Summary
Although it has long been known that microbes can generate energy using diverse strategies, only recently has it become clear that a growing number involve electron transfer to or from extracellular substrates. The best-known example of what we will term ‘extracellular respiration’ is electron transfer between microbes and minerals, such as iron and manganese (hydr)oxides. This makes sense, given that these minerals are sparingly soluble. What is perhaps surprising, however, is that a number of substrates that might typically be classified as ‘soluble’ are also respired at the cell surface. There are several reasons why this might be the case: the substrate, in its ecological context, might be associated with a solid surface and thus effectively insoluble; the substrate, while soluble, might simply be too large to transport inside the cell; or the substrate, while benign in one redox state, might become toxic after it is metabolized. In this review, we discuss various examples of extracellular respiration, paying particular attention to what is known about the molecular mechanisms underlying these processes. As will become clear, much remains to be learned about the biochemistry, cell biology and regulation of extracellular respiration, making it a rich field of study for molecular microbiologists.
Introduction
Beginning in the 1880s when Martinus Beijerinck and Serge Winogradsky began studying nitrogen fixation and chemolithotrophy based on the oxidation of inorganic compounds such as sulphur and iron (Beijerinck, 1888; Winogradsky, 1889), an astounding number of metabolic pathways have been described for microorganisms. Today, it is well recognized that the capacity to derive energy for growth by catalysing diverse chemical reactions is one of the hallmarks of the microbial world. Many of the most important discoveries in biochemistry during the past century have stemmed from mechanistic studies of the enzymes and protein complexes involved in these metabolisms. For example, the first crystal structure of a membrane-bound protein came from the work of Johann Deisenhofer, Robert Huber, Hartmut Michel, who studied the photosynthetic reaction centre from Rhodopseudomonas viridis (Deisenhofer and Michel, 1989), and much of what we know about proton movements coupled to ATP synthesis stems from early work by Peter Mitchell and colleagues on chromatophores from Rhodospirillum rubrum (Scholes et al., 1969).
In recent years, more and more attention has been paid to microbial metabolisms that involve electron transfer between cells and extracellular substrates, such as minerals. In part, this subject has become popular thanks to the interest and support of the earth sciences community, in recognition of the key roles such metabolisms play in weathering reactions today (Druschel et al., 2004) and might have played in ore deposit formation in the past (Johnson et al., 2003). Environmental microbiologists have contributed greatly to the identification of both bacterial and archaeal strains that respire iron and manganese (hydro)oxides (Lovley and Phillips, 1988; Myers and Nealson, 1988), and microbial ecologists have documented that they are widespread in nature (Ghiorse, 1984; Lovley, 1991; Nealson and Saffarini, 1994; Ehrlich, 2002; Lovley et al., 2004). Only relatively recently, however, have molecular microbiologists turned their attention to them, and our understanding of how organisms catalyse mineral transformations at the molecular level is still incomplete and, thus, a promising area for future biochemical, genetic and cell biological research.
In this MicroReview, we will describe several examples of what we term ‘extracellular respiration’. By this we mean the use of a substrate (be it a solid or a solute) that interacts with the cellular electron transport chain through a protein that resides on either the cell surface, a cellular appendage that protrudes into the extracellular space, or a small molecule that traverses the space between the cell and the extracellular substrate (Fig. 1). The substrate is either used as an electron donor or electron acceptor to power oxidative phosphorylation. As will be detailed in the sections that follow, these substrates run the gamut from poorly soluble minerals to electrodes to solvents such as dimethyl sulphoxide (DMSO). Our objective is to highlight recent work that has advanced our understanding of extracellular respiration at the molecular genetic and/or biochemical level. Beyond the intriguing molecular and cell biological challenges extracellular respiration poses, this processes is also of applied interest, particularly in the context of microbial fuel cells (MFCs) (Rabaey and Verstraete, 2005; Gorby et al., 2006).
Fig. 1.
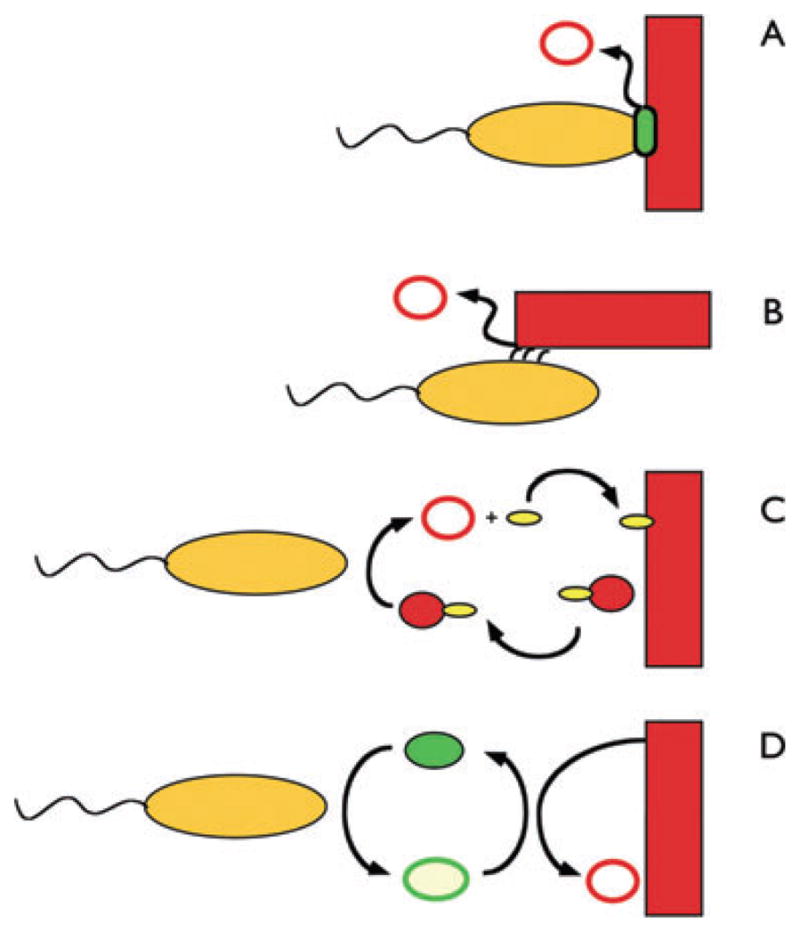
Four potential strategies for extracellular respiration. In this cartoon, the substrate is depicted as a solid red rectangle [e.g. an iron (hydr)oxide] and its reduced product (e.g. ferrous iron) as an open red circle. As discussed in the text, this is but one example of extracellular respiration. Note that these strategies can apply to other substrates, and the flow of electrons can also be reversed. A. A protein that resides on the cell surface (green oval) directly interacts with the extracellular substrate to transfer electrons from the cell to the substrate.
B. A cellular appendage (such as an electrically conductive pilus) forms a bridge between the cell and the substrate, catalysing electron transfer.
C and D. A small molecule (such as a chelator, C) or an electron shuttle (D) interacts with the substrate outside the cell. In the case of a chelator (small yellow oval), the substrate is brought to the cell for reduction (either on the outside or on the inside); in the case of an electron shuttle (green oval; closed oval is in the oxidized state, open oval is in the reduced state), the substrate remains at a distance from the cell, with the shuttle catalysing the transfer of electrons between the cell and the substrate. The shuttle, in principle, could be reduced on the outside or inside of the cell.
Extracellular respiration of ‘insoluble’ substrates
Minerals
To date, the subject of extracellular respiration has been most thoroughly explored through studies concerning the reduction or oxidation of iron and manganese minerals. As several reviews have been published on this topic recently (Croal et al., 2004; Tebo et al., 2005; Weber et al., 2006), here, we restrict our discussion to a few general comments.
Thanks to numerous biochemical and genetic studies over the past two decades in model organisms such as Shewanella oneidensis, Geobacter sulfurreducens, Acidithiobacillus ferroxidans, Bacillus sp. strain SG-1 and Pseudomonas putida, the general scheme for electron transfer in iron/manganese-oxidizing/reducing bacteria is now known. Diverse membrane-bound and -soluble (i.e. periplasmic) electron carriers, such as c-type cytochromes, quinones and multicopper oxidases, can play a role in these systems. Commonly, proteins involved in electron transfer to or from iron/manganese (hydr)oxides are localized to the outer membrane of Gram-negative bacteria or the exosporium of spores from Gram-positive bacteria (Myers and Myers, 1992; Francis et al., 2002; Yarzabal et al., 2002; Mehta et al., 2005; 2006). It is generally assumed that this subcellular location facilitates direct interaction with the metal substrate. In addition to redox-active proteins, extracellular polymeric substances may also affect adherence to mineral surfaces and mediate metal reactivity, as seen in acidophilic iron-oxidizing bacteria (Sand and Gherke, 2006). Biophysical experiments with S. oneidensis have shown that specific attractive forces are induced between the cell and a mineral surface during conditions in which electron transfer from the cell to the mineral is expected (Lower et al., 2001). Although these forces have not yet been shown to be due to any particular protein, recent biochemical experiments with OmcA, an outer membrane c-type cytochrome from S. oneidensis (Myers and Myers, 1998), have indicated that it can bind hematite with high affinity and transfer electrons to it directly (Xiong et al., 2006). Interestingly, however, OmcA did not bind goethite, the mineral used in the Lower et al. study. How different minerals are recognized by the cell is unknown, but may involve a number of subtle effects, as S. oneidensis appears capable of discriminating even between different single crystal faces (Neal et al., 2005). In a separate study, scanning tunnelling microscopy and tunnelling spectroscopy were used to characterize the electron tunnelling properties of OmcA and MtrC (also known as OmcB, another outermembrane c-type cytochrome) immobilized as molecular monolayers on Au(III) surfaces (Wigginton et al., 2007). In this case, modified versions of the native proteins containing a tetracysteine sequence were used, and it remains to be seen whether the conclusions from these in vitro biophysical experiments apply in vivo in the presence of iron oxides. For more details on this topic, see the review by Shi et al. (2007).
In addition to direct electron transfer to minerals, it has also been established that indirect electron transfer can occur via small molecules that act either to chelate metals and deliver them to an intracellular metal oxidoreductase, or by themselves serving as electron shuttles. A large number of research groups have made important contributions to this area; for a thorough review of this topic, we refer the reader to Kappler and Straub (2005). In the case of metal chelators, ligands such as citrate and nitro-triacetic acid (NTA) are able to deliver soluble iron to c-type cytochromes inside the cell, although it is clear that some metal chelates are also reduced extracellularly (Beliaev and Saffarini, 1998; Beliaev et al., 2001; Myers and Myers, 2002; Lies et al., 2005). In the latter case, a host of molecules – organic and inorganic, endogenously or exogenously produced – can shuttle electrons back and forth between cells and mineral surfaces. This might be particularly relevant in the context of biofilms, where the majority of cells are not in direct contact with the mineral surface (Hernandez and Newman, 2001). Although the oxidoreductases that act on these molecules and their respective subcellular locations have not been determined for the majority of the known electron shuttles, it was recently shown that at least some of these molecules require the same outer membrane proteins for their reduction that are required for mineral reduction, which blurs the distinction between the ‘direct’ and ‘indirect’ pathways (Lies et al., 2005).
Irrespective of the potential substrate promiscuity of the oxidoreductases involved in these processes (see below), a novel mechanism of extracellular electron transfer has been described recently in studies with G. sulfurreducens (Reguera et al., 2005) and S. oneidensis (Gorby et al., 2006) that invokes electron transfer through microbial ‘nanowires’ (Fig. 2). In the case of G. sulfurreducens, these nanowires appear to comprise a new class of pili (the so-called ‘geopilins’) that are required for Fe(III) oxide reduction. Interestingly, these pili appear to localize to one side of the cell and are induced only under certain conditions. Twitching motility does not appear to occur under these conditions; thus, Reguera et al. (2005) concluded that the geopili do not play an indirect role in Fe(III) oxide reduction by mediating surface motility. Moreover, the geopili are not required for Fe(III) oxides to attach to the cell (i.e. mutants lacking geopili can still bind mineral particles on the cell surface), yet when they are expressed they have a high affinity for Fe(III) oxides. Based on measurements of their electrical conductivity using atomic force microscopy, the geopili were shown to be electrically conductive. From this, it was suggested that geopili serve as direct electron conduits between the cell and the mineral surface. How the amino acids that comprise the pili conduct electrons is not yet understood, nor is it known with which electron-donating proteins they interact inside the cell.
Fig. 2.
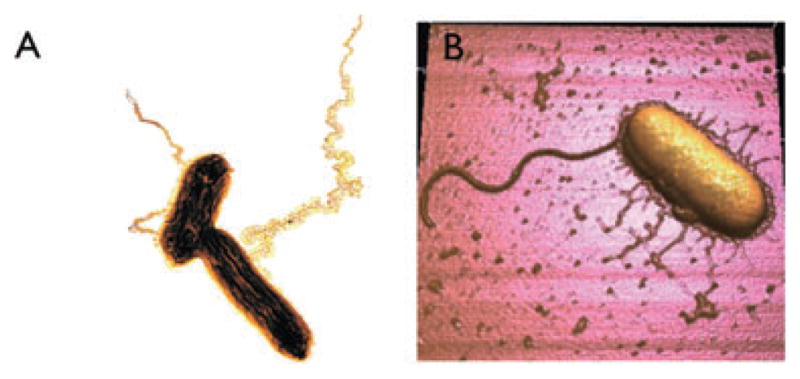
‘Nanowires’ produced by two different Fe(III)-reducing bacteria.
A. Transmission electron micrograph of platinum-shadowed ‘geopili’ produced by Geobacter sulfurreducens (courtesy of Gemma Reguera, Michigan State University). The length of the longer cell is approximately 2 μm. The image has been colorized to enhance contrast.
B. Atomic force micrograph of Shewanella oneidensis (courtesy of Pamela Gross, University of Southern California). The large appendage is the flagellum; the shorter pili are electrically conductive nanowires. The length of the cell is approximately 3 μm.
Geobacter sulfurreducens is not alone in its ability to produce nanowires. Gorby et al. (2006) showed that S. oneidensis produces electrically conductive nanowires in response to electron acceptor limitation. In comparing the electrical conductivity of the nanowires produced by the wild type with that of mutants defective in the production of the outer membrane c-type cytochromes OmcB (also known as MtrC) and OmcA by scanning tunnelling microscopy, evidence was found that suggested the nanowires were poor electrical conductors in the absence of these cytochromes. Whether these cytochromes are physically incorporated into the nanowires themselves, or whether they facilitate electron transfer through the nanowires indirectly, is not known. An important additional finding of the Gorby et al. study was that nanowires can also be produced by other bacterial genera in addition to mineral reducers. They identified electrically conductive nanowires between cells of the photosynthetic cyanobacterium Synechocystis strain PCC 6803 when they were cultivated under conditions of CO2 limitation. In addition, electrically conductive nanowires were also produced by the thermophilic fermentative bacterium Pelotomaculum thermopropionicum when grown in monocultures on fumarate or in co-cultures with Methanothermobacter thermoautotropicus on propionate. It seems possible therefore that nanowire production might serve as a general mechanism for electron transfer both within and between species under certain conditions. Elucidation of what exactly these conditions are, how nanowire production serves the physiological needs of the cell under these conditions, and the mechanisms of their biosynthesis and cellular localization, are priorities for future work.
Electrodes
Electrodes are an important substrate for extracellular respiration in a more applied context. Since the 1970s, understanding how microbes catalyse the conversion of substrates directly into electricity has been relevant to optimizing the performance of MFCs (Rabaey and Verstraete, 2005). Electrodes can serve either as electron donors or as electron acceptors for microorganisms, depending on whether the electrode is functioning as a cathode or anode respectively. Many different types of microorganisms with different physiological capabilities (some fermentative, others respiratory) have been shown to catalyse electricity generation in fuel cells. These organisms have been enriched from diverse environments, ranging from organic wastewaters to aquatic sediments (Bond et al., 2002; Tender et al., 2002; Holmes et al., 2004; Kim et al., 2004; Rabaey et al., 2004; Reimers et al., 2006). The manner in which they generate electricity can vary from catalysing electron transfer directly via outer membrane proteins, to oxidizing/reducing soluble redox mediators that shuttle electrons to/from the electrode, in direct analogy to what occurs for minerals. The potential for artificial mediators to facilitate electron transfer in MFCs has been known for a long time (Roller et al., 1984; Park et al., 2000), but more recently, some MFC bacteria have been shown to produce mediators themselves (Bond and Lovley, 2005; Rabaey et al., 2005). A recent study of electricity production by G. sulfurreducens growing on graphite electrodes demonstrated that the same ‘geopilins’ that play a role in Fe(III) oxide reduction by this organism also contribute to maximum power output when G. sulfurreducens is growing as a multilayered biofilm (Reguera et al., 2006). Interestingly, if G. sulfurreducens grows on electrodes under conditions where cells do not stack on top of each other, the geopili do not appear to affect current production (Holmes et al., 2006). Although there are obvious parallels between electron transfer to/from electrodes and to/from minerals, it appears that the particular proteins needed to catalyse electron transfer reactions in these two contexts differ (Holmes et al., 2006). Moreover, in natural environments, with mixed communities colonizing deployed electrodes, a variety of mechanisms might operate simultaneously, with both organic and inorganic substrates (e.g. sulphur species in the marine environment) potentially participating in electron transfer reactions at both the anode and the cathode (Reimers et al., 2006).
Extracellular respiration of ‘soluble’ substrates
As described above, extracellular respiration pathways exist for utilization of insoluble substrates as electron acceptors for anaerobic respiration. Mounting evidence suggests that soluble substrates can also be utilized extracellularly. In this section we will discuss this less intuitive class of extracellular respiration.
Dimethyl sulphoxide
Laboratory perceptions of what is ‘soluble’ might not reflect the ecological perspective of microorganisms. To illustrate this point, we will discuss DMSO, which can be found in significant concentrations (μM to nM) in aquatic environments (Lee and de Mora, 1999). There are several natural sources for DMSO: photochemical (Brimblecombe and Shooter, 1986) and biological (Hanlon et al., 1994) oxidation of dimethyl sulphide (DMS) and production, either directly or indirectly, from algae (Andreae, 1980; Lee and de Mora, 1999). Although DMSO is considered to be a robust solvent (David, 1972), because it is a highly polar molecule it readily adsorbs to active surfaces such as glass (Simo et al., 1998). In aquatic environments, these surfaces might be the silica frustules of diatoms or the gel-like matrices of marine snow. Such active surfaces might provide a reservoir of ‘insoluble’ DMSO in the environment that an extracellular respiratory mechanism would allow a bacterium to exploit. What evidence is there that this is the case?
Many organisms are known to utilize DMSO as a terminal electron acceptor for anaerobic growth. In the systems best studied for DMSO respiration, Escherichia coli and Rhodobacter spp., the terminal reductase is located in the periplasm (McEwan et al., 1985; Stanley et al., 2002). However, our work recently indicated that the DMSO reductase of S. oneidensis is localized to the outside of the cell (Gralnick et al., 2006). In support of this, strains defective in type II secretion had a decreased capacity to respire DMSO. Type II secretion was first suggested to be required for the extracellular respiration of iron and manganese by Shewanella putrefaciens (DiChristina et al., 2002). Type II secretion is the process of protein translocation across the outer membrane of an organism, and has been extensively studied in systems such as pullulanase from Klebsiella oxytoca (d’Enfert et al., 1987) and cholera toxin from Vibrio cholerae (Davis et al., 2000). The DMSO reduction defect of type II secretion mutants suggested that respiration required an extra-cellular or surface-attached component. Immunogold labelling of one of the DMSO reductase subunits indicated that it resides on the outer leaflet of the outer membrane under anaerobic conditions (Gralnick et al., 2006). Like other surface-attached proteins such as pullulanase and the outer membrane decahaem c-type cytochromes OmcA and OmcB/MtrC from S. oneidensis (Myers and Myers, 2003), the catalytic subunit of the S. oneidensis DMSO reductase is predicted to be a lipoprotein. Although these different lines of evidence are consistent with the model that DMSO is reduced extracellularly, more work is necessary to conclusively demonstrate this.
Humic substances and other organic compounds
DMSO provides an example of a compound we might typically think of as soluble that might be insoluble in some environments. But for compounds that truly are soluble, what would motivate a cell to localize its enzymatic machinery to the cell surface in order to access them? One reason might simply be that the compounds are too large to be transported into the cell. To illustrate this, let us consider the case of humic substances.
Humic substances (hereafter, ‘humics’) are high-molecular-weight mixtures of lignin, proteins, carbohydrates and aliphatic polymers that primarily derive from the degradation of microbial and plant matter (McKnight and Aiken, 1998). Humics are among the most widely distributed class of organic compounds on Earth (Stevenson, 1994), accounting for at least half of the organic carbon found in soil (Lal, 2004). These substances can act as electron acceptors for microbial respiration coupled to the oxidation of short-chain fatty acids (Lovley et al., 1996) or pollutants such as toluene (Cervantes et al., 2001) and carbon tetracholoride (Cervantes et al., 2004). In addition, they can also serve as electron donors. Electron spin resonance studies suggested that quinone moieties within humics are the redox-active centres (Scott et al., 1998). A correlation was found between the semiquinone content of a variety of bacterially reduced humics and the amount of reduced iron produced when they were placed in contact with iron oxides. Furthermore, the small quinoid compound anthraquinone-2,6-disulphonate (AQDS) has been used successfully as a proxy for the reactive moieties of humic substances (Lovley et al., 1996; Coates et al., 1998).
Considerable debate exists regarding the structure of humic substances due to their extraordinarily heterogeneous nature (see reviews by Swift, 1999; Piccolo, 2001; Sutton and Sposito, 2005). A recent view of humic substances describes a micell-like structure, comprised of low-molecular-mass components where hydrophobic constituents are centrally located and hydrophilic substances comprise the surface (Sutton and Sposito, 2005). Though soluble in nature, the size and colloidal nature of these compounds would make it difficult or impossible for bacteria to uptake humics actively, motivating their utilization as electron sources/sinks extracellularly. Although humic substances themselves have yet to be tested, experiments with AQDS are consistent with the hypothesis that humic substances are indeed substrates for extracellular respiration in Shewanella.
Mutant strains of S. oneidensis lacking various steps in the pathway of metal reduction are defective in reducing AQDS (Shyu et al., 2002; Lies et al., 2005). The degree of the AQDS reduction defect differed from mutant to mutant, with strains lacking menaquinone biosynthetic genes or the mtrB gene showing the strongest defects (Lies et al., 2005). MtrB is an outer membrane protein that is required for the proper localization of both OmcA and OmcB (Myers and Myers, 2002). In addition, an OmcB mutant was seriously impaired in AQDS reduction, although not as severely as the MtrB or menaquinone mutants (Lies et al., 2005). These results suggest that most, if not all, of AQDS reduction is catalysed by the same proteins that catalyse metal reduction, which implies that the MtrB/OmcB complex is promiscuous in its choice of substrates (see below). But promiscuity is not necessarily a hallmark of extracellular respiration, as the DMSO pathway in Shewanella appears to be specific.
In addition to humics, we predict that melanins might be an important candidate for extracellular respiration. Melanins are produced both intra- and extracellularly by a wide variety of microorganisms, including several species of pathogenic bacteria, fungi and worms (Nosanchuk and Casadevall, 2003) as well as by metal-reducing bacteria (Turick et al., 2002). The production of melanin by S. oneidensis is stimulated by high concentrations of tyrosine (Turick et al., 2002), which is not surprising given that tyrosine is a precursor of melanin. In many respects, melanins are structurally similar to humic substances. Although there is a strong connection between melanization and virulence, very little is understood about the cell biology of this pigment system and even less about whether these pigments are involved in extracellular respiration. We hope that the similarity between melanin and humics will prompt increased attention to this important and enigmatic class of polymers.
Soluble metal ions
Lastly, a final consideration in predicting whether a soluble compound might be a candidate for extracellular respiration is whether the respired product is also soluble. If not, a cell might be wise to localize its respiratory machinery to the cell surface to avoid intracellular precipitation of the product. To illustrate this, we consider the case of certain redox-active metals.
Redox state often has a profound influence on the solubility of metal ions. Many metals used by bacteria for anaerobic respiration exist naturally in a soluble state. Examples of metal and radionuclide species that are soluble in the oxidized state and can be substrates for anaerobic respiration are Uranium, Vanadium, Arsenic, Chromium, Technetium and Selenium (Lovley, 1991; Turick et al., 1996; Lloyd et al., 2000; Carpentier et al., 2003; Stolz et al., 2006). Even though these substrates are soluble and could be respired inside or outside of the cell, some of these metals (Se) and radionuclides (U, Tc) precipitate during microbial reduction due to the low solubility of their reduced forms. Acidithiobacillus ferroxidans, for example, uses Fe(II) as an electron donor in its metabolism, and precipitates Fe(III) oxides extracellularly. A high-molecular-weight c-type cytochrome has been localized to the outer membrane, and is presumed to facilitate Fe(II) oxidation on the cell surface (Yarzabal et al., 2002). Studies of metal reduction in Shewanella species support the notion that extracellular respiration/reduction occurs in these cases. For example, mutant strains lacking the extracellular metal reductase complex (OmcA, OmcB/MtrC) have a decreased capacity to reduce U(VI) compared with wild type (Marshall et al., 2006). Purified OmcB/MtrC is able to reduce U(VI) in vitro when artificially reduced with dithionite. Consistent with this, another study found an mtrB mutant to exhibit a partial defect in U(VI) reduction (Bencheikh-Latmani et al., 2005). Similarly, vanadate {V(V)} reduction proceeds through the extracellular metal reductase pathway, requiring all of the same components as iron and manganese reduction (Myers et al., 2004; Carpentier et al., 2005). While evidence for extracellular V(V) reduction is clear, the evidence that selenite {Se(IV)} reduction in S. oneidensis takes place extracellularly is based only on the localization of Se(0) nanoparticles to the outside of the cell (Klonowska et al., 2005). Interestingly, a mutant strain lacking CymA still retains the capacity to reduce SeO3. If true, this would indicate a process independent of the main metal reductase pathway, as CymA is essential for iron and manganese respiration (Myers and Myers, 1997). However, it is unclear if selenite reduction in Shewanella can be coupled to anaerobic growth, as has been seen in other organisms (Stolz et al., 2006). The poor solubility of the reduced forms of V, Se and U is consistent with the strategy of extracellular respiration in preventing precipitation of metals and metalloids in cellular compartments.
Promiscuity of the mtr/omc pathway in S. oneidensis
Through the efforts of many research groups, it has become clear that the Mtr/Omc electron transfer pathway in S. oneidensis is involved in the reduction of a variety of substrates. While mutant strains were initially observed to be defective in the reduction of iron and manganese oxide minerals, other substrates are now recognized including: AQDS, chelated Fe(III), V(V) and U(IV) (Shyu et al., 2002; Croal et al., 2004; Myers et al., 2004; Bencheikh-Latmani et al., 2005; Carpentier et al., 2005; Lies et al., 2005; Marshall et al., 2006; Weber et al., 2006). Neither the extent nor the physiological benefit of this promiscuity is clear, but remains an important area for future research. Moreover, elucidating the mechanism of this promiscuity presents an exciting challenge for biochemists. It is possible that particular proteins (e.g. MtrB) play a generic role in targeting different oxidoreductases (e.g. OmcB, OmcA) to the outer membrane, which in turn are specific for particular substrates; alternatively, it is possible that a single oxidoreductase catalyses the reduction of diverse substrates, either because it itself can recognize and directly reduce them, or because it uses a single mechanism to indirectly reduce them (e.g. an endogenous electron shuttle).
Common pathways used in extracellular respiration
In general, studies with Shewanella and Geobacter species show that electron transfer proceeds through a sequence of soluble and membrane-bound electron carriers from the cytosol to the outside of the cell. Early steps in this process contribute to the generation of a proton motive force (Δp) around the inner membrane, but later steps are uncoupled from proton pumping, and merely serve to deliver electrons to the terminal oxidant. In many of these systems, menaquinone is a key inner membrane-bound electron carrier that presumably contributes to the establishment of a Δp (Lovley et al., 1993; Myers and Myers, 1993; Newman and Kolter, 2000; Saffarini et al., 2002). Many proteins involved in extracellular respiration are c-type cytochromes. CymA, a tetrahaem c-type cytochrome, interacts with the menaquinone pool directly in Shewanella species. This protein is required for almost all forms of anaerobic respiration in Shewanella, with the exception of thiosulphate and TMAO reduction (Myers and Myers, 1997; Saffarini et al., 2002). In each extracellular respiration system described so far, an electron carrier protein is involved in moving electrons across the periplasm. In the metal reduction pathway in Shewanella this protein is MtrA, and in DMSO reduction the protein is DmsE. MtrA is a decahaem c-type cytochrome (Pitts et al., 2003), while DmsE is also predicted to be one, based on the presence of conserved haem binding motifs (Gralnick et al., 2006). The small periplasmic c-type cytochrome PpcA from G. sulfurreducens might play a similar electron-carrying role (Lloyd et al., 2003). The terminal reductases used by the extracellular respiratory pathways in Shewanella differ, however: in the case of metal reduction, the terminal reductases appear to be decahaem c-type cytochromes (Shi et al., 2006), whereas a putative molybtopterin-containing protein is used in DSMO reduction (Gralnick et al., 2006). Outer membrane c-type cytochromes have also been identified in G. sulfurreducens that are required for metal reduction (Leang et al., 2003; Kim et al., 2005; 2006; Mehta et al., 2005).
Although less is understood regarding the mechanisms involved in localizing the components of the extracellular respiratory systems to the outside of the cell, it appears that type II secretion plays an important role in both Shewanella and Geobacter. Interestingly, the defect seen in Shewanella type II secretion mutants is not as profound as the defect seen in G. sulfurreducens. Shewanella mutants retain some DMSO and Fe(III) reduction activity (DiChristina et al., 2002; Gralnick et al., 2006), whereas a G. sulfurreducens type II secretion mutant is completely defective (Mehta et al., 2006). This could indicate the existence of an additional system of protein translocation independent of type II secretion in Shewanella. Another difference is that the defect in G. sulfurreducens is specific to insoluble Fe(III), whereas type II secretion in Shewanella is required for maximal reduction of both soluble and insoluble metals. Moreover, G. sulfurreducens has two gene clusters encoding type II secretion systems, a so-called ‘classical’ cluster which is similar to the Gsp system of many Gram-negative bacteria (including Shewanella) and one similar to the non-classical Xcm secretion system of P. putida (de Vrind et al., 2003) that is required for insoluble Fe(III) reduction (Mehta et al., 2006). What role, if any, the classical type II secretion system has in the ability of Geobacter to reduce metals is unknown.
Perspectives for future research
Unknown substrates
In this review, we have discussed various substances that are substrates for extracellular respiration, but in all likelihood this set is far from complete. In addition to melanins, it seems reasonable that hydrophobic substrates such as oil, or mineral deposits such as elemental sulphur, or compounds whose processing gives rise to a toxic product, might be metabolized on the cell surface. Even when we restrict our focus to the genomes of Shewanella species in search of homologues to proteins known to be required for extracellular respiration, it is clear that there are several gene clusters of unknown function that likely encode novel extracellular respiratory processes. For example, S. oneidensis contains two such clusters. One cluster is similar to the metal reductase gene cluster, but is not required for metal reduction (Myers and Myers, 2002; Marshall et al., 2006), while the other cluster is similar to the DMSO reductase cluster, but likely does not play a role in DMSO reduction (Gralnick et al., 2006). As more environments and metabolically diverse bacteria are explored, we expect that additional substrates will be discovered.
Elucidating the mechanisms of extracellular respiration
Many interesting mechanistic questions about extracellular respiration remain to be explored. It is striking that many of the outer membrane proteins in Gram-negative bacteria that are known to be involved in extracellular respiration are lipoproteins. How are they transported to the external face of the outer membrane? After being exported across the inner membrane, do they insert into the outer membrane on the periplasmic side, and then flip from one face to the other via a Lol-dependent mechanism (Tokuda and Matsuyama, 2004)? Or does the type II secretory system directly target them to the external face of the outer membrane? While genetic evidence suggests the latter pathway is involved (DiChristina et al., 2002; Gralnick et al., 2006), the former pathway cannot yet be excluded because detailed biochemical studies of the transport machinery have not yet been performed. Even in the well-studied pullulanase system, we do not yet know where the lipids of pullulanase are located. In addition, although bioinformatic predictions suggest that many of these outer membrane proteins are decahaem cytochromes, this awaits confirmation from structural biological studies. Once this is known, it will open up the possibility of identifying the sequence of electron transfer events both within these proteins, and between them and their substrate(s) or other proteins in the electron transport chain. Similarly, much remains to be learned regarding the assembly and electron-transfer mechanisms of nanowires. With increased attention from molecular and cellular biologists, as well as bioinorganic chemists, we anticipate exciting advances will be made in the coming decade. Lastly, recent studies of transcription factors, such as ArcA from S. oneidensis (Gralnick et al., 2005) and RpoS from G. sulfurreducens (Nunez et al., 2006), have shown that their regulons are notably different from those that have been established for E. coli, and thus our appreciation of the diversity of biological networks will likely be enhanced by studying how extracellular substrates are sensed. Given the wide distribution of organisms with the capacity for extracellular respiration in the environment, insights gained from detailed mechanistic studies could one day facilitate their use for bioremediation or energy generation in many different contexts.
Acknowledgments
We would like to thank Doug Lies and Lars Dietrich for stimulating discussions, and Ken Nealson for comments on the manuscript. D.K.N. is an Investigator of the Howard Hughes Medical Institute. We are grateful to the ONR, Luce Foundation and Packard Foundation for supporting our work on extracellular respiration over the years.
References
- Andreae MO. Dimethylsulfoxide in marine and freshwaters. Limnol Oceanogr. 1980;25:1054–1063. [Google Scholar]
- Beijerinck MW. Die bacterien der papilion-aceenknöllchen. Bot Ztg. 1888;46:725–804. [Google Scholar]
- Beliaev AS, Saffarini DA. Shewanella putrefaciens mtrB encodes an outer membrane protein required for Fe(III) and Mn(IV) reduction. J Bacteriol. 1998;180:6292–6297. doi: 10.1128/jb.180.23.6292-6297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beliaev AS, Saffarini DA, McLaughlin JL, Hunnicutt D. MtrC, an outer membrane decahaem c cytochrome required for metal reduction in Shewanella putrefaciens MR-1. Mol Microbiol. 2001;39:722–730. doi: 10.1046/j.1365-2958.2001.02257.x. [DOI] [PubMed] [Google Scholar]
- Bencheikh-Latmani R, Williams SM, Haucke L, Criddle CS, Wu L, Zhou J, Tebo BM. Global transcriptional profiling of Shewanella oneidensis MR-1 during Cr(VI) and U(VI) reduction. Appl Environ Microbiol. 2005;71:7453–7460. doi: 10.1128/AEM.71.11.7453-7460.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond DR, Lovley DR. Evidence for involvement of an electron shuttle in electricity generation by Geothrix fermentans. Appl Environ Microbiol. 2005;71:2186–2189. doi: 10.1128/AEM.71.4.2186-2189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond DR, Holmes DE, Tender LM, Lovley DR. Electrode-reducing microorganisms that harvest energy from marine sediments. Science. 2002;295:483–485. doi: 10.1126/science.1066771. [DOI] [PubMed] [Google Scholar]
- Brimblecombe P, Shooter D. Photooxidation of dimethylsulfide in aqueous solution. Mar Chem. 1986;19:343–353. [Google Scholar]
- Carpentier W, Sandra K, De Smet I, Brige A, De Smet L, Van Beeumen J. Microbial reduction and precipitation of vanadium by Shewanella oneidensis. Appl Environ Microbiol. 2003;69:3636–3639. doi: 10.1128/AEM.69.6.3636-3639.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier W, De Smet L, Van Beeumen J, Brige A. Respiration and growth of Shewanella oneidensis MR-1 using vanadate as the sole electron acceptor. J Bacteriol. 2005;187:3293–3301. doi: 10.1128/JB.187.10.3293-3301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes FJ, Dijksma W, Duong-Dac T, Ivanova A, Lettinga G, Field JA. Anaerobic mineralization of toluene by enriched sediments with quinones and humus as terminal electron acceptors. Appl Environ Microbiol. 2001;67:4471–4478. doi: 10.1128/AEM.67.10.4471-4478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes FJ, Vu-Thi-Thu L, Lettinga G, Field JA. Quinone-respiration improves dechlorination of carbon tetrachloride by anaerobic sludge. Appl Microbiol Biotechnol. 2004;64:702–711. doi: 10.1007/s00253-004-1564-z. [DOI] [PubMed] [Google Scholar]
- Coates JD, Ellis DJ, Blunt-Harris EL, Gaw CV, Roden EE, Lovley DR. Recovery of humic-reducing bacteria from a diversity of environments. Appl Environ Microbiol. 1998;64:1504–1509. doi: 10.1128/aem.64.4.1504-1509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croal LR, Gralnick JA, Malasarn D, Newman DK. The genetics of geochemistry. Ann Rev Gen. 2004;38:175–202. doi: 10.1146/annurev.genet.38.072902.091138. [DOI] [PubMed] [Google Scholar]
- David NA. Pharmacology of dimethyl sulfoxide. Ann Rev Pharm. 1972;12:353. doi: 10.1146/annurev.pa.12.040172.002033. [DOI] [PubMed] [Google Scholar]
- Davis BM, Lawson EH, Sandkvist M, Ali A, Sozhamannan S, Waldor MK. Convergence of the secretory pathways for cholera toxin and the filamentous phage, CTXphi. Science. 2000;288:333–335. doi: 10.1126/science.288.5464.333. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J, Michel H. The photosynthetic reaction center from the purple bacterium Rhodopseudomonas viridis. EMBO J. 1989;8:2149–2170. doi: 10.1002/j.1460-2075.1989.tb08338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vrind J, De Groot A, Brouwers GJ, Tommassen J, De Vrind-De Jong E. Identification of a novel Gsp-related pathway required for the secretion of the manganese-oxidizing factor of Pseudomonas putida strain GB-1. Mol Microbiol. 2003;47:993–1006. doi: 10.1046/j.1365-2958.2003.03339.x. [DOI] [PubMed] [Google Scholar]
- DiChristina TJ, Moore CM, Haller CA. Dissimilatory Fe(III) and Mn(IV) reduction by Shewanella putrefaciens requires ferE, a homolog of the pulE (gspE) type II protein secretion gene. J Bacteriol. 2002;184:142–151. doi: 10.1128/JB.184.1.142-151.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druschel GK, Baker BJ, Gihring TM, Banfield JF. Acid mine drainage biogeochemistry at Iron Mountain, California. Geochem Trans. 2004;5:13–32. doi: 10.1186/1467-4866-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich HL. Geomicrobiology. New York: Dekker; 2002. [Google Scholar]
- d’Enfert C, Chapon C, Pugsley AP. Export and secretion of the lipoprotein pullulanase by Klebsiella pneumoniae. Mol Microbiol. 1987;1:107–116. doi: 10.1111/j.1365-2958.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- Francis CA, Casciotti KL, Tebo BM. Localization of Mn(II)-oxidizing activity and the putative multicopper oxidase, MnxG, to the exosporium of the marine Bacillus sp strain SG-1. Arch Microbiol. 2002;178:450–456. doi: 10.1007/s00203-002-0472-9. [DOI] [PubMed] [Google Scholar]
- Ghiorse WC. Biology of iron-depositing and manganese-depositing bacteria. Ann Rev Microbiol. 1984;38:515–550. doi: 10.1146/annurev.mi.38.100184.002503. [DOI] [PubMed] [Google Scholar]
- Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A, et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci USA. 2006;103:11358–11363. doi: 10.1073/pnas.0604517103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralnick JA, Brown CT, Newman DK. Anaerobic regulation by an atypical Arc system in Shewanella oneidensis. Mol Microbiol. 2005;56:1347–1357. doi: 10.1111/j.1365-2958.2005.04628.x. [DOI] [PubMed] [Google Scholar]
- Gralnick JA, Vali H, Lies DP, Newman DK. Extracellular respiration of dimethyl sulfoxide by Shewanella oneidensis strain MR-1. Proc Natl Acad Sci USA. 2006;103:4669–4674. doi: 10.1073/pnas.0505959103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon SP, Holt RA, Moore GR, McEwan AG. Isolation and characterization of a strain of Rhodobacter sulfidophilus – a bacterium which grows autotrophically with dimethylsulfide as electron donor. Microbiology-UK. 1994;140:1953–1958. [Google Scholar]
- Hernandez ME, Newman DK. Extracellular electron transfer. Cell Mol Life Sci. 2001;58:1562–1571. doi: 10.1007/PL00000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes DE, Bond DR, O’Neill RA, Reimers CE, Tender LR, Lovley DR. Microbial communities associated with electrodes harvesting electricity from a variety of aquatic sediments. Microbiol Ecol. 2004;48:178–190. doi: 10.1007/s00248-003-0004-4. [DOI] [PubMed] [Google Scholar]
- Holmes DE, Chaudhuri SK, Nevin KP, Mehta T, Methe BA, Liu A, et al. Microarray and genetic analysis of electron transfer to electrodes in Geobacter sulfurreducens. Environ Microbiol. 2006;8:1805–1815. doi: 10.1111/j.1462-2920.2006.01065.x. [DOI] [PubMed] [Google Scholar]
- Johnson CM, Beard BL, Beukes NJ, Klein C, O’Leary JM. Ancient geochemical cycling in the Earth as inferred from Fe isotope studies of banded iron formations from the Transvaal Craton. Contrib Mineral Petrol. 2003;144:523–547. [Google Scholar]
- Kappler A, Straub KL. Geomicrobiological cycling of iron. Rev Mineral Geochem. 2005;59:85–108. [Google Scholar]
- Kim BC, Leang C, Ding YH, Glaven RH, Coppi MV, Lovley DR. OmcF, a putative c-type mono-heme outer membrane cytochrome required for the expression of other outer membrane cytochromes in Geobacter sulfurreducens. J Bacteriol. 2005;187:4505–4513. doi: 10.1128/JB.187.13.4505-4513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BC, Qian X, Leang C, Coppi MV, Lovley DR. Two putative c-type multiheme cytochromes required for the expression of OmcB, an outer membrane protein essential for optimal Fe(III) reduction in Geobacter sulfurreducens. J Bacteriol. 2006;188:3138–3142. doi: 10.1128/JB.188.8.3138-3142.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BH, Park HS, Kim HJ, Kim GT, Chang IS, Lee J, Phung NT. Enrichment of microbial community generating electricity using a fuel-cell-type electrochemical cell. Appl Microbiol Biotechnol. 2004;63:672–681. doi: 10.1007/s00253-003-1412-6. [DOI] [PubMed] [Google Scholar]
- Klonowska A, Heulin T, Vermeglio A. Selenite and tellurite reduction by Shewanella oneidensis. Appl Environ Microbiol. 2005;71:5607–5609. doi: 10.1128/AEM.71.9.5607-5609.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal R. Carbon sequestration impacts on global climate change and food security. Science. 2004;304:1623–1627. doi: 10.1126/science.1097396. [DOI] [PubMed] [Google Scholar]
- Leang C, Coppi MV, Lovley DR. OmcB, a c-type polyheme cytochrome, involved in Fe(III) reduction in Geobacter sulfurreducens. J Bacteriol. 2003;185:2096–2103. doi: 10.1128/JB.185.7.2096-2103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PA, de Mora SJ. Intracellular dimethylsulfoxide (DMSO) in unicellular marine algae: speculations on its origin and possible biological role. J Phycol. 1999;35:8–18. [Google Scholar]
- Lies DP, Hernandez ME, Kappler A, Mielke RE, Gralnick JA, Newman DK. Shewanella oneidensis MR-1 uses overlapping pathways for iron reduction at a distance and by direct contact under conditions relevant for biofilms. Appl Environ Microbiol. 2005;71:4414–4426. doi: 10.1128/AEM.71.8.4414-4426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd JR, Sole VA, Van Praagh CV, Lovley DR. Direct and Fe(II)-mediated reduction of technetium by Fe(III)-reducing bacteria. Appl Environ Microbiol. 2000;66:3743–3749. doi: 10.1128/aem.66.9.3743-3749.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd JR, Leang C, Hodges Myerson AL, Coppi MV, Cuifo S, Methe B, et al. Biochemical and genetic characterization of PpcA, a periplasmic c-type cytochrome in Geobacter sulfurreducens. Biochem J. 2003;369:153–161. doi: 10.1042/BJ20020597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley DR. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991;55:259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley DR, Phillips EJP. Novel mode of microbial energy metabolism – organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988;54:1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley DR, Giovannoni SJ, White DC, Champine JE, Phillips EJ, Gorby YA, Goodwin S. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch Microbiol. 1993;159:336–344. doi: 10.1007/BF00290916. [DOI] [PubMed] [Google Scholar]
- Lovley DR, Woodward JC, Chapelle FH. Rapid anaerobic benzene oxidation with a variety of chelated Fe(III) forms. Appl Environ Microbiol. 1996;62:288–291. doi: 10.1128/aem.62.1.288-291.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley DR, Holmes DE, Nevin KP. Dissimilatory Fe(III) and Mn(IV) reduction. In: Poole RK, editor. Advances in Microbial Physiology. Vol. 49. London: Academic Press; 2004. pp. 219–286. [DOI] [PubMed] [Google Scholar]
- Lower SK, Hochella MF, Beveridge TJ. Bacterial recognition of mineral surfaces: nanoscale interactions between Shewanella and alpha-FeOOH. Science. 2001;292:1360–1363. doi: 10.1126/science.1059567. [DOI] [PubMed] [Google Scholar]
- McEwan AG, Wetzstein HG, Ferguson SJ, Jackson JB. Periplasmic location of the terminal reductase in trimethylamine N-oxide and dimethylsulphoxide respiration in the photosynthetic bacterium Rhodopseudomonas capsulata. Biochim Biophys Acta. 1985;806:410–417. [Google Scholar]
- McKnight DM, Aiken GR. Sources and ages of aquatic humus. In: Hessen DO, Tranvik LJ, editors. Aquatic Humic Substances: Ecology and Biogeochemistry. Berlin: Springer; 1998. pp. 9–39. [Google Scholar]
- Marshall MJ, Beliaev AS, Dohnalkova AC, Kennedy DW, Shi L, Wang Z, et al. c-Type cytochrome-dependent formation of U(IV) nanoparticles by Shewanella oneidensis. PLoS Biol. 2006;4:e268. doi: 10.1371/journal.pbio.0040268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta T, Coppi MV, Childers SE, Lovley DR. Outer membrane c-type cytochromes required for Fe(III) and Mn(IV) oxide reduction in Geobacter sulfurreducens. Appl Environ Microbiol. 2005;71:8634–8641. doi: 10.1128/AEM.71.12.8634-8641.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta T, Childers SE, Glaven R, Lovley DR, Mester T. A putative multicopper protein secreted by an atypical type II secretion system involved in the reduction of insoluble electron acceptors in Geobacter sulfurreducens. Microbiology. 2006;152:2257–2264. doi: 10.1099/mic.0.28864-0. [DOI] [PubMed] [Google Scholar]
- Myers CR, Myers JM. Localization of cytochromes to the outer-membrane of anaerobically grown Shewanella putrefaciens MR-1. J Bacteriol. 1992;174:3429–3438. doi: 10.1128/jb.174.11.3429-3438.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CR, Myers JM. Role of menaquinone in the reduction of fumarate, nitrate, iron(III) and manganese(IV) by Shewanella putrefaciens MR-1. FEMS Microbiol Let. 1993;114:215–222. [Google Scholar]
- Myers CR, Myers JM. Cloning and sequence of cymA, a gene encoding a tetraheme cytochrome c required for reduction of iron(III), fumarate, and nitrate by Shewanella putrefaciens MR-1. J Bacteriol. 1997;179:1143–1152. doi: 10.1128/jb.179.4.1143-1152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CR, Myers JM. Isolation and sequence of omcA, a gene encoding a decaheme outer membrane cytochrome c of Shewanella putrefaciens MR-1, and detection of omcA homologs in other strains of S. putrefaciens. Biochim Biophys Acta. 1998;1326:307–318. doi: 10.1016/s0005-2736(98)00111-4. [DOI] [PubMed] [Google Scholar]
- Myers CR, Myers JM. MtrB is required for proper incorporation of the cytochromes OmcA and OmcB into the outer membrane of Shewanella putrefaciens MR-1. Appl Environ Microbiol. 2002;68:5585–5594. doi: 10.1128/AEM.68.11.5585-5594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CR, Myers JM. Cell surface exposure of the outer membrane cytochromes of Shewanella oneidensis MR-1. Let Appl Microbiol. 2003;37:254–258. doi: 10.1046/j.1472-765x.2003.01389.x. [DOI] [PubMed] [Google Scholar]
- Myers CR, Nealson KH. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science. 1988;240:1319–1321. doi: 10.1126/science.240.4857.1319. [DOI] [PubMed] [Google Scholar]
- Myers JM, Antholine WE, Myers CR. Vanadium(V) reduction by Shewanella oneidensis MR-1 requires menaquinone and cytochromes from the cytoplasmic and outer membranes. Appl Environ Microbiol. 2004;70:1405–1412. doi: 10.1128/AEM.70.3.1405-1412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal AL, Bank TL, Hochella MF, Rosso KM. Cell adhesion of Shewanella oneidensis to iron oxide minerals: effect of different single crystal faces. Geochem Trans. 2005;6:77–84. doi: 10.1186/1467-4866-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson KH, Saffarini D. Iron and manganese in anaerobic respiration – environmental significance, physiology, and regulation. Ann Rev Microbiol. 1994;48:311–343. doi: 10.1146/annurev.mi.48.100194.001523. [DOI] [PubMed] [Google Scholar]
- Newman DK, Kolter R. A role for excreted quinones in extracellular electron transfer. Nature. 2000;405:94–97. doi: 10.1038/35011098. [DOI] [PubMed] [Google Scholar]
- Nosanchuk JD, Casadevall A. The contribution of melanin to microbial pathogenesis. Cell Microbiol. 2003;5:203–223. doi: 10.1046/j.1462-5814.2003.00268.x. [DOI] [PubMed] [Google Scholar]
- Nunez C, Esteve-Nunez A, Giometti C, Tollaksen S, Khare T, Lin W, et al. DNA microarray and proteomic analyses of the RpoS regulon in Geobacter sulfurreducens. J Bacteriol. 2006;188:2792–2800. doi: 10.1128/JB.188.8.2792-2800.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DH, Kim SK, Shin IH, Jeong YJ. Electricity production in biofuel cell using modified graphite electrode with Neutral Red. Biotechnol Let. 2000;22:1301–1304. [Google Scholar]
- Piccolo A. The supramolecular structure of humic substances. Soil Sci. 2001;166:810–832. [Google Scholar]
- Pitts KE, Dobbin PS, Reyes-Ramirez F, Thomson AJ, Richardson DJ, Seward HE. Characterization of the Shewanella oneidensis MR-1 decaheme cytochrome MtrA. J Biol Chem. 2003;278:27758–27765. doi: 10.1074/jbc.M302582200. [DOI] [PubMed] [Google Scholar]
- Rabaey K, Verstraete W. Microbial fuel cells: novel biotechnology for energy generation. Trend Biotechnol. 2005;23:291–298. doi: 10.1016/j.tibtech.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Rabaey K, Boon N, Siciliano SD, Verhaege M, Verstraete W. Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl Environ Microbiol. 2004;70:5373–5382. doi: 10.1128/AEM.70.9.5373-5382.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabaey K, Boon N, Hofte M, Verstraete W. Microbial phenazine production enhances electron transfer in biofuel cells. Environ Sci Technol. 2005;39:3401–3408. doi: 10.1021/es048563o. [DOI] [PubMed] [Google Scholar]
- Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR. Extracellular electron transfer via microbial nanowires. Nature. 2005;435:1098–1101. doi: 10.1038/nature03661. [DOI] [PubMed] [Google Scholar]
- Reguera G, Nevin KP, Nicoll JS, Covalla SF, Woodard TL, Lovley DR. Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl Environ Microbiol. 2006;72:7345–7348. doi: 10.1128/AEM.01444-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers CE, Girguis P, Stecher HA, Tender LM, Ryckelynck N, Whaling P. Microbial fuel cell energy from an ocean cold seep. Geobiology. 2006;4:123–136. [Google Scholar]
- Roller SD, Bennetto HP, Delaney GM, Mason JR, Stirling JL, Thurston CF. Electron transfer coupling in microbial fuel cells – comparison of redox mediator reduction rates and respiratory rates of bacteria. J Chem Technol Biotechnol. 1984;34:3–12. [Google Scholar]
- Saffarini DA, Blumerman SL, Mansoorabadi KJ. Role of menaquinones in Fe(III) reduction by membrane fractions of Shewanella putrefaciens. J Bacteriol. 2002;184:846–848. doi: 10.1128/JB.184.3.846-848.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand W, Gherke T. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron(III) ions and acidophilic bacteria. Res Microbiol. 2006;157:49–56. doi: 10.1016/j.resmic.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Scholes P, Mitchell P, Moyle J. The polarity of proton translocation in some photosynthetic microorganisms. Euro J Biochem. 1969;8:450–454. doi: 10.1111/j.1432-1033.1969.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Scott DT, McKnight DM, Blunt-Harris EL, Kolesar SE, Lovley DR. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ Sci Technol. 1998;32:2984–2989. [Google Scholar]
- Shi L, Chen B, Wang Z, Elias DA, Mayer MU, Gorby YA, et al. Isolation of a high-affinity functional protein complex between OmcA and MtrC: two outer membrane decaheme c-type cytochromes of Shewanella oneidensis MR-1. J Bacteriol. 2006;188:4705–4714. doi: 10.1128/JB.01966-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Squier TC, Zachara JM, Fredrickson JK. Bacterial metal (hydr)oxide respiration: a key role for multiheme c-type cytochromes. Mol Microbiol. 2007;65 doi: 10.1111/j.1365-2958.2007.05783.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu JB, Lies DP, Newman DK. Protective role of tolC in efflux of the electron shuttle anthraquinone-2,6-disulfonate. J Bacteriol. 2002;184:1806–1810. doi: 10.1128/JB.184.6.1806-1810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo R, Hatton AD, Malin G, Liss PS. Particulate dimethyl sulphoxide in seawater: production by microplankton. Mar Ecol Prog Ser. 1998;167:291–296. [Google Scholar]
- Stanley NR, Sargent F, Buchanan G, Shi J, Stewart V, Palmer T, Berks BC. Behaviour of topological marker proteins targeted to the Tat protein transport pathway. Mol Microbiol. 2002;43:1005–1021. doi: 10.1046/j.1365-2958.2002.02797.x. [DOI] [PubMed] [Google Scholar]
- Stevenson FJ. Humus Chemistry: Genesis, Composition, Reactions. New York: John Wiley & Sons; 1994. [Google Scholar]
- Stolz JF, Basu P, Santini JM, Oremland RS. Arsenic and selenium in microbial metabolism. Ann Rev Microbiol. 2006;60:107–130. doi: 10.1146/annurev.micro.60.080805.142053. [DOI] [PubMed] [Google Scholar]
- Sutton R, Sposito G. Molecular structure in soil humic substances: the new view. Environ Sci Technol. 2005;39:9009–9015. doi: 10.1021/es050778q. [DOI] [PubMed] [Google Scholar]
- Swift RS. Macromolecular properties of soil humic substances: fact, fiction, and opinion. Soil Sci. 1999;164:790–802. [Google Scholar]
- Tebo BM, Johnson HA, McCarthy JK, Templeton AS. Geomicrobiology of manganese(II) oxidation. Trend Microbiol. 2005;13:421–428. doi: 10.1016/j.tim.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Tender LM, Reimers CE, Stecher HA, Holmes DE, Bond DR, Lowy DA, et al. Harnessing microbially generated power on the seafloor. Nat Biotechnol. 2002;20:821–825. doi: 10.1038/nbt716. [DOI] [PubMed] [Google Scholar]
- Tokuda H, Matsuyama S. Sorting of lipoproteins to the outer membrane in E. coli. Biochim Biophys Acta Mol Cell Res. 2004;1693:5–13. doi: 10.1016/j.bbamcr.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Turick CE, Apel WA, Carmiol NS. Isolation of hexavalent chromium-reducing anaerobes from hexavalent-chromium-contaminated and noncontaminated environments. Appl Microbiol Biotechnol. 1996;44:683–688. doi: 10.1007/BF00172503. [DOI] [PubMed] [Google Scholar]
- Turick CE, Tisa LS, Caccavo F., Jr Melanin production and use as a soluble electron shuttle for Fe(III) oxide reduction and as a terminal electron acceptor by Shewanella algae BrY. Appl Environ Microbiol. 2002;68:2436–2444. doi: 10.1128/AEM.68.5.2436-2444.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KA, Achenbach LA, Coates JD. Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol. 2006;4:752–764. doi: 10.1038/nrmicro1490. [DOI] [PubMed] [Google Scholar]
- Wigginton NS, Rosso KM, Lower BH, Shi L, Hochella MF. Electron tunneling properties of outer-membrane decaheme cytochromes from Shewanella oneidensis. Geochim Cosmochim Acta. 2007;71:543–555. [Google Scholar]
- Winogradsky S. Recherches physiologiques sur les sulfobacteries. Annals l’Institut Pasteur. 1889;3:49–60. [Google Scholar]
- Xiong Y, Shi L, Chen B, Mayer MU, Lower BH, Londer Y, et al. High-affinity binding and direct electron transfer to solid metals by the Shewanella oneidensis MR-1 outer membrane c-type cytochrome OmcA. J Am Chem Soc. 2006;128:13978–13979. doi: 10.1021/ja063526d. [DOI] [PubMed] [Google Scholar]
- Yarzabal A, Brasseur G, Ratouchniak J, Lund K, Lemesle-Meunier D, DeMoss JA, Bonnefoy V. The high molecular weight cytochrome c Cyc2 of Acidithiobacillus ferrooxidans is an outer membrane protein. J Bacteriol. 2002;184:313–317. doi: 10.1128/JB.184.1.313-317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


