Abstract
Introduction
Solid tumors, such as neuroblastoma (NB), are associated with a heterogeneous cell environment. Multicellular tumor spheroid (MCTS) cultures have been shown to better mimic growth characteristics of in vivo solid tumors. Because tumor spheroid growth patterns may be quite different from standard two-dimensional culture systems, we sought to compare the protein expression profiles of two- and three-dimensional in vitro NB cultures, i.e., monolayers and MCTS.
Materials and methods
Human NB cells were grown as both monolayers and spheres. Nuclear and cytosolic proteins were analyzed for differentially secreted proteins by two-dimensional polyacrylamide gel electrophoresis (2-D PAGE) and selected polypeptides were identified by mass spectrometry (LC-MS/MS).
Results
Several metabolic (transketolase, triosephosphate isomerase, pyruvate kinase M1/M2, alpha enolase, and phosphoglycerate mutase-1), cell stress response (heat shock proteins (HSP) 90, 70, and 60; antioxidant, thioredoxin), cell structure (septin 2, adenyl cyclase-associated protein-1), tubulin β-2 chain, actin, translationally controlled tumor protein and cofilin), signal transduction (peptidyl prolyl cis/trans isomerase A), biosynthetic (phosphoserine aminotransferase) and transport (cellular retinoic acid binding protein 1) polypeptides were over-expressed in spheroids. Several protein groups were differentially expressed between NB monolayers and spheroids.
Conclusion
The altered proteins among NB spheroids may represent an important link between monolayer cell cultures and in vivo experiments and thus a more ideal in vitro culture system for determining the precise three-dimensional microenvironment of NB.
Keywords: Neuroblastoma, Monolayers, 3-D Culture, Multicellular spheroid, Proteomics, Tumor microenvironment
Introduction
Neuroblastoma (NB) is a solid tumor characterized by heterogeneous cell subpopulations and microenvironments. These microregions of tumor diversity contain varying levels of hypoxia, decreased pH, and nutrient deprivation which may contribute to the development of chemoresistance and thus, tumor aggressiveness or reoccurrence [1]. For example, it has been shown that solid tumors contain significant areas of chronic or transient hypoxia [2]. Evidence suggests that hypoxia may have a profound impact on malignant progression and on responsiveness to therapy [3–5]. For these reasons, accurate tumor microenvironment is important to consider when evaluating experimental in vitro NB models.
Monolayer or two-dimensional (2D) cell culture methods are a traditional tissue culture model that provides a well-defined environment to study NB biology. This widely used analytical technique has increased the understanding of many aspects NB cell proliferation including immunology, metastasis, apoptosis, and gene expression. Despite these advances, monolayer culture methods do not adequately represent the three-dimensional (3D) in vivo physiological behavior of NB. Moreover, because optimal treatment for NB depends on the comprehension of the interaction between tumor cell growth and microenvironment, utilizing cell culture models that best recapitulate the biochemical and morphological features of corresponding in vivo tumors is required to improve patient mortality. Thus, multicellular tumor spheroids (MCTS) have been developed in an attempt to recreate a more suitable in vitro model to investigate the 3D arrangement of solid tumors [6, 7]. As such, the use of MCTS serve as an important intermediate adjunct to monolayer cultures and animal models and have been used in a variety of therapeutic studies including radiotherapy, chemotherapy, radioimmunotherapy, immunotherapy, hyperthermia, gene therapy, and photodynamic treatment [8–15].
MCTS show characteristics commonly associated with in vivo solid tumors. Sutherland et al. initially demonstrated strong similarities between cancer spheres and solid tumors. For example, growth patterns of tumor spheres reflect an outer rim of proliferating cells, an inner area of quiescent/differentiating cells, and a region of necrotic cells in the center [16, 17]. Additionally, tumor sphere models resemble micrometastases during the avascular phase of their development [18]. These functional properties make spheroids a powerful model to investigate the intercellular relationships in solid tumors. As a consequence, sphere culture models have been described for a variety of tumor types, including NB [19–26].
Because of the distinct structural differences between monolayers and spheres, it is logical to consider the functional differences between these two models. Reports indicate that loss of tumor antigen expression occurs when cancer cells are grown as monolayers and the re-expression of these antigens when the cells are grown as spheres [27]. A similar feature has been described with regard to multidrug and radiation resistance on cancer cell behavior. Tumor cell resistance to chemotherapeutics or irradiation was lost in the conventional 2D culture technique, but was re-established when cells were grown as MCTS [28–30]. These contrasting functional observations further underscore the importance of 3D tumor cell culture for investigating tumorigenic events in preclinical oncology.
To date, NB 3D culture studies have not addressed the biologic differences responsible for the functional properties between monolayers and spheres. In order to gain insight into these growth differences, we analyzed and compared the protein expression profiles of NB cultured in traditional 2D conditions and 3D multicellular spheroids. The identification of proteins differentially expressed between these two distinct cell culture methods may contribute to the understanding of the complex microenvironment contributing to NB pathogenesis in vivo.
Materials and methods
NB 2D and 3D cell culture
Human NB cell lines SK-N-AS, SK-N-DZ, and IMR-32 were initially grown as monolayers in Dulbecco’s modified Eagle’s medium with 4 mM L-glutamine adjusted to contain 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, and10% fetal bovine serum at 37°C in 5% CO2. Cells were transitioned into MCTS in plastic culture flasks (T75) following coating with a 50 μg/mL poly-(2-hydroxyethyl methacrylate, polyHEMA, Sigma, St Louis, MO) solution, preventing cell binding, as described by Folkman and Moscona [31]. NB MCTS were propagated in growth media consisting of DMEM/HAMS F12 (3:1), penicillin G, streptomycin sulfate, amphotericin B (1:100; Gibco), B27 (1:50; Gibco), human recombinant FGF-2 and EGF (both at 20 ng/mL; R&D Systems) and heparin (5 μg/mL; Sigma). Spheres approximately ≥500 μm (most spheroids from established cell lines develop a necrotic core with a surrounding viable rim of cells at this critical size) were expanded by an automated sectioning technique as described by Svendsen et al. [32]. Additionally, all 3 NB cell lines were grown as monolayers in the same culture medium as described for spheroids. Both NB monolayer cells and MCTS were harvested at 80–90% confluence (monolayers), or when spheres reached ≥500 μm, washed twice with PBS, pelleted by low-speed centrifugation, flash frozen in liquid nitrogen, and stored at −80°C until ready for cell fractionation and protein extraction.
Cell fractionation and protein extraction
In order to reduce protein sample complexity, a combined enriched nuclear and cytosolic protein fraction (NE/S3) was utilized to evaluate differential protein expression between NB monolayers and MCTS. Cell pellets from both 2D and 3D culture methods were homogenized and a NE/S3 protein fraction was isolated and stored according to Sandoval et al. [33].
Two-dimensional polyacrylamide gel electrophoresis (2D-PAGE)
Protein, measuring 500 μg, was rehydrated into 11 cm pH 3–10 ReadyStrips (Bio-Rad, Hercules, CA) for 12 h at 20°C in a PROTEAN IEF Cell (Bio-Rad) prior to focusing. Focusing was performed at 8,000 V for 30,000 h at 20°C. Reduction and alkylation were accomplished by following the manufacturer’s instructions (Bio-Rad) and the second dimension 8–16% PAGE was accomplished using a Criterion cell (Bio-Rad).
Staining, imaging, and analysis
After SDS-PAGE, the gels were fixed in 50% methanol (v/v) and 5% acetic acid (v/v) for 1 h and subsequently stained overnight with Colloidal Coomassie Blue (CCB, GelCode Blue Stain Reagent) according to the manufacturer’s instruction (Pierce, Rockford, IL). Imaging was accomplished using a GS-710 Calibrated Imaging Densitometer (Bio-Rad), and analysis performed using Phoretix 2D Evolution (Nonlinear Dynamics, Inc, Durham, NC).
In-gel trypsin digestion and mass spectrometry (MS)
Differentially expressed protein spots were digested and identified by liquid chromatography linked tandem mass spectrometry (LC-MS/MS) as previously described by Sandoval et al. [34].
Results
Two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) combined with MS is the most widely used technique for proteomics. Briefly, complex protein mixtures such as whole cell lysates are first separated by their charge in the IEF step and then separated by their molecular size using a polyacrylamide gel. Following 2D-gel electrophoresis, Coomassie brilliant blue stain is used to visualize proteins and create a 2D map for each sample. The 2D maps demonstrate intact proteins, which reflect changes in protein expression level, isoforms, or post-translational modifications. Analysis of these 2D protein maps between samples relies on computer-assisted programs that are based on distinguishing quantitative tests at three different levels of standard 2-DE analysis: spot detection, gel matching and spot quantitation. Differentially expressed spots from these analyses are excised, proteins are subjected to in-gel digestion, and the resulting peptide fragments are identified by MS.
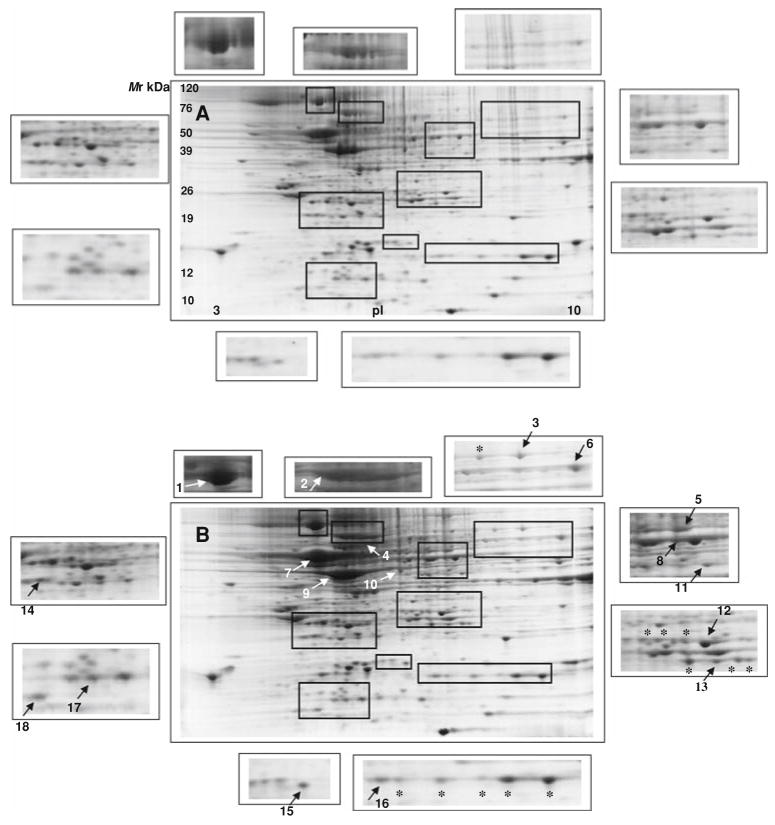
In our study, the proteomes from NB cells grown in culture as monolayers or spheres were compared to identify a global protein response between differing in vitro conditions. Because of the inherent protein differences in each NB cell line tested, each cell line was separated in triplicate and the spots common to monolayers were analyzed against common proteins derived from spheres using computer-assisted image evaluation. We concentrated on polypeptides that were upregulated in spheres relative to monolayers at least 2.5-fold (P ≤ 0.05). Figure 1 shows representative 2D gels of solubilized protein extracts from NB grown as either monolayers (Fig. 1a) or MCTS (Fig. 1b). Of the spots observed between both culture systems, little change is appreciated. In total, 18 spots were detected to be differentially over expressed in NB spheres. The identified protein spots were categorized by functional class. These include metabolism (glycolytic proteins; transketolase, triosephosphate isomerase, pyruvate kinase M1/M2, alpha enolase, and phosphoglycerate mutase-1), cell stress response (heat shock proteins (HSP) 90, 70, and 60; antioxidant, thioredoxin), cell organization/structure (septin 2, adenyl cyclase-associated protein-1 (adenyl CAP-1), tubulin β-2 chain, actin, translationally controlled tumor protein and cofilin), signal transduction (peptidyl prolyl cis/trans isomerase A (Pin1), biosynthetic (phosphoserine aminotransferase) and transport (cellular retinoic acid binding protein 1). Table 1 summarizes identified protein data from mass spectrometry and database search for NB monolayers and spheres. Collectively, the unique proteins identified between monolayers and MCTS have varied functional roles that support tumor progression, protect against intracellular stress, promote structural integrity, and facilitate cell signaling.
Fig. 1.
Global comparative 2-D PAGE patterns of neuroblastoma cell lines (SK-N-DZ, SK-N-AS and IMR-32) isolated from monolayer (a) or spheroid (b) cultures. Eighteen intracellular protein spots were identified by peptide mass fingerprinting and are listed in Table 1. Magnified regions show differentially expressed protein spots between monolayers and spheres. Arrows (numbers 1–18) indicate relative over expressed proteins (>2.5-fold). Asterisk denotes multiple protein isomers
Table 1.
Identification results of proteins differentially expressed between neuroblastoma monolayer and sphere cell lines
| Number | Protein | Swissprot accession number | Molecular weight (kDa) | Isoelectric point (pI) | Functional association |
|---|---|---|---|---|---|
| 1 | HSP 90-β | P08238 | 83.1 | 4.97 | Cell stress induced |
| 2 | HSC 71 | P11142 | 70.8 | 5.37 | Cell stress induced |
| 3 | Transketolase | P29401 | 67.8 | 7.58 | Hexose monophosphate pathway |
| 4 | HSP 60 | P10809 | 61.0 | 5.24 | Cell stress induced |
| 5 | Pyruvate kinase, isozymes M1/M2 | P14618 | 57.7 | 7.95 | Glycolysis |
| 6 | Adenyl CAP-1 | Q01518 | 51.5 | 8.12 | Actin monomer binding protein |
| 7 | Tubulin β-2 chain | P68371 | 49.8 | 4.79 | Microtubule component |
| 8 | Alpha enolase | P60712 | 47.0 | 6.99 | Glycolysis |
| 9 | Actin, cytoplasmic 1 | P60712 | 41.7 | 5.29 | Cell motility |
| 10 | Septin 2 | Q15019 | 40.4 | 6.15 | Cytoskelton organization |
| 11 | PSAT | Q9Y617 | 40.3 | 7.56 | De novo serine biosynthesis |
| 12 | PGAM1 | P18669 | 28.7 | 6.75 | Glycolysis |
| 13 | TPI | P60174 | 26.5 | 6.51 | Glycolysis |
| 14 | TCTP | P13693 | 19.5 | 4.84 | Microtubule/calcium binding |
| 15 | Cofilin | P23528 | 18.3 | 8.26 | Actin polymerization/depolymerization |
| 16 | Pin-1 | P62937 | 17.9 | 7.82 | Signal transduction |
| 17 | CRABP1 | P29762 | 15.4 | 5.31 | Cytoplasmic transport |
| 18 | Thioredoxin | P10599 | 11.6 | 4.82 | Antioxidant |
HSP heat shock protein, Adenyl CAP-1 adenyl cyclase-associated protein 1, PSAT phosphoserine aminotransferase, PGAM1 phosphoglycerate mutase 1, TPI triosephosphate isomerase, TCTP translationally controlled tumor protein, Pin-1 peptidyl prolyl cis-trans isomerase A, CRAB1 cellular retinoic acid binding protein 1
Discussion
Treatment strategies for advanced stage NB have not succeeded in significantly extending patient survival. This failure likely originates from the lack of information on NB physiology, attributable to the difficulties in developing relevant preclinical models that accurately reflect NB heterogeneity and the interactions of the tumor-microenvironment. To overcome these limitations in the tissue culture setting, tridimensional MCTS have become a well established model which recapitulates histological and architectural organization comparable to tissue. Indeed, 3D NB spheroids have been used extensively in the studies of experimental radiobiology and radioimmunotherapy. Because intercellular interactions between cancer cells act as an important microenvironmental control during cancer development, the focus of this study was on comparing the proteomic differences between traditional monolayer cell culture and MCTS. Defining intercellular proteins that regulate neuroblastomagenesis in vitro may provide key information for the development of novel treatment strategies that target functional tumor formation and growth.
We show several protein groups differentially expressed between NB monolayers and spheroids. These protein classes have important roles in glycolysis (transketolase, triosephosphate isomerase, pyruvate kinase M1/M2, alpha enolase, and phosphoglycerate mutase-1), cell stress (heat shock proteins (HSPs) 90, 70, 60, and antioxidant thioredoxin), cell structure (septin 2, adenyl CAP-1, tubulin β-2 chain, actin, translationally controlled tumor protein and cofilin), signal transduction [peptidyl prolyl cis/trans isomerase A (Pin1)], biosynthetic (phosphoserine amino-transferase) and transport (cellular retinoic acid binding protein 1). Evaluating the changes in protein expression between spheres and monolayers, we gain an understanding of the role these polypeptides play with regard to contributing to the NB microenvironment. For instance, the observation of increased glycolytic proteins in spheres versus monolayer NB cultured cells is consistent with the well characterized metabolic property of malignant cells known as the glycolytic phenotype. Increased glycolysis is a known phenomenon of invasive cancer cells in part, due to the development of intratumor areas of hypoxia and on the reliance on anaerobic pathways for glucose metabolism even in the presence of oxygen (Warburg effect) [35]. Our data support the view that sphere-generated NB cells more closely mirror the altered in vivo glucose activity of human cancer as we show the upregulation of glycolytic proteins in NB spheres.
Correlating our data to the relative over abundance of HSPs 90, 70, and 60 in NB spheres as compared to monolayers, we gain further insight into the functional significance of tumor spheres. The HSP family of molecules was initially shown to be induced by environmental and pathophysiologic stress and is implicated in protein–protein interactions, such as folding, translocation, and prevention of inappropriate protein aggregation. Additionally, HSPs have been identified to be overexpressed in many malignancies and have been suggested to be contributing factors in tumorigenesis. Studies have confirmed that increased expression of HSPs support the various roles involved in establishing the malignant phenotype; these include inhibiting programmed cell death, sustaining angiogenesis, and promoting chemoresistance [36]. The over abundance of HSPs in NB spheres facilitates the notion that the tumor microenvironment is better accounted for in vitro through the adaptation of cancer sphere culture methods.
Cytoskeletal components, such as actin filaments and microtubules, play an important role in integrating and propagating signaling circuits and dynamic morphological alterations in normal and malignant cells. It is not surprising that a host of cytoskeletal proteins (adenyl CAP-1, tubulin β-2, actin, translationally controlled tumor protein and cofilin) were observed to be increased in NB MCTS over monolayer cells. We further reason that these differences are indicative of the reorganization and distribution of cytoskeletal elements required for the formation of MCTS. Other upregulated proteins identified in NB spheres such as phosphoserine aminotransferase, thioredoxin, retinoic acid binding protein and peptidyl prolyl cis/trans isomerase A (Pin1) all have anti-apoptotic, neural cell growth promoting effects [37–40], and to this effect, may aid in modulating the intracellular microenvironment in NB MCTS to realistically mimic in vivo tumors.
Taken altogether, the proteomic data in this report reveal a dynamic alteration between protein profiles in NB tumor cells grown as traditional 2D culture and 3D spheres. The differential proteins identified denote NB spheroids more likely recapitulate the salient aspects of in vivo neoplasm growth and better reflect solid tumor physiology. Underscoring these findings is the observation that maintenance of 3D cultures of some cancers is required to retain their tumorigenic properties [41]. Translating our findings into clinical application, the utilization of MCTS provides an attractive model to assess molecular events associated with NB pathogenesis. This proteomic approach establishes the basis for understanding NB MCTS and constitutes a starting point for further work on uncovering innovative routes for therapeutic and diagnostic impact against advanced stage disease. Future studies characterizing NB spheroids include investigation of global oncogene expression, assessment of migration and invasion potential, and examining in vivo growth in nude mice to explore the invasive behavior of these 3D tumor cells.
Acknowledgments
We thank Dr. Clive Svendsen and Kyle Wallace (University of Wisconsin, Madison, WI) for site training and instruction in sphere cell culture and propagation methodologies. The authors would finally like to thank the A.N.N.A. foundation (India-napolis, IN) for their continuous support of our research.
References
- 1.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 2.Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955;9:539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teicher BA. Hypoxia and drug resistance. Cancer Metastasis Rev. 1994;13:139–168. doi: 10.1007/BF00689633. [DOI] [PubMed] [Google Scholar]
- 4.Subarsky P, Hill RP. The hypoxic tumour microenvironment and metastatic progression. Clin Exp Metastasis. 2003;20:237–250. doi: 10.1023/A:1022939318102. [DOI] [PubMed] [Google Scholar]
- 5.Hockel M, et al. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 6.Sutherland RM, McCredie JA, Inch WR. Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J Natl Cancer Inst. 1971;46:113–120. [PubMed] [Google Scholar]
- 7.Sutherland RM. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 1988;240:177–184. doi: 10.1126/science.2451290. [DOI] [PubMed] [Google Scholar]
- 8.Genc M, et al. Enhancement of effects of irradiation by gemcitabine in a glioblastoma cell line and cell line spheroids. J Cancer Res Clin Oncol. 2004;130:45–51. doi: 10.1007/s00432-003-0506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xing H, et al. Effect of the cyclin-dependent kinases inhibitor p27 on resistance of ovarian cancer multicellular spheroids to anticancer chemotherapy. J Cancer Res Clin Oncol. 2005;131:511–519. doi: 10.1007/s00432-005-0677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDevitt MR, et al. An alpha-particle emitting antibody ([213Bi]J591) for radioimmunotherapy of prostate cancer. Cancer Res. 2000;60:6095–6100. [PubMed] [Google Scholar]
- 11.Senekowitsch-Schmidtke R. Binding of EGF peptide and EGF receptor antibodies and its fragments in different tumor models. Hybridoma. 1999;18:29–35. doi: 10.1089/hyb.1999.18.29. [DOI] [PubMed] [Google Scholar]
- 12.Fullerton NE, et al. Application of targeted radiotherapy/gene therapy to bladder cancer cell lines. Eur Urol. 2005;47:250–256. doi: 10.1016/j.eururo.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Boyd M, et al. Transfectant mosaic spheroids: a new model for evaluation of tumour cell killing in targeted radiotherapy and experimental gene therapy. J Gene Med. 2002;4:567–576. doi: 10.1002/jgm.293. [DOI] [PubMed] [Google Scholar]
- 14.Sutherland R, Macfarlane W. Cytotoxicity of radiosensitizers in multicell spheroids: combination treatment with hyperthermia. Br J Cancer Suppl. 1978;37:168–172. [PMC free article] [PubMed] [Google Scholar]
- 15.Dubessy C, et al. Spheroids in radiobiology and photodynamic therapy. Crit Rev Oncol Hematol. 2000;36:179–192. doi: 10.1016/S1040-8428(00)00085-8. [DOI] [PubMed] [Google Scholar]
- 16.Freyer JP, Sutherland RM. Selective dissociation and characterization of cells from different regions of multicell tumor spheroids. Cancer Res. 1980;40:3956–3965. [PubMed] [Google Scholar]
- 17.Sutherland RM, Durand RE. Growth and cellular characteristics of multicell spheroids. Recent Results Cancer Res. 1984;95:24–49. doi: 10.1007/978-3-642-82340-4_2. [DOI] [PubMed] [Google Scholar]
- 18.Knuchel R, et al. Interactions between bladder tumor cells as tumor spheroids from the cell line J82 and human endothelial cells in vitro. J Urol. 1988;139:640–645. doi: 10.1016/s0022-5347(17)42550-x. [DOI] [PubMed] [Google Scholar]
- 19.Sminia P, et al. Oxygenation and response to irradiation of organotypic multicellular spheroids of human glioma. Anticancer Res. 2003;23:1461–1466. [PubMed] [Google Scholar]
- 20.McLoughlin P, et al. Transcriptional responses to epigal-locatechin-3 gallate in HT 29 colon carcinoma spheroids. Genes Cells. 2004;9:661–669. doi: 10.1111/j.1356-9597.2004.00754.x. [DOI] [PubMed] [Google Scholar]
- 21.Rofstad EK, et al. Apoptosis, energy metabolism, and fraction of radiobiologically hypoxic cells: a study of human melanoma multicellular spheroids. Int J Radiat Biol. 1996;70:241–249. doi: 10.1080/095530096144978. [DOI] [PubMed] [Google Scholar]
- 22.Griffon-Etienne G, Merlin JL, Marchal C. Evaluation of Taxol in head and neck squamous carcinoma multicellular tumor spheroids. Anticancer Drugs. 1997;8:48–55. doi: 10.1097/00001813-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Dunkern TR, Mueller-Klieser W. Quantification of apoptosis induction by doxorubicin in three types of human mammary carcinoma spheroids. Anticancer Res. 1999;19:3141–3146. [PubMed] [Google Scholar]
- 24.Minard KR, Guo X, Wind RA. Quantitative 1H MRI and MRS microscopy of individual V79 lung tumor spheroids. J Magn Reson. 1998;133:368–373. doi: 10.1006/jmre.1998.1493. [DOI] [PubMed] [Google Scholar]
- 25.Cunningham S, et al. A gene therapy approach to enhance the targeted radiotherapy of neuroblastoma. Med Pediatr Oncol. 2000;35:708–711. doi: 10.1002/1096-911X(20001201)35. 6<708:: AID-MPO49>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 26.Weber W, Weber J, Senekowitsch-Schmidtke R. Therapeutic effect of m-[131I]-and m-[125I]iodobenzylguanidine on neuroblastoma multicellular tumor spheroids of different sizes. Cancer Res. 1996;56:5428–5434. [PubMed] [Google Scholar]
- 27.Horan Hand P, et al. Influence of spatial configuration of carcinoma cell populations on the expression of a tumor-associated glycoprotein. Cancer Res. 1985;45:833–840. [PubMed] [Google Scholar]
- 28.Desoize B, Jardillier J. Multicellular resistance: a paradigm for clinical resistance? Crit Rev Oncol Hematol. 2000;36:193–207. doi: 10.1016/S1040-8428(00)00086-X. [DOI] [PubMed] [Google Scholar]
- 29.Mueller-Klieser W. Tumor biology and experimental therapeutics. Crit Rev Oncol Hematol. 2000;36:123–139. doi: 10.1016/S1040-8428(00)00082-2. [DOI] [PubMed] [Google Scholar]
- 30.Mueller-Klieser W. Three-dimensional cell cultures: from molecular mechanisms to clinical applications. Am J Physiol. 1997;273:1109–1123. doi: 10.1152/ajpcell.1997.273.4.C1109. [DOI] [PubMed] [Google Scholar]
- 31.Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 32.Svendsen CN, et al. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods. 1998;85:141–152. doi: 10.1016/S0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 33.Sandoval JA, Hickey RJ, Malkas LH. Isolation and characterization of a DNA synthesome from a neuroblastoma cell line. J Pediatr Surg. 2005;40:1070–1077. doi: 10.1016/j.jpedsurg.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 34.Sandoval JA et al. Novel peptides secreted from human neuroblastoma: useful clinical tools? J Pediatr Surg. 2006;41:245–251. doi: 10.1016/j.jpedsurg.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 35.Warburg O. The metabolism of tumours. Constable Press; London: 1930. [Google Scholar]
- 36.Calderwood SK, et al. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Snell K. Enzymes of serine metabolism in normal and neoplastic rat tissues. Biochim Biophys Acta. 1985;843:276–281. doi: 10.1016/0304-4165(85)90149-7. [DOI] [PubMed] [Google Scholar]
- 38.Backman E, et al. Thioredoxin, produced by stromal cells retrieved from the lymph node microenvironment, rescues chronic lymphocytic leukemia cells from apoptosis in vitro. Haematologica. 2007;92:1495–1504. doi: 10.3324/haematol.11448. [DOI] [PubMed] [Google Scholar]
- 39.Lu KP, et al. Targeting carcinogenesis: a role for the prolyl isomerase Pin1? Mol Carcinog. 2006;45:397–402. doi: 10.1002/mc.20216. [DOI] [PubMed] [Google Scholar]
- 40.Sani BP, et al. Retinoic acid binding protein in experimental and human colon tumor. Cancer. 1980;190:60–61. doi: 10.1002/1097-0142(19800315)45:5+<1199::aid-cncr2820451326>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 41.Friedrich J, Ebner R, Kunz-Schughart LA. Experimental anti-tumor therapy in 3-D: spheroids-old hat or new challenge? Int J Radiat Biol. 2007;83:849–871. doi: 10.1080/09553000701727531. [DOI] [PubMed] [Google Scholar]