Abstract
There is growing evidence that the host innate immune system has a critical role in regulating carcinogenesis, but the specific receptors involved and the importance of their interaction with commensal bacteria need to be elucidated. Two major classes of innate immune receptors, the Toll-like receptors (TLRs) and Nod-like receptors (NLRs), many of which are upstream of NFκB, are involved in the detection of intestinal bacteria. The TLRs have been implicated in promoting colon tumorigenesis, but the role of NLRs in regulating tumorigenesis remains unclear. Using an established mouse model system of colitis-associated colon tumorigenesis, we show that Nod1-deficiency results in increased development of both colitis-associated and Apc tumor suppressor-related colon tumors. In the absence of Nod1 signaling, there is greater disruption of the intestinal epithelial cell barrier due to chemically-induced injury as manifested by increased surface epithelial apoptosis early on during chemically-induced colitis and increased intestinal permeability. The increased intestinal permeability is associated with enhanced inflammatory cytokine production and epithelial cell proliferation in Nod1-deficient mice as compared to wildtype. Depletion of the gut microbiota suppressed tumor development in Nod1-deficient mice, thus highlighting a link between the commensal bacteria within the intestine and the host innate immune Nod1 signaling pathway in the regulation inflammation-mediated colon cancer development.
Keywords: colon cancer, colitis, inflammation, NLR
Introduction
The association between inflammation and cancer has long been recognized, while the molecular basis for this is only beginning to be understood (1) (2). One of the best known examples of inflammation-associated carcinogenesis is the significantly increased risk of colon cancer development in patients with inflammatory bowel disease (3). Innate immune signaling has been implicated in the pathogenesis of inflammatory bowel disease, and there is also growing evidence that it has a critical role in both intestinal homeostasis and tumorigenesis (4–8). However, the specific innate immune receptors involved in the regulation of inflammation-induced colon carcinogenesis remain to be elucidated.
Two major classes of innate immune receptors, the Toll-like receptors (TLRs), located on the extracellular surface, and the cytoplasmic Nod-like receptors (NLRs) are involved in the detection of intestinal bacteria (reviewed in (9) and (10)). These pattern-recognition receptors, or PRRs, recognize conserved microbial components, known as pathogen-associated molecular patterns (PAMPS), such as peptidoglycan or LPS, and upon stimulation by their respective agonist, result in the activation of inflammatory pathways (9). NF-κB is one of the key downstream effectors of PRR signaling (11, 12) and therefore, activation of the innate immune system likely has a critical role in inflammation-associated colon tumorigenesis. Understanding how innate immune signaling pathways within the gut respond to bacteria and modulate NF-κB activation during the inflammatory response may provide clues to the process of colon cancer initiation and progression associated with inflammatory bowel disease.
Nod1 is an NLR that is expressed ubiquitously in both intestinal epithelial and immune cells and recognizes a peptidoglycan-related moiety produced by many bacteria (13, 14). Nod1 agonists have also been detectable in intestinal luminal contents, likely representing secreted products from the gut microbiota (15). Nod1 has, for example, a significant role in the host defense against pathogenic agents such as Helicobacter pylori (16, 17). Interestingly, in certain populations, Nod1 polymorphisms have been associated with either an increased susceptibility to as well as protection against the development of inflammatory bowel disease (18), suggesting a possible role for Nod1 in the pathogenesis of inflammatory bowel disease. Nod1 stimulation results in the activation of both NF-κB and mitogen-activate protein kinase (MAPK) pathways (19, 20), and we therefore hypothesized that it may play a role in colitis-related tumorigenesis. Here, we report that Nod1 deficiency results in increased inflammation-induced colon tumor formation in mice. In the absence of Nod1 signaling, there is increased intestinal permeability and inflammation as a result of chemically-induced epithelial injury by dextran-sulfate sodium (DSS). The increased intestinal inflammation is associated with increased epithelial proliferation within colonic crypts. Together, our results suggest that Nod1 may be important in maintaining the integrity of the intestinal epithelium to protect it against injury, inflammation, and subsequent carcinogenesis.
Materials and Methods
Mice
ApcMin/+ (Jackson Laboratories, Bar Harbor, ME) mice on a C57BL/6 background were crossed to Nod1−/+ mice (at least N5 on C57BL/6 background) to generate Apc+/−Nod1+/+ and ApcMin/+Nod1−/− littermates. Nod1−/− mice in C57BL/6 background were previously described (21). Mice were bred and maintained under specific pathogen-free conditions (SPF), and all animal experiments were approved by the University’s Committee on Use and Care of Animals (UCUCA).
Inflammation-induced colon tumorigenesis
8–12 week old mice were injected intraperitoneally (i.p.) with 10mg/kg azoxymethane (Sigma). Water containing 2% dextran sulfate sodium (MP Biomedicals, m.w.=36,000–50,000) was administered on day 5 for 5 days followed by 16 days of water. This was repeated twice. Mice were sacrificed 3 weeks after the end of the third cycle of DSS or at the end of 10 weeks. For ApcMin/+ or ApcMin/+Nod1−/− mice, only 2% DSS was given for 7 days, and mice sacrificed 5 weeks later for tumor counting. After sacrifice, colons were harvested, flushed of feces and longitudinally slit open to grossly count tumors with the aid of a magnifier and stereomicroscope.
Inflammation
Colons were harvested from mice, flushed free of feces and jelly-rolled for formalin fixation and paraffin embedding. 5 um sections were used for hematoxylin and eosin staining. Histologic assessment was performed in a blinded fashion by using a scoring system as described previously with some modifications (22). Briefly, a 3–4 point scale was used to denote the severity of inflammation (0=none, 1=mild, 2=moderate, and 3=severe), the level of involvement (0=none, 1=mucosa, 2=mucosa and submucosa and 3=transmural) and extent of epithelial/crypt damage (0=none, 1=basal 1/3, 2=basal 2/3, 3=crypt loss, 4=crypt and surface epithelial destruction). Each parameter was then multiplied by a factor reflecting the percentage of the colon involved (0–25%, 26–50%, 51–75%, and 76–100%), and then summed to obtain the overall score. Changes in the colon length with DSS treatment was determined by measuring the length from the anus to cecum and comparing with the colon length in age- and sex-matched untreated mice.
Proliferation
Mice were injected with 100mg/kg BrdU (B.D. Pharmingen) i.p. 2.5 hours prior to sacrifice. Colons were then dissected free and jellyrolled, formalin-fixed, and paraffin-embedded. Sections were subsequently stained for BrdU using the BrdU in situ detection kit (BD Biosciences).
Apoptosis
Colons sections were assessed for apoptotic cells using the ApoAlert DNA fragmentation assay kit.
RNA isolation and real-time PCR
Colonic tissue was homogenized, and total RNA isolated using the Nucleospin RNA kit (Macherey-Nagel). cDNA synthesis was performed using iScript (BioRad) and the cDNA was then used for quantitative PCR using either SYBR Green or Taqman Gene Expression Assay on the ABI 7900HT.
Organ Cultures
Fifty to one hundred milligrams of colon tissue from individual mice were cultured in 1 ml of serum-free RPMI containing penicillin and streptomycin. After 24 hours, the supernatants were harvested for ELISA measurements of IL-6 and MIP-2.
Western blot analysis
Colonic protein was extracted using the Nucleospin RNA/protein kit (Macherey-Nagel). Proteins were separated by SDS-PAGE., and immunoblotting was performed by using antibodies against p-IκB-α (Cell Signaling), p-ERK (Cell Signaling) and tubulin (Sigma).
Intestinal Permeability
Mice were fasted for four hours with the exception of drinking water prior to the administration of 0.6 mg/kg FITC-dextran (4kD, Sigma). Serum was collected 4 hours later retro-orbitally, diluted 1:3 in PBS and the amount of fluorescence measured using a fluorescent spectrophotometer with emission at 488 nm, absorption at 525 nm.
Depletion of gut microbiota
Mice were initially given a four week treatment of 1 g/L ampicillin, 0.5 g/L vancomycin, 1 g/L metronidazole and 1g/L neomycin for 4 weeks. Due to only modest intestinal depletion as assayed by fecal cultures, mice were subsequently switched over to a different antibiotic cocktail of 2 g/L streptomycin, 0.17 g/L gentamycin, 0.125 mg/L ciprofloxacin and 1g/L bacitracin which was maintained for the duration of the experiment. Intestinal depletion was assessed by collecting feces, homogenizing in PBS, serially diluting and plating on trypticase soy agar with 10% sheep blood (Fisher Scientific) for 48 hours at 37° aerobically and in anaerobic chamber (AnaeroPack System).
Statistical Analysis
All statistics were performed using the two-tailed Student’s t-test. Errors are expressed as S.E.M, and statistical significance was defined as p<0.05.
Results
Nod1 deficiency results in increased inflammation-induced colon tumors
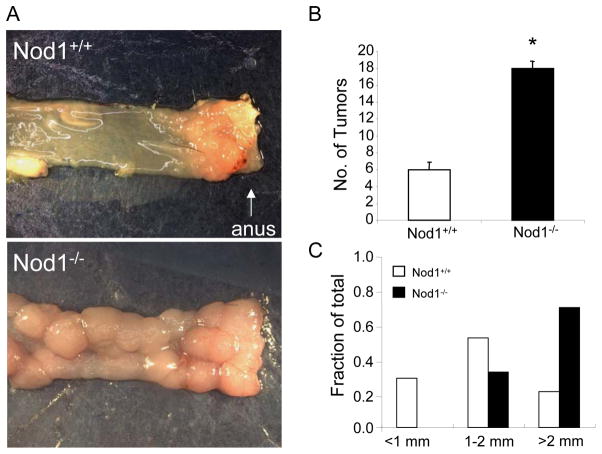
To determine the role of Nod1 in inflammation-associated colon tumorigenesis, we used a mouse model which recapitulates the progression from chronic colonic inflammation to dysplasia to adenoma and finally to adenocarcinoma in humans (23, 24). In this model, wildtype B6 and Nod1−/− B6 mice were injected with the chemical carcinogen, azoxymethane (AOM), followed by repeated administration of water containing 2% dextran sodium sulfate (DSS), which causes intestinal epithelial cell injury resulting in colonic inflammation. Since Nod1 stimulation results in the activation of NF-κB, which has previously been shown to promote colitis-associated tumorigenesis (23), we hypothesized that the lack of Nod1 signaling would result in decreased tumor formation in the setting of chronic inflammation. Surprisingly, we observed a 200% increase in tumor incidence in Nod1-deficient mice compared with the wildtype group (Fig. 1B). The tumors were broad-based non-invasive adenomas with dysplastic changes (Supplementary Fig. 1) and were located primarily in the distal colon and rectum in both Nod1-deficient and wildtype mice. Furthermore, tumors that developed in Nod1-deficient mice were also significantly larger (Fig. 1A, C). Thus, these results suggest that Nod1 signaling may be important in suppressing the development of inflammation-induced colon tumors.
Figure 1. Nod1 protects against inflammation-induced colon tumor development.
(A) Mice were treated with AOM on day 0 followed by 3 cycles of water containing DSS (AOM/DSS protocol). Mice were sacrificed at the end of 10 weeks from the start of experiment and tumors grossly counted. Representative images of tumors in the rectum of age- (8–16 weeks) and sex-matched wildtype (N=22) and Nod1−/− (N=17) mice are shown. (B) Number of colonic tumors between wildtype and Nod1−/− mice. Tumors within the colon were counted with the assistance of stereomicroscopy. (C) Measurement of largest dimension of tumor (mm) was performed using calipers. Statistical analysis was performed using two-tailed Student’s t-test. Error bars, +/− S.E.M. *, p< 0.05.
Nod1 and the Apc tumor suppressor cooperate in colitis-associated tumorigenesis
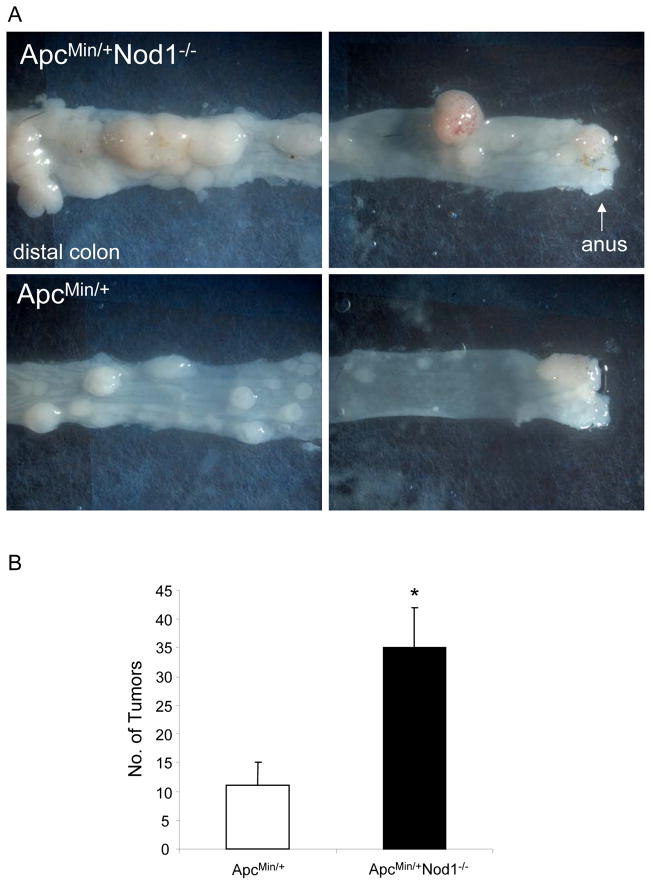
Mutations in the Apc tumor suppressor gene occur in approximately 80% of sporadic human colon cancers and results in the constitutive activation of the Wnt pathway that regulates intestinal epithelial stem cell renewal and proliferation (25). ApcMin/+ mice, which harbor a mutation in Apc, develop spontaneous tumors primarily within the small intestine; however, when treated with DSS for 7 days, the number of tumors that form in the colon significantly increase and form within they develop significantly increased colonic tumors (26, 27). With DSS treatment, colonic tumor formation is accelerated and develops within 5 weeks. In this model, colon tumors form within 5 weeks and do not require the use of azoxymethane. To determine whether the Wnt pathway contributes to the increased tumorigenicity seen in Nod1-deficient mice, we generated ApcMin/+ mice that were also deficient in Nod1. These mice were treated only with DSS for 7 days and sacrificed 5 weeks later to count the number of visible tumors in the colon. Similar to that observed with Nod1−/− mice treated with AOM followed by DSS, ApcMin/+Nod1−/− mice developed more tumors compared to those in ApcMin/+ mice, suggesting that Nod1 deficiency potentiates the tumor-promoting effect of dysregulated Wnt signaling and that both pathways play a role in inflammation-induced colon tumorigenesis (Fig. 2). Thus, these results indicate that Nod1 signaling limits inflammation-related colon tumor development in response to genomic stress from chemical carcinogenesis or through loss of the Apc tumor suppression function as seen in sporadic human colon cancers.
Figure 2. Nod1 increases susceptibility of ApcMin/+ mice to colon tumorigenesis.
(A) Mice are treated with one cycle of DSS (no AOM) for 1 week and sacrificed 5 weeks later for tumor counting within the colon. Number of colonic tumors in age-(6–8 weeks) and sex-matched ApcMin/+ (N=5) and ApcMin/+Nod1−/− (N=6) littermates are shown. (C) Representative images of tumors within the descending colon/proximal rectum (left), and distal rectum (right) of ApcMin/+ and ApcMin/+Nod1−/− mice. Statistical analysis was performed using two-tailed Student’s t-test. Error bars, +/− S.E.M. *p<0.01
Severity of chemically-induced colitis is increased with Nod1 deficiency
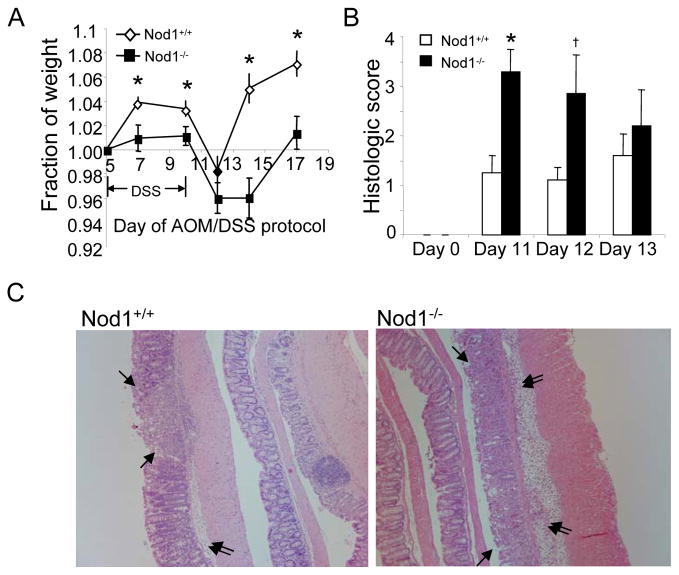
To investigate how Nod1 protects against colon tumor development, we examined whether wildtype and Nod1-deficient mice exhibited differences in the acute inflammatory response during the first two weeks after initiation of treatment with AOM followed by 2% DSS. Compared to wildtype mice, Nod1-deficient mice had greater weight loss and were slower to recover their weight after DSS treatment, suggesting that Nod1−/− mice developed colitis of greater severity compared to wildtype (Fig. 3A). Also, the colons of Nod1-deficient mice were shortened to a greater extent compared with wildtype mice (Supplementary Fig. 2A). Consistently, Nod1-deficient mice had higher histologic scores of inflammation (Fig. 3B, C, and Supplementary Fig. 2B).
Figure 3. Greater severity of DSS-induced colitis in Nod1-deficient mice.
(A) Mice were weighed on sequential days after start of first cycle of DSS of the tumor induction protocol and plotted as a fraction of baseline weight just prior to starting the 5 day course of DSS. (B) Severity of colitis of Nod1−/− and wildtype mice on days 11 through 13 of AOM/DSS protocol was assessed by histological scoring on three major categories, encompassing the extent of epithelial damage, inflammatory cell infiltration and extent of colon involved. (N=5/group). (C) Representative images of mucosal injury and superficial mucosal erosion (single arrowheads) and inflammatory cell infiltration with submucosa edema (double arrowheads) in Nod1−/− and wildtype mice. Notice increased edema and number of inflammatory cells in the submucosa of Nod1−/− mice as well as areas of mucosal hemorrhage. Images are at 100X. Statistical analyses were performed using two-tailed Student’s t-test. Error bars, +/− S.E.M. *p<0.05, †p=0.09
Nod1 deficiency results in increased proinflammatory mediator production and increased intestinal epithelial proliferation
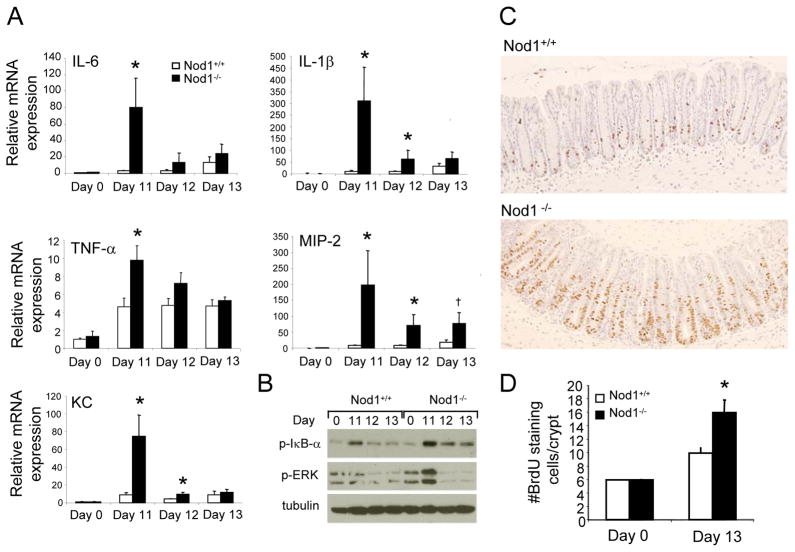
To determine the mechanism governing the increased severity of colitis observed with loss of Nod1 signaling, colonic mRNA expression levels of various inflammatory cytokines and chemokines in wildtype and Nod1−/− mice were compared after completion of the first cycle of DSS. As shown in Figure 4A, induction of proinflammatory mediators was generally greater in Nod1-deficient mice compared to wildtype mice especially on days 11 and 12, consistent with the increased severity of colitis observed histologically on those days. Since the transcription of inflammatory cytokines is largely mediated by the NF-κB and MAPK signaling pathways (9), the activation of NF-κB and MAPK signaling pathways was also assessed on days 11 through 13. In Nod1−/− mice, there were greater levels of p-IκB-α and pERK, suggesting increased activation of NF-κB and MAPK pathways, respectively, which is consistent with the observed pattern of cytokine production (Fig. 4A, B). Similarly, colon organ cultures demonstrate increased protein levels of the inflammatory cytokine IL-6 and MIP-2 in Nod-deficient mice compared to that in wildtype (Supplementary Figure 3). These results suggest that in the absence of Nod1, there is an overexuberant inflammatory response associated with NF-κB and MAPK activation by Nod1-independent signaling pathways.
Figure 4. Nod1 deficiency results in increased proinflammatory mediator production followed by increased intestinal epithelial proliferation.
(A) Relative colonic mRNA expression of various proinflammatory mediators in Nod1−/− and wildtype mice (N=5 per group) on days 11 through 13 of AOM/DSS protocol. Relative expression of genes was normalized to two housekeeping genes (α-actin and HPRT). (B) Western blot analysis of p-IκB-α and p-ERK on days 11 to 13 of AOM/DSS protocol. (C) Representative images of BrdU staining in Nod−/− and wildtype mice on day 13 by immunohistochemistry. Images are at 200×. (D) Number of BrdU-staining epithelial cells per crypt in a 200 crypt count in Nod1−/− and wildtype mice (N=4 per group) on day 13 of the AOM/DSS protocol. Statistical analyses were performed using two-tailed Student’s t-test. Error bars, +/− S.E.M., * p<0.05; †p=0.05.
To investigate whether the increased inflammatory response promotes greater cellular proliferation, thereby increasing susceptibility to tumor formation, mice were injected with bromodeoxyuridine (BrdU) 2.5 hours prior to sacrifice on day 13, three days after the completion of the first cycle of DSS when mice exhibit recovery in their weight and histologically, regenerating colonic crypts are present. Based on immunohistochemical analysis, greater levels of proliferating intestinal epithelial cells were observed within the colonic crypts in Nod1−/− mice compared to that in wildtype (Fig. 4C, D). Thus, these results suggest that Nod1 signaling is important in protecting against chemically-induced injury such that its absence leads increased inflammatory cytokine production and epithelial proliferative activity.
A role for Nod1 in maintaining integrity of the intestinal epithelial barrier
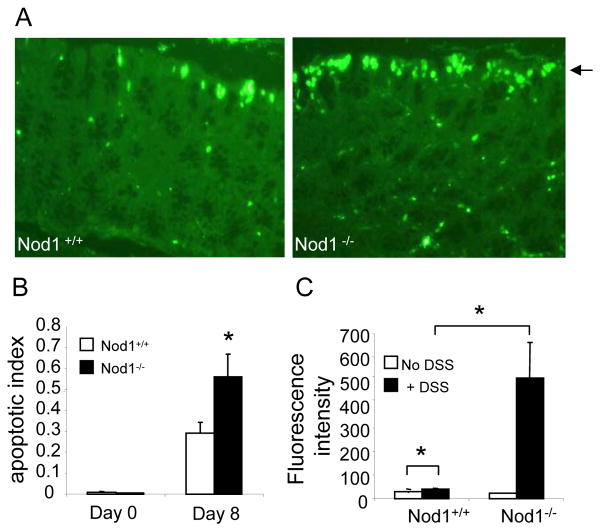
Given the increased epithelial destruction seen in Nod1−/− mice histologically, we subsequently investigated whether AOM/DSS treatment resulted in increased intestinal epithelial apoptosis and intestinal permeability in Nod1-deficient mice. Indeed, higher levels of surface intestinal epithelial cell apoptosis were observed in Nod1−/− mice compared to that in wild-type mice by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) early on during DSS treatment (day 8) prior to the development of histologically-discernible inflammation (Fig. 5A, B). The increased apoptosis was observed primarily in intestinal epithelial cells along the luminal surface of the colon (Fig. 5A), and therefore, likely reflects an increased susceptibility to DSS-induced epithelial cell injury that occurs in the absence of Nod1 signaling.
Figure 5. Nod1 deficiency results in increased surface epithelial apoptosis and enhanced intestinal permeability.
(A) TUNEL stain of colonic tissue from age- and sex-matched wildtype and Nod1−/− mice on day 0 (untreated, N=5 per group) and day 8 mice (N=10 per group). Images are at 400× magnification. Single arrowhead denotes surface epithelium lining the lumen of the colon. (B) Apoptotic index was assessed by counting number of apoptotic surface epithelial cells per crypt in a 500 crypt count on day 8 of the AOM/DSS protocol. (C) Serum fluorescence intensity in age- and sex-matched Nod1−/− and wildtype mice (N=5 per group) after administration of FITC-dextran on day of completion of the first cycle of DSS (day 10). Statistical analyses were performed using two-tailed Student’s t-test. Error bars, +/− S.E.M. *p<0.05
We next hypothesized that the increased levels of apoptotic cells within the luminal surface epithelium would be associated with enhanced intestinal permeability. To directly test this hypothesis in vivo, FITC-labeled dextran was administered to Nod1−/− and wildtype mice on day 10 after completion of a 5 day course of DSS. As FITC-dextran is not actively absorbed by the gut, the amount of fluorescence detected in the serum is a direct measure of intestinal permeability in vivo. As shown in Figure 5C, Nod1−/− mice demonstrated greater intestinal permeability with higher levels of fluorescence measured in the serum compared to wildtype animals. Altogether, these results suggest that the increased colonic inflammation seen in Nod1-deficient mice is associated with a compromise in the integrity of the intestinal epithelial barrier as reflected by the increased levels of surface epithelial apoptosis and increased intestinal permeability.
The gut microbiota contributes to increased colitis-associated tumorigenesis with Nod1 deficiency
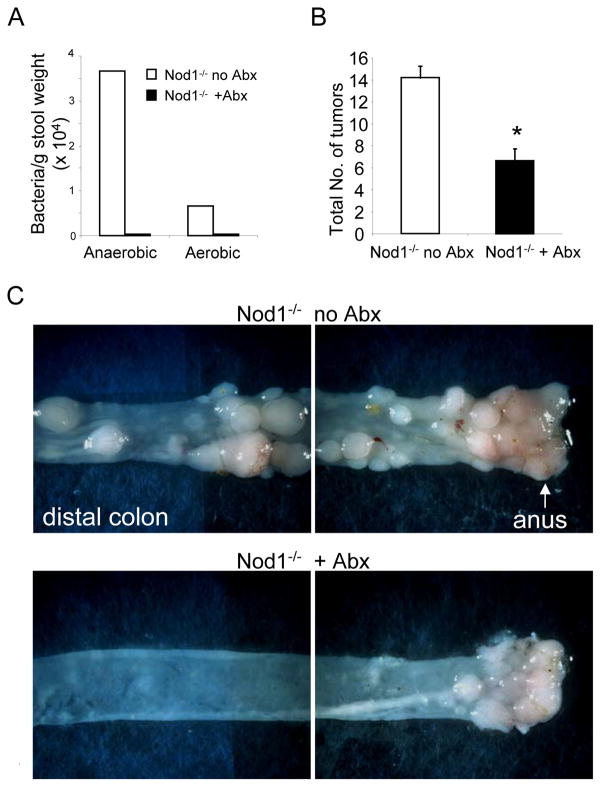
The previous data indicate that loss of Nod1 signaling leads to disruption of the intestinal epithelium after DSS treatment, which is associated with greater intestinal permeability (Fig. 5C). Therefore, whether depletion of the gut microbiota in Nod1−/− mice ameliorates the process of inflammation and tumorigenesis was examined. First, mice were treated with an antibiotic cocktail for two months, resulting in a greater than two log-fold depletion of anaerobic intestinal bacteria, which comprise the majority of the gut microbiota (Fig. 6A). Next, mice were treated with AOM and DSS to induce tumor formation as previously described. Interestingly, Nod1−/− mice treated with antibiotics developed cecal swelling with thinning of the colonic mucosa similar to that observed in mice raised in germ-free conditions (data not shown). There was subsequently greater mortality with DSS-induced colitis likely resulting from severe intestinal hemorrhage and confirming the importance of the gut microbiota in intestinal homeostasis (6, 28). As seen in Figure 6B and C, intestinal depletion of bacteria by antibiotic treatment of Nod1-deficient mice resulted in a dramatic suppression of intestinal tumor formation, especially within the transverse colon. These results indicate that in the context of Nod-1 deficiency, the gut microbiota contributes to the increased tumorigenesis.
Figure 6. Intestinal depletion of bacteria reduces tumor formation in Nod1-deficient mice.
(A) Bacterial counts from cultures of feces procured from Nod1−/− mice that were or were not treated with antibiotics (Abx). (B) Number of tumors in colons from Nod1−/− mice treated with antibiotics (N=20 with 55% mortality during DSS treatment, final N=9) and Nod1−/− mice not treated with antibiotics (N=15). (C) Representative images of the descending colon/proximal rectum and distal rectum/anus. Statistical analyses were performed using two-tailed Student’s t-test. Error bars, +/− S.E.M. *p<0.05
Discussion
In this study, we demonstrate for the first time a role for an NLR family member, specifically Nod1, in the regulation of colitis-associated colon tumorigenesis. Nod1 deficiency promotes increased tumor initiation and progression in the context of inflammation and dysregulated Wnt signaling, which is seen in the majority of human colon cancers. The data further support a role for Nod1 in maintenance of the intestinal epithelial barrier such that in the absence of Nod1, a series of events occur in response to chemical injury, namely: 1) increased surface epithelial apoptosis associated with enhanced intestinal permeability, 2) runaway inflammation with upregulated proinflammatory cytokine production likely in response to bacterial translocation across a disrupted epithelial barrier, and 3) increased cellular proliferation that can predispose to tumorigenesis.
Although Nod1 was previously suggested to have tumor suppressor properties, as demonstrated in a breast cancer xenograft mouse model, the mechanism for tumor suppression in that system appears different from that observed in the current study of colon tumors (29). In the breast cancer model, a retrovirally-mutated MCF-7 clone that disrupted Nod1 expression showed enhanced tumor growth when implanted subcutaneously in SCID mice (29). This phenotype was related to the fact that the xenograft was less responsive to estrogen-induced tumor growth and that the MCF-7 clone with disrupted Nod1 expression was less sensitive to apoptosis in vitro (29). Our results point to a crucial role for Nod1 in protecting against tumor development within the colon through an entirely different mechanism, that is, by protecting the intestinal epithelial barrier against injury, bacterial translocation, and colitis. This function is likely organ-specific since the colon requires the coexistence of commensal bacteria and the host, and therefore represents a profoundly different environment from that of the mammary gland. Thus far, the protective role of Nod1 against colonic tumor development is evident only in the context of chronic injury and inflammation since no differences in spontaneous colonic tumor development have been observed between Nod1−/− and wildtype mice (data not shown). Whether Nod1-deficiency results in increased spontaneous intestinal tumor formation in ApcMin+/− mice is under current investigation.
Nod1 is expressed in both intestinal epithelial and non-epithelial cells (30–32); however, our data support an important role for Nod1 signaling primarily in the intestinal epithelial cell although we cannot exclude the possibility that Nod1 also exerts its protective effect through non-epithelial cells. Given the increased levels of DSS-induced surface epithelial apoptosis, the current data suggests that Nod1 promotes survival in the intestinal epithelial cell and protects the colonic lining from chemically-induced injury. The precise mechanism by which Nod1 regulates intestinal epithelial cell apoptosis is as yet unclear. One possibility is that, since Nod1 stimulation results in the activation of NF-κB(12), its protective function is mediated by NF-κB. NF-κB is also an important regulator of cell survival (33, 34), and more importantly, has previously been shown to have a crucial role in maintaining the integrity of the intestinal epithelium, specifically through the adaptor molecule NEMO, such that its deletion in mice resulted in increased intestinal epithelial apoptosis and spontaneous colitis (35). Similarly, in a different study evaluating the role of NF-κB in colitis-induced tumorigenesis, downregulation of NF-κB in intestinal epithelial cells also promoted apoptosis (23). Nod1 may also protect the intestinal epithelium through other mechanisms that are not necessarily NF-κB-dependent. For example, Nod1 has been demonstrated to regulate the induction of antimicrobial peptides such as the defensins (16) (32), which have been implicated in having an important role in epithelial wound repair and injury as well as in the host defense against invasive bacteria in the intestinal tract (8, 36).
As a consequence of increased intestinal permeability after DSS treatment, we hypothesize that bacteria can translocate into the intestinal mucosa of Nod1-deficient mice and induce an exacerbated inflammatory cytokine response. Consistently, antibiotic treated Nod1-deficient mice develop fewer tumors compared to untreated Nod1-deficient mice. We have demonstrated that the increased inflammatory response in Nod1-deficient mice is associated with activation of both NF-κB and MAPK pathways, which can lead to increased tumorigenesis (23). Our data suggest that Nod1 signaling limits the inflammatory response by protecting the intestinal epithelium against DSS-induced apoptosis and subsequent penetration by luminal bacteria. This mechanism does not preclude the possibility that once the epithelial barrier is breached by DSS treatment, innate immune signaling through Nod1 limits the number of bacteria that permeate the barrier. Regardless, the upregulation of NF-κB and MAPK during the inflammatory response in Nod1-deficient mice obviously occurs independently of Nod1 signaling, and therefore, is most likely mediated by other PRRs, which include the TLRs and other NLRs. One hypothesis is that the responsible PRRs behind the inflammatory response to microbial infiltration are associated with the myeloid rather than the epithelial cell. Consistent with this hypothesis, it was previously demonstrated that after AOM/DSS treatment, inactivation of NF-κB in myeloid cells rather than intestinal epithelial cells resulted in decreased inflammatory cytokine production and tumor progression (23). Thus, our results suggest the possibility that different innate immune pathways function differently to regulate colitis-associated tumor development. On the one hand, innate immune signaling through Nod1 is important for intestinal epithelial integrity and protection against bacterial translocation, while other innate immune pathways, such as the TLRs, may be involved in the inflammatory response to microbiota and further promote tumor formation. In fact, a colitis-associated tumor-promoting effect by TLRs has recently been demonstrated in a similar colitis-associated tumorigenesis mouse model (37).
Our results support the concept that the gut microbiota can have dichotomic roles in that it can promote inflammation and tumorigenesis in the context of an impaired epithelial barrier, but by providing signals to an intact Nod1, may also protect against colon tumorigenesis. It has previously been demonstrated that individual strains of commensal bacteria have differential effects on promoting or suppressing inflammation and consequently, changes in the constitution of the commensal bacterial such as by the administration of probiotics or selective antibiotics can change host susceptibility to inflammation and tumorigenesis in experimental colitis models (38–40). The molecular mechanisms by which certain commensal bacteria influence the development of colitis and tumorigenesis are not well-understood, and may include increasing barrier function, altering the composition of the gut microbiota, and modulating innate immune and T-cell responses (39, 41–43). The intestinal luminal contents contain high levels of Nod1-stimulating activity likely produced by the gut microbiota (15). Our data suggests that commensal bacteria are capable of protecting the intestinal epithelial barrier through Nod1 stimulation to limit injury to the intestinal epithelium. Our results also raise the interesting possibility that commensal bacteria which can specifically activate Nod1 are more protective against the development of colonic tumors than bacteria that have low Nod1 activity. Therefore, an important implication of this study is to investigate, in the future, the use of Nod1 agonists or probiotics with high Nod1-stimulatory activity to reduce colon cancer in the setting of inflammatory bowel disease.
Supplementary Material
Acknowledgments
We thank J. Macdonald of the Affymetrix and Microarray Core Facility for assistance in the statistical analysis of real-time PCR data and B. Henry for critical reading of the manuscript. We would also like to thank B. Henry and T. Kanneganti-Devi for helpful discussions. G. Chen was supported by a grant from the National Cancer Institute T32 CA009357 and the Pilot feasibility Grant from the University of Michigan Gastrointestinal Peptide Research Center. M. Shaw was supported by NIH training grant T32 HL007517-26A2. This work was also supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases R01 DK61707 to G. Núñez.
Grant support: NIH grant R01-DK61707 (GN) and Pilot feasibility Grant from the University of Michigan Gastrointestinal Peptide Research Center (GC). GC is also supported by training grant NCI T32 CA009357. MS is supported by training grant NIH T32 HL007517-26A2
References
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. The Journal of clinical investigation. 2007;117:1175–83. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–33. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 4.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. The Journal of clinical investigation. 2007;117:514–21. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maeda S, Hsu LC, Liu H, et al. Nod2 mutation in Crohn’s disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–8. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 6.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–7. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi KS, Chamaillard M, Ogura Y, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–4. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 9.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Franchi L, McDonald C, Kanneganti TD, Amer A, Nunez G. Nucleotide-binding oligomerization domain-like receptors: intracellular pattern recognition molecules for pathogen detection and host defense. J Immunol. 2006;177:3507–13. doi: 10.4049/jimmunol.177.6.3507. [DOI] [PubMed] [Google Scholar]
- 11.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 12.Inohara, Chamaillard, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–83. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- 13.Chamaillard M, Hashimoto M, Horie Y, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–7. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 14.Girardin SE, Boneca IG, Carneiro LA, et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003;300:1584–7. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa M, Yang K, Hashimoto M, et al. Differential release and distribution of Nod1 and Nod2 immunostimulatory molecules among bacterial species and environments. The Journal of biological chemistry. 2006;281:29054–63. doi: 10.1074/jbc.M602638200. [DOI] [PubMed] [Google Scholar]
- 16.Boughan PK, Argent RH, Body-Malapel M, et al. Nucleotide-binding oligomerization domain-1 and epidermal growth factor receptor: critical regulators of beta-defensins during Helicobacter pylori infection. The Journal of biological chemistry. 2006;281:11637–48. doi: 10.1074/jbc.M510275200. [DOI] [PubMed] [Google Scholar]
- 17.Viala J, Chaput C, Boneca IG, et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5:1166–74. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 18.McGovern DP, Hysi P, Ahmad T, et al. Association between a complex insertion/deletion polymorphism in NOD1 (CARD4) and susceptibility to inflammatory bowel disease. Hum Mol Genet. 2005;14:1245–50. doi: 10.1093/hmg/ddi135. [DOI] [PubMed] [Google Scholar]
- 19.Inohara N, Koseki T, del Peso L, et al. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kappaB. The Journal of biological chemistry. 1999;274:14560–7. doi: 10.1074/jbc.274.21.14560. [DOI] [PubMed] [Google Scholar]
- 20.Girardin SE, Tournebize R, Mavris M, et al. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2001;2:736–42. doi: 10.1093/embo-reports/kve155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JH, Kim YG, McDonald C, et al. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol. 2007;178:2380–6. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- 22.Dieleman LA, Palmen MJ, Akol H, et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clinical and experimental immunology. 1998;114:385–91. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greten FR, Eckmann L, Greten TF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–73. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–70. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 26.Cooper HS, Everley L, Chang WC, et al. The role of mutant Apc in the development of dysplasia and cancer in the mouse model of dextran sulfate sodium-induced colitis. Gastroenterology. 2001;121:1407–16. doi: 10.1053/gast.2001.29609. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka T, Kohno H, Suzuki R, et al. Dextran sodium sulfate strongly promotes colorectal carcinogenesis in Apc(Min/+) mice: inflammatory stimuli by dextran sodium sulfate results in development of multiple colonic neoplasms. Int J Cancer. 2006;118:25–34. doi: 10.1002/ijc.21282. [DOI] [PubMed] [Google Scholar]
- 28.Kitajima S, Morimoto M, Sagara E, Shimizu C, Ikeda Y. Dextran sodium sulfate-induced colitis in germ-free IQI/Jic mice. Exp Anim. 2001;50:387–95. doi: 10.1538/expanim.50.387. [DOI] [PubMed] [Google Scholar]
- 29.da Silva Correia J, Miranda Y, Austin-Brown N, et al. Nod1-dependent control of tumor growth. Proc Natl Acad Sci U S A. 2006;103:1840–5. doi: 10.1073/pnas.0509228103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- 31.Kim JG, Lee SJ, Kagnoff MF. Nod1 is an essential signal transducer in intestinal epithelial cells infected with bacteria that avoid recognition by toll-like receptors. Infect Immun. 2004;72:1487–95. doi: 10.1128/IAI.72.3.1487-1495.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uehara A, Fujimoto Y, Kawasaki A, Kusumoto S, Fukase K, Takada H. Meso-diaminopimelic acid and meso-lanthionine, amino acids specific to bacterial peptidoglycans, activate human epithelial cells through NOD1. J Immunol. 2006;177:1796–804. doi: 10.4049/jimmunol.177.3.1796. [DOI] [PubMed] [Google Scholar]
- 33.Chen C, Edelstein LC, Gelinas C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L) Mol Cell Biol. 2000;20:2687–95. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maeda S, Chang L, Li ZW, Luo JL, Leffert H, Karin M. IKKbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFalpha. Immunity. 2003;19:725–37. doi: 10.1016/s1074-7613(03)00301-7. [DOI] [PubMed] [Google Scholar]
- 35.Nenci A, Becker C, Wullaert A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–61. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 36.Peyrin-Biroulet L, Vignal C, Dessein R, Simonet M, Desreumaux P, Chamaillard M. NODs in defence: from vulnerable antimicrobial peptides to chronic inflammation. Trends Microbiol. 2006;14:432–8. doi: 10.1016/j.tim.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Fukata M, Chen A, Vamadevan AS, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–81. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Mahony L, Feeney M, O’Halloran S, et al. Probiotic impact on microbial flora, inflammation and tumour development in IL-10 knockout mice. Aliment Pharmacol Ther. 2001;15:1219–25. doi: 10.1046/j.1365-2036.2001.01027.x. [DOI] [PubMed] [Google Scholar]
- 39.Kokesova A, Frolova L, Kverka M, et al. Oral administration of probiotic bacteria (E. coli Nissle, E. coli O83, Lactobacillus casei) influences the severity of dextran sodium sulfate-induced colitis in BALB/c mice. Folia microbiologica. 2006;51:478–84. doi: 10.1007/BF02931595. [DOI] [PubMed] [Google Scholar]
- 40.Rath HC, Schultz M, Freitag R, et al. Different subsets of enteric bacteria induce and perpetuate experimental colitis in rats and mice. Infect Immun. 2001;69:2277–85. doi: 10.1128/IAI.69.4.2277-2285.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe T, Asano N, Murray PJ, et al. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. The Journal of clinical investigation. 2008;118:545–59. doi: 10.1172/JCI33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–5. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 43.Madsen K, Cornish A, Soper P, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–91. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.