Abstract
Rmi1 is a conserved OB-fold protein that is associated with RecQ DNA helicase complexes from humans (BLM-TOP3α) and yeast (Sgs1-Top3). Although human Rmi1 stimulates the dissolution activity of BLM-TOP3α, its biochemical function is unknown. Here we examined the role of Rmi1 in the yeast complex. Consistent with the similarity of top3Δ and rmi1Δ phenotypes, we find that a stable Top3-Rmi1 complex can be isolated from yeast cells overexpressing these two subunits. Compared to Top3 alone, this complex displays increased superhelical relaxation activity. The isolated Rmi1 subunit also stimulates Top3 activity in reconstitution experiments. In both cases, elevated temperatures are required for optimal relaxation unless the substrate contains a ssDNA bubble. Interestingly, Rmi1 binds only weakly to ssDNA on its own, but it stimulates the ssDNA binding activity of Top3 five-fold. Top3 and Rmi1 also cooperate to bind the Sgs1 N-terminus and promote its interaction with ssDNA. These results demonstrate that Top3-Rmi1 functions as a complex and suggest that Rmi1 stimulates Top3 by promoting its interaction with ssDNA.
INTRODUCTION
Mutations in BLM result in Bloom Syndrome (BS), a rare autosomal disease characterized by a variety of symptoms including a predisposition to cancer (1). Cells from BS patients display genomic instability characterized by elevated rates of sister chromatid exchange (SCE) (2). BLM is a RecQ-family DNA helicase that forms a complex with DNA topoisomerase IIIα (Top3α) (1,3,4). This complex is conserved throughout eukaryotes where it acts to suppress recombination - especially in response to DNA damaging agents such as inter-strand crosslinkers (e.g., mitomycin C) or the alkylating agent methylmethanesulfonate (5–10). The fact that the lesions created by these agents are known to impede replication forks has led to the notion that the hyper-SCE phenotype of BS cells is a consequence of an alternative repair pathway for replication-induced DNA damage.
Although the molecular function of BLM-TOP3α is not completely understood, the ability of the helicase-topoisomerase complex to “dissolve” double Holliday junction (HJ) substrates in vitro has suggested a compelling mechanism by which it could suppress crossing over during recombinational repair (11,12). Studies of orthologous RecQ complexes from model systems such as budding yeast (Sgs1-Top3), fission yeast (Rqh1-Top3) and Drosophila have provided genetic insight into the function of these proteins as well as support for the HJ dissolution model (12–19).
Rmi1/BLAP75 is a conserved protein that was recently identified based on its association with BLM, Sgs1, and Rqh1. In human cells, Rmi1 co-purifies with a complex of proteins including BLM and Top3α (20). This complex has not been purified to homogeneity from yeast, but Rmi1 co-fractionates with a complex containing Sgs1 and Top3 (21,22). Human Rmi1 is a 625 aa protein that contains a predicted oligonucleotide and oligosaccharide binding fold (OB-Fold) between residues 115 and 192 (Fig. 1A) (20). OB-folds are found in a large number of proteins that interact with single stranded DNA (ssDNA) and RNA (23,24). Although yeast Rmi1 is smaller than its human homolog and contains no obvious OB-fold motif, amino acid sequence similarity between these proteins is greatest in the region predicted to contain the OB-fold (22). This similarity suggests that Rmi1 should interact with ssDNA. Although some support for this idea was previously reported, UV-crosslinking was required to detect a specific interaction between Rmi1 and ssDNA (22). Moreover, the interaction between Rmi1 and several branched DNA stubstrates using an Electrophoretic Mobility Shift Assay (EMSA) (22) suggests that structure-specific DNA binding could be influenced by additional proteins in the complex.
Fig. 1.
Purification and characterization of recombinant Top3-Rmi1 complex. (A) Comparison of full-length amino acid sequences of human Rmi1 and budding yeast Rmi1. Gray shading represents the predicted OB-fold domain while crosshatch represents the region of greatest amino acid sequence similarity. (B) Approximately 1.5 μg Top3-Rmi1, 1μg Top3, and 1μg Rmi1 were resolved by 17% SDS-PAGE and subjected to Coomassie blue staining. (C) Forty μg of purified Top3-Rmi1 complex was subjected to Superose 6 size exclusion chromatography and analyzed as in above. (D) Thirty μg of Top3-Rmi1 complex was subjected to 15–35% glycerol gradient sedimentation and analyzed as above.
In-vitro studies have shown that human Rmi1 can stimulate the BLM-Top3α-dependent HJ dissolution assay and that it interacts with human Top3α (6,7,25,26). Consistent with these results, co-immunoprecipitation studies have shown that Rmi1 and Top3 can interact in yeast extracts even in the absence of the Sgs1 DNA helicase (21,22). While these biochemical assays reveal a functional role for Rmi1 in stimulating BLM-Top3 activity, the mechanism by which it stimulates dissolution and the nature of the interaction between Top3 and Rmi1 has not been investigated.
To determine whether Top3 and Rmi1 formed a stable sub-complex with unique activities, we expressed and purified them from yeast. We found that the two proteins formed a stable complex and that the superhelical relaxation activity of Top3 was stimulated by the Rmi1 subunit. Interestingly, Rmi1 did not show appreciable ssDNA binding activity on its own, but it did stimulate the ssDNA binding activity of Top3. The two subunits also displayed cooperative binding to the Sgs1 N-terminal domain. These data demonstrate that Rmi1 and Top3 form a functional sub-complex and suggest a mechanism by which Rmi1 can regulate Top3 activity.
EXPERIMENTAL PROCEDURES
Yeast strains, plasmids, and antibodies
Yeast strains were constructed and maintained following standard procedures (27). Strain JEL1 (MATa leu2 trp1 ura3-52 prb1-1122 pep4-3 Δhis::PGAL10-GAL4::ura3) (28) was used as the host for expressing galactose-induced proteins. Derivative NJY2063 expresses a previously-described C-terminal V5-His6-tagged Top3 (Top3-V5) (29) under the control of the GAL1-10 promoter on each of two plasmids, pNJ2585 and pNJ2588. Derivative NJY2062 contains plasmids pNJ2585 and pNJ2586, and was used for the purification of Top3-Rmi1 complex. Plasmid pNJ2586 expresses Top3-V5 and wild type Rmi1 under the control of the GAL1-10 promoter. A rabbit anti-serum was raised against recombinant yeast His6-Rmi1 (Covance). Antibodies against the following epitopes were obtained commercially: V5 (Invitrogen), Sgs1 N-terminus (Santa Cruz Biotechnology), and HA (Roche).
Expression and purification of recombinant proteins
Top3-Rmi1 was expressed in yeast strain NJY2062 by growing it at 30°C in 100 ml of synthetic complete medium lacking leucine and uracil and supplemented with 2% (w/v) glucose. After reaching saturation, the culture was diluted 20-fold into the same medium and grown until OD600= 1.25. Cells from 2 L of culture were collected by centrifugation, washed with sterile distilled water, and resuspended in 4 L synthetic complete medium lacking leucine and uracil and containing 2% galactose and 2% sucrose. At OD600 = 1.5 the cells were harvested by centrifugation, washed as above, and resuspended in an equal volume of 2X buffer N (1X = 25 mM Tris·HCl [pH 7.5], 0.1 mM phenylmethylsulfonyl fluoride [PMSF], 0.01% NP-40, 1 mM dithiothreitol [DTT], 10% glycerol, and 500 mM NaCl) and the following protease inhibitors: pepstatin, 10 μg/ml; leupeptin, 5 μg/ml; benzamidine, 10 mM; and bacitracin, 100 μg/ml. Cells were packed into a syringe and extruded through an 18-gauge needle into liquid nitrogen. The frozen material was then ground in a coffee grinder with dry ice for 3 min. This mixture was placed on ice, allowed to sublime, and diluted with 1 volume of cold 2X buffer N plus protease inhibitors. The insoluble material was pelleted by centrifugation at 13,000 RPM in an SS34 rotor for 20 min at 4°C, and the soluble portion was taken as extract. The extract was filtered through a 0.45 μm syringe filter (Nalgene) and made 10 mM in imidazole before loading onto a 5 ml Ni His-Trap column attached to an AKTA FPLC (GE Healthcare). The column was washed with 10 column-volumes (CVs) of Buffer N plus 10 mM imidazole and eluted with a six CV gradient from 10 to 500 mM imidazole in Buffer N. The Top3-Rmi1 complex eluted at 170 mM imidazole based on SDS-PAGE and Coomassie blue staining. The peak fractions were pooled and dialyzed against buffer B (25 mM HEPES [pH 6.9], 1 mM EDTA, 0.01% NP-40, 10% glycerol, 0.1 mM PMSF, 1 mM DTT) containing 50 mM NaCl. The dialyzed pool was loaded onto a 1 ml MonoS column, washed with Buffer B plus 50 mM NaCl and resolved into 0.3 ml fractions across a 6 CV gradient from 50 to 1000 mM NaCl. Peak fractions eluted at 375 mM NaCl.
Top3 was expressed in yeast strain NJY2063 and purified by Ni-column chromatography as described above, except that Top3 eluted from the Ni-column at 200 mM imidazole. Following dialysis, the sample was fractionated by Mono S column chromatography as described above. Peak fractions (425 mM NaCl) were identified by SDS-PAGE and Coomassie blue staining. Recombinant His6-Rmi1-HA protein was expressed in E. coli BL21(DE3)-RIL cells (Stratagene) and purified as described (22).
The N-terminal 652 amino acids of Sgs1 was expressed as a (His)6-Sgs11-652-V5 fusion protein from plasmid pKR6318, which was transformed into E. coli BL21(DE3)-RIL cells. Freshly transformed colonies were pooled and grown in 1L LB media containing 0.1 mg/ml ampicillin at 37°C until OD600 = 0.4. The recombinant protein was induced by addition of 0.4 mM isopropyl-1-thio-D-galactopyranoside and the cells were grown at 16°C for 16 hours. Induced cells were pelleted and resuspended in 40 ml Buffer N containing 10 mM imidazole and protease inhibitors as above. The cells were sonicated for 2 min with a Branson sonifer 450 microtip at setting 2 and 25% duty cycle. The lysate was centrifuged at 13,500 rpm in an SS34 rotor at 4°C for 15 min and the supernatant was filtered before loading onto a 5 ml Ni column. The column was washed with 10 CVs of Buffer N plus 10 mM imidazole and eluted with a 6 CV gradient from 10 to 500 mM imidazole in Buffer N. Peak fractions were pooled and dialyzed against buffer B plus 50 mM NaCl. The dialyzed pool was loaded onto a 1 ml HiTrap SP column (Amersham Bioscience) and washed with 10 CVs Buffer B plus 50 mM NaCl and then eluted into 0.3 CV fractions across an 8 CV gradient from 50 to 1000 mM NaCl and 2 additional CVs of 1000 mM NaCl in Buffer B. Peak fractions were pooled and dialyzed against Buffer A (25 mM Tris-HCl [pH 7.5], 1 mM EDTA, 0.01% NP-40, 10% glycerol, 0.1 mM PMSF, and 1 mM DTT) containing 50 mM NaCl. The dialyzed pool was loaded onto a HiTrap Q HP 1 ml column (GE Healthcare), washed with 10 CVs Buffer A plus 50 mM NaCl and eluted with an 8 CV gradient from 50 to 1000 mM NaCl in Buffer A. Peak fractions were pooled based on SDS-PAGE and Coomassie blue staining.
Superose 6 HR10/30 chromatography was performed on an AKTA FPLC system in the presence of Buffer A containing 150 mM NaCl but lacking glycerol. Approximately 40 μg protein was fractionated at a flow rate of 0.4 ml/min. Thirty μg of Top3-Rmi1 complex was subjected to 15–35% glycerol gradient sedimentation by centrifugation for 24 h at 45,000 RPM in a Beckman SW55 Ti rotor. 0.2 ml fractions were collected from the top. Fractions from both treatments were analyzed by SDS-PAGE and Coomassie blue staining.
GST pulldown assay
For GST-pulldown experiments, a GST-fusion to the N-terminal 158 amino acids of Sgs1 (29) was incubated with various amounts of Rmi1-HA and Top3-V5 in 30 μl of Buffer A containing 150 mM NaCl at 4°C for 30 min. Protein solutions were mixed by rotation with 10 μl glutathione-Sepharose beads (GE Healthcare) for 30 min at 4°C. Beads were washed twice with 150 μl of Buffer A containing 150 mM NaCl. Bound proteins were eluted by SDS-PAGE loading buffer, resolved by 17% SDS-PAGE, and visualized via western blotting as described (22).
Preparation of DNA substrates
The pKS bubble substrate was prepared by annealing and linking the plus- and minus-strands of pBlueScript KS using ADP and Archaeoglobus fulgidus reverse gyrase (30), a kind gift of Dr. Tao Hsieh. Negatively supercoiled bubble DNA was prepared by incubating circular bubble DNA (50 ng/μl) with yeast DNA topoisomerase I (5 ng/μl) and ethidium bromide (EtBr; 1.5 μg/ml) in DNA topoisomerase I relaxation assay buffer : 35 mM Tris-HCl [pH 8.0], 72 mM KCl, 5 mM MgCl2, 5 mM DTT, 5 mM spermidine, and 0.01% bovine serum albumin. The reaction was incubated at 37°C for 1 hr and stopped by treatment with 0.5% SDS and 0.05 mg/ml proteinase K at 37°C for 20 min. DNA was extracted by phenol/chloroform, precipitated, and redissolved in TE buffer.
Relaxation of negatively supercoiled DNA
The standard DNA topoisomerase III reaction contained 40 mM HEPES (pH 7.0), 40% glycerol, 5 mM sodium acetate, and 10 μg/ml bovine serum albumin in a final volume of 20 μl. The reactions were assembled on ice, initiated by shifting to 30°C for 20 min, and stopped by treatment with SDS and proteinase K as above. Loading dye was added and the samples were resolved on a 0.8% agarose gel by overnight electrophoresis at 1.5 V/cm in 1X TBE buffer at room temperature. The gel was stained with 0.5 μg/ml EtBr and visualized with UV light.
Relaxation of supercoiled bubble DNA
Standard assays were performed as above, stopped by the addition of EDTA to 25 mM and incubation at room temperature for 2 min. SDS was then added to 0.5% for a 2 min incubation, followed by the addition of proteinase K to 0.05 mg/ml and incubation at 37°C for 15 min. The samples were analyzed by electrophoresis through 0.8% agarose containing ½ X TBE buffer. For positively supercoiled bubble DNA, EtBr was first added to 1.5 μg/ml.
Electrophoretic mobility shift assay (EMSA)
32P-labeled DNA substrates were prepared and assayed in a 20 μl reaction volume as previously described (22). The sequences of the oligonucleotide substrates were taken from (31) as follows: ssDNA, oligo 1253 (5′-TGGGTCAACGTGGGCAAAGATGTCCTA GCAATGTAATCGTCTATGACGTT-3′); linear dsDNA, oligos 1253 and 2148 (5′-AACGTCATAGACGATTACATTGCTAGG ACATCTTTGCCCACGTTGACCCA-3′); or branch-migratable Holliday junction, oligos 1253, 1254 (5′-TGCCGAATTCTACCA GTGCCAGTGATGGACATCTTTGCCCAC GTTGACCC-3′), 1255 (5′-GTCGGATCCTCTAGACAGCTCCATGAT CACTGGCACTGGTAGAATTCGGC-3′), and 1256 (5′-CAACGTCATAGACGATTACATTGCTAC ATGGAGCTGTCTAGAGGATCCGA-3′). Also used was oligo 1313 (5′-TGGCGTTAGGAGATACCGATAAGCTTC GGC-3′).
RESULTS
Isolation of the Top3-Rmi1 complex
In order to test whether Top3 and Rmi1 formed a stable complex we simultaneously overexpressed both proteins in yeast. Top3 complexes were then affinity purified via a C-terminal hexahistidine tag. This Top3-V5His6-tagged protein was expected to be functional in-vitro because it had previously been shown to complement a variety of top3Δ phenotypes in yeast (22,29). Rmi1 was found to co-purify with Top3 following Ni-affinity and Mono-S chromatography. The resulting complex of 68- and 33-kD proteins was purified to apparent homogeneity based on Coomassie blue staining (Fig. 1B). A similar approach was used to purify Top3, which was overexpressed alone in yeast, while recombinant Rmi1 was purified from E. coli as previously described (22). The Top3-Rmi1 complex was stable to gel filtration chromatography (Fig. 1C) and glycerol gradient sedimentation (Fig. 1D).
Rmi1 stimulates Top3 superhelical relaxing activity
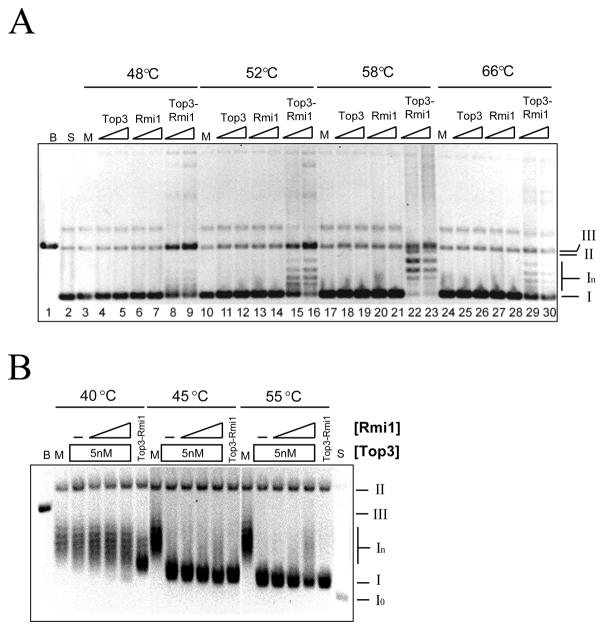
These proteins were tested for the ability to relax negatively supercoiled plasmid DNA. Top3 is most active when provided with ssDNA substates or incubated at elevated temperature (32,33) so we assayed at several temperatures. As shown in Fig. 2A, Top3-Rmi1 displayed a peak of relaxation activity at 58°C. Under the conditions used in this experiment, similar concentrations of Top3 or Rmi1 displayed no relaxation activity at any temperature. As shown below, the Top3 preparation used in this assay was active but required Rmi1 and a more sensitive assay to detect relaxation. This suggests that the activity of Top3 in the complex was stimulated by Rmi1.
Fig. 2.
Relaxation of negatively supercoiled DNA by Top3, Rmi1, and Top3-Rmi1. (A) Relaxation reactions containing 200 ng pKS+ supercoiled plasmid DNA were incubated at the indicated temperature for 20 min. Following resolution on a native agarose gel, the products were stained with EtBr and photographed. Presented is the negative image. Top3 concentrations were 75 nM or 150 nM; Rmi1 concentrations were 75 nM or 150 nM; and the concentrations of Top3-Rmi1 complex were 45 nM or 90 nM. (B) Relaxation reactions containing 200 ng negatively supercoiled pKS+ plasmid DNA were incubated at the indicated temperature with 5 nM Top3-Rmi1, or 5 nM Top3 together with increasing concentrations of Rmi1. Following the reactions, the products were resolved by 0.8% agarose gel electrophoresis in presence of 5 μg/ml chloroquine and the gel was processed as above. The concentrations of Rmi1 were 25 (lanes 4, 10, 16), 100 (lanes 5, 11, 17), or 200 nM (lanes 6, 12, 18). The various topological states of the plasmid are indicated to the right of the gel as follows: I, negatively supercoiled DNA; In, topoisomers of negatively supercoiled DNA; II, open circle and nicked circle forms; III, linear DNA. B, pKS+ plasmid digested with BamHI (lane 1); M, mock incubation; S, substrate relaxed with DNA topoisomerase I.
To confirm this idea, we assayed Top3 in the presence of increasing amounts of Rmi1 and used chloroquine gel electrophoresis to detect any partial relaxation activity. Under these conditions negatively supercoiled DNA is resolved into a ladder of bands (In) (Fig. 2B, lane 2) while fully relaxed DNA migrates as a faster-moving species (Io) that accumulates due to the intercalation of chloroquine (Fig. 2B, lane 20). Partial relaxation of negatively supercoiled DNA could be detected when Top3 was assayed on its own at 45°C or 55°C (Fig. 2B, lanes 9 and 15), but not at 40°C. Increasing levels of Rmi1 stimulated Top3 relaxation of negatively supercoiled DNA at 40°C as revealed by the accumulation of Form I products (Fig. 2B lanes 4 – 6). At 45°C and 55°C Rmi1 had a small effect of accelerating the migration of the Form I product (e.g., Fig. 2B, lanes 10–12). This result confirms that 100 to 200 nM Rmi1 can stimulate 5 nM Top3 in the relaxation of negatively supercoiled plasmid DNA. However, at 40°C this activity was far less than that obtained with 5 nM Top3-Rmi1 complex. This suggests that in vitro reconstitution of the Top3-Rmi1 complex from the individual subunits is inefficient.
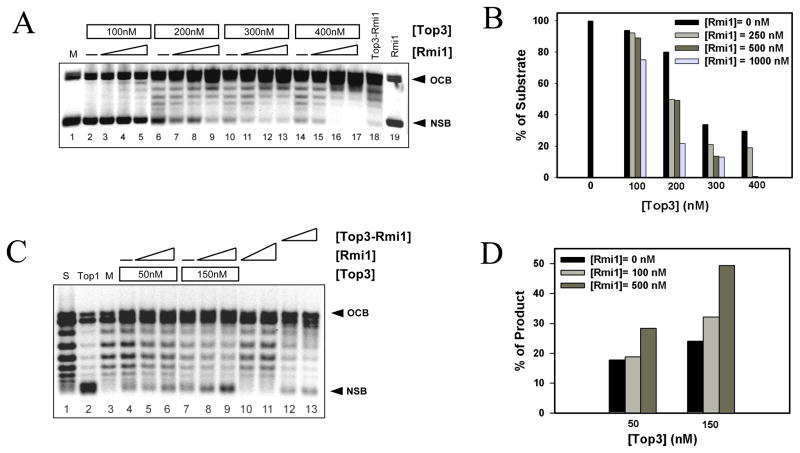
The inability of Top3 to completely relax supercoiled DNA is likely due to its inability to gain access to ssDNA like its bacterial counterpart DNA topoisomerase III (34,35). The stimulation of Top3 by high temperature or its ability to relax hyper-negatively supercoiled DNA is consistent with this idea (32,33). Further, it has been shown that Top3 is capable of relaxing positively supercoiled DNA if the enzyme is provided acess to ssDNA strands via ssDNA extrusions or bubble DNA (36). We tested the abilility of Rmi1 to stimulate Top3 using a negatively supercoiled duplex DNA containing a 500 bp bubble (36). As previously demonstrated (36), Top3 was able to partially relax this bubble substrate when assayed at low temperature (30°C) (Fig. 3A, lanes 2, 6, 10, and 14). Increasing concentrations of Rmi1 stimulated this activity especially when assayed in the presence of 200 – 400 nM Top3. The ability of Rmi1 to stimulate relaxation was confirmed by quantifying the level of unreacted negatively supercoiled bubble DNA (NSB) in the titrations. As shown in Fig. 3B, Rmi1 stimulated relaxation at all concentrations of Top3 that were used. Complete relaxation was obtained with 400 nM Top3 and 500 nM Rmi1 (Fig. 3A, lane 16). As before, Top3-Rmi1 complex (125 nM) was more active than Top3 alone (400 nM).
Fig. 3.
Rmi1 stimulates Top3-dependent relaxation of supercoiled bubble DNA at 30°C. (A) Negatively supercoiled DNA containing a 500 bp bubble (NSB) was prepared as described (30) and incubated with the indicated concentrations of Top3 together with increasing concentrations of Rmi1 (0, 250, 500, or 1000 nM) for 20 min at 30°C. Alternatively, NSB was mock treated (lane 1), or treated with 125 nM Top3-Rmi1 (lane 18) or 1000 nM Rmi1 (lane 19). OCB, Open circular bubble DNA. The products were analyzed by native gel electrophoresis as in Fig 2A. (B) The percent of initial substrate DNA (NSB; mock = 100%) remaining in each reaction was quantified and is presented as a function of Top3 concentration. (C) Positively supercoiled DNA containing a 500 bp bubble (PSB) was prepared by pre-incubating OCB with EtBr. This substrate was then incubated with the indicated concentration of Top3 together with increasing amounts of Rmi1 (0, 100, 500 nM) for 20 min at 30°C. Alternatively, PSB substrate (S, lane 1) was treated with DNA topoisomerase I (Top1, lane 2), mock treated (M, lane 3), or treated with 50 nM (lane 12) or 100 nM (lane 13) Top3-Rmi1. Following incubation, the DNA was purified away from protein and EtBr by phenol/chloroform extraction and analyzed by native agarose gel electrophoresis. Note that the relaxation of PSB DNA produces NSB DNA. (D) The NSB product DNA was quantified for each reaction and is presented as percent maximal relaxation (where M = 0% and Top1 = 100%).
We used the bubble substrate to test whether Rmi1 could stimulate Top3 to relax positively supercoiled DNA. Bubble DNA was first incubated with 1.5 μg/ml EtBr to positively supercoil the DNA, and then incubated with either Top3 alone, or Top3 plus Rmi1. Following incubation the reaction was extracted and the product was analyzed by agarose gel electrophoresis. Under these conditions, the substrate DNA migrates as a ladder of bands between NSB and OCB, while Top1 treatment, which is known to relax positively supercoiled DNA, produces Form I DNA (Fig 3C, lane 2). Although Top3 or Rmi1 alone had little or no effect on the substrate, Rmi1 stimulated Top3 to relax the positively supercoiled substrate as indicated by the appearance of NSB (Fig. 3C, lanes 5 & 6, and 8 & 9). Quantifying the NSB confirmed this interpretation (Fig. 3D). Finally, the specificity of the Top3-Rmi1 interaction was determined by showing that Rmi1 failed to stimulate the relaxation activity of DNA topoisomerase I (Sup. Fig. S1).
Rmi1 stimulates the ssDNA binding activity of Top3
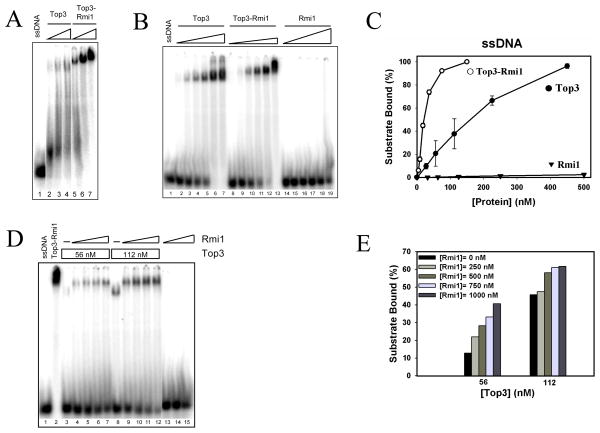
Given the possibility of an OB-fold within the yeast Rmi1 subunit, we investigated whether the Rmi1-Top3 complex displayed specific DNA binding activity. Using an EMSA assay we first tested the ability of the Rmi1 or Top3 subunits to bind 32P-labeled ssDNA, dsDNA, or HJ probes that were 50 nt in length. Consistent with previous data (22), high concentrations of Rmi1 interacted weakly with ssDNA, producing a diffuse smear following gel electrophoresis (Fig. 4A, lanes 2–6). Also, some signal was observed in the well at maximal Rmi1 concentrations. Small amounts of Rmi1 bound the dsDNA and HJ probes, although a majority of these complexes could not enter the gel, suggesting that it was aggregated (Fig. 4A, lanes 7 – 17). As previously reported (16), low concentrations of Top3 bound the ssDNA probe and formed a discrete band in the gel (Fig. 4B, lane 2). At higher concentrations, Top3 bound the dsDNA and HJDNA probes (Fig. 4B) with evidence of aggregation at the highest concentrations. Like Top3, the Top3-Rmi1 complex bound the ssDNA probe at low concentrations and produced a single band within the gel (Fig. 4C, lanes 1 – 11). Higher concentrations of Top3-Rmi1 complex were required for dsDNA and HJ binding and a specific band was obtained (Fig. 4C, right panels). Under these conditions little or no signal was observed in the well suggesting that Top3-Rmi1 is resistant to aggregation.
Fig. 4.
DNA binding specificity of Rmi1, Top3, and the Top3-Rmi1 complex. EMSA probes were made by gel-purifying 32P-labeled DNA substrates that were assembled from 50-mer oligonucleotides. Standard binding reactions (Experimental Procedures) were carried out at a final DNA concentration of 0.25 nM. Following incubation, the reactions were subjected to 5% PAGE after which the gel was dried and analyzed on a phosphorimager. (A) The indicated substrates were incubated with Rmi1 at the following concentrations: 0 (lanes 1, 7, 13), 162 (lanes 2, 8, 14), 325 (lanes 3, 9, 15), 650 (lanes 4, 10, 16), 1300 (lanes 5, 11, 17), or 2600 nM (lanes 6, 12). (B) Top3 was incubated with the indicated substrates exactly as in (A). (C) Top3-Rmi1 complex was incubated with either the ssDNA substrate (0, 0.037, 0.075, 0.15, 0.3, 0.75, 1.5, 2.25, 3, 4.5, or 9 nM protein; lanes 1 – 11), the HJ substrate (0, 1.5, 3, 6, 15, 23, 30, 45, or 90 nM protein; lanes 12 – 20), or the dsDNA substrate (0, 6, 15, 23, 30, 45, 60, 90, or 150 nM protein; lanes 21 – 29). The fraction of free and bound probe was then determined using IP LabGel software. (D) Quantification of ssDNA binding. (E) Quantification of dsDNA binding. (F) Quantification of HJDNA binding. Each data point represents the mean of three independent experiments presented along with the standard deviation as error. (G) The specificity of ssDNA binding by Top3-Rmi1 was confirmed by competition assay. Six nM Top3-Rmi1 was incubated together with 0.25 nM 32P-labeled (dT)60 ssDNA in a 20 μl reaction volume. Where indicated, reactions included unlabeled competitor DNA consisting of either boiled pKS+ plasmid (ssDNA) at 0.00064, 0.0032, 0.0016, 0.08, 0.4, 2, 10 nM or untreated pKS+ plasmid (dsDNA) at 0.0032, 0.0016, 0.08, 0.04, 0.2, 1, 5, 25 nM. The products were analyzed as above. For each reaction, the percentage of initial ssDNA binding (obtained in the absence of unlabeled DNA) was determined.
To measure substrate preference, we quantified the amount of each substrate that was bound by Top3, Rmi1, or Top3-Rmi1 as a function of protein concentration (Figures 4D–F). These results indicated that Top3 and Top3-Rmi1 complex bound preferentially to the ssDNA probe. In the case of the Top3-Rmi1 complex, this preference is reflected by a Kd (0.18 nM) that was four to ten-fold lower than those obtained for HJDNA or dsDNA (Table 1). Consistent with this result, unlabelled ssDNA was a better competitor than dsDNA (Fig. 4G). Using this assay, 50% of the ssDNA binding was competed by 0.12 nM ssDNA or 6.5 nM dsDNA which is a a 50-fold difference.
Table 1.
DNA binding affinity of Top3-Rmi1
| Substrate | Kd (nM) |
|---|---|
| ssDNA 50 | 0.18 ± 0.038 |
| HJDNA 50 | 0.72 ± 0.11 |
| dsDNA 50 | 1.5 ± 0.14 |
Using a 50 nt substrate there was little if any difference in ssDNA binding affinity between Top3 and the Top3-Rmi1 complex (Fig. 4D). Because Top3 was previously shown to bind a 41 nt substrate (16), we tested whether shorter ssDNA substrates could distinguish these proteins. A difference between Top3 and the Top3-Rmi1 complex was observed using a low concentration of a 30 nt substrate (Fig. 5A). To compare binding affinities we performed titration experiments using a higher concentration of the 30 nt ssDNA probe as this resulted in detectable Top3 band-shifts by EMSA (Fig. 5B). Under these conditions ssDNA binding required significantly less Top3-Rmi1 complex than Top3 alone (Fig. 5C). This difference was reflected in a dissociation constant for Top3-Rmi1 (2.25 nM) that was five-fold lower than that of Top3 (Table 2). Taken together, these results indicate that the Top3-Rmi1 complex has a reduced tendency to aggregate on DNA and that it has a higher affinity for ssDNA than Top3 alone.
Fig. 5.
Top3-Rmi1 displays enhanced ssDNA binding activity. (A) A 32P-labeled 30-mer oligonucleotide (0.25 nM) was incubated under standard conditions with either no protein (lane 1), Top3 protein (7.5, 30, or 60 nM; lanes 2–4) or Top3-Rmi1 complex (7.5, 30, or 60 nM; lanes 5–7). Products were then analyzed by EMSA as in Fig. 4, except for use of a 4% polyacrylamide gel. (B) The 32P-labeled 30-mer oligonucleotide (100 nM) was incubated with either no protein (ssDNA; lane 1), Top3 (28, 56, 112, 225, 450, 670 nM; lanes 2 – 7), Top3-Rmi1 (4.5, 9, 18, 38, 75, 150 nM; lanes 8 – 13), or Rmi1 (32, 64, 125, 250, 500, 1000 nM; lanes 14 – 19). (C) The experiment shown in panel (B) was repeated and the signal corresponding to bound DNA was quantified for each protein. Presented is the mean value obtained from three experiments together with its standard deviation. (D) The 32P-labeled 30-mer oligonucleotide (30 nt) was incubated with either no protein (ssDNA; lane 1), Top3-Rmi1 (112 nM; lane 2), or the indicated concentrations of Top3 together with increasing concentrations of Rmi1 (0, 250, 500, 750, or 1000 nM). As control, the probe was incubated with Rmi1 alone (500, 750, or 1000 nM; lanes 13 – 15). (E) The signal corresponding to bound ssDNA in panel (D) was quantified and is presented graphically.
Table 2.
ssDNA (30nt) binding affinity of Top3-Rmi1 and its subunits
| Protein | Kd (nM) |
|---|---|
| Top3-Rmi1 | 2.25 ± 0.05 |
| Top3 | 11.7 ± 1.5 |
| Rmi1 | 227 ± 19 |
To confirm this result, we tested whether exogenous Rmi1 could directly stimulate ssDNA binding by Top3. For this analysis increasing amounts of Rmi1 were incubated together with a fixed concentration of Top3 and the 30 nt probe. As shown in Fig. 5D (lanes 3–7), increasing levels of Rmi1 resulted in increased probe being retarded in the gel. The migration of this band was retarded relative to that of Top3 alone and its position approximated that of the purified Top3-Rmi1 complex (Fig. 5D, lane 2). This is consistent with the idea that Top3-Rmi1 binds the probe as a complex. This experiment was repeated with increasing Top3 concentrations and the results were quantified. As shown in Figure 5E, Rmi1 stimulated Top3-dependent ssDNA binding with maximal stimulation occurring at lower concentrations of Top3.
Top3 and Rmi1 cooperate to bind Sgs1
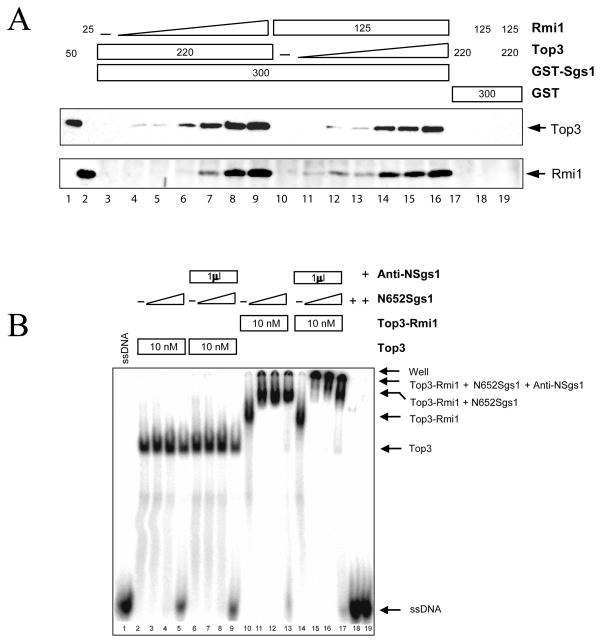
Top3 has been shown to interact directly with N-terminal domain of Sgs1 (16,29) and fractionation of yeast extracts indicates that these proteins exist in a complex with Rmi1 (21,22). To examine these subunit interactions more completely, we tested whether Rmi1 modulated the interaction between Top3 and the Sgs1 N-terminus. To do this, we exploited a GST-fusion protein consisting of GST plus residues 1 – 158 of Sgs1 (29). Constant amounts of Top3 and GST-Sgs11-158 were first incubated together with increasing levels of Rmi1, and the amount of Top3 or Rmi1 that bound to GST-Sgs11-158 was then measured by glutathione-bead pull down and immunoblotting. Under the conditions used here, Top3 failed to interact with Sgs1 alone (Fig. 6A, lane 3). However increasing levels of Rmi1 stimulated the binding of Top3 to Sgs1 (lanes 4–9). As expected, Rmi1 precipitated along with GST- Sgs11-158 and Top3 consistent with the formation of a ternary complex. Control reactions showed that neither Rmi1 nor Top3 bound to GST alone (Fig. 6A, lanes 17 – 19). In the reciprocal experiment, increasing levels of Top3 were found to stimulate the binding of Rmi1 to GST-Sgs11-158 (Fig. 6A, lanes 10–16). We conclude that Rmi1 and Top3 act together as a complex to bind Sgs1.
Fig. 6.
Top3 and Rmi1 cooperate to bind the N-terminus of Sgs1 and promote its interaction with ssDNA. (A) Constant amounts of Top3 (220 ng) and GST-Sgs11-158 (300 ng) were incubated on ice together with increasing amounts of Rmi1 (0, 3.1, 6.3, 12.5, 25, 50, or 100 ng; lanes 3 – 9). Alternatively, constant amounts of Rmi1 (125 ng) and GST-Sgs11-158 (300 ng) were incubated with increasing amounts of Top3 (0, 6.8, 13.8, 27.5, 55, 110, or 220 ng; lanes 10 – 16). Glutathione beads were added to the mixture and bound proteins were detected by immunoblot. Top3 and Rmi1 markers are shown in lanes 1 and 2. Control reactions (lanes 17 – 19) were performed as above by incubating GST together with the indicated proteins in ng. (B) Constant amounts of Top3 (lanes 2 – 9) or Top3-Rmi1 (lanes 10 – 17) were incubated with increasing concentrations of Sgs11-652 (10, 40, or 160 nM) on ice. After 15 min, a 32P-labeled oligonucleotide (50 nt) was added to a final concentration of 0.25 nM and the incubation was continued at room temperature for 15 min. Where indicated, an antibody to the Sgs1 N-terminus was added and the incubation continued for 30 min. The probe was also left untreated (ssDNA; lane 1) or incubated with Sgs11-652 (lane 18) or Sgs11-652 followed by anti-Sgs1 antibody (lane 19). Following the incubation the samples were subjected to standard EMSA analysis.
Given the ability of Top3-Rmi1 to bind ssDNA and Sgs1, we asked whether the Top3-Rmi1 complex could mediate the binding of Sgs1 to ssDNA. It has previously been shown that the N-terminal 640 amino acids of BLM are dispensible for ssDNA binding by BLM (37). Consistent with this result, the Sgs1 N-terminal domain (Sgs11-652) did not bind ssDNA on its own (Fig. 6B, lanes 18–19) and increasing concentrations of this domain had no effect on the binding of a 50 nt ssDNA probe by Top3 (Fig. 6B, lanes 2 – 5). However, when incubated in the presence of Top3-Rmi1 and the probe, Sgs11-652 promoted the formation of a slower-migrating band (Fig. 6B, lane 10 – 13). The migration of this band is consistent with a quaternary complex of Top3-Rmi1-Sgs11-652-ssDNA. To confirm that the Sgs11-652 protein was present in the complex we incubated the mixture with antibodies against the Sgs1 N-terminal 18 amino acids. As shown in Fig. 6B (lanes 14 – 17), this antibody supershifted the complex into the wells. Taken together, we conclude that Top3-Rmi1 functions as a complex to bind ssDNA and Sgs1.
DISCUSSION
In this study we report that Rmi1 interacts stably with DNA topoisomerase III and that the Top3-Rmi1 complex displays enhanced superhelical relaxation activity compared to Top3 alone. The Top3-Rmi1 complex is stable to low concentrations of SDS (22) and displays improved solubility compared to its individual subunits (CFC and SJB, unpublished results). In addition to its enhanced relaxation activity, the complex binds a variety of DNA structures without aggregation and displays unique functional interactions with the Sgs1 N-terminus. Unfortunately, the full activity of the Top3-Rmi1 complex could not be reconstituted from its individual subunits. Perhaps this is because a fraction of these proteins had folded inappropriately and lacked accessible interaction domains. Although we have not systematically tested this idea by prolonged incubation or refolding experiments, such behavior is not uncommon with proteins that form tight complexes. Thus, the stimulation of Top3 activity by Rmi1 may be due in large part to its role in maintaining the structure of Top3.
EMSA experiments with the Top3 and Rmi1 subunits have previously suffered from the fact that much of the signal is retained in the well of the gel (Figure 4) (22). Thus, it appears that the Top3-Rmi1 complex is an improved reagent to identify biologically relevant substrates for Top3 activity. So, which substrate is bound preferentially? Our data on Top3-Rmi1 do not support preferential binding to either dsDNA or a single HJ. First, although Top3-Rmi1 binds HJDNA with twice the affinity of dsDNA, this can be explained by the fact that our HJ probe contains twice the amount of DNA as the dsDNA probe (i.e., approximately two 50-mer duplexes). Second, competition experiments confirmed that Top3-Rmi1 preferred ssDNA over dsDNA. Third, the preference for ssDNA is apparent from its 10-fold lower Kd. It will be interesting to test whether the Sgs1-Top3-Rmi1 complex retains this specificity given that its DNA helicase domain has been shown to bind a variety of branched DNAs including HJs (38). Since there are multiple enzymatic activities in the Sgs1-Top3-Rmi1 complex, we would not be surprised to find that the full complex recognizes a variety of DNA structures. Future studies should be able to address this question by measuring the interaction of Top3-Rmi1 or Sgs1-Top3-Rmi1 with more complex substrates including those with multiple HJs (11,39).
Amino acid sequence analysis predicts that human Rmi1 contains a single OB-fold (20). OB-folds are found in numerous proteins that interact with ssDNA or RNA (23). This includes a number of well-characterized DNA replication proteins such as the ssDNA binding protein Replication Protein A (RPA), BRCA2 and POT1 (40–42). Although little evidence for an OB-fold can be extracted from yeast Rmi1 sequences, OB-folds are known to tolerate considerable sequence variation (43). In addition, we think it is revealing that the region of human Rmi1 with greatest amino acid sequence similarity to its homologs overlaps significantly with the predicted OB-fold (20–22). Thus, the ability of Rmi1 to stimulate ssDNA binding by Top3 may be due to the presence of an OB-fold. Structural studies of Rmi1 will be necessary to make this conclusion definitively, but other results are consistent with this idea. In the case of RPA, a pair of OB-folds (the A–B dimer) binds stably to 8 nt of ssDNA (44). In contrast, the interaction between a solitary OB-fold (A or B) and ssDNA is not stable to EMSA analysis and requires UV crosslinking to fix the interaction (45). Interestingly, we previously observed weak ssDNA binding by the isolated Rmi1 subunit that could be stabilized by UV crosslinking (22). Taking RPA as a model, we suggest that Rmi1’s ssDNA binding activity is enhanced in the Top3-Rmi1 complex where it synergizes with that of Top3.
Like DNA topoisomerase III from E. coli, the eukaryotic enzyme is limited in its ability to relax superhelical stress at low temperature (33–35). The requirement for elevated temperatures has been taken as evidence of a requirement for ssDNA access by Top3, and a number of elegant studies have proved this idea by demonstrating that DNA topoisomerase III is capable of decatenating circular DNA and relaxing negative or positive supercoils if the protein is given access to ssDNA (32–34,36). In vitro, this access can be provided by altering the secondary structure of the substrate using hyper-negative supercoiling or by engineering non-homologous bubbles or hairpins into the DNA. In the case of decatenation, which does not require elevated temperatures, Top3 can be stimulated by RecQ DNA helicases (11,12,46). In turn, RPA has been shown to stimulate this reaction (12). Taken together, these findings suggest that Rmi1 may have evolved to assist eukaryotic Top3 in binding ssDNA present in its substrates. Future experiments might address this idea by identifying mutants of Rmi1 that lack ssDNA binding activity. Interestingly, Rmi1 stimulated Top3’s relaxation activity but only slightly reduced the temperature needed for this reaction. Indeed, we were surprised to find that a ssDNA bubble was necessary for relaxation at 30°C. This may reflect the fact that relaxation of superhelical stress is not a biological activity of Top3. In decatenation reactions, such as the dissolution assay, Rmi1 has been shown to stimulate at 37°C (25,26).
An alternative model for Rmi1 function is that it regulates Top3’s access to ssDNA. It has previously been shown that the N-terminal domain of Sgs1 inhibits the ssDNA binding activity of Top3 (16). In contrast to this result, we have shown that the Top3-Rmi1 complex binds ssDNA stably in the presence of the Sgs1 N-terminus. In fact, the N-terminal domain of Sgs1 forms a quaternary complex with the Top3-Rmi1-ssDNA assembly. The ability of Rmi1 to simultaneously stimulate binding to ssDNA and Sgs1 places it in a position to regulate Top3’s interaction with the helicase or the DNA substrate. At present it is unclear how Rmi1 mediates these two interactions. It will be interesting to identify the domains of Rmi1 required for these interactions and test whether they are regulated by proteins involved in homologous recombination or if they are modified in response to DNA damage. In addition, the robust interaction we have identified between the Top3-Rmi1 complex and Sgs1 places Rmi1 in a position to directly regulate Sgs1. Additional studies will be required to test whether Rmi1 modulates the substrate recognition or DNA helicase activity of Sgs1.
Supplementary Material
The abbreviations used are
- ssDNA
single-stranded DNA
- dsDNA
double-stranded DNA
- HJ
Holliday junction
- HA
hemmaglutinin
- DTT
dithiothreitol
- Top3
DNA topoisomerase III
- SCE
sister chromatid exchange
- OB-fold
oligonucleotide and oligosaccharide binding fold
- EMSA
electrophoretic mobility shift assay
- PMSF
phenylmethylsulfonyl fluoride
- CV
column volume
- EtBr
ethidium bromide
Footnotes
The authors are grateful to Bill Fricke, Hiroshi Hiasa, Tao Hsieh, Janet Lindsley, John Nitiss and Weidong Wang for strains, plasmids, and other reagents. We also thank Leroy Liu and Jan Mullen for comments on the manuscript, and Ferez Nallaseth for technical assistance during the early stages of this work. This study was supported by NIH grant R01GM072569.
References
- 1.Ellis NA, Groden J, Ye TZ, Straughen J, Lennon DJ, Ciocci S, Proytcheva M, German J. Cell. 1995;83:655–666. doi: 10.1016/0092-8674(95)90105-1. [DOI] [PubMed] [Google Scholar]
- 2.German J. Science. 1964;144:298–301. doi: 10.1126/science.144.3616.298. [DOI] [PubMed] [Google Scholar]
- 3.Wu L, Davies SL, North PS, Goulaouic H, Riou JF, Turley H, Gatter KC, Hickson ID. J Biol Chem. 2000;275(13):9636–9644. doi: 10.1074/jbc.275.13.9636. [DOI] [PubMed] [Google Scholar]
- 4.Karow JK, Chakraverty RK, Hickson ID. J Biol Chem. 1997;272(49):30611–30614. doi: 10.1074/jbc.272.49.30611. [DOI] [PubMed] [Google Scholar]
- 5.Bachrati CZ, Hickson ID. Biochem J. 2003;374(Pt 3):577–606. doi: 10.1042/BJ20030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sung P, Klein H. Nat Rev Mol Cell Biol. 2006;7(10):739–750. doi: 10.1038/nrm2008. [DOI] [PubMed] [Google Scholar]
- 7.Cheok CF, Bachrati CZ, Chan KL, Ralf C, Wu L, Hickson ID. Biochem Soc Trans. 2005;33(Pt 6):1456–1459. doi: 10.1042/BST0331456. [DOI] [PubMed] [Google Scholar]
- 8.Krepinsky AB, Heddle JA, German J. Human Genetics. 1979;50(2):151–156. doi: 10.1007/BF00390236. [DOI] [PubMed] [Google Scholar]
- 9.Hook GJ, Kwok E, Heddle JA. Mutat Res. 1984;131(5–6):223–230. doi: 10.1016/0167-8817(84)90029-4. [DOI] [PubMed] [Google Scholar]
- 10.Shiraishi Y. EMBO J. 1985;4(10):2553–2560. doi: 10.1002/j.1460-2075.1985.tb03970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu L, Hickson ID. Nature. 2003;426(6968):870–874. doi: 10.1038/nature02253. [DOI] [PubMed] [Google Scholar]
- 12.Plank JL, Wu J, Hsieh TS. Proc Natl Acad Sci USA. 2006;103(30):11118–11123. doi: 10.1073/pnas.0604873103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gangloff S, McDonald JP, Bendixen C, Arthur L, Rothstein R. Mol Cell Biol. 1994;14(12):8391–8398. doi: 10.1128/mcb.14.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watt PM, Louis EJ, Borts RH, Hickson ID. Cell. 1995;81(2):253–260. doi: 10.1016/0092-8674(95)90335-6. [DOI] [PubMed] [Google Scholar]
- 15.Bennett RJ, Sharp JA, Wang JC. J Biol Chem. 1998;273(16):9644–9650. doi: 10.1074/jbc.273.16.9644. [DOI] [PubMed] [Google Scholar]
- 16.Bennett RJ, Noirot-Gros MF, Wang JC. J Biol Chem. 2000;275(35):26898–26905. doi: 10.1074/jbc.M003137200. [DOI] [PubMed] [Google Scholar]
- 17.Davey S, Han CS, Ramer SA, Klassen JC, Jacobson A, Eisenberger A, Hopkins KM, Lieberman HB, Freyer GA. Mol Cell Biol. 1998;18(5):2721–2728. doi: 10.1128/mcb.18.5.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maftahi M, Han CS, Langston LD, Hope JC, Zigouras N, Freyer GA. Nucleic Acids Res. 1999;27(24):4715–4724. doi: 10.1093/nar/27.24.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ira G, Malkova A, Liberi G, Foiani M, Haber JE. Cell. 2003;115(4):401–411. doi: 10.1016/s0092-8674(03)00886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin J, Sobeck A, Xu C, Meetei AR, Hoatlin M, Li L, Wang W. EMBO J. 2005;24(7):1465–1476. doi: 10.1038/sj.emboj.7600622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang M, Bellaoui M, Zhang C, Desai R, Morozov P, Delgado-Cruzata L, Rothstein R, Freyer GA, Boone C, Brown GW. EMBO J. 2005;24 (11):2024–2033. doi: 10.1038/sj.emboj.7600684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullen JR, Nallaseth FS, Lan YQ, Slagle CE, Brill SJ. Mol Cell Biol. 2005;25(11):4476–4487. doi: 10.1128/MCB.25.11.4476-4487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theobald DL, Mitton-Fry RM, Wuttke DS. Annu Rev Biophys Biomol Struct. 2003;32:115–133. doi: 10.1146/annurev.biophys.32.110601.142506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murzin AG. EMBO J. 1993;12:861–867. doi: 10.1002/j.1460-2075.1993.tb05726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raynard S, Bussen W, Sung P. J Biol Chem. 2006;281(20):13861–13864. doi: 10.1074/jbc.C600051200. [DOI] [PubMed] [Google Scholar]
- 26.Wu L, Bachrati CZ, Ou J, Xu C, Yin J, Chang M, Wang W, Li L, Brown GW, Hickson ID. Proc Natl Acad Sci USA. 2006;103(11):4068–4073. doi: 10.1073/pnas.0508295103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose MD, Winston F, Hieter P. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1990. [Google Scholar]
- 28.Lindsley JE. Methods Mol Biol. 1999;94:187–197. doi: 10.1385/1-59259-259-7:187. [DOI] [PubMed] [Google Scholar]
- 29.Fricke WM, Kaliraman V, Brill SJ. J Biol Chem. 2001;276(12):8848–8855. doi: 10.1074/jbc.M009719200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsieh TS, Plank JL. J Biol Chem. 2006;281(9):5640–5647. doi: 10.1074/jbc.M513252200. [DOI] [PubMed] [Google Scholar]
- 31.Whitby MC, Lloyd RG. J Biol Chem. 1998;273(31):19729–19739. doi: 10.1074/jbc.273.31.19729. [DOI] [PubMed] [Google Scholar]
- 32.Wilson TM, Chen AD, Hsieh T. J Biol Chem. 2000;275(3):1533–1540. doi: 10.1074/jbc.275.3.1533. [DOI] [PubMed] [Google Scholar]
- 33.Kim RA, Wang JC. J Biol Chem. 1992;267(24):17178–17185. [PubMed] [Google Scholar]
- 34.DiGate RJ, Marians KJ. J Biol Chem. 1988;263(26):13366–13373. [PubMed] [Google Scholar]
- 35.Srivenugopal KS, Lockshon D, Morris DR. Biochemistry. 1984;23(9):1899–1906. doi: 10.1021/bi00304a002. [DOI] [PubMed] [Google Scholar]
- 36.Plank JL, Chu SH, Pohlhaus JR, Wilson-Sali T, Hsieh TS. J Biol Chem. 2005;280(5):3564–3573. doi: 10.1074/jbc.M411337200. [DOI] [PubMed] [Google Scholar]
- 37.Cheok CF, Wu L, Garcia PL, Janscak P, Hickson ID. Nucleic Acids Res. 2005;33(12):3932–3941. doi: 10.1093/nar/gki712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett RJ, Keck JL, Wang JC. J Mol Biol. 1999;289(2):235–248. doi: 10.1006/jmbi.1999.2739. [DOI] [PubMed] [Google Scholar]
- 39.Plank JL, Hsieh TS. J Biol Chem. 2006;281(25):17510–17516. doi: 10.1074/jbc.M602933200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bochkarev A, Bochkareva E. Curr Opin Struct Biol. 2004;14(1):36–42. doi: 10.1016/j.sbi.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Lei M, Podell ER, Baumann P, Cech TR. Nature. 2003;426(6963):198–203. doi: 10.1038/nature02092. [DOI] [PubMed] [Google Scholar]
- 42.Yang H, Jeffrey PD, Miller J, Kinnucan E, Sun Y, Thoma NH, Zheng N, Chen PL, Lee WH, Pavletich NP. Science. 2002;297(5588):1837–1848. doi: 10.1126/science.297.5588.1837. [DOI] [PubMed] [Google Scholar]
- 43.Agrawal V, Kishan KV. Curr Protein Pept Sci. 2003;4(3):195–206. doi: 10.2174/1389203033487207. [DOI] [PubMed] [Google Scholar]
- 44.Bochkarev A, Pfuetzner RA, Edwards AM, Frappier L. Nature. 1997;385:176–181. doi: 10.1038/385176a0. [DOI] [PubMed] [Google Scholar]
- 45.Philipova D, Mullen JR, Maniar HS, Gu C, Brill SJ. Genes and Development. 1996;10:2222–2233. doi: 10.1101/gad.10.17.2222. [DOI] [PubMed] [Google Scholar]
- 46.Harmon FG, DiGate RJ, Kowalczykowski SC. Mol Cell. 1999;3(5):611–620. doi: 10.1016/s1097-2765(00)80354-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.