Abstract
Background
Rat lung allograft rejection is mediated by collagen type V (col(V)) specific Th17 cells. Adoptive transfer of these cells is sufficient to induce rejection pathology in isografts, whereas tolerance to col(V) suppresses allograft rejection. We therefore tested if regulatory T cells from tolerant rats could suppress the Th17 mediated rejection in the syngeneic model of lung transplantation.
Methods
Rats were subjected to syngeneic left lung transplantation and acute rejection was induced by adoptive transfer of lymph node cells from col(V)-immunized rats. Tolerance was induced by intravenous (iv) injection of col(V) and spleen lymphocytes were used for adoptive transfer. CD4+ T cells were depleted using magnetic beads. Lung isografts were analyzed using micro-PET imaging and histochemistry. The transvivo delayed type hypersensitivity (TV-DTH) assay was used to analyze the Th17 response.
Results
Adoptive co-transfer of col(V)-specific effector cells with cells from col(V) tolerized rats suppressed severe vasculitis and bronchiolitis with parenchymal inflammation, and the expression of IL-17 transcripts in mediastinal lymph nodes induced by effector cells alone. Analysis by TV-DTH showed that the reactivity to col(V) was dependent on the presence of TNF-α and IL-17, but not IFN-γ. Depletion of CD4+ T cells from the suppressor cell population abrogated the col(V)-specific protection.
Conclusion
Th17 mediated acute rejection after lung transplantation is ameliorated by CD4+ col(V)-specific regulatory T cells. The mechanism for this Th17 suppression is consistent with tolerance induction to col(V). The goal of transplantation treatment therefore should target Th17 development and not suppression of T cell activation by suppressing IL-2.
Keywords: lung transplantation, animal model, IL-17, autoimmunity, adoptive transfer
Introduction
Studies in the rat lung model for bronchiolitis obliterans (BO) have demonstrated a central role for collagen type V (col(V)). Transfer of col(V)-specific T cells into rats after syngeneic lung transplantation led to acute rejection and ultimately “Bronchiolitis Obliterans Syndrome (BOS)-like” injury and airway obliteration (1,2). Similarly, autoimmunity to col(V) is a major risk factor in the development of BOS after lung transplantation in humans (2). In contrast, the induction of tolerance in rats by inducing anergy or regulation to this antigen, prevented rejection in a model of orthotopic lung allo-transplantation (3).
Col(V) is a minor collagen but abundant in skin, placenta and lung and essential for tissue elasticity and compliance (4,5). This antigen is cryptogenic in normal collagen fibril structure and thus not normally recognized by the immune system (4). Little is known about the structure of collagen fibrils in the repaired matrix of the lung. An exuberant repair after diffuse lung injury, as in transplanted lung allografts, may result in excess col(V) α1-chain production. This has been observed in ischemic heart injury models, where production of several matrix proteins including col(V) α1 and col(V) α2 were markedly upregulated (6). Dysregulated synthesis of col(V) can lead to formation of col(V) α1 homotrimers that are not concealed in the molecular structure of the growing collagen fibril leading to exposure long after the transplant allograft has recovered from the post implantation response.(7).
Col(V)-specific T cells appear in human and rat lung transplant recipients. This occurs prior to the clinical onset and pathological manifestation of BOS. Adoptive transfer of col(V) reactive T cells induce rejection in transplanted rat lung allografts or isografts (2,8). In experimental models, oral administration of col(V) prior to lung transplantation, prevents acute rejection and the onset of BO in rat allografts. Preliminary analysis of the mechanism of col(V)-induced transplant tolerance suggests TGF-β-dependent immune regulation, mediated by allo- and auto-antigen-specific Tregs (3).
In contrast to Tregs protecting lung transplants from BO, rejection is mediated by IL-17 expressing col(V)-specific T effector cells (1,2). IL-17 is a potent proinflammatory cytokine mainly produced by CD4+ T cells in humans, termed Th17, that are distinct and develop independent from Th1 and Th2 subpopulations (9–11). The differentiation and maturation of Th17 cells is regulated by several cytokines with TGF-β and IL-6 being the differentiation factors, IL-21 the amplification factor and IL-23 the stabilization factor resulting in an expanded Th17 population producing IL-17A, IL-17F, and IL-22 (12). IL-17 plays both protective and pathogenic roles in the immune system, and it is important for host defense against various infectious organisms (13–15). Development of rejection-like pathology after adoptive transfer of col(V)-specific effector lymphocytes is characterized by the presence of IL-17 producing T cells (1), and IL-17 expression is associated with acute rejection and BO in clinical transplantation (2). IL-17 is a proinflammatory cytokine that is significantly upregulated in several diseases and in allograft rejection and induces the expression of a variety of inflammatory mediators (16).
Induction of tolerance can be achieved by oral, intranasal, subcutaneous, or intravenous application of the antigen. Intravenous administration of autoantigen is an effective method to induce Ag-specific tolerance against experimental autoimmune encephalomyelitis (EAE) by inhibiting classical macrophage activation and APC function and increasing alternative macrophage activation (17,18). Oral tolerance induces regulatory T cells which function as an interplay between mucosal immunity in the gut and colonic dendritic cells that present the antigen in a tolerogenic manner to T cells (19). Orally induced tolerance to col(V) down-regulates acute rejection, inhibits the development of BO in rats, and is mediated by CD4+/CD45RChi T cells that have regulatory function (20). The spleen has a critical role in the generation of induced Tregs (iTregs) and T cells migrate to regional lymphoid tissues and the spleen after iv injection. The appearance of regulatory T cells in the spleen has been described as a function of various routes of antigen application including oral, sublingual, intraocular, and intravenous (21–24); this observation suggests that induction of tolerance is not specific neither to the antigen or the route of administration (25).
Here we used intravenous induced tolerance to col(V) to investigate the mechanisms of rejection and tolerance after syngeneic rat lung transplantation and adoptive transfer of col(V)-specific cells. We tested the hypothesis that T cells derived from intravenous, non-inflammatory response to col(V) (tolerant T cells) are able to suppress the acute rejection mediated by col(V)-specific lymphocytes (sensitized T cells) derived from an inflammatory response (1).
Methods
Animals
All experiments were carried out under an approved animal protocol with F344 and WKY rats weighing 300–350 g (Harlan, Indianapolis, IN). The animals were housed with 2–3 animals per cage that were maintained in environmentally controlled rooms at 22°C and 50–55% humidity, with a 12/12h light/dark cycle. All rats were fed a standard laboratory diet (NIH-07 Rat and Mouse Chow) and were provided water ad libitum. The animal resource services facilities are accredited by the American Association for the Accreditation of Laboratory Animal Care. Animals were euthanized with an overdose of inhaled isoflurane.
Lung Transplantation
The orthotopic transplantation of left lung was performed as previously reported using the cuff technique (26–28). Briefly: rats were anaesthetized by inhalation of a mixture of isoflurane and oxygen, intubated and ventilated with a mixture of isoflurane and oxygen to maintain anesthesia. The donor rat was placed in a supine position and the heart and lungs were removed en bloc. The left lung was resected and the pulmonary vein, bronchus, and pulmonary artery were passed through teflon cuffs and the proximal ends were everted over the cuffs. On the recipient rat a left thoracotomy was performed. The donor pulmonary vein, bronchus, and pulmonary artery were inserted into the corresponding recipient hilar structures, and fixed with separate circumferential ligatures of silk. The chest wall was closed, and the isoflurane was stopped.
Immunization of rats
Human col(V) was purified from placenta as reported (29). Two hundred microgram_human col(V) was injected intravenously into recipient Wistar Kyoto (WKY) rats two times in 1 ml saline 20 and 10 days prior to the transplantation. This dose has been used to induce tolerance to other proteins (30). A second group of rats was sensitized to col(V) or hen egg lysozyme (HEL) control antigen by injecting 100 μg col(V) or HEL in complete Freunds adjuvants (CFA) subcutaneously at the base of the tail and on the back between the front legs. Animals were boosted with 100 μg antigen in IFA 10 days later and draining lymph nodes were removed 10 days after that.
FDG-PET of rat lungs after transplantation
18F-fluorodeoxyglucose positron emission tomography ([F-18]FDG-PET) imaging was performed using a dedicated small animal scanner (UW Micropet P4, Concorde Microsystems, Knoxville, TN) at the Keck Laboratory for Functional Brain Imaging (UW-Madison, WI). An attenuation scan was performed prior to injection of the FDG. Imaging was performed beginning prior to injection of the tracer and data was collected over 90 min to detect late trapping of the glucose analogue. FDG injections of 0.5 – 0.9 mCi were delivered intravenously. Ordered subsets expectation maximization (OSEM) images from 45–90 min were used for registration. The appropriate attenuation correction data for the 2 and 4 animal time frames was applied for dynamic filtered back-projection images at 10 × 2 min and 8 × 10 min to a resolution of 0.63 × 0.63 × 1.23 mm. The time resolved filtered back-projection images were used to perform Patlak analysis using Spamalize software (Waisman Imaging Lab, Madison, WI). Arterial input function was determined graphically using an ROI placed in the liver to determine the tracer uptake wash-in (31).
In vivo delayed type hypersensitivity (DTH) assay
Animals were anesthetized by isoflurane inhalation. 25 μl saline with 100 μg col(V) was injected into the left ear of the animal. 25 μl saline without the antigen was injected into the right ear. Swelling of the ear was determined 24 hours later and values were compared to the thickness before injection.
Preparation of lymph node and spleen cells for adoptive transfer
Animals were sacrificed by isoflurane inhalation and pentobarbital injection. Draining mesenteric and axilar lymph nodes were removed from animals immunized with col(V) in CFA/IFA and the spleen was removed from animals that were injected with col(V) intravenously. Cell suspensions were prepared using tissue strainers from BD Bioscience (40 μm; San Jose, CA) and separated over Ficoll (MP Biomedicals, Inc., Solon, OH). Mononuclear cells were washed and injected intravenously at 1×107 lymph node cells and 4×107 spleen cells in a total of 1 ml saline. In some experiments, CD4+ T cells were depleted from the tolerant splenocytes using a MACs sorter and anti-CD4 beads prior to co-adoptive transfer with sensitized lymph node cells (LNCs).
Preparation of lung, lymph nodes and blood
After sacrifice blood was drawn from the abdominal vein into a heparinized tube. BAL was performed separately in the left transplanted lung and the right native lung. Lungs were then removed en bloc and fixed via tracheal infusion of a 1% paraformaldehyde solution at 30 cm H2O pressure overnight. Lungs were then embedded in paraffin and 5μm sections were stained with H&E and trichrome. The classification of pulmonary allograft rejection was done based on the recommendation of the lung rejection study group (32). Mediastinal lymph nodes and spleen were removed and cell suspensions were prepared using cell strainers from BD Bioscience (40 μm; San Jose, CA). Red blood cells were lysed with ammonium chloride.
Transvivo delayed type hypersensitivity assay (TV-DTH)
CB-17 SCID (severe combined immunodeficiency) mice were bred at the University of Wisconsin Gnotobiotics Laboratory facility. All animals were housed and treated in accordance with guidelines outlined by the University of Wisconsin and the National Institute of Health. To assess DTH reactivity, 8–10×106 LNCs from col(V) or HEL-sensitized rats were injected, along with 20 μg antigen, into the footpads of naive SCID mice in a total volume of 40 μL saline. To assess regulation, spleen cells from col(V) tolerant rats were co-injected at 2×106 cells. Antigen-driven swelling was measured after 24 hours using a dial thickness gauge. Post-injection measurements were compared with pre-injection measurements to obtain specific swelling. DTH reactivity is shown as the change in thickness, using units of 10−4 inches. In some assays, 10 μg antibodies to neutralize IL-17 (eBio64Dec17, eBioscience), IFN-γ (4S.B3, eBioscience), IL-1β (B122, eBioscience) or TNF-α (TN3-19.12, eBioscience) were co-injected. In some experiments, CD25+ cells from spleens of col(V) tolerant animals were purified using immunomagnetic beads (MACS Beads; Miltenyi Biotec) and co-injected with the effector LNCs at a ratio of 1:4 of Treg to TE.
Cytokine mRNA analysis
For the measurement of mRNA steady state levels in immune cells, 106 splenocytes or lymphocytes were lysed in Lysis buffer (Stratagene, La Jolla, CA) then stored at −80°C. Total RNA was extracted as described by the manufacturer. The synthesis of first strain cDNA was performed using the manufacturer’s protocol (Stratagene, La Jolla, CA). Briefly, total RNA (20 μl) was incubated with 1 μl of random hexamer primers (27 OD/μl; Invitrogen, Carlsbad, CA) for 10 minutes at 65°C. After 10 minutes incubation at room temperature, 5 μl 10x RT buffer, 2 μl of 10mM dNTP, and 1 μl Stratascript (50 U/μl) were added and incubated for 60 min at 42°C. 4 μl of the RT products were used for the real time PCR (qPCR). The primers were selected using the Primer3 software (Whitehead Institute for Biomedical Research, Cambridge, MA) and the iScript One-Step RT-PCR mix with SYBR Green from BioRad was used as recommended by the manufacturer using the following primer pairs: rat IL-17 primers: F: 5′-ACTTTCCGGGTGGAGAAGAT; R: 5′-TGGCGGACAATAGAGGAAAC. Rat IL-23 primers: F: 5′-ACACACACCAGTGGGACAAA; R: 5′-ACAACCATCACCACACTGGA. Rat FoxP3 primers: F: 5′ CAGGCTGATCCCTCTCTGTC; R: GTTGGGCATCAGGTTCTTGT. Rat Cyclophilin primers: F: 5′ GCGTCTCCTTCGAGCTGT; R: 5′ GGAACCCTTATAGCCAAATCC. For each set of primers, the efficiency of the PCR was determined after serial dilutions of the cDNA. The PCR efficiencies were > 90% and allowed the ΔΔCT calculations relative to cyclophilin in order to calculate the fold change (2−ΔΔCT) in mRNA steady state levels between the different groups.
Statistics
Quantitative data were expressed as the mean ± standard deviation (SD). To compare the data between different time points the Wilcoxon signed ranks test was used. Two-sided t-test p values were calculated, and p values of <0.05 were considered significant.
Results
Inhibition of co(V)-induced acute rejection pathology by splenocytes from col(V) tolerant mice
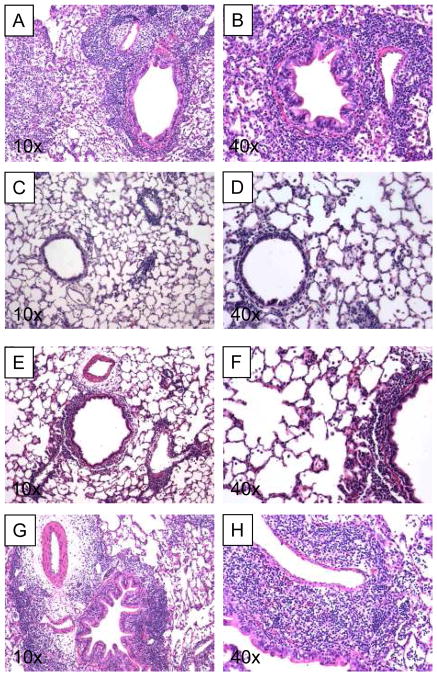
Adoptive transfer of 107 col(V)-specific sensitized LNCs led to extensive and dense mononuclear inflammation with perivascular and peribronchiolar cuffing in recipients of lung isografts. Alveolar parenchyma and spaces were densely infiltrated by the inflammatory process. Some small bronchioles showed concentric increase of fibrous tissue with mild occlusion of the airways. The inflammatory patterns resembled a grade A3.7±0.6/B4.0±0.0 (n=3) acute cellular rejection (Figure 1A and 1B). We then injected 4×107 spleen lymphocytes from a col(V) tolerant rat together with the 107 col(V)-specific sensitized LNCs one day prior to the syngeneic lung transplantation to determine if cells from col(V) tolerant mice could protect from col(V)-induced rejection pathology. One week after transplantation (Figure 1C/D) the lung architecture was well preserved and infiltration was limited to mild peribronchiolar and perivascular mononuclear cell inflammatory cuffing. Cellular inflammation did not infiltrate the alveolar parenchyma. Occasional interstitial lymphoid aggregates were seen. The pattern was consistent with minimal rejection-like pathology A0.7±0.6/B1.0±0.0. After two weeks the lung architecture was still preserved, with mild peribronchiolar and perivascular mononuclear cell inflammatory cuffing (Figure 1E/F). Cellular infiltrate minimally infiltrated the alveolar parenchyma forming interstitial lymphoid aggregates. The pattern was consistent with a mild rejection-like pathology A1.7±0.6/B1.0±1.0 (n=3; Figure 1E/F).
Figure 1. H & E staining of lung after left lung transplantation and adoptive transfer of col(V)-specific effector and regulator T cells.
A and B: 7 days after transfer of 1×107 effector cells into rats subjected to lung isograft transplantation. C and D: 7 days after adoptive transfer of 1×107 effector cells together with 4×107 regulator cells into rats subjected to lung isograft transplantation. E and F: 14 days after adoptive transfer of 1×107 effector cells together with 4×107 regulator cells into rats subjected to lung isograft transplantation. G and H: 7 days after adoptive transfer of 1×107 effector cells together with 4×107 CD4+-T-cell-depleted regulator cells. All slides are H&E stained 5 μm sections of paraformaldehyde fixed lungs.
Inflammation after lung transplantation was monitored by positron emission tomography (PET) scanning with the tracer (18F) fluorodeoxyglucose (FDG, FDG-PET). We found that rats that received sensitized LNCs showed a strong inflammation in the transplanted left lung covering the whole lung (Figure 2A). In contrast, the transplanted left lung of rats that received a combination of sensitized LNCs and tolerant splenocytes showed some residual inflammation near the incision, where often tissue adhesion occurs, but very low signals in the apical and caudal region (Figure 2B), suggesting that the anti-inflammatory action of the transferred tolerant splenocytes is able to suppress the col(V) specific immune response but not the inflammation caused by surgical incision. No inflammation is detected in healthy control lungs (Figure 2C).
Figure 2. Inflammatory response analyzed by PET 7 days after syngeneic transplanted lungs and adoptive transfer of col(V)-specific cells.
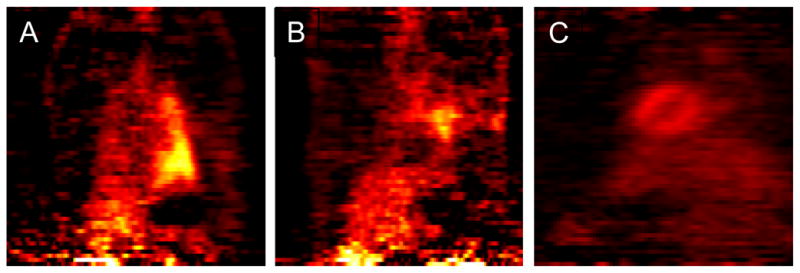
(A) Transfer of 107 effector cells (Te). (B) Transfer of 107 effector cells together with 4×107 regulatory cells (Te + Tr). (C) Uninflammed normal lung. No inflammatory signals are detected in normal lungs. No transplantation has been performed in this rat.
Since suppression of inflammation can be mediated by several cell types, we next analyzed if CD4+ T cells are responsible for the reduced response. Tolerant splenocytes were depleted of the CD4+ T cells with magnetic beads and adoptive transferred together with the sensitized effector LNCs into lung isograft recipients. The depletion of the CD4+ T cell population from the tolerized donor cells negated the protection, resulting in a dense mononuclear inflammatory cell infiltrate involving both alveolar parenchyma and peribronchiolar and perivascular areas, along with peribronchiolar and perivascular edema and young fibroblast proliferation (Figure 1G and 1H). Occasional polymorphonuclear cell and intra-alveolar monocytes/macrophages were seen. The pattern was consistent with a rejection-type histology grade A3.0±0.0/B3.3±0.6 (n=3). Since the transfer of effector cells leads to col(V) reactivity in the recipient animal (1), we measured the DTH response to col(V) by direct ear challenge. Animals that received sensitized lymph node cells (TE) showed a strong DTH reaction to col(V). Co-transfer of tolerant splenocytes blocked this direct DTH response completely. No DTH reactivity was seen to the control antigen HEL in animals that received col(V)-specific sensitized LNCs (Figure 3A). Together, these data show that anti-col(V)-specific response induced by the transfer of col(V)-reactive T cells can be suppressed by CD4+ splenocytes from col(V) tolerant rats.
Figure 3. Physiologic effects of adoptive transfer of col(V) specific effector and regulator cells.
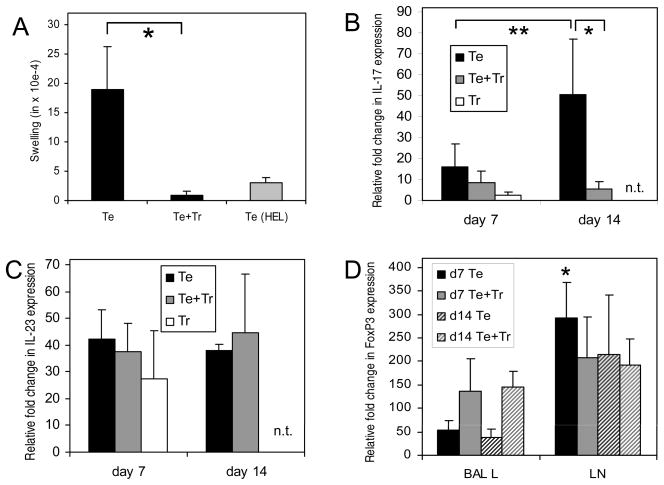
(A) Swelling of the rat ear 24 hours after injection of col(V) into the ear and adoptive transfer of Te or Te+Tr cells. HEL was used as a control antigen. (B, C) Expression of IL-17 (B) and IL-23 (C) in mediastinal lymph nodes 7 and 14 days after transplantation and adoptive transfer of LNCs and SPCs. qPCR for the cytokines IL-17 (B) and IL-23 (C). (D) FoxP3 expression in BAL and mediastinal lymph node cells from animals that obtained effector cells (Te) or effector cells (black solid and black hatched bars) and regulatory cells (Te + Tr) (gray solid and grey hatched bars). ΔΔCT with an external standard was calculated relative to cyclophilin and the fold change (2−ΔΔCT) in mRNA steady state levels between the different groups was calculated. * and ** indicates a significant difference between the data denoted (p<0.05). All values are shown as the mean of 3 to 5 independent experiments ± standard deviation.
Expression of IL-17 during acute rejection
Development of rejection-like pathology after adoptive transfer of col(V)-specific effector lymphocytes is characterized by the presence of IL-17 producing T cells (1). Further, IL-17 expression is associated with acute rejection and BO in clinical transplantation (2). We therefore utilized real time PCR to quantitate expression of IL-17 and IL-23 transcripts in the mediastinal lymph node lymphocytes. IL-23 provides the signals in the late development of Th17 cells. Developing Th17 cells upregulate the inducible component of the IL-23 receptor (IL-23R) downstream of RORγt to confer responsiveness to IL-23 (33). Lymph node cells from control rats that did not obtain a lung transplant or adoptive transferred cells showed no expression of IL-17 (data not shown). Rats that received sensitized LNCs alone showed significantly increased amounts of IL-17 mRNA between day 7 and day 14 post transplantation (p=0.03; Figure 3B). At day 7 rats that received effector and suppressor cells had significantly less IL-17 mRNA and showed no increase at day 14, at which time IL17 expression was significantly lower than in rats receiving effector cells only (P=0.04). Even less IL-17 mRNA was found at day 7 in rats that received suppressor cells only. In contrast to the concentration of IL-17 mRNA, IL-23 mRNA was expressed in all animals without a significant difference between the two time points and the treatment groups (Figure 3C).
Since Th17 and regulatory T cells are in a critical balance we analyzed the expression of FoxP3 in BAL lymphocytes and mediastinal lymph node cells by real time PCR. The expression of FoxP3 was elevated in BAL lymphocytes in the mouse receiving both sensitized and tolerant cell types (Figure 3D). At day 7 the mediastinal lymph node cells in rats that received sensitized LNCs showed significantly higher expression of FoxP3 than BAL cells at the same time point (black bars). No significant difference between BAL and mediastinal lymph node cells were evident although the expression of FoxP3 in the lymph nodes was generally higher compared to the BAL cells independent on the type of cells transferred. The expression of FoxP3 in BAL cells was increased after transfer of Te and Tr compared to Te alone at both time points whereas no such difference was observed in the draining lymph nodes (Figure 3D). These data suggest that FoxP3 expression in the lung reflects the immunosuppression achieved by the cotransfer of Te and Tr cells whereas in the lymph node no such correlation was seen.
IL-17 mediates the reactivity to col(V)
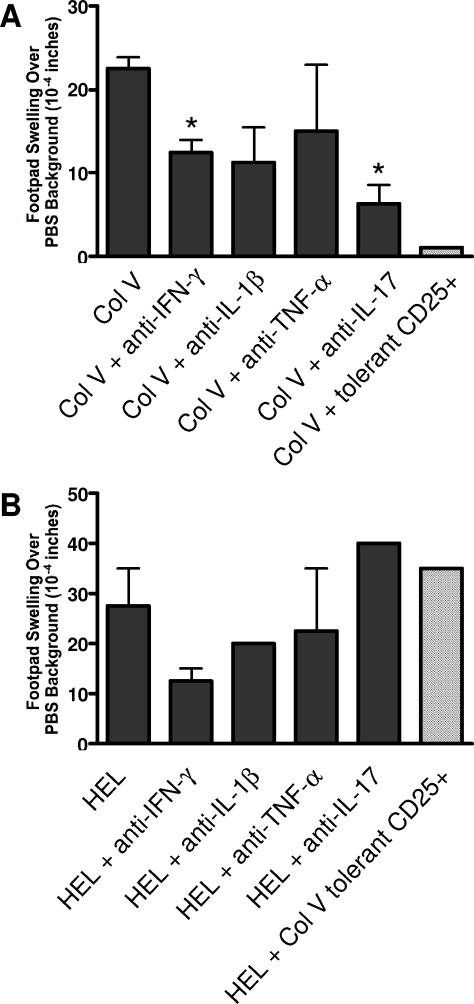
The presence of IL-17 transcripts in the mediastinal lymph nodes in inflamed lungs suggests that the response leading to chronic rejection is driven by Th17 cells. However, the co-injection of effector and suppressor cells in the transplant recipients reduced the amount of IL-17 message in the mediastinal lymph nodes. This suggests that the effector T cells are either functionally suppressed or are still functional but do not migrate to the lung. We tested the role of IL-17 in the response to col(V) by using the trans-vivo delayed-type hypersensitivity (TV-DTH) assay in SCID mice. Co-injection of col(V) sensitized rat LNCs with the antigen col(V) caused robust swelling of the footpad of recipient naive SCID mice (Figure 4A). This col(V) swelling response was markedly reduced in the presence of anti-IL17 and anti-IFN-γ antibodies [p<0.05]. The presence of anti-IL-1β or anti-TNF-α also reduced the response but the effect was highly variable (not significant). The TV-DTH response with HEL sensitized rat LNCs to the control antigen HEL in contrast was markedly inhibited by anti-IFN-γ and anti-IL-1β antibodies but not at all by anti-IL-17 (Figure 4B). By far the greatest level of inhibition of the col(V) DTH swelling response was achieved by adding sorted CD25+ spleen T cells from col(V) tolerized rats. This effect was quite antigen specific in that TV-DTH response to HEL was not inhibited (Figure 4B). These data show that the immune response induced by the transfer of col(V)-specific effector cells is dominated by IL-17 and in part by IFN-γ compared to the response to HEL that is dominated by IFN-γ. Moreover it shows that the inhibition achieved by col(V)-specific tolerance is antigen specific.
Figure 4. Th17- effector response to col(V) in Col(V) sensitized mice.
Inguinal lymph node cells were collected from rats immunized with col(V) (A) or control HEL (B) and were co-injected into CB17. SCID recipient footpads with PBS or sensitizing antigen. To determine the cytokines responsible for mounting a positive swelling response, cells were co-injected with sensitizing antigen and neutralizing antibodies directed against rat IFN-γ, IL-1β, TNF-α, or IL-17 and CD25 positive cells from col(V) tolerant animals. Results are shown as the 24-h net swelling response subtracting the PBS background net swelling.
Discussion
Cellular immunity to col(V) mediated by CD4+ T cells and monocytes has a detrimental role in the process of chronic rejection after lung transplantation in humans (2). Col(V)-specific IL17-dependent autoimmunity drives human BO/BOS and IL-17 is upregulated during acute rejection (2,34). Our data are consistent with the finding that adoptive transfer of col(V)-reactive lymphocytes express transcripts for IL-17 and induce grade 2 rejection in rat lung isografts if transferred at the time of transplantation. The pathology is more severe (grade 3) when transferred to isograft recipients 30 days post-transplantation (1). We showed that intravenous injection of col(V) generated a CD4+ T cell population that was able to suppress the rejection induced by adoptive transfer of IL-17 expressing col(V)-specific TE cells. The co-transfer of splenocytes from rats tolerized to col(V) reduced the expression of IL-17 at day 7, and completely blocked it 2 weeks post transplant.
Clonal deletion, anergy of Ag-specificT cells and induction of Th2 or Treg cells are the main mechanismsknown to be involved in the induction of intravenous tolerance (35). The intravenous injection of col(V) induces tolerance that can be transferred and is associated with CD4+ T cells. We did not characterize the regulatory function of these cells but we observed an increase in FoxP3 expression in the transplanted lung after transfer of regulatory cells. If these cells develop de novo or are the transferred cells will be addressed in future work.
Extended expression of IL-17 is strongly associated with the development of autoimmunity and IL-17 is expressed in target tissues of patients with various autoimmune diseases (10), including experimental autoimmune encephalitis (EAE) and collagen-induced arthritis (CIA). In these diseases, blocking IL-17 production alleviates the pathology (36,37). The model of adoptive transfer devised by Yoshida et al (1) allowed us to confirm, that increased IL-17 mRNA is associated with acute rejection. The increase in IL-17 indicates a key role for the Th17 effector cell subset in BOS (38). The increase in IL-17 is in line with the finding in BOS of increased numbers of neutrophils and of IL-8, a potent neutrophil-attracting chemokine that is potently induced by IL-17. The goal of transplantation treatment therefore should target Th17 development and not suppression of T cell activation by suppressing IL-2. This in view that blockade of IL-2 signaling is unable to prevent rejection in which Th17 cells are likely to play a crucial role.
Activation and the development of Th17 cells may be inhibited at several levels by inhibiting cytokines and signal transduction pathways. Th1 and Th2 cytokines IFN-γ and IL-4 suppress the development of Th17 cells (9) as does IL-27 (39). Blocking of IFN-γ partially reduced the response to col(V) in the TV-DTH assay. This differs from data in human lung transplant recipients where no influence of anti-IFN-γ antibodies on the DTH swelling reaction was seen in association of a significant reduction by anti-IL-1β, anti-TNF-α, and anti-IL-17 antibodies (40). The decrease of the col(V)-specific response in the presence of anti-IFN-γ antibodies may indicate the presence of a Th1 mediated response in parallel to the Th17 reaction (Figure 4). However, the production of IL-17 does not exclude the production of IFN-γ and both cytokines can be produced by the same cell. In our system it is possible that IFN-γ is produced by already differentiated Th17 cells. Similarly, production of IL-4 together with IFN-γ has been observed. Therefore, the inhibition by anti-IL-17 and anti-IFN-γ may inhibit the same cells (41–43). In our experiments we injected activated and differentiated effector cells that were inhibited by the regulatory cells. It is not clear how this inhibition happens. Antigen specific T cells inhibit by acting on antigen presenting dendritic cells (44–47). Adoptive transfer of regulatory T cells that suppress lung rejection in our model is not always able to suppress the targeted disease. Korn et al. showed that in experimental autoimmune encephalomyelitis (EAE), Tregs were unable to abrogate IL-17 production by effector T cells that produced high amounts of IL-6 and TNF-α (48). This finding is compatible with the enhanced expression of IL-17 since in the presence of IL-6 and the expression of TGF-β by Tregs is sufficient to induce Th17 development or even differentiation of Tregs into Th17 that still express FoxP3 (49). The presence of regulatory T cells characterized by the expression of FoxP3 was found in a mouse model of acutely rejecting mouse islet allografts and in human renal allograft patients where the expression of FoxP3 was highest in the graft (50,51). However, the relationship between Tregs and Th17 cells is not a simple reciprocal one. There seems to be some plasticity in both the Treg and the Th17 population (33,52,53). These findings have significant implications for the possible use of transfer of ex vivo generated Tregs in the induction of transplant tolerance. A controlled immune response depends on the balanced presence of effector cells and Tregs. This also supports the hypothesis that the chronic immune response directed at an allograft is neither monotonic nor exclusively proinflammatory. In contrast, the immune response during an episode of acute rejection includes the generation of both graft-destructive T cells and graft-protective Treg cells (51). The balance between effector cells (i.e. col(V)-sensitized lymph node cells) and regulator cells (col(V)-specific spleen cells) determines the fate of the transplanted lung (54).
Maintaining the balance between effecter and regulator cells has specific clinical implications: col(V)-specific regulatory T cells specifically suppress the Th17 response to the autoantigen col(V). The presence of a col(V)-directed Th17 response post-transplant in humans is a high risk factor to develop BOS. Potentially, this can be inhibited by inducing a regulatory response to col(V). Since BOS is the major adverse event for lung transplant recipients this may offer a successful treatment of this disease. The understanding of the role of col(V) in the development of acute rejection may furthermore provide a base for a therapy to prevent acute rejection after lung transplantation and the development of chronic rejection. Targeting lung transplant candidateswith col(V)-based tolerance induction strategies before transplantation may reduce the risk and intensity of acute and chronic rejection. Such strategies should be implemented together with adaptations in immunosuppression to enhance Treg development.
Acknowledgments
DSW is supported by grants HL60797 and HL/Al67177 from the National Institutes of Health
Abbreviations
- BO
bronchiolitis obliterans
- BOS
Bronchiolitis Obliterans Syndrome
- cDNA
complementary deoxyribonucleic acid
- CFA
complete Freund’s adjuvant
- col(V)
collagen type V
- EAE
experimental autoimmune encephalomyelitis
- [F-18]FDG-PET
F-18 fluorodeoxyglucose positron emission tomography
- IFN-γ
interferon-γ
- IL-17
interleukin-17
- LNCs
lymph node cells
- mRNA
messenger ribonucleic acid
- OSEM
ordered subsets expectation maximization
- PET
Positron emission tomography
- RT-PCR
reverse transcription polymerase chain reaction
- SCID
severe combined immunodeficiency
- SPC
spleen cell
- TNF- α
tumor necrosis factor-α
- TV-DTH
trans-vivo delayed type hypersensitivity
- TGF-β
transforming growth factor-β
- WKY
Wistar Kyoto
- TR or Treg
regulatory T cell
- TE
effector T cell
- iTreg
induced regulatory T cell
- HEL
hen egg lysozyme
Reference List
- 1.Yoshida S, Haque A, Mizobuchi T, et al. Anti-Type V Collagen Lymphocytes that Express IL-17 and IL-23 Induce Rejection Pathology in Fresh and Well-Healed Lung Transplants. Am J Transplant. 2006;6:724–735. doi: 10.1111/j.1600-6143.2006.01236.x. [DOI] [PubMed] [Google Scholar]
- 2.Burlingham WJ, Love RB, Jankowska-Gan E, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yasufuku K, Heidler KM, Woods KA, et al. Prevention of bronchiolitis obliterans in rat lung allografts by type V collagen-induced oral tolerance. Transplantation. 2002;73:500–505. doi: 10.1097/00007890-200202270-00002. [DOI] [PubMed] [Google Scholar]
- 4.Linsenmayer TF, Gibney E, Igoe F, et al. Type V collagen: molecular structure and fibrillar organization of the chicken alpha 1(V) NH2-terminal domain, a putative regulator of corneal fibrillogenesis. J Cell Biol. 1993;121:1181–1189. doi: 10.1083/jcb.121.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwarze U, Atkinson M, Hoffman GG, Greenspan DS, Byers PH. Null alleles of the COL5A1 gene of type V collagen are a cause of the classical forms of Ehlers-Danlos syndrome (types I and II) Am J Hum Genet. 2000;66:1757–1765. doi: 10.1086/302933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalasani G, Li Q, Konieczny BT, et al. The allograft defines the type of rejection (acute versus chronic) in the face of an established effector immune response. J Immunol. 2004;172:7813–7820. doi: 10.4049/jimmunol.172.12.7813. [DOI] [PubMed] [Google Scholar]
- 7.Chanut-Delalande H, Bonod-Bidaud C, Cogne S, et al. Development of a functional skin matrix requires deposition of collagen V heterotrimers. Mol Cell Biol. 2004;24:6049–6057. doi: 10.1128/MCB.24.13.6049-6057.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haque MA, Mizobuchi T, Yasufuku K, et al. Evidence for immune responses to a self-antigen in lung transplantation: role of type V collagen-specific T cells in the pathogenesis of lung allograft rejection. J Immunol. 2002;169:1542–1549. doi: 10.4049/jimmunol.169.3.1542. [DOI] [PubMed] [Google Scholar]
- 9.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 11.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 12.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly MN, Kolls JK, Happel K, et al. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect Immun. 2005;73:617–621. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye P, Garvey PB, Zhang P, et al. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 15.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71:1–8. [PubMed] [Google Scholar]
- 17.Zhang GX, Xu H, Kishi M, Calida D, Rostami A. The role of IL-12 in the induction of intravenous tolerance in experimental autoimmune encephalomyelitis. J Immunol. 2002;168:2501–2507. doi: 10.4049/jimmunol.168.5.2501. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Ciric B, Yang J, et al. Intravenous tolerance modulates macrophage classical activation and antigen presentation in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2009;208:54–60. doi: 10.1016/j.jneuroim.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizobuchi T, Yasufuku K, Zheng Y, et al. Differential expression of Smad7 transcripts identifies the CD4(+)CD45RC(high) regulatory T cells that mediate type V collagen-induced tolerance to lung allografts. J Immunol. 2003;171:1140–1147. doi: 10.4049/jimmunol.171.3.1140. [DOI] [PubMed] [Google Scholar]
- 21.Thorstenson KM, Khoruts A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J Immunol. 2001;167:188–195. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- 22.Sun JB, Cuburu N, Blomquist M, Li BL, Czerkinsky C, Holmgren J. Sublingual tolerance induction with antigen conjugated to cholera toxin B subunit induces Foxp3+CD25+CD4+ regulatory T cells and suppresses delayed-type hypersensitivity reactions. Scand J Immunol. 2006;64:251–259. doi: 10.1111/j.1365-3083.2006.01823.x. [DOI] [PubMed] [Google Scholar]
- 23.Seidel-Guyenot W, Perschon S, Dechant N, Alt R, Knop J, Steinbrink K. Low zone tolerance induced by systemic application of allergens inhibits Tc1-mediated skin inflammation. J Allergy Clin Immunol. 2006;117:1170–1177. doi: 10.1016/j.jaci.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Keino H, Takeuchi M, Kezuka T, et al. Induction of eye-derived tolerance does not depend on naturally occurring CD4+CD25+ T regulatory cells. Invest Ophthalmol Vis Sci. 2006;47:1047–1055. doi: 10.1167/iovs.05-0110. [DOI] [PubMed] [Google Scholar]
- 25.Kamphorst AO, da Silva MF, da Silva AC, Carvalho CR, Faria AM. Genetic selection for resistance or susceptibility to oral tolerance to ovalbumin affects general mechanisms of tolerance induction in mice. Ann N Y Acad Sci. 2004;1029:350–354. doi: 10.1196/annals.1309.018. [DOI] [PubMed] [Google Scholar]
- 26.Mizuta T, Nakahara K, Shirakura R, et al. Total nonmicrosuture technique for rat lung transplantation. J Thorac Cardiovasc Surg. 1991;102:159–160. [PubMed] [Google Scholar]
- 27.Mizuta T, Kawaguchi A, Nakahara K, Kawashima Y. Simplified rat lung transplantation using a cuff technique. J Thorac Cardiovasc Surg. 1989;97:578–581. [PubMed] [Google Scholar]
- 28.Mizobuchi T, Sekine Y, Yasufuku K, Fujisawa T, Wilkes DS. Comparison of surgical procedures for vascular and airway anastomoses that utilize a modified non-suture external cuff technique for experimental lung transplantation in rats. J Heart Lung Transplant. 2004;23:889–893. doi: 10.1016/j.healun.2003.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Yasufuku K, Heidler KM, O’Donnell PW, et al. Oral tolerance induction by type V collagen downregulates lung allograft rejection. Am J Respir Cell Mol Biol. 2001;25:26–34. doi: 10.1165/ajrcmb.25.1.4431. [DOI] [PubMed] [Google Scholar]
- 30.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 31.Green LA, Gambhir SS, Srinivasan A, et al. Noninvasive methods for quantitating blood time-activity curves from mouse PET images obtained with fluorine-18-fluorodeoxyglucose. J Nucl Med. 1998;39:729–734. [PubMed] [Google Scholar]
- 32.Yousem SA, Berry GJ, Cagle PT, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15:1–15. [PubMed] [Google Scholar]
- 33.Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009 doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 34.Vanaudenaerde BM, Dupont LJ, Wuyts WA, et al. The role of interleukin-17 during acute rejection after lung transplantation. Eur Respir J. 2006;27:779–787. doi: 10.1183/09031936.06.00019405. [DOI] [PubMed] [Google Scholar]
- 35.Hilliard BA, Kamoun M, Ventura E, Rostami A. Mechanisms of suppression of experimental autoimmune encephalomyelitis by intravenous administration of myelin basic protein: role of regulatory spleen cells. Exp Mol Pathol. 2000;68:29–37. doi: 10.1006/exmp.1999.2290. [DOI] [PubMed] [Google Scholar]
- 36.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koenders MI, Kolls JK, Oppers-Walgreen B, et al. Interleukin-17 receptor deficiency results in impaired synovial expression of interleukin-1 and matrix metalloproteinases 3, 9, and 13 and prevents cartilage destruction during chronic reactivated streptococcal cell wall-induced arthritis. Arthritis Rheum. 2005;52:3239–3247. doi: 10.1002/art.21342. [DOI] [PubMed] [Google Scholar]
- 38.Vanaudenaerde BM, De Vleeschauwer SI, Vos R, et al. The role of the IL23/IL17 axis in bronchiolitis obliterans syndrome after lung transplantation. Am J Transplant. 2008;8:1911–1920. doi: 10.1111/j.1600-6143.2008.02321.x. [DOI] [PubMed] [Google Scholar]
- 39.Stumhofer JS, Hunter CA. Advances in understanding the anti-inflammatory properties of IL-27. Immunol Lett. 2008;117:123–130. doi: 10.1016/j.imlet.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bobadilla JL, Love RB, Jankowska-Gan E, et al. TH-17, Monokines, Collagen Type V, and Primary Graft Dysfunction in Lung Transplantation. Am J Respir Crit Care Med. 2008;177:660–668. doi: 10.1164/rccm.200612-1901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mills KH. Induction, function and regulation of IL-17-producing T cells. Eur J Immunol. 2008;38:2636–2649. doi: 10.1002/eji.200838535. [DOI] [PubMed] [Google Scholar]
- 42.Messi M, Giacchetto I, Nagata K, Lanzavecchia A, Natoli G, Sallusto F. Memory and flexibility of cytokine gene expression as separable properties of human T(H)1 and T(H)2 lymphocytes. Nat Immunol. 2003;4:78–86. doi: 10.1038/ni872. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Lee YS, Yu CR, Egwuagu CE. Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J Immunol. 2008;180:6070–6076. doi: 10.4049/jimmunol.180.9.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DiPaolo RJ, Brinster C, Davidson TS, Andersson J, Glass D, Shevach EM. Autoantigen-specific TGFbeta-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J Immunol. 2007;179:4685–4693. doi: 10.4049/jimmunol.179.7.4685. [DOI] [PubMed] [Google Scholar]
- 45.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Veldhoen M, Moncrieffe H, Hocking RJ, Atkins CJ, Stockinger B. Modulation of dendritic cell function by naive and regulatory CD4+ T cells. J Immunol. 2006;176:6202–6210. doi: 10.4049/jimmunol.176.10.6202. [DOI] [PubMed] [Google Scholar]
- 47.Stummvoll GH, DiPaolo RJ, Huter EN, et al. Th1, Th2, and Th17 effector T cell-induced autoimmune gastritis differs in pathological pattern and in susceptibility to suppression by regulatory T cells. J Immunol. 2008;181:1908–1916. doi: 10.4049/jimmunol.181.3.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korn T, Reddy J, Gao W, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 50.Muthukumar T, Dadhania D, Ding R, et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 2005;353:2342–2351. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 51.Yang H, Ding R, Sharma VK, et al. Hyperexpression of Foxp3 and IDO during acute rejection of islet allografts. Transplantation. 2007;83:1643–1647. doi: 10.1097/01.tp.0000263991.74052.46. [DOI] [PubMed] [Google Scholar]
- 52.Mitchell P, Afzali B, Lombardi G, Lechler RI. The T helper 17-regulatory T cell axis in transplant rejection and tolerance. Curr Opin Organ Transplant. 2009 doi: 10.1097/MOT.0b013e32832ce88e. [DOI] [PubMed] [Google Scholar]
- 53.Beriou G, Costantino CM, Ashley CW, et al. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–4249. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng XX, Sanchez-Fueyo A, Sho M, Domenig C, Sayegh MH, Strom TB. Favorably tipping the balance between cytopathic and regulatory T cells to create transplantation tolerance. Immunity. 2003;19:503–514. doi: 10.1016/s1074-7613(03)00259-0. [DOI] [PubMed] [Google Scholar]