL5 antibody treatments, suggesting a direct rather than a secondary effect (supplementary fig. 4A, 4B and 4C). Since CXCL5 is secreted in normal mice we tested the effect of the treatments in these mice. Even in these SB225002- and anti CXCL5 treated lean mice a significant increase in glucose clearance was observed (suppl. fig.5B).
Figure 5. Inhibition of CXCR2 signaling improves insulin sensitivity.
A-B. Fasting glucose (A) and insulin (B) in 18-week-old HFD fed mice after 9 days of treatment with CXCR2 antagonist, SB225002 (100mg/kg) or vehicle (n=10 mice per group).
C-D. Glucose clearance after intraperitoneal injection of insulin (0.75U/kg) (C) or glucose (D) in animals treated with SB225002 or vehicle. Area under the curve was analyzed (AUC).
To unequivocally demonstrate the participation of the CXCL5-CXCR2 signaling axis in insulin sensitivity we next analyzed the phenotype of CXCR2 −/− mice. The most concluding observation was the protection of CXCR2−/− mice to obesity-induced insulin resistance (fig. 6). Fasting glucose concentration remained normal in CXCR2−/− mice fed high fat diet, compared to CXCR2+/+ mice (fig. 6A). Surprisingly, an increase in fasted insulin levels was observed suggesting that the genetic deletion of CXCR2 could also affect other pathways (fig. 6B). Moreover, no differences in body weight or food intake were observed (fig. 6C and 6D) whereas improved insulin sensitivity and glucose tolerance in both chow and high fat diet CXCR2−/−, compared to +/+ mice were observed (fig. 6E and 6F). Interestingly, genetic CXCR2 ablation or CXCR2 antagonist treatment are more effective than CXCL5 blocking, suggesting that other CXCR2 ligands could be involved in this pathway. Strikingly, circulating levels of CXCL1 and CXCL2, which are also CXCR2 ligands, are increased in mice models of obesity, although to a lesser extent than CXCL5. Taken together these results show that CXCL5 plays a major role in insulin resistance.
Figure 6. CXCR2 −/− mice improve insulin sensitivity.
A-B. Fasting glucose (A) and insulin (B) in 10-weeks-old CXCR2 −/− and control mice before (chow) and after 7 weeks of high fat diet (HFD)(n=10 mice per group).
C. Body weight in control and CXCR2 −/− mice in chow and high fat diet.
D. Food intake in control and CXCR2 −/− mice.
E-F. Glucose clearance after intraperitoneal injection of insulin (E) or glucose (F) in control and CXCR2 −/− mice under chow or high fat diet. Area under the curve was analyzed (AUC).
CXCL5 is regulated by TNFα
We finally analyzed the molecular mechanisms implicated in the observed increase in CXCL5 expression in the macrophage fraction of WAT. WAT secretes large amounts of TNFα in obese states, suggesting that this cytokine could regulate the expression of CXCL5. This was shown by the observation that CXCL5 secretion by human WAT explants was increased upon treatment with TNFα for 24h (fig.7A). TNFα is a key factor regulating CXCL5 level in WAT since we demonstrated that CXL5 mRNA expression is strongly decreased in WAT from obese ob/ob TNFα receptor −/− mice compared to the ob/ob mice (fig.7B). Furthermore, we showed in WAT explants from mice that TNFα-induced CXCL5 secretion were reduced by cotreatment with the PPARγ agonist rosiglitazone, which is known to antagonize the effects of TNFα (fig.7C). Similar results were observed when THP-1 macrophages, instead of WAT explants were treated with TNFα (fig.7D). Moreover, increased CXCL5 secretion in response to TNFα correlated to increased CXCL5 mRNA expression in these cells (fig. 7E). Rosiglitazone cotreatment of the cells also resulted in a reduction of TNFα pro-secretory (fig. 7D) and mRNA stimulation effects (fig. 7E). This suggested that TNFα regulation of CXCL5 expression is at the transcriptional level. Computational analysis of the human CXCL5 promoter sequence revealed the presence of several NFκB binding sites located upstream of the transcription start site, which could mediate the effects of TNFα. Transient transfection experiments using the CXCL5 promoter driving the expression of the luciferase gene demonstrated a 6.5-fold increase in CXCL5 promoter activity upon stimulation of the transfected cells with TNFα (fig. 7F). Similar to that observed when mRNA levels were analyzed (fig. 7E) the induction of CXCL5 promoter activity by TNFα was reduced by rosiglitazone co-treatment in cells cotransfected with PPARγ (fig. 7F). To demonstrate that the regulation of the CXCL5 promoter in response to TNFα is mediated by NFκB, chromatin immunoprecipitation (ChIP) studies were performed using a specific anti-p65 antibody, or preimmune serum in THP-1 cells. No amplification of the CXCL5 promoter was observed when non-specific antibodies were used to immunoprecipitate the chromatin (IgG, Figure 7G), whereas a specific fragment corresponding to the NFκB binding site of the CXCL5 promoter was amplified when chromatin of TNFα-treated cells was immunoprecipitated with an anti-NFkB antibody (fig. 7G). Furthermore, rosiglitazone treatment resulted in decreased binding of p65 to this site, which was consistent with the transfection data (fig. 7F). Altogether, these results suggest that TNFα triggers the expression of CXCL5 in WAT through the NFκB pathway.
Figure 7. CXCL5 expression is regulated by TNFα and rosiglitazone.
A. CXCL5 level in conditioned medium of human subcutaneous adipose tissue explants after treatment with vehicle (control) or TNFα (10ng/ml) for 24h (n=3 per group).
B. Quantification of relative CXCL5 mRNA levels in WAT from obese ob/ob TNFα receptor −/− mice compared to the control obese mice (n=3 per group) .
C-D. CXCL5 levels in WAT (A) from C57Bl/6 mice or THP1 macrophages (B) after treatment with vehicle (control), TNFα (10ng/ml), rosiglitazone (10−6M) or co-treated with TNFα/rosiglitazone for 6h. Secreted CXCL5 in medium was analyzed by ELISA and normalized by DNA tissue content.
E. Q-PCR analysis of CXCL5 mRNA in THP-1 macrophages treated as in (C)
F. Luciferase activity expressed as relative luciferase units of a 1422 bp DNA fragment of the CXCL5 promoter coupled to the luciferase gene in the absence or in the presence of a PPARγ expression vector in 293T cells treated with vehicle, TNFα (10ng/ml), rosiglitazone (10−6M) or both TNFα/rosiglitazone. Results were normalized to ß galactosidase activity. Values in (C), (D), (E) and (F), represent means of three independents experiments.
G. ChIP analysis of the NFkB binding site of the CXCL5 promoter in immunoprecipitated chromatin from THP-1 macrophages either non-treated (cont.) or treated with TNFα, rosi, or a combination of both (upper panel). Chromatin was immunoprecipitated with mock antibody (IgG) or anti p65 antibody as indicated. Quantification of the ChIP analysis in lower panel. Image J software was used to measure the optical density of the bands. Results are corrected by the signal of the corresponding input lanes. Blot is representative of 2 independents experiments.
Discussion
The most important finding in this study is the insulin sensitizing effects of both anti-CXCL5 neutralizing antibodies, and CXCR2 antagonists. This finding underscores a role of WAT-secreted CXCL5 in the development of insulin resistance in obese subjects, and validates an anti-CXCL5 therapy for the treatment or prevention of obesity-induced insulin resistance.
WAT participation in metabolic control goes beyond its well known task as a lipid storage organ. Two major physiological functions of WAT account for this participation. First, under normal conditions WAT participates in the clearance of circulating TG and FFA. Increased circulating FFA, such as observed during obesity leads to accumulation of TG in non WATs, such as muscle, liver or pancreas, resulting in decreased glucose utilization in these tissues and pancreatic toxicity, finally leading to insulin resistance and diabetes (Waki and Tontonoz, 2007). The second major role of WAT in the control of metabolism is the secretion of adipose tissue derived factors, such as leptin, resistin, adiponectin, or TNFα, which may have both paracrine and endocrine effects on several biological functions including energy homeostasis, lipid and glucose metabolism, insulin sensitivity, and inflammation (reviewed in (Badman and Flier, 2007; Rosen and Spiegelman, 2006)). Obesity is characterized by progressive macrophage infiltration in WAT, which is positively correlated with insulin resistance (Clement et al., 2004; Di Gregorio et al., 2005; Weisberg et al., 2003; Wellen and Hotamisligil, 2005). Although the relative contribution of macrophages, compared to the contribution of adipocytes, to the development of obesity-related insulin resistance is still not clear, recent studies have underscored the important role of macrophage-secreted cytokines in insulin resistance (Wellen and Hotamisligil, 2005). Such is the case for the well-known effects of TNFα, IL-6, MCP-1, or IL-1b (reviewed in (Waki and Tontonoz, 2007; Wellen and Hotamisligil, 2005)). More recently, MCP-1 cytokine and its receptor CCR2 have been shown to play a major role in macrophage infiltration in WAT, and in the development of diabetes (Sartipy and Loskutoff, 2003; Takahashi et al., 2003). Reduced macrophage accumulation, as observed in CCR2 receptor-deficient mice results in increased insulin sensitivity in these mice (Weisberg et al., 2006). A recent study demonstrated, however that the absence of MCP-1 does not attenuate obesity-associated macrophage recruitment into WAT or improve metabolic function, suggesting that MCP-1 is not critical for obesity-induced insulin resistance (Inouye et al., 2007). The dominant factor for macrophage recruitment to WAT and signaling to insulin sensitive tissues remains, therefore to be identified. CXCL5 could be, from our data one of these macrophage recruitment and insulin desensitizing WAT-secreted factors. In our study we have demonstrated increased expression of CXCL5, which is a WAT-secreted cytokine in obese human and mice. Similar to other adipokines, CXCL5 is expressed mainly in the macrophage fraction of WAT, and its role goes beyond the participation in the typical local inflammatory reaction that is characteristic of WAT in obese subjects. Interestingly we show that TNFα, which is secreted by both macrophages and adipocytes from WAT in obesity, controls the expression of CXCL5 in mouse and human. No CXCL5 regulation could be identified in Thp-1 macrophages upon treatment with sex steroids, dexamethasone, IBMX, saturated FFA or unsaturated FFA (suppl.fig. 7). Moreover, deletion of TNFα receptor in WAT from obese mice abolished CXCL5 mRNA expression in this tissue, proving that TNFα is a master regulator of CXCL5 during obesity. In addition this suggests that CXCL5 mediates the effects of TNFα on insulin resistance. Thiazolidinediones, which are PPARγ agonists used clinically as insulin sensitizers, reduce CXCL5 expression, likely through direct reduction of NFκB transcriptional regulation of CXCL5 promoter in response to TNFα stimulation. This is consistent with previously reported effects of PPARγ in the inhibition of the NFkB pathway (Ricote et al., 1998).
Our results show that CXCL5 promotes insulin resistance. This is supported, by the following data: First, increased circulating CXCL5 in human obese subjects correlates with increased insulin resistance. Second, amelioration of insulin sensitivity, such as observed during a very low calorie diet in human obese subjects, or as a response to rosiglitazone treatment in mice correlates with decreased levels of CXCL5. Third, CXCL5 impairs glucose uptake in isolated muscles. Fourth, treatment of obese, insulin resistant mice with anti-CXCL5 blocking antibodies results in modest, but consistent amelioration of insulin sensitivity in these mice. Finally, and most importantly, CXCR2(−/−) mice show improved insulin sensitivity and are resistant to diet-induced insulin resistance and diabetes.
These effects of CXCL5 are not related, or at least not only related to neutrophil migration or the control of local inflammatory reaction in WAT. CXCL5 function is rather related to the negative modulation of the insulin signaling pathway in distant muscle cells. In line with this hypothesis is the observation that CXL5 inhibits Akt phosphorylation in isolated muscles in response to insulin, which correlates with decreased glucose uptake in these CXCL5-treated muscle cells. This decrease of insulin-induced phospho-Akt is the result of the activation of the Jak2/STAT5 pathway by CXCL5 and the induction of SOC2 expression, which is well known to inhibit insulin signaling (Starr et al., 1997). The effects of CXCL5 in muscle are likely mediated by the CXCR2 receptor. This is consistent with the insulin sensitizing effects that result from the treatment of insulin resistant mice with CXCR2 antagonists. Interestingly this receptor is expressed in cells other than muscle cells, such as endothelial, pulmonary, or intestinal epithelial cells suggesting that increased CXCL5 circulating levels, such as observed in obesity could be involved in the development of obesity-related pathologies other than insulin resistance, such as atherosclerosis and other inflammatory diseases. Studies aiming to elucidate the role of WAT-secreted CXCL5 in all these obesity-related pathologies are likely to be forthcoming in the near future. Inhibiting CXCL5 secretion or function in obese individuals not only ameliorate their insulin sensitivity, but could also decrease the risk of developing other major obesity-related pathologies. Inhibition of CXCL5 signaling should be therefore considered as a therapeutic tool for the treatment of the metabolic syndrome.
Materials and Methods
Materials
All chemicals, except if stated otherwise, were purchased from Sigma (St-Louis, Missouri). Recombinant human TNFα and recombinant mouse CXCL5 were purchased from AbCys s.a. (France) and R&D systems (France), respectively. Rosiglitazone was purchased from Molekula (France).
Animals and Experimental Design
Male ob/ob mice, male db/db mice and male C57BL/6 mice were purchased at 8-9 weeks of age from Charles River laboratory. Male CXCR2 −/− mice were purchased at 9 weeks of age from Jackson Laboratory. Animals were maintained according to European Union guidelines for use of laboratory animals. In vivo experiments were performed in compliance with the French guidelines for experimental animal studies (Agreement No. B-34-172-27). For the diet-induced obesity model, 6-weeks-old C57BL/6 mice were fed ad libitum with a diet including 45% from fat (TestDiet) for 12 weeks. For antibody treatment, the neutralizing CXCL5 antibody we purchased from Dr. Strieter and injected every to other day as described previously (Arenberg et al., 1998). The neutralizing CXCL5 antibody has been shown to inhibit rat peritoneal neutrophil recruitment to recombinant CXCL5 in vivo (Halloran et al., 1999). For antagonist treatment, SB225002 was synthetized as previously described (White et al., 1998) and injected in intraperitoneal everyday for 9 days (100mg/kg). For rosiglitazone treatment, 11 weeks-old db/db mice were gaved with 20mg/kg everyday for 12 days. Body weights and glycemia was measured every 3 days and blood samples were collected. Food intake was measured every second day for 6 consecutive days.
For serum preparation, blood samples were collected. The blood let to clot for at least 30 minutes before centrifugation for 10 minutes at 1000xg.
For glucose tolerance test, 18h-fasted mice were injected i.p. with glucose (1.7g/kg) and glycemia were measured at time 0, 30, 60 and 90 min after injection. For insulin tolerance test, 8h-fasted mice were injected i.p. with insulin (0.75U/kg) and glucose level was measured at time 0, 20 and 40 min after injection.
Human studies
Serum samples, and subcutaneous abdominal WAT were obtained from subjects in agreement with French, Czech and English laws on biomedical research. Human adipocytes in primary culture were differentiated as described (Tiraby et al., 2003). The different cell types of human subcutaneous WAT were isolated as previously described (Curat et al., 2006). Human white adipose tissue was isolated as described (Tiraby et al., 2003). Results are expressed in pg/ml/g of tissue.
WAT explants culture
Fat pads from male C57BL/6 mice and/or db/db mice were dissected under sterile conditions, washed in Krebs Ringer Bicarbonate Hepes buffer (KRBH), minced finely, and incubated in 12-well tissue culture plates containing KRBH supplemented with 1% bovine serum albumin (BSA). The fat tissue samples were left unstimulated at 37°C under 5% CO2 for indicated time or were stimulated with vehicle (DMSO), TNFα (10ng/ml), rosiglitazone (10-6M) or co-treated with TNFα / rosiglitazone. At different time point, conditioned medium was removed and CXCL5 was measured by ELISA assay. At the end of experiment, fat tissue was removed and DNA extraction was done to normalize. Results are expressed in pg/ml/cells.
Protein extraction
WAT, liver and muscle were removed from C57BL/6 mice and were homogenized by Polytron in TEG buffer (10mM Tris-Hcl, pH 7.4, 1.5mM EDTA and 10% glycerol) containing protease inhibitors cocktail (Roche). The mixture was then sonicated and the cellular debris were pelleted by centrifugation at 13,000g for 10 minutes at 4°C. CXCL5 level was analyzed by ELISA assay.
ELISA and Bio-plex assay
CXCL5 concentration in cellular extracts, conditioned medium and serum was determined by ELISA with Duoset kit (DY254 and DY443, R&D Systems) as recommended by the manufacturer. CXCL1 and CXCL2 concentrations in serum were evaluated by Bio-Plex Cytokine Assay (Bio-Rad) as recommended by the manufacturer.
RNA Extraction and Reverse Transcriptase, Quantitative PCR
Total RNA from cells and tissues were isolated with TRIzol reagent (Invitrogen) as described by the manufacturer. Reverse transcription was performed using 5 μg total RNA, random primers and MMLV enzyme (Invitrogen). Quantitative PCR was performed with FastStart DNA Master SYBR Green I kit (Roche, Manheim, Germany) on a Light Cycler instrument (Roche) as specified by the manufacturer. Ribosomal protein S9 (rS9) was used as an internal control. The sequence of the primers used is available upon request.
Cell culture, transient transfection, and glucose transport
Preadipocytes 3T3-L1 cells were grown in DMEM supplemented with 10% foetal bovine serum (FBS), 100U/ml penicillin and 100□g/ml streptomycin. 3T3-L1 cells were differentiated as previously described (Sarruf et al., 2005). To analyze CXCL5 effect on adipocyte differentiation, 3T3-L1 preadipocytes were differentiated with mix supplemented with recombinant mouse CXCL5 (50ng/ml). Glucose transport experiments were performed as previously described (Abella et al., 2005). Human monocytic cell line Thp-1 were grown in RPMI 1640 supplemented with 10% FBS, 100U/ml penicillin and 100□g/ml streptomycin. Macrophage differentiation was induced by stimulating cells with TPA 100nM for 48h. Then, macrophages Thp-1 were maintained in DMEM 10% FBS and stimulated or not with TNFα (10ng/ml), rosiglitazone (10-6M) or co-treated with TNFα/rosiglitazone for 18h. CXCL5 mRNA level was analyzed by Q-PCR. Secreted CXCL5 was measured by ELISA assay and normalized by DNA content. Fibroblastic 293T cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% FBS, 100U/ml penicillin and 100□g/ml streptomycin. Transient transfections were performed as described previously (Annicotte et al., 2005) and luciferase activity measurements were normalized for ß-galactosidase activity to correct for differences in transfection efficiency. Graph values represent the mean of three independent experiments.
Primary MEFs were obtained from embryos at day 13.5 as previously described (Abella et al., 2005)and maintained in DMEM supplemented with 25mM glucose and 10% FBS. MEFs were starved 1h in DMEM containing 0.2%BSA and then treated 30min with Jak2 inhibitor AG490 (50μM) or CXCR2 antagonist SB225002 (10−6M) before to stimulate 1h with CXCL5 (300ng/ml). For insulin signaling, cells were stimulated with insulin (10−9M) for 10min.
Chromatin immunoprecipitation (ChIP)
ChIP assays were performed as described previously (Annicotte et al., 2006). Briefly, proteins from Thp-1 cells were formaldehyde cross-linked to DNA. After homogenization, lysis and DNA sonication, proteins were then immunoprecipitated using anti-p65 antibody. After washing, DNA-protein-complexes were subsequently eluted and cross-linking was reversed by heating the samples at 65°C for 16 h. DNA was then purified using Qiagen PCR purification kit (Qiagen, Courtaboeuf, France) and PCR amplification was performed using cxcl5 promoter-specific oligonuleotide primers.
Plasmids
Human CXCL5 promoter corresponding to −1359/+63 was cloned from breast cancer cell line MCF-7 using 5′-TCA GAA CCA GCC AGA AGA GGA-3′ and 5′-AGC GGA GAT TGG AGG AGC GAA GAT-3′ and subcloned in xp-2 luciferase reporter vector. CMV-GAL corresponds to the β-galactosidase gene under the control of the cytomegalovirus (CMV) promoter.
Statistical analysis
Data are presented as means ± SEM. Group means were compared by factorial analysis of variance (ANOVA). Upon significant interactions, differences between individual group means were analyzed by Fisher’s protected least squares difference (PLSD) test. Differences were considered statistically significant at p < 0.05 (*), 0,01≤ p≤0,001 (**), p≤0,001 (***).
In human studies, statistical comparisons were performed using non parametric Wilcoxon-Mann Whitney test (p values < 0.05 were considered to be statistically significant).
Supplementary Material
Acknowledgements
We are grateful to Drs. Anne Bouloumié, Alexia Zakaroff-Girard, Virginie Bourlier and Marie Marques (Inserm U858) for the preparation of human adipose tissue macrophages. Corinne Henriquet, Zohra Benfodda, Sébastien Hure (Inserm U896), Carine Valle (Inserm U858) and Lionel Valera (CNRS/Bio-Rad) are acknowledged for technical assistance. Denis Greuet is acknowledged for his excellent animal expertise. This work was supported by grants of INSERM, Fondation pour la Recherche Médicale (équipe FRM), the European Commission (HEPADIP project, Contract LSHM-CT-2005-018734) and ANR (PHYSIO 2006). C.C is granted by Agence National pour la Recherche. GSH is supported by a grant from the National Institutes of Health USA (DK052539).
References
- Abella A, Dubus P, Malumbres M, Rane SG, Kiyokawa H, Sicard A, Vignon F, Langin D, Barbacid M, Fajas L. Cdk4 promotes adipogenesis through PPARgamma activation. Cell Metab. 2005;2:239–249. doi: 10.1016/j.cmet.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327–332. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- Annicotte JS, Chavey C, Servant N, Teyssier J, Bardin A, Licznar A, Badia E, Pujol P, Vignon F, Maudelonde T, et al. The nuclear receptor liver receptor homolog-1 is an estrogen receptor target gene. Oncogene. 2005;24:8167–8175. doi: 10.1038/sj.onc.1208950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annicotte JS, Iankova I, Miard S, Fritz V, Sarruf D, Abella A, Berthe ML, Noel D, Pillon A, Iborra F, et al. Peroxisome proliferator-activated receptor gamma regulates E-cadherin expression and inhibits growth and invasion of prostate cancer. Mol Cell Biol. 2006;26:7561–7574. doi: 10.1128/MCB.00605-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Morris SB, Xue YY, Burdick MD, Glass MC, Iannettoni MD, Strieter RM. Epithelial-neutrophil activating peptide (ENA-78) is an important angiogenic factor in non-small cell lung cancer. J Clin Invest. 1998;102:465–472. doi: 10.1172/JCI3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badman MK, Flier JS. The adipocyte as an active participant in energy balance and metabolism. Gastroenterology. 2007;132:2103–2115. doi: 10.1053/j.gastro.2007.03.058. [DOI] [PubMed] [Google Scholar]
- Clement K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, Sicard A, Rome S, Benis A, Zucker JD, et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. Faseb J. 2004;18:1657–1669. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- Curat CA, Wegner V, Sengenes C, Miranville A, Tonus C, Busse R, Bouloumie A. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49:744–747. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, Ranganathan G, Peterson CA, McGehee RE, Kern PA. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54:2305–2313. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- Halloran MM, Woods JM, Strieter RM, Szekanecz Z, Volin MV, Hosaka S, Haines GK, 3rd, Kunkel SL, Burdick MD, Walz A, Koch AE. The role of an epithelial neutrophil-activating peptide-78-like protein in rat adjuvant-induced arthritis. J Immunol. 1999;162:7492–7500. [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Inouye KE, Shi H, Howard JK, Daly CH, Lord GM, Rollins BJ, Flier JS. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes. 2007;56:2242–2250. doi: 10.2337/db07-0425. [DOI] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor g is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarruf DA, Iankova I, Abella A, Assou S, Miard S, Fajas L. Cyclin D3 promotes adipogenesis through activation of peroxisome proliferator-activated receptor gamma. Mol Cell Biol. 2005;25:9985–9995. doi: 10.1128/MCB.25.22.9985-9995.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Mizuarai S, Araki H, Mashiko S, Ishihara A, Kanatani A, Itadani H, Kotani H. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. J Biol Chem. 2003;278:46654–46660. doi: 10.1074/jbc.M309895200. [DOI] [PubMed] [Google Scholar]
- Tiraby C, Tavernier G, Lefort C, Larrouy D, Bouillaud F, Ricquier D, Langin D. Acquirement of brown fat cell features by human white adipocytes. J Biol Chem. 2003;278:33370–33376. doi: 10.1074/jbc.M305235200. [DOI] [PubMed] [Google Scholar]
- Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. 2006;27:762–778. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- Vitkova M, Klimcakova E, Kovacikova M, Valle C, Moro C, Polak J, Hanacek J, Capel F, Viguerie N, Richterova B, et al. Plasma levels and adipose tissue messenger ribonucleic acid expression of retinol-binding protein 4 are reduced during calorie restriction in obese subjects but are not related to diet-induced changes in insulin sensitivity. J Clin Endocrinol Metab. 2007;92:2330–2335. doi: 10.1210/jc.2006-2668. [DOI] [PubMed] [Google Scholar]
- Waki H, Tontonoz P. Endocrine functions of adipose tissue. Annu Rev Pathol. 2007;2:31–56. doi: 10.1146/annurev.pathol.2.010506.091859. [DOI] [PubMed] [Google Scholar]
- Walz A, Burgener R, Car B, Baggiolini M, Kunkel SL, Strieter RM. Structure and neutrophil-activating properties of a novel inflammatory peptide (ENA-78) with homology to interleukin 8. J Exp Med. 1991;174:1355–1362. doi: 10.1084/jem.174.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz A, Schmutz P, Mueller C, Schnyder-Candrian S. Regulation and function of the CXC chemokine ENA-78 in monocytes and its role in disease. J Leukoc Biol. 1997;62:604–611. doi: 10.1002/jlb.62.5.604. [DOI] [PubMed] [Google Scholar]
- Walz A, Strieter RM, Schnyder S. Neutrophil-activating peptide ENA-78. Adv Exp Med Biol. 1993;351:129–137. doi: 10.1007/978-1-4615-2952-1_14. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JR, Lee JM, Young PR, Hertzberg RP, Jurewicz AJ, Chaikin MA, Widdowson K, Foley JJ, Martin LD, Griswold DE, Sarau HM. Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J Biol Chem. 1998;273:10095–10098. doi: 10.1074/jbc.273.17.10095. [DOI] [PubMed] [Google Scholar]
- Wislez M, Philippe C, Antoine M, Rabbe N, Moreau J, Bellocq A, Mayaud C, Milleron B, Soler P, Cadranel J. Upregulation of bronchioloalveolar carcinoma-derived C-X-C chemokines by tumor infiltrating inflammatory cells. Inflamm Res. 2004;53:4–12. doi: 10.1007/s00011-003-1215-3. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 2006;7:243. doi: 10.1186/gb-2006-7-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.