Abstract
The nature of the rapid action of aldosterone on blood vessels, whether endothelium-dependent dilation or smooth muscle-dependent constriction is predominant, is still in dispute. In this study, we administered aldosterone intraluminally or extraluminally to isolated mesenteric and cerebral arterioles of male Wistar rats. Extraluminal administration of aldosterone (10−11 or 10−7M) elicited a transient vasodilatation. The peak response appeared at ~5 minutes. In contrast, intraluminal administration of aldosterone elicited a greater and sustained dilation. When aldosterone (10−12 to 10−7M) was administered extraluminally in a cumulative manner, dose-dependent vasodilator responses were elicited, except a reduced dilation was observed to 10−7M aldosterone. The dilations were significantly inhibited by spironolactone (10−7M), a mineralocorticoid receptor antagonist or L-NAME (3×10−4M), a NO synthesis inhibitor. In endothelium-denuded vessels, extraluminal aldosterone induced a dose-dependent vasoconstrictor response. Scavenging superoxide with Tempol (10−4M) sustained the extraluminal aldosterone (10−11 or 10−7M)-induced dilation; whereas inhibition of NO synthesis or removal of the endothelium abolished intraluminal aldosterone-induced dilation. Dilation to 10−7M aldosterone was significantly enhanced after inhibition of NAD(P)H-oxidase with apocynin (10−5M). Furthermore, in the presence of endothelial dysfunction, induced by chronic inhibition of NO synthesis, intraluminal administration of aldosterone failed to dilate the arterioles. We conclude that in physiological conditions, acute elevation of aldosterone will evoke mainly an endothelium-dependent, nitric oxide-mediated dilation.
Keywords: endothelium, aldosterone, oxidative stress
Introduction
A growing body of evidence suggests that pathological excess of aldosterone induces endothelial dysfunction through increased vascular reactive oxygen species (ROS) and decreased nitric oxide bioavailability 1. However, the nature of the acute effect of aldosterone on vascular function is still in dispute. In coronary and renal arteries of animals 2–4 and forearm arteries of humans 5, acute administration of aldosterone elicits concentration-dependent vasoconstrictor responses. It was demonstrated that the aldosterone-induced vasoconstriction was independent of the mineralocorticoid receptor but dependent on activation of phospholipase C and protein kinase C, and a subsequent calcium mobilization thorough voltage-dependent calcium channels 2,3. In contrast to vasoconstrictor responses to aldosterone, it has also been reported that aldosterone increases forearm blood flow in healthy subjects 6 and inhibits vasoconstrictor responses induced by KCl in renal arteries 7 and by phenylephrine in aorta of rats 8. Removal of the endothelium or inhibition of nitric oxide synthesis enhanced aldosterone-induced vasoconstriction 9 or eliminated the attenuation of agonist-induced constriction by aldosterone 7,8. These results suggest that aldosterone is able to modulate the endothelial function of blood vessels. In cultured vascular smooth muscle cells, aldosterone dose-dependently increased intracellular inositol 1,4,5-trisphosphate and calcium 10,11, mechanisms which may underlie the rapid nongenomic vasoconstrictor responses to aldosterone. On the other hand, in cultured endothelial cells, acute administration of aldosterone time- and dose-dependently increased nitric oxide synthase activity via mineralocorticoid receptor and phosphatidylinositol 3-kinase-dependent mechanisms 8. Taken together, aldosterone may affect vascular tone directly via both endothelium- and smooth muscle-dependent mechanisms.
Plasma aldosterone rises rapidly and substantially in response to a variety of stresses 12. Therefore, the increased aldosterone may modify tissue perfusion via increasing or decreasing the tone of blood vessels. However, it is not clear how vessels other than coronary and renal arteries will respond to aldosterone. Here, we studied the nongenomic effects of aldosterone on the tone of mesenteric and cerebral arterioles of rats, aimed to explore the physiological role of aldosterone-induced endothelium-dependent dilation and smooth muscle constriction by comparing the different responses induced by its intraluminal and extraluminal administration.
Materials and Methods
Isolated mesenteric arterioles
All protocols were approved by the Institutional Animal Care and Use Committee of New York Medical College and conformed to the current guidelines of the National Institutes of Health and the American Physiological Society for the use and care of laboratory animals. Eight-week-old male Wistar rats were anesthetized with intraperitoneal injections of pentobarbital sodium (50 mg/kg). The mesentery was removed and placed in a dissecting dish containing cold (0–4°C) MOPS-buffered physiological salt solution (PSS; pH 7.4). The solution contained (in mM) 142 NaCl, 5 KCl, 2CaCl2, 1.2 MgSO4, 1.2 NaH2PO4, 5 dextrose, 2 pyruvate, 0.02 EDTA and 3 MOPS. The mesentery was pinned to the bottom of the silicone-lined Petri dish. Third-order mesenteric arterioles were isolated and cannulated by two glass pipettes in a vessel chamber (4 ml in volume) and then secured with sutures. The vessels were perfused with PSS at 37°C and the intravascular pressure was maintained at 80 mmHg in a no-flow condition. Only vessels that developed spontaneous tone to pressure were used.
Aldosterone-induced response
In the first series of experiments, responses of third-order mesenteric arterioles to intraluminal and extraluminal administration of aldosterone were compared. A single dose of aldosterone, at a concentration of 10−11 or 10−7M, was administered to the vessels and the response was monitored continuously for 30 minutes. For extraluminal administration, aldosterone was added into the perfusion chamber directly. To deliver aldosterone intraluminally, aldosterone was injected into vessels with a syringe pump at a low flow rate of 2µl/min for 30 minutes. 2µl/min PSS did not cause a measurable flow-induced dilation. A thin tube, filled with aldosterone, was advanced into to the tip of inflow pipette to ensure delivery of aldosterone into the vessel immediately after the onset of the infusion 13. Vessels were further perfused with PSS before additional experiments. Time-dependent experiments were also performed. Three sequential administrations of 10−11M intraluminal aldosterone, in 30-minute each and one hour apart between the two deliveries, were performed. Responses were not different amount administrations. In the second series of experiments, aldosterone (10−12 to 10−7M) was administered into the vessel chamber (extraluminally) in a cumulative manner and a 5-minute interval was allowed between each administration to obtain peak vasodilator responses. Aldosterone-induced responses were assessed in control and after administration of Nω-nitro-L-arginine methyl ester (L-NAME, 3 × 10−4M), an inhibitor of NOS, or spironolactone (10−7M), a non specific blocker of mineralocorticoid receptors or after removal of the endothelium. Vessels were incubated with inhibitors for at least 30 minutes before additional treatments. Endothelial denudation was accomplished by injection of air into the vessel lumen, as described previously 14. The efficacy of removal of endothelium and intact function of smooth muscle were assessed by loss of dilation to acetylcholine (ACh, 10−7M) and maintenance of dilation to sodium nitroprusside (SNP, 10−7M). In another series of experiments, vasodilatation to 10−11 or 10−7M of extraluminal aldosterone was assessed in control and after scavenging superoxide with Tempol (10−4M). The response was continuously recorded for 30 minutes. In another series of experiments, vasodilation to 10−7M of aldosterone was assessed before and after administration of acetovanillone (apocynin, 10−5M), an inhibitor of NADPH oxidase. L-NAME was also used to assess changes in NO-mediated responses in the presence of apocynin. In the final series of experiments, the effects of intraluminal administration of aldosterone (10−7M) were assessed in control and after inhibition of NO synthesis with L-NAME (3 × 10−4M) or removal of the endothelium. Responses were recorded for 30 minutes in each experimental condition.
Aldosterone-induced responses in cerebral arterioles
Branches of the middle cerebral arteries were isolated from eight week old normal male Wistar rats. Vasodilator responses to intraluminal and extraluminal administrations of aldosterone (10−7M), in control and after inhibition of NO synthesis with L-NAME (3 × 10−4M), were assessed.
Chronic inhibition of NO synthesis
L-NAME was administered to rats in the drinking water (50 mg/100 ml) for 4 weeks. Third-order mesenteric arterioles were then isolated to assess responses to intraluminal and extraluminal administrations of aldosterone (10−7M). ACh (10−7M), SNP (10−7M) was used to evaluate NO-mediated and smooth muscle-dependent vasodilator responses after inhibition of NO synthesis.
Passive diameter
To assess the active tone generated by arterioles in response to intravascular pressure, as well as to normalize the changes in diameter in response to the agents administered, at the conclusion of each experiment, the suffusion solution was changed to a Ca2+-free solution containing 1mM EGTA. The vessels were incubated for 10 min, and then the passive diameter of arterioles at 80 mmHg perfusion pressure was obtained.
Chemicals
All chemicals were obtained from Sigma (St. Louis. MO), except for aldosterone, which was obtained from Fisher Scientific (Pittsburgh, PA). Drugs were dissolved in distilled water except for apocynin which was dissolved in DMSO and aldosterone which was dissolved in ethanol. The highest concentration of DMSO or ethanol in the chamber was 0.01 % (vol/vol), which has no effect on vessel tone. Drugs were freshly prepared on the day of the experiments.
Data calculation and statistical analysis
Data are expressed as means ± SE; n refers to the number of rats from which mesenteric arteries were taken. Change in diameter refers to the increase or decrease of vessel diameter from the basal diameter (BD) which is defined as the vessel diameter before the onset of an experimental procedure. Changes in diameter were further normalized by the passive diameter of each vessel. Statistical analysis was performed using repeated-measures of one-way and two-way ANOVA followed by Bonferroni post-test and Student's t-test. Statistical significance was accepted at a level of P < 0.05.
Results
Characteristics of mesenteric arterioles
Isolated third order mesenteric arterioles developed spontaneous constriction during an equilibration period at 80 mmHg of pressure without the use of any vasoactive agent. Upon the addition of inhibitors, the mean basal diameter, passive diameter and basal tone of arterioles were not statistically different from control (Table 1).
Table 1.
Characteristics of mesenteric arterioles after administration of agents.
| Spiro (n=7) | L-NAME (n=7) | Removal Endo (n=8) |
Tempol (n=7) | |||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| BD, µm | 75 ±9 | 73 ± 7 | 91 ± 11 | 103 ± 12 | 94 ± 8 | 103 ± 9 | 65 ± 5 | 63 ± 5 |
| Basal tone, % | 56 ± 3 | 56 ± 2 | 61 ± 4 | 69 ± 4 | 64 ± 3 | 69 ± 4 | 50 ± 2 | 49 ± 2 |
| PD, µm | 131 ± 11 | 148 ± 12 | 148 ± 9 | 130 ± 7 | ||||
Values are means ± SE ; BD, basal diameter ; PD, passive diameter; L-NAME, Nω-nitro-L-arginine methyl ester (3 ×10−4 M); Spiro, spironolactone (10−7 M); Removal Endo, removal endothelium; Tempol (10−4 M).
Vasodilatation of mesenteric arterioles to aldosterone
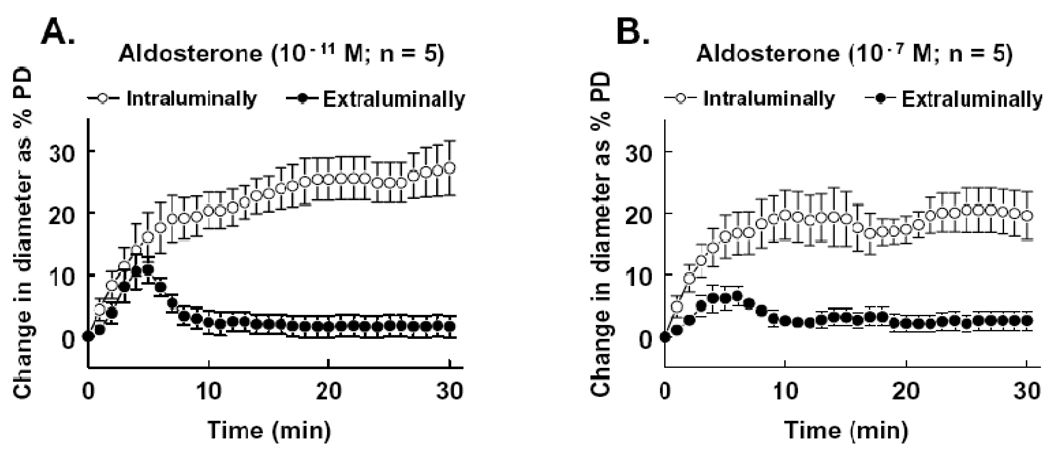
Two concentrations of aldosterone (10−11 and 10−7M) were administrated intraluminally or extraluminally to the isolated mesenteric arterioles. Changes in diameter were continuously recorded for 30 minutes. Figure 1 shows that extraluminal administration of aldosterone elicited a transient vasodilator response (15 ± 3 and 7 ± 3 µm to 10−11 and 10−7M aldosterone, respectively). The peak response appeared 4–5 minutes after administering aldosterone and the dilation lasted for less than 10 minutes. After that the diameter decreased to the control level. Intraluminal administration of aldosterone, however, elicited a significantly greater vasodilatation (42 ± 8 and 28 ± 5 µm to 10−11 and 10−7M aldosterone, respectively). The dilation was sustained during the perfusion and ceased after the washout of aldosterone. The dilation was greater in response to 10−11M than to 10−7M of intraluminal administration of aldosterone.
Figure 1.
Change in diameter as percentage of passive diameter (PD) of mesenteric arterioles in response to intraluminal and extraluminal administration of aldosterone at concentrations of 10−11M (A) and 10−7M (B). Aldosterone was administered at time 0 and the changes in diameter of vessels were continuously recorded for 30 minutes. The curves between intraluminal and extraluminal aldosterone were statistically different (p<0.05), determined by repeated measurements of two-way ANOVA.
Endothelial mechanisms of aldosterone-induced dilation in mesenteric arterioles
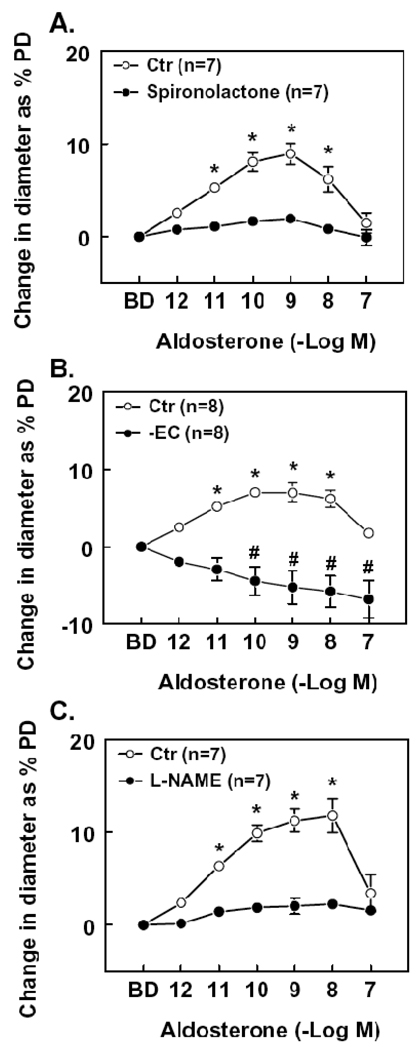
Based on the findings shown in Figure 1, extraluminal aldosterone (10−12 to 10−7M) was administered to the vessels in a cumulative manner with a 5-minute interval between each administration. Figure 2 shows peak increases in diameter in response to extraluminal administration of aldosterone. Compared to the basal diameter (86 ± 5 µm, n=23), aldosterone increased the diameter significantly at concentrations of 10−11 – 10−8M (94 ± 5 and 99 ± 6 µm at 10−11 and 10−8M, respectively). Further increasing the concentration to 10−7M, the diameter of the dilated vessels decreased to a level (90 ± 5 µm) that was not significantly different from the basal diameter. The mechanism of vasodilator response to extraluminal administration of aldosterone was also assessed. Figure 2A shows that in the presence of spironolactone, responses to aldosterone (10−11 to 10−8M) were significantly inhibited, suggesting that the aldosterone-induced arteriolar dilation is mediated through activation of mineralocorticoid receptors. In endothelium-denuded arterioles, aldosterone (10−10 to 10−7M) induced a concentration dependent constriction (Figure 2B), suggesting dual actions, an endothelium-dependent dilation and a smooth muscle-dependent constriction. To clarify whether nitric oxide is the endothelium-derived mediator responsible of the aldosterone-induced dilation, L-NAME was administered; the results that L-NAME inhibited aldosterone-induced dilation (10−11 to 10−8M; Figure 2C) demonstrate that the aldosterone-induced dilation is mediated by NO.
Figure 2.
Change in diameter as percentage of passive diameter (PD) of mesenteric arterioles in response to extraluminal administration of aldosterone (10−12 to 10−7M) in control (Ctr) condition and in presence of spironolactone (10−7M), a non specific blocker of mineralocorticoid receptors (A), or after endothelial removal (EC-) (B) or after administration of Nω-nitro-L-arginine methyl ester (L-NAME, 3 × 10−4M), an inhibitor of NOS (C). Aldosterone was administered into the vessel chamber in a cumulative manner. A 5-minute interval was given and a maximal dilation was recorded at each concentration. BD, basal diameter. Spironolactone, L-NAME or removal of the endothelium significantly inhibited aldosterone induced dilation (two-way ANOVA). * or # p < 0.01 vs. BD (Bonferroni procedure).
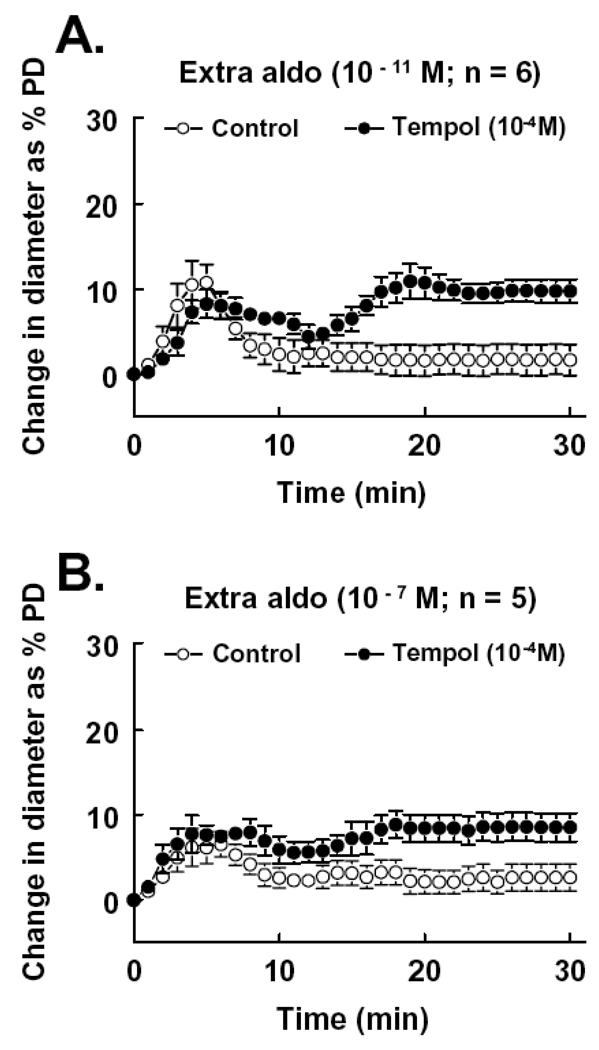
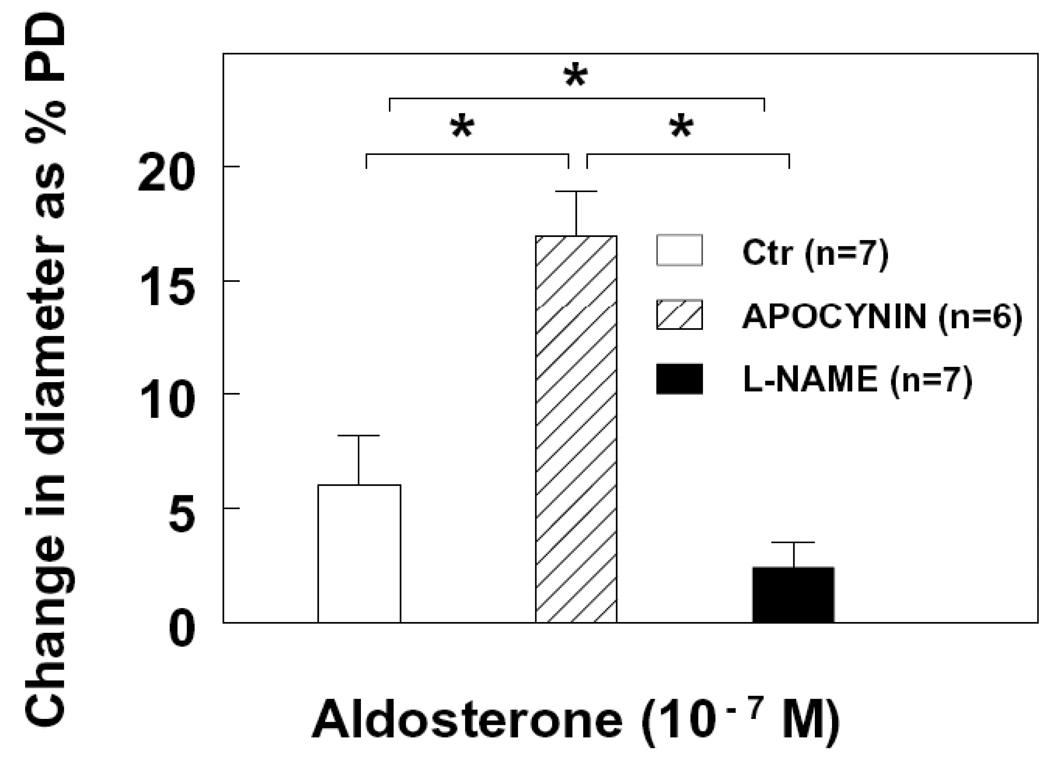
To elucidate whether the transient dilation induced by extraluminal aldosterone was due to a diminished NO bioavailability, vessels were incubated with Tempol (10−4M), prior and during administration of aldosterone to scavenge intracellular superoxide. Figure 3 shows that in the presence of Tempol, the late phase of the extraluminal administration of aldosterone-induced response, at both concentrations of 10−11 and 10−7M, was converted to a significant vasodilator response (Figure 3A and 3B). In connection with the results of Figure 2B, these data support the notion that the reduced and short-lasting endothelial NO-mediated dilation in response to extraluminal aldosterone resulted from combined effects of a direct smooth muscle-dependent vasoconstriction and a reduced NO bioavailability due to an increased superoxide formation. We also used apocynin (10−5M) to inhibit NAD(P)H oxidase. Figure 4 shows that in the presence of apocynin, the extraluminal aldosterone (10−7M)-induced dilation increased three fold. This increased dilation was abolished by L-NAME (3 × 10−4M), confirming that aldosterone, at an increased concentration, enhances NADPH oxidase-dependent superoxide production and decreases NO bioavailability.
Figure 3.
Change in diameter as percentage of passive diameter (PD) of mesenteric arterioles in response to extraluminal administration of aldosterone at concentrations of 10−11M (A) and 10−7M (B) in control, and after scavenging superoxide with Tempol (10−4M). The curves with and without Tempol are significantly different (p<0.05; two-way ANOVA).
Figure 4.
Change in diameter as percentage of passive diameter (PD) of mesenteric arterioles in response to extraluminal administration of aldosterone (10−7M) before (Ctr) and after inhibition of NADPH oxidase with acetovanillone (apocynin), and additional inhibition of NO synthesis (L-NAME). * p < 0.05.
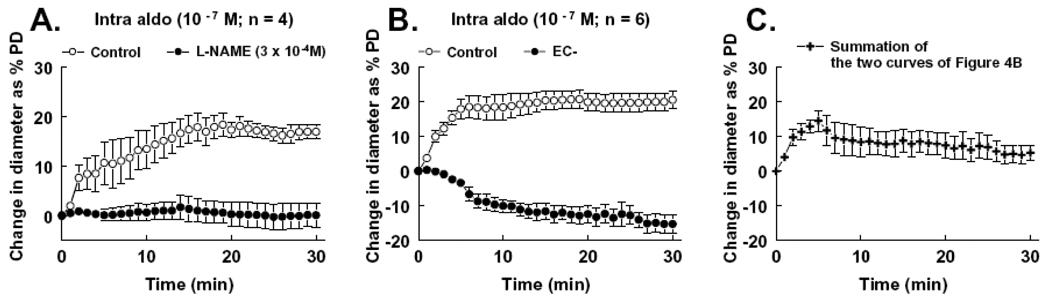
We further assessed the underlying mechanism of the enhanced dilation to intraluminal administration of aldosterone. Figure 5A shows that dilation induced by intraluminal administration of aldosterone was abolished after inhibition of NO synthesis with L-NAME (3 × 10−4M), suggesting that the enhanced dilation is a NO-mediated response. Removal of the endothelium converted the dilation to a vasoconstrictor response (Figure 5B), further confirming that the enhanced dilation is an endothelium-dependent response and that the existence of the endothelium effectively prevents the direct action of aldosterone on vascular smooth muscle cells, hence, reducing smooth muscle-dependent vasoconstriction and superoxide formation. Furthermore, when the two curves shown in Figure 5B were mathematically combined, the newly created curve (Figure 5C) shows a pattern similar to that induced by extraluminal administration of aldosterone. Therefore, these results clearly show the differences between intraluminal and extraluminal administration of aldosterone and suggest that in a physiological condition, an acute rise of aldosterone in plasma will mainly elicit an endothelial NO-mediated dilation in mesenteric arterioles of rats.
Figure 5.
Change in diameter as percentage of passive diameter (PD) of mesenteric arterioles in response to intraluminal administration of aldosterone (Intro aldo, 10−7M) in control and after inhibition of NO synthesis with L-NAME (A), and in control and after removal of the endothelium (EC-) (B). Inhibition of NO synthesis or removal of the endothelium significantly decreased intraluminal aldosterone-induced dilation (two-way ANOVA). Figure 4C shows combined data of the two curves, in control and after endothelial removal, of Figure 4B. The curve was calculated by summation of the corresponding data points of the two curves from each vessel.
Vasodilatation of cerebral arterioles to aldosterone
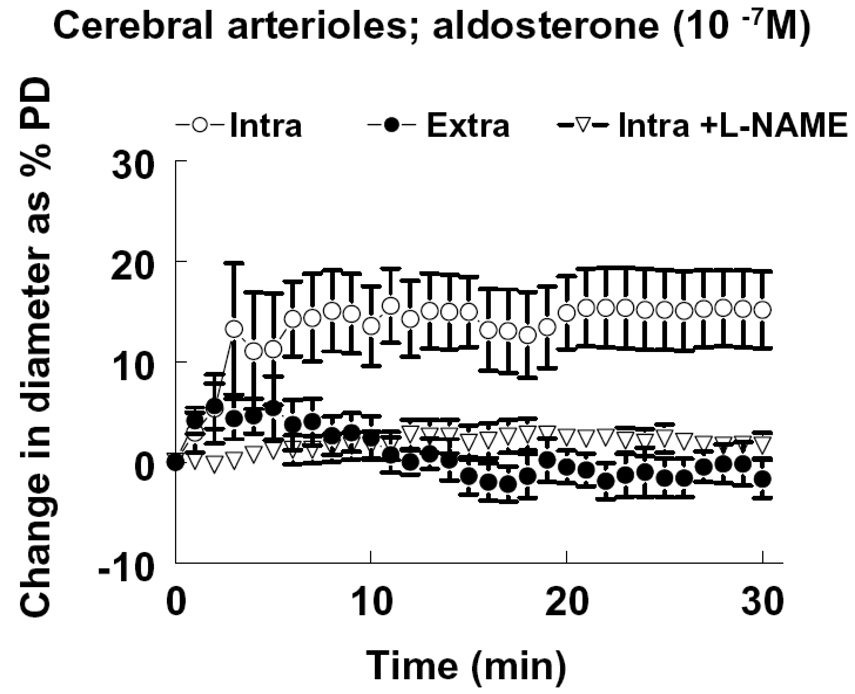
The average passive and basal diameters of middle cerebral arterioles, isolated and pressurized at 80 mmHg of intravascular pressure, were 152 ± 12 and 82 ± 5 µm, respectively. Intraluminal and extraluminal administrations of aldosterone (10−7M) to the vessels elicited responses similar to those of mesenteric arterioles of rats (Figure 6). L-NAME abolished the intraluminal administration of aldosterone-induced dilation, indicating that aldosterone-induced dilation in cerebral vessels of rats is also an endothelial NO-mediated response.
Figure 6.
Change in diameter as a percentage of passive diameter (PD) of cerebral arterioles in response to intraluminal (Intra) and extraluminal (Extra) administration of aldosterone at concentration of 10−7M. L-NAME (3 × 10−4M) was used to inhibit NO synthesis. The response curve of “Intra” is significantly different (p<0.05) from the curve of “Extra” or “Intra + L-NAME”, by two-way ANOVA (n=6 per group).
Impaired aldosterone-induced dilation in endothelial dysfunction
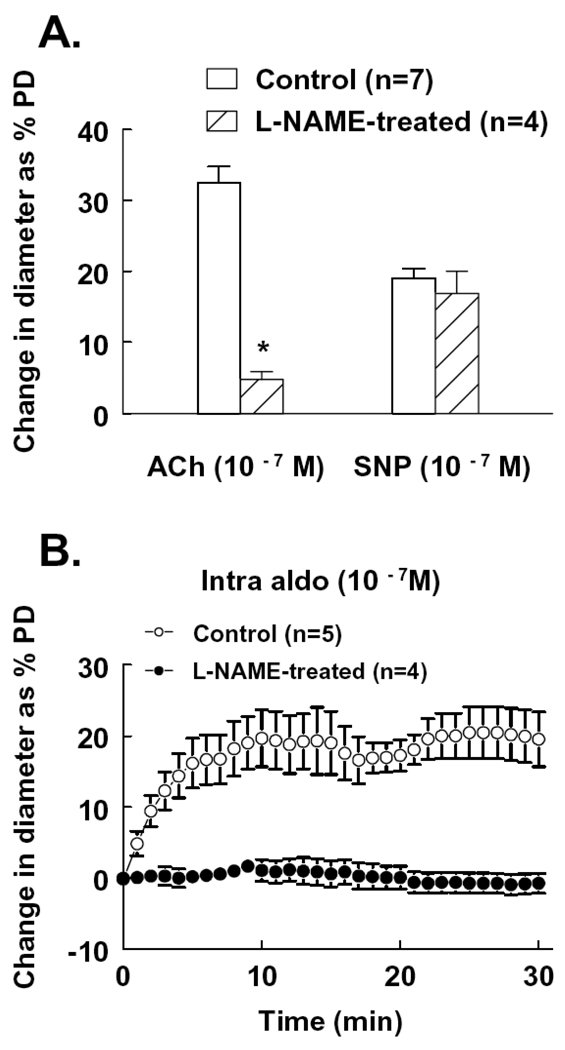
NO synthesis was inhibited by administration of L-NAME in the drinking water to rats for four weeks. Mesenteric arterioles were then isolated to assess endothelial function and intraluminal aldosterone-induced dilation. The basal tone of the vessels, developed under 80 mmHg of intravascular pressure, was not different from control rats. Figure 7A shows that ACh-induced dilation was significantly reduced while SNP-induced dilation was not affected, confirming the inhibition of NO synthesis and maintained smooth muscle function in these vessels . In the presence of this endothelial dysfunction, intraluminal administration of aldosterone (10−7M) failed to initiate a significant vasodilator response (Figure 7B).
Figure 7.
Change in diameter of mesenteric arterioles to acetylcholine (ACh) and sodium nitroprusside (SNP) (A) and intraluminal administration of aldosterone (B) in control rats and rats treated with L-NAME in the drinking water for four weeks. * p<0.05 from control.
Discussion
In this study, we have demonstrated vasodilatation of mesenteric and cerebral arterioles of rats in response to various concentrations of aldosterone and have characterized the effects of intraluminal and extraluminal administrations of aldosterone on these vessels. Acute administration of aldosterone to endothelium-intact mesenteric and cerebral arterioles, using either intraluminal or extraluminal administration, elicited an endothelium-dependent and nitric oxide-mediated dilation. In endothelium-denuded vessels, aldosterone elicited a vasoconstrictor response. Compared to extraluminal administration, intraluminal administration of aldosterone elicits a greater and sustained dilation. The reduced extraluminal aldosterone-induced dilation is a consequence of a direct stimulation of smooth muscle and an increased superoxide production, which decreases NO bioavailability. We have also demonstrated that endothelial dysfunction impairs aldosterone-induced dilation.
The rapid onset and sustained endothelium-dependent dilation to intraluminal administration of aldosterone likely represent the physiological role of aldosterone in thecirculation. Using an intraluminal delivery technique, we can clearly depict this action, distinguishing it from flow-induced dilation. Intraluminal administration of aldosterone only elicits an endothelium-dependent and NO-mediated dilation, as demonstrated in Figure 5A and 5B. The time course of aldosterone-induced dilation found in our study is consistent with studies 7 of the vasodilator action of aldosterone on rabbit renal afferent arterioles. The effect was potent, already manifest at a picomolar range which is well within the physiological concentration of aldosterone in blood. Moreover, we also treated arterioles with relatively high concentrations of aldosterone, as high as 10−7M, a level that has been reported in individuals with congestive heart failure 15. With this concentration, the acute effect of intraluminal administration of aldosterone was still a vasodilator response (Figure 1B and Figure 5).
When aldosterone was administered extraluminally, the smooth muscle cell-dependent response became more prominent. The dilation that was significantly smaller than that induced by intraluminal aldosterone diminished within 10 minutes and remained so as the vessels were exposed continuously to aldosterone for 30 minutes (Figure 1). However, the dilation was still an endothelium-dependent and NO-mediated response (Figure 2B and 2C). In endothelium-denuded vessels, both intraluminal (Figure 5B) and extraluminal (Figure 2B) aldosterone caused vasoconstrictor responses which in 5–10 minutes reached a plateau. The time course of this response was similar to the vasoconstrictor effect of aldosterone in the afferent and efferent arterioles of rabbit kidney 2 and in the superior mesenteric artery of rats 16. These results suggest that the direct effect of aldosterone on vascular smooth muscle is a vasoconstrictor response.
In recent years, it has become clear that one of the important pathways by which aldosterone exerts its harmful effects on the cardiovascular system is through the production of reactive oxygen species. Several studies reported that treatment of cultured human umbilical vein endothelial cells or vascular smooth muscle cells with aldosterone results in an enhanced generation of reactive oxygen species through activation of NADPH oxidase, mainly via enhanced transcription of the subunit p47phox and its translocation to membrane sites containing the NOX subunits 17 or via c-Src 18. In the presence of high aldosterone levels, the increased formation of reactive oxygen species can lead to an uncoupling of eNOS, thereby further reducing NO synthesis and enhancing radical oxygen species 19. Our results show that in resistance vessels incubated with Tempol, a superoxide scavenger, extraluminal aldosterone caused a greater and sustained vasodilatation than that observed without Tempol (Figure 3). Furthermore, Figure 4 shows that inhibition of NADPH oxidase with apocynin increased extraluminal aldosterone (10−7M)-induced dilation via a NO-dependent mechanism, suggesting that extraluminal aldosterone stimulates superoxide formation which reduces NO bioavailability, hence, reduces dilation. However, owing to the fact that we performed functional studies, we were not able to measure superoxide production directly in vascular endothelium or smooth muscle cells. It should also be noted that the specificity of apocynin as an NADPH oxidase inhibitor has been challenged by recent studies 20. Nevertheless, our studies identify the increase in superoxide production as an important factor in the reduction of NO bioavailability to account for the responses to aldosterone. Also, in Tempol-treated vessels, the overall dilatation to aldosterone was still lower than after intraluminal application and the antioxidant effect was not apparent until after 10 minutes. Other mechanisms besides aldosterone-induced smooth muscle activation and superoxide formation may be responsible for this occurrence.
Together, our data clearly indicate that the overall vascular effect of aldosterone depends on its concentration. Thus physiological concentrations will elicit a vasodilator response or provide for an inhibition of vasoconstrictor responses via a mechanism of enhanced endothelial NO formation; whereas supraphysiologic concentrations may have opposite effects 7,8. We propose that the detrimental vascular effects of aldosterone are counterbalanced by activation of endothelial NO synthesis. Especially, in the presence of endothelial dysfunction, as the results show in Figure 7, the effects of pathologic concentrations of aldosterone are unopposed, resulting in a reduced vasodilatation or even a vasoconstriction. Our results also indicate that aldosterone-induced vascular responses depend on the route of the administration. Intraluminal administration of aldosterone to isolated arterioles mimics an increase of plasma aldosterone in vivo and the response is a potent NO-mediated dilation; whereas extraluminal administration of aldosterone results in a response greatly different from that observed after intraluminal aldosterone. Therefore, extraluminal administration of aldosterone might produce a response that may be misleading as to the physiological role of aldosterone.
Compared to extraluminal administration of aldosterone, in endothelium-intact vessels, intraluminal aldosterone induced a potent and sustained dilation (Figure 1), which resulted mainly from the direct exposure of the endothelium to aldosterone and a greater NO-mediated dilation (Figure 5A). Furthermore, in endothelium-intact vessels, L-NAME only blocked intraluminal aldosterone-induced dilation without causing vasoconstriction; whereas in endothelium-denuded vessels, intraluminal aldosterone induced a sustained vasoconstriction (Figure 5B). These data further indicate that the integrity of vascular endothelium and maintained endothelial function prevents the vasoconstriction and allows mainly a vasodilator response to an elevated plasma aldosterone. The phenomenon of aldosterone-induced vasodilatation and vasoconstriction is reminiscent of the pattern of responses to acetylcholine in endothelium-intact versus denuded vessels. Liu et al. also found an increase in NO-synthase activity within minutes after administration of a physiological concentration aldosterone to bovine aortic endothelial cells, suggesting that this is a nongenomically mediated effect 8.
The ability of spironolactone to inhibit the vascular effects of aldosterone suggests that the vasodilation is due to binding of aldosterone to the mineralocorticoid receptor (Figure 2A). Our results are consistent with previous studies of others 7,8,21. However, this is in contrast to the effects observed by yet other investigators, showing that the rapid effects of aldosterone were not blocked by spironolactone treatment 2,11. Those studies in which the nongenomic effects of aldosterone were not inhibitable were presumed to be associated with the PKC/Ca2+ pathway in vascular smooth muscle.
In summary, we have demonstrated aldosterone-induced endothelial NO-dependent dilation in mesenteric and cerebral arterioles of rats. The rapid vascular effects of aldosterone reflect a balance between endothelial and vascular smooth muscle actions, as well as vasodilator and vasoconstrictor mechanisms, similar to those observed to the administration of several agonists, such as acetylcholine and endothelin, that act on multiple targets in blood vessels. These rapid effects of aldosterone may be beneficial or detrimental, depending on the integrity of endothelial cells. Thus, with endothelial dysfunction, the vasodilative effect of aldosterone may be reduced, resulting in a vasoconstriction and enhanced peripheral resistance.
Acknowledgments
The work reported here was supported by NIH grants HL43023 and HL070653
Footnotes
Disclosures
We have no conflicts to disclose.
Reference List
- 1.Schiffrin EL. Effects of aldosterone on the vasculature. Hypertension. 2006;47:312–318. doi: 10.1161/01.HYP.0000201443.63240.a7. [DOI] [PubMed] [Google Scholar]
- 2.Arima S, Kohagura K, Xu HL, Sugawara A, Abe T, Satoh F, Takeuchi K, Ito S. Nongenomic vascular action of aldosterone in the glomerular microcirculation. J Am Soc Nephrol. 2003;14:2255–2263. doi: 10.1097/01.asn.0000083982.74108.54. [DOI] [PubMed] [Google Scholar]
- 3.Fujita M, Minamino T, Asanuma H, Sanada S, Hirata A, Wakeno M, Myoishi M, Okuda H, Ogai A, Okada K, Tsukamoto O, Koyama H, Hori M, Kitakaze M. Aldosterone nongenomically worsens ischemia via protein kinase C-dependent pathways in hypoperfused canine hearts. Hypertension. 2005;46:113–117. doi: 10.1161/01.HYP.0000171184.84077.80. [DOI] [PubMed] [Google Scholar]
- 4.Kushibiki M, Yamada M, Oikawa K, Tomita H, Osanai T, Okumura K. Aldosterone causes vasoconstriction in coronary arterioles of rats via angiotensin II type-1 receptor: influence of hypertension. Eur J Pharmacol. 2007;572:182–188. doi: 10.1016/j.ejphar.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Romagni P, Rossi F, Guerrini L, Quirini C, Santiemma V. Aldosterone induces contraction of the resistance arteries in man. Atherosclerosis. 2003;166:345–349. doi: 10.1016/s0021-9150(02)00363-5. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt BM, Oehmer S, Delles C, Bratke R, Schneider MP, Klingbeil A, Fleischmann EH, Schmieder RE. Rapid nongenomic effects of aldosterone on human forearm vasculature. Hypertension. 2003;42:156–160. doi: 10.1161/01.HYP.0000083298.23119.16. [DOI] [PubMed] [Google Scholar]
- 7.Uhrenholt TR, Schjerning J, Hansen PB, Norregaard R, Jensen BL, Sorensen GL, Skott O. Rapid inhibition of vasoconstriction in renal afferent arterioles by aldosterone. Circ Res. 2003;93:1258–1266. doi: 10.1161/01.RES.0000106135.02935.E1. [DOI] [PubMed] [Google Scholar]
- 8.Liu SL, Schmuck S, Chorazcyzewski JZ, Gros R, Feldman RD. Aldosterone regulates vascular reactivity: short-term effects mediated by phosphatidylinositol 3-kinase-dependent nitric oxide synthase activation. Circulation. 2003;108:2400–2406. doi: 10.1161/01.CIR.0000093188.53554.44. [DOI] [PubMed] [Google Scholar]
- 9.Arima S, Kohagura K, Xu HL, Sugawara A, Uruno A, Satoh F, Takeuchi K, Ito S. Endothelium-derived nitric oxide modulates vascular action of aldosterone in renal arteriole. Hypertension. 2004;43:352–357. doi: 10.1161/01.HYP.0000111138.78714.1a. [DOI] [PubMed] [Google Scholar]
- 10.Christ M, Douwes K, Eisen C, Bechtner G, Theisen K, Wehling M. Rapid effects of aldosterone on sodium transport in vascular smooth muscle cells. Hypertension. 1995;25:117–123. doi: 10.1161/01.hyp.25.1.117. [DOI] [PubMed] [Google Scholar]
- 11.Wehling M, Neylon CB, Fullerton M, Bobik A, Funder JW. Nongenomic effects of aldosterone on intracellular Ca2+ in vascular smooth muscle cells. Circ Res. 1995;76:973–979. doi: 10.1161/01.res.76.6.973. [DOI] [PubMed] [Google Scholar]
- 12.Stier CT, Jr, Serova LI, Singh G, Sabban EL. Stress triggered rise in plasma aldosterone is lessened by chronic nicotine infusion. Eur J Pharmacol. 2004;495:167–170. doi: 10.1016/j.ejphar.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 13.Sun D, Huang A, Koller A, Kaley G. Endothelial K(ca) channels mediate flow-dependent dilation of arterioles of skeletal muscle and mesentery. Microvasc Res. 2001;61:179–186. doi: 10.1006/mvre.2000.2291. [DOI] [PubMed] [Google Scholar]
- 14.Sun D, Huang A, Smith CJ, Stackpole CJ, Connetta JA, Shesely EG, Koller A, Kaley G. Enhanced release of prostaglandins contributes to flow-induced arteriolar dilation in eNOS knockout mice. Circ Res. 1999;85:288–293. doi: 10.1161/01.res.85.3.288. [DOI] [PubMed] [Google Scholar]
- 15.Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345:1689–1697. doi: 10.1056/NEJMra000050. [DOI] [PubMed] [Google Scholar]
- 16.Michea L, Delpiano AM, Hitschfeld C, Lobos L, Lavandero S, Marusic ET. Eplerenone blocks nongenomic effects of aldosterone on the Na+/H+ exchanger, intracellular Ca2+ levels, and vasoconstriction in mesenteric resistance vessels. Endocrinology. 2005;146:973–980. doi: 10.1210/en.2004-1130. [DOI] [PubMed] [Google Scholar]
- 17.Nagata D, Takahashi M, Sawai K, Tagami T, Usui T, Shimatsu A, Hirata Y, Naruse M. Molecular mechanism of the inhibitory effect of aldosterone on endothelial NO synthase activity. Hypertension. 2006;48:165–171. doi: 10.1161/01.HYP.0000226054.53527.bb. [DOI] [PubMed] [Google Scholar]
- 18.Callera GE, Touyz RM, Tostes RC, Yogi A, He Y, Malkinson S, Schiffrin EL. ZAldosterone activates vascular p38MAP kinase and NADPH oxidase via c-Src. Hypertension. 2005;45:773–779. doi: 10.1161/01.HYP.0000154365.30593.d3. [DOI] [PubMed] [Google Scholar]
- 19.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 21.Gros R, Ding Q, Armstrong S, O'Neil C, Pickering JG, Feldman RD. Rapid effects of aldosterone on clonal human vascular smooth muscle cells. Am J Physiol Cell Physiol. 2007;292:C788–C794. doi: 10.1152/ajpcell.00407.2006. [DOI] [PubMed] [Google Scholar]