Abstract
Bipolar disorder (BPD) is a devastating psychiatric illness marked by recurrent episodes of mania and depression. While the underlying pathophysiology of BPD remains elusive, an abnormal hypothalamic–pituitary–adrenal (HPA) axis and dysfunctional glucocorticoid receptor (GR) signaling are considered hallmarks. This review will examine how targeting resiliency signaling cascades at the cellular level may serve as a mechanism to treat BPD. Here, cellular resiliency is defined as the ability of a cell to adapt to an insult or stressor. Such resiliency at the cellular level could confer resiliency at the systems level and, ultimately, help individuals to cope with stressors or recover from depressive or manic states. This review will focus on four molecular targets of mood stabilizers that are known to play integral roles in these cellular resiliency signaling pathways: (1) B-cell CLL/lymphoma 2 (Bcl-2), (2) Bcl-2-associated athanogene (BAG-1), (3) glucocorticoid receptors (GRs), and (4) 51 kDa FK506-binding protein (FKBP5). These targets have emerged from neurobiological and human genetic studies and employ mechanisms that modulate GR function or promote anti-apoptotic processes critical to affective resilience. Future research should focus on elucidating sustainable treatments that target resiliency factors—such as BAG-1 or FKBP5—which could ultimately be used to treat individuals suffering from BPD and prevent relapses in afflicted individuals. Further identification of resiliency and susceptibility factors will also be vital. Ultimately, these developments would allow for the treatment of susceptible individuals prior to the development of BPD.
Keywords: Resiliency, Bipolar disorder, Bcl-2, BAG-1, FKBP5, Glucocorticoid receptor
1. Introduction
1.1. The burden of bipolar disorder and lack of effective treatments
Bipolar disorder (BPD) is a common, severe, chronic, and sometimes life-threatening illness that places an enormous economic and emotional burden on society in addition to the patients and families directly affected (Goodwin and Jamison, 2007). The disease is characterized by recurrent episodes of mania and depression. A variety of pharmacological therapies are available to treat BPD; for acute manic episodes and its subtypes, these options consist mainly of lithium, anticonvulsants (e.g., valproate), and both typical and atypical antipsychotic agents (Baldessarini et al., 2003; Tohen et al., 2001; Zarate and Tohen, 2000). However, for most patients with BPD, monotherapy is often insufficient and combination treatment is required (Zarate and Quiroz, 2003). Furthermore, it has become apparent in recent years that these options are far from adequate in treating recurrences, relapses, and acute episodes of the illness in addition to restoring premorbid functioning. For long-term prophylaxis, far fewer agents are available; they include lithium, aripiprazole, and lamotrigine, but of these only lamotrigine has been shown to help prevent depressive relapses (McElroy et al., 2004). In addition, some patients are unable to tolerate existing therapies for BPD, which leads to frequent changes in medications or non-adherence (Sajatovic et al., 2006; Zarate et al., 1999; Zarate and Tohen, 2004). Thus, currently available therapies for BPD are insufficient, and demand the development of new therapeutics that are more effective and better tolerated.
One possible avenue for the development of novel therapies may come from studying the mechanisms of affective resilience. This review will consider some of the cellular substrates that are thought to underlie this phenomenon. While most of our discussion will examine cellular resiliency pathways with a focus on anti-apoptotic, glucocorticoid, and neurotrophic signaling cascades, it is important to acknowledge that other pathways involving biogenic amines, glutamate, etc. also play important roles in this disease (interested readers, see Manji et al. (2003)). This review, however, will focus on recent developments that support the role for specific glucocorticoid receptor (GR)-related proteins and B-cell CLL/lymphoma 2 (Bcl-2) family members as resiliency factors in BPD.
1.2. Human genetic and neurobiological studies implicating resiliency factors relating to GR function
While the etiology of BPD is unknown, a variety of environmental and genetic factors have been implicated. For instance, family, twin and adoption studies have revealed a familial aggregation of BPD—there is a ten-fold higher risk for the disorder in individuals with an affected first-degree relative compared to individuals with unaffected first-degree relatives (Smoller and Finn, 2003). While it is still challenging to quantitatively assign the heritability of BPD, many studies support the hypothesis that a variety of genetic risk factors exist for the disease.
Broadly speaking, these genetic risk factors may lead to dysfunction of the stress response. Following an acute stressor, a multitude of biochemical and physiological changes occur. Some of these biochemical changes include release of catecholamines (epinephrine and norepinephrine) from the sympathetic nervous system. In addition to activation of the sympathetic nervous system, the hypothalamic–pituitary–adrenal (HPA) axis is also activated following stress by release of corticotropin releasing hormone (CRH) from the hypothalamus to the pituitary gland which releases adrenocorticotrophic hormone (ACTH) that activates the adrenal gland's release of glucocorticoids. In turn, these biochemical signals initiate a host of physiological changes ranging from enhanced mobilization of energy stores to muscles, increased cardiovascular tone, and cognitive function while decreased digestion, immune function, growth, and reproduction (for a more complete review see Sapolsky et al. (2000)).
Problems arise when this system is unable to turn off, which can occur during chronic stress. GR signaling provides an important negative feedback loop to the HPA axis' regulation of the stress response. Dysfunctional GR activity (either hypersensitivity or resistance to glucocorticoids) may contribute toward hyperactivation of the HPA axis, which is one of the most consistent biological disruptions noted in patients with depression.
Patient-based studies have shown that genetic components of the GR signaling pathway may contribute to some individuals' predisposition to mood disorders or to the efficacy of certain medications. Genetic studies looking at single nucleotide polymorphisms (SNPs), single base pair changes in a DNA nucleotide sequence that often influences a gene's activity and expression, in the GR gene have implicated this gene as a risk factor for major depressive disorder (MDD). Van West et al. analyzed SNPs in the GR gene (Nuclear Receptor Subfamily 3, Group C Member 1; NR3C1) and discovered that polymorphisms in the 5′ region of the NR3C1 gene conferred genetic risk for MDD (van West et al., 2006). Further research is required to perform functional studies that will look at how SNPs influence GR expression and function.
Another group looked at different polymorphisms in the GR gene. These polymorphisms had functional changes in the GR gene by either being associated with hypersensitivity (BclI, polymporphism in the BclI restriction site in intron 2 and a C to G nucleotide change 646 base pairs downstream from exon 2) or resistance (ER22/23EK, polymorphism in exon 2 consisting of 2 linked nucleotide changes in codons 22 and 23 GAG AGG → GAA AAG where the first nucleotide change in codon 22 is silent, coding for glutamic acid, E, and the second change arginine, R, to lysine, K) to glucocorticoids. Interestingly, this group found an increased risk of depression in homozygous carriers of BclI and certain ER22/23EK polymorphisms, due to glucocorticoid hypersensitivity or resistance within these respective genotypes (van Rossum et al., 2006). This study supports the concept that GR signaling dysfunction can lead to depression through either glucocorticoid hypersensitivity or resistance. Yet simple increases or decreases in GR expression cannot be attributable to either dysfunction or enhanced function. Furthermore, the ER22/23EK carriers had a faster response to antidepressants, suggesting that in the future, genetics may be used to predict a patient's therapeutic response to treatments dependant upon glucocorticoid hypersensitivity or resistance.
Additional biochemical studies have found that GR function (measured as the response to steroid manipulations of either GR binding and translocation to the nucleus or of peripheral cell functions) is decreased in major depression without consistently showing a concomitant decrease in GR expression. Some antidepressants improve GR function and increase its expression (reviewed in Pariante and Miller (2001)). Wassef et al. found that, following oral dexamethasone (a synthetic glucocorticoid) administration, control subjects had a suppression of GR expression while depressed patients failed to exhibit this response (Wassef et al., 1990). An additional study found that depressed patients had higher plasma cortisol concentrations relative to control patients, but they did not show any increase in the GR-responsive enzyme sialytransferases, nor were there any changes in GR binding (Maguire et al., 1997).
Mechanisms that may underlie GR dysfunction have been suggested to include: (1) downregulation of GR even with elevated cortisol levels, (2) genetic alterations in GR influencing function, and (3) ligand-independent mechanisms impacting GR function. While most studies have not shown a consistent downregulation of GR in depressed patients, the elevated cortisol levels may ultimately overburden the recycling capacity of GR and dampen its negative feedback. We have already considered several genetic polymorphisms (i.e. ER22/23EK) that may impact GR function, however, further studies are required to elucidate the exact mechanisms. In terms of ligand-independent mechanisms, factors such as proinflammatory cytokine interleukin 1 and factors involved in the cyclic adenosine monophosphate (cAMP) cascade such as protein kinase A (PKA) have both been shown to impact GR function. Miller et al. show interleukin 1 has an inhibitory effect on GR translocation and hormone-induced GR-mediated gene transcription (Miller et al., 1999). Interestingly, antidepressants have been reported to upregulate GR protein and mRNA in addition to facilitating GR translocation and enhancing HPA axis negative feedback mechanisms (reviewed in Pariante and Miller (2001)). These effects may ultimately restore GR negative feedback mechanisms in depressed patients.
GRs are modulated by a variety of factors/mechanisms including antidepressants, cytokines, and intracellular signaling cascades as mentioned above. Another important regulator is the chaperone protein 51 kDa FK506-binding protein (FKBP5). FKBP5 acts as a negative feedback regulator through its association with heat shock protein 90 (Hsp90) and p23 (see Fig. 1) of GR activity (Vermeer et al., 2003). The TT homozygous FKBP5 polymorphism has been associated with an increased number of depressive episodes in patients with various mood disorders, as well as enhanced response to antidepressant treatment (Binder et al., 2004). In addition, genetic variation within FKBP5 correlates with the number of suicide attempts and the number of depressive episodes in BPD (Willour et al., 2008). Further clinical evidence comes from Lekman et al. (2008), who found markers in FKBP5 associated with either disease status (rs1360780) or remission (rs4713916) in white, non-Hispanic patients suffering from MDD (Lekman et al., 2008).
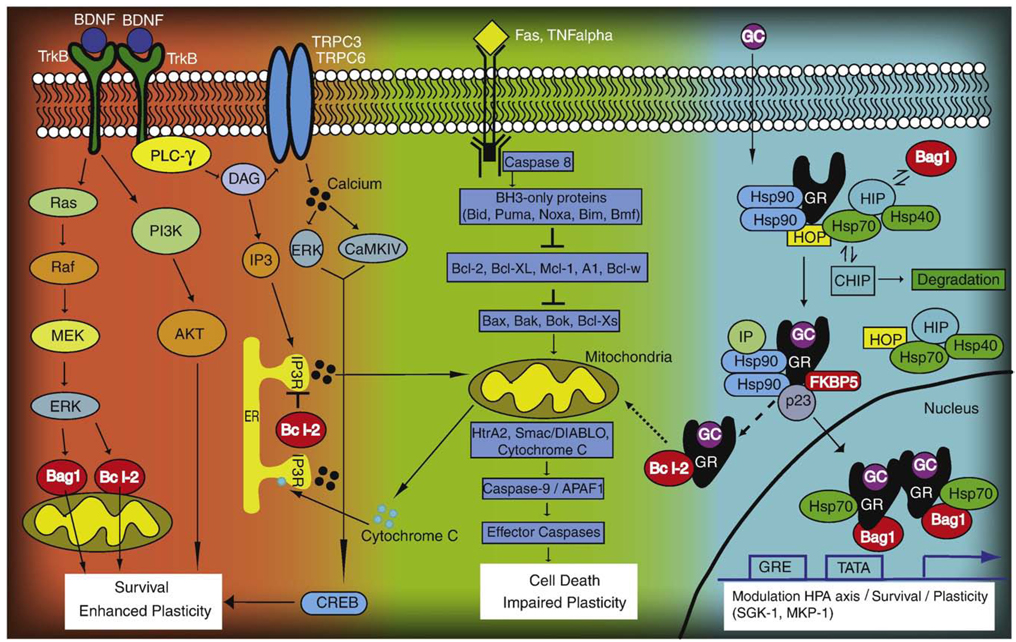
Fig. 1.
Cellular resiliency signaling pathways targeted by mood stabilizers. (1) Neurotrophic factor signaling (left). BDNF activates TrkB receptors; phosphorylation can then activate either the ERK signaling cascade, PI3K, or PLC-γ. Ultimately, these independent pathways converge to enhance plasticity and cell survival. (2) Anti-apoptotic signaling (center). Following activation of pro-caspases (e.g. Caspase 8), pro-apoptotic factors are activated (BH3-only proteins), which in turn inhibit anti-apoptotic proteins such as Bcl-2. This enables pro-apoptotic members to form pores on the outer mitochondrial membrane, ultimately leading to the release of cytochrome C, activation of effector caspases (e.g. Caspase 3) and, eventually, impaired plasticity and cell death. (3) Glucocorticoid receptor signaling (right). Following a stress response, glucocorticoids (GC) are released and downregulate the HPA axis, turning off the stress response after a period of time. At the cellular level, GCs bind to their receptor (GR), whereby different co-chaperones can modulate GR nuclear trafficking. FKBP5 and BAG-1 are two such co-chaperones that have opposing roles in either attenuating or enhancing GR nuclear trafficking, respectively. Once inside the nucleus, GRs bind to glucocorticoid responsive elements (GRE) and turn on downstream gene targets (e.g. SGK-1 and MKP-1), leading to enhanced survival and plasticity mechanisms. Alternatively, the GRs can associate with Bcl-2 (dashed line) following acute doses of corticosterone. This complex translocates (dotted line) to the mitochondria to enhance survival, leading to enhanced cellular plasticity and resiliency. Mood stabilizer targets are depicted in red.
In addition to the association between BPD and dysfunctional GR signaling, grey matter volume and glial reductions in the prefrontal cortex (PFC) have also been noted, suggesting a deficit in survival, neurotrophic, and plasticity mechanisms (Drevets et al., 1998). These deficits may be facilitated by impaired cellular function and decreased resistance to cellular insults following prolonged exposure to glucocorticoids. Very recent findings from two independent samples identified an association between BPD and a SNP in the anti-apoptotic gene BCL-2 (Chen et al, unpublished data). This BCL-2 SNP was associated with lower Bcl-2 mRNA and protein levels. Furthermore, lymphoblasts collected from probands in these families with the BCL-2 risk allele showed an increased rate of cell death in the presence of cytotoxic calcium levels. This diverse group of studies has shown that impaired GR function is correlated with a multitude of factors that influence behavior states, including reduced GR-mediated negative feedback of the HPA axis, diminished sensitivity to cortisol, impaired cell survival mechanisms, and reductions in brain area volume. In this manner, behavioral and cellular resilience can be achieved by either enhancing GR's negative feedback mechanisms or reducing prolonged exposure to glucocorticoids by enhancing survival mechanisms.
2. Resiliency as a targeted mechanism
Targeting resiliency at the cellular level can be accomplished through a variety of mechanisms, including (but not exclusively) neurotrophic, survival, and GR signaling pathways (see Fig. 1). Here, cellular resiliency is broadly defined as a cell's ability to adapt to different insults that are caused by the environment, which may be exacerbated (or attenuated) by certain inherited or developed factors. Furthermore, targeting resiliency as a mechanism to treat BPD is well-supported in the literature through neuroimaging and neuropathological studies. The neuroimaging and neuropathological findings reviewed below suggest that, in BPD, loss of cellular plasticity may be due to loss of trophic support or survival factors coupled with additional mechanisms such as disrupted intracellular signaling cascades.
Specifically, neuroimaging data support reductions in gray matter in the subgenual anterior cingulated cortex (sgACC) of patients with MDD or BPD, regardless of mood state (Drevets et al., 2008). Additional imaging studies have also shown abnormalities in the PFC, ventral striatum, hippocampus, and third ventricle (Beyer and Krishnan, 2002; Drevets, 2001; Strakowski et al., 2002). In patients with BPD, decreased regional cerebral blood flow (rCBF) in the orbitofrontal cortex coupled with increased rCBF in the dorsal anterior cingulated cortex associates with biological correlates of emotional vulnerability (Kruger et al., 2006). Interestingly, hypoactivity of the orbitofrontal cortex has been associated with shifts between euphoric and dysphoric mood states as well as the inability to distinguish between relevant and irrelevant emotional stimuli (Angrilli et al., 1999; Grafman et al., 1986; Joseph, 1999).
Neuropathological studies of individuals with MDD or BPD indicate decreased gray matter volume and reduced glia in the sgACC, but no impact on neuronal number (Drevets et al., 2008). Rajkowska et al. (2001), concentrating on the dorsal lateral PFC, found reduced neuronal density in layer III, and reduced pyramidal cell densities in layers III and V, in addition to reductions in glial densities in sublayer IIIC and enlarged glial shapes in patients with BPD compared to controls (Rajkowska et al., 2001). Because these changes may be progressive over the course of the illness, preventing these reductions by engaging resiliency mechanisms could slow or disrupt the progression of BPD.
2.1. Common molecular targets of mood stabilizers
The two mood stabilizers most often used to treat BPD are lithium and valproate. These structurally dissimilar molecules (cation versus fatty acid) share common targets that may underlie their clinical benefits. Chronic lithium treatment has been shown to upregulate Bcl-2 (Manji et al., 2000), Bag-1 (Zhou et al., 2005), and Fkbp5 (McQuillin et al., 2007) in addition to downregulating mRNA coding for post-synaptic 5-HT(1A) receptors in the rat hippocampus (McQuade et al., 2004). Similarly, chronic valproate treatment upregulates BAG-1 (Zhou et al., 2005) and Bcl-2 (Chen et al., 1999), although the direct mechanisms remain unclear. Treatment of mouse fibroblast cells (L929), stably transfected with mouse mammary tumor virus (MMTV)-chloramphenicol acetyltransferase reporter plasmid (LMCAT), with lithium (1–4 mM) or valproate (0.1–3 mM) inhibits corticosterone-induced activity of the reporter gene (Basta-Kaim et al., 2004). This further supports lithium and valproate's inhibitory effects on corticosterone-induced mechanisms that may enhance GR function in suppressing over activity of the HPA axis. Fig. 1 highlights three prominent signaling cascades that are affected by mood stabilizer treatment (e.g., the Bcl-2 family, brain-derived neurotrophic factor (BDNF) neurotrophic/neuroprotection, and glucocorticoid receptor signaling cascades). Many examples of pathway interaction and convergence are demonstrated, especially surrounding mitochondrial membrane integrity, which is a critical target of cell survival and death mechanisms.
2.2. Anti-apoptotic function
Apoptosis is an active form of cell death that depends on new gene expression and is characterized by nuclear condensation, DNA strand breaks, cellular fragmentation, and phagocytosis by adjacent cells (Steller, 1995). Preclinical studies suggest that chronic stress can precipitate cell death in stress-sensitive hippocampal neurons (Magarinos et al., 1996). In addition, clinical studies have found cell atrophy and death in the hippocampus and PFC of depressed patients (Duman et al., 1999); the hypersecretion of glucocorticoids during stress may contribute to this cell death (Lee et al., 2002). Therefore, enhancing cell survival mechanisms that oppose these stress-induced changes could serve as a novel therapeutic approach to treating BPD.
Bcl-2 protein is a key regulator of apoptosis through its interaction with mitochondria-altering members of the Bcl-2 family. As noted previously, Bcl-2 is regulated by both lithium and valproate (Chen et al., 1999) in addition to antidepressants (Murray and Hutson, 2007). Animal studies have demonstrated several key findings: Bcl-2 knockout mice: 1) have altered oxidative metabolism and antioxidant levels in kidney, liver, and brain (Hochman et al., 2000); 2) exhibit increased levels of anxiety (Einat et al., 2005); 3) show an altered response to amphetamine, resembling the clinical phenomena found in euthymic patients with BPD treated with amphetamine (Chen et al., unpublished data); and 4) have an increased tendency to be helpless and have slower spontaneous recovery in learned helplessness tasks (Chen et al, unpublished data). Clinical studies further implicate lower Bcl-2 levels as a risk factor for BPD. For instance, Bcl-2 protein levels were found to be increased in lymphocytes from lithium-treated patients with BPD. In addition, a SNP of BCL-2 was associated with increased risk for BPD; this risk allele was also associated with the lowest Bcl-2 mRNA and protein levels, and enhanced cell death to elevated calcium levels in lymphoblast cells (Chen et al, unpublished data).
Similarly, BAG-1 appears to enhance the anti-apoptotic properties of Bcl-2 through direct interaction (Fig. 1), although BAG-1 shares no sequence homology with Bcl-2 family's conserved domains (Takayama et al., 1995). BAG-1 does have sequence similarity with several ubiquitin and ubiquitin-like proteins, and some researchers have speculated that BAG-1 may orchestrate the interaction between Bcl-2 and proteosome complexes that participate in cell death regulation (Alberti et al., 2003). As with Bcl-2, animal studies investigating the function of BAG-1 in affective resilience offer further evidence for this factor's potential as a therapeutic target for BPD (Maeng et al., 2008).
2.3. Neurotrophic/neuroprotective effects
Chronic treatment with lithium and valproate activate the neurotrophic/neuroprotective signaling pathways highlighted in Fig. 1, including the BDNF/extracellular signal regulated kinase (ERK) pathway, the PI3K/AKT pathway, and the glycogen synthase kinase 3 (GSK-3) pathway (Shaltiel et al., 2007). Therefore, targeting neurotrophic signaling cascades has been suggested as a putative treatment for BPD.
For example, lithium and valproate both increase BDNF in the rat frontal cortex (Einat et al., 2003; Fukumoto et al., 2001). BDNF, in turn, has been implicated in enhancing synaptic plasticity, cell survival, and antidepressant actions when infused directly into the hippocampus in rodent models used to screen for antidepressant efficacy (Shirayama et al., 2002). Interestingly, lithium and valproate both protect against oxidative stress (Lai et al., 2006), but lithium also has neuroprotective properties in a rodent cerebral ischemic model (Bian et al., 2007). Lithium also increases brain N-acetylaspartate (NAA) concentrations (Moore et al., 2000), a marker of neuronal viability. BAG-1 has also been shown to activate the ERK mitogen-activated protein (MAP) kinase cascades (Kermer et al., 2002), which are key signaling cascades upstream of BDNF. Taken together, this evidence suggests that the neuroprotective effects of mood stabilizers may in part be mediated through enhancement of BDNF/ERK signaling.
2.4. Glucocorticoid/HPA axis regulation
GR signaling provides a negative feedback mechanism for the HPA axis. GRs have a lower affinity than mineralocorticoid receptors (MRs) so they are activated at higher levels of cortisol, such as during a stress response. Interestingly, very recent data support the notion that GRs can modulate mitochondrial function (specifically, mitochondrial oxidation, membrane potential, and mitochondrial calcium holding capacity) by forming an association with Bcl-2 and translocating to the mitochondria to enhance neuroprotection (Du et al., in press). By enhancing mitochondrial calcium buffering, GR in concert with Bcl-2 may increase cell survival mechanisms and reduce cell death following prolonged exposure to cytotoxic calcium levels (Fig. 1). Thus, GR signaling appears to play a multifaceted role, including acting not only as a negative modulator of the HPA axis, but also interacting directly with Bcl-2 to provide neuroprotection.
In animal models, overexpression of GRs in mouse forebrain leads to increased anxiety-like and depressive-like behaviors, increased sensitivity to antidepressants, and enhanced cocaine sensitization (Wei et al., 2004). In addition, overexpression of GRs in mouse forebrain leads to mild cognitive dysfunction as well as an initial blunted response to stress followed by a delay in turning off the stress response. Wei et al. (2007) also found that overexpression of GRs—even in the absence of stress—alters hippocampal molecular signaling, especially glutamate signaling, in addition to impairing hippocampal spatial memory tasks in the Morris water maze (Wei et al., 2007).
BAG-1 is a Hsp70/Hcs70-regulating co-chaperone protein that can interact with GRs (Fig. 1) and attenuate their nuclear trafficking and function (Schneikert et al., 1999). Interestingly, glucocorticoids are one of the few agents that can promote either depressive or manic episodes in patients with BPD. BAG-1 overexpressing mice showed faster recovery in the amphetamine-induced hyperlocomotion test, as well as resistance to cocaine-induced behavioral sensitization (Maeng et al., 2008); these two animal models are used to screen potential anti-manic agents. Lithium-treated animals also showed similar behavioral responses as BAG-1 overexpressing mice. Reduction of BAG-1 in heterozygous knockout mice had the opposite effect, leading to enhanced response to cocaine-induced behavioral sensitization (Maeng et al., 2008). In terms of anxiety and depression, BAG-1 overexpressing mice demonstrated a less anxious behavioral phenotype in the elevated plus maze test and had facilitated spontaneous recovery rates from helplessness behavior compared with wild-type littermates (Maeng et al., 2008). Small interfering RNA (siRNA) studies found that BAG-1 blocked the lithium-induced attenuation of GR activity, suggesting that mood stabilizers may be acting via BAG-1 (Zhou et al., 2005), and lending further support to the notion that BAG-1 may be a future therapeutic target in developing novel medications for BPD.
FKBP5 also modulates GR function through its association with Hsp-90. Hsp-90 is a molecular chaperone with a central role in steroid hormone signaling. In animal studies, squirrel monkeys have high levels of cortisol, and normal expression of GR. They also possess an ortholog of FKBP5 (FKBP51). FKBP51 effectively lowers the corticosteroid binding affinity of the GR in squirrel monkeys by 11-fold and, interestingly, is expressed 13-fold higher in squirrel monkeys than in humans (Reynolds et al., 1999). This study indicates one mechanism whereby a GR chaperone can lead to glucocorticoid resistance. Taken together, the evidence suggests that BAG-1, FKBP5, and Hsp-90 support the GR network by enhancing its function during stress-induced events by increasing negative feedback to the HPA axis or promoting cell survival mechanisms. In this manner, these modulators of glucocorticoid receptors provide another layer of control that enables GRs to have such diverse functions in regulation and survival.
3. Future directions
3.1. Developing new treatments to improve resiliency
Recent findings from both clinical and preclinical studies suggest that certain mood stabilizers and antidepressants regulate GR negative feedback mechanisms as well as anti-apoptotic proteins. Perturbations in these pathways lead to altered behavior and deficiencies in coping with manic or depressive challenges. Concurrently, GR and Bcl-2 protein families use a variety of mechanisms to promote cell survival and confer resiliency to certain cytotoxic and stress-induced insults. Drugs that enhance GR negative feedback mechanisms and cell survival functions, either directly or indirectly through upregulation of chaperone/associated proteins, could provide effective alternative treatments to conventional mood stabilizers (Oral and Vahip, 2004). The question remains whether varying intracellular signaling cascades can ultimately provide resiliency or susceptibility to stressful stimuli on a systemic level and contribute to the ability of some individuals to cope with challenging conditions.
Modulating these pathways via novel therapeutic strategies could provide a new way to treat individuals who are sensitive to stressful stimuli. As this review has shown, numerous targets could be selected based on their relative importance in regulating GR or apoptotic cascades. It would be interesting to determine whether altering BAG-1, which mediates nuclear receptor trafficking, could translate to a noticeable change in an individual's response to certain stresses that rely on GR trafficking. Alternatively, enhancing molecules that are chaperones to GR production, like FKBP51 or Hsp70, could lead to a different resiliency profile for patients who are more susceptible to certain types of stressors. This approach would be tailored to treat those affected in an individual-specific manner.
3.2. Need for identification of both resiliency and susceptibility factors
The idea that more refined, tailored medications can boost resiliency demands an improved characterization of the biological underpinnings of both resiliency and susceptibility factors. The diverse set of responses to currently available antidepressants and mood stabilizers (Post et al., 2003), demonstrates that a great deal of heterogeneity exists at the biological level. Strong evidence also exists that dysfunctional GR negative feedback contributes to mood disorders (McEwen, 2005), but it is not clear how different resiliency and susceptibly constituents interact within the glucocorticoid system. Future efforts should focus on further identifying and characterizing these factors, and also attempt to elucidate how they contribute to patient response to particular therapies. Doing so would improve the treatment of mood disorders in general, and could also provide a critical set of biomarkers that would improve both diagnosis and intervention at earlier stages of the disease. Biological markers, or biomarkers, are quantitative measurements that provide information about biological processes, a disease state, or response to treatment (Institute of Medicine, 2008). They thus hold the potential to provide a better understanding of the etiology and pathophysiology of a complex and heterogeneous disorder like BPD.
3.3. Treating susceptible individuals before disease manifestation onset
The ability to identify, much less treat, susceptible individuals prior to illness onset is still in its infancy; however, the development of biomarkers is currently underway in an effort to achieve this goal. Notably, any biomarker or cognitive marker that would help to identify susceptible individuals would be invaluable in efforts to transition toward the prevention of BPD. Identifying such biomarkers would lead to earlier stage interventions, prior to the onset of debilitating and recurrent symptoms, and with a concomitant long-term impact on course of illness, morbidity, and mortality.
HPA axis function has been one area of focus for potential biomarkers. For example, one study has suggested that measuring levels of dexamethasone/corticotrophin-releasing hormone (DEX/CRH) can quantify HPA axis function. Notably, another study found hyper-arousal of the HPA axis in depressed BPD patients compared with either patients with MDD or control subjects (Rybakowski and Twardowska, 1999). HPA axis dysfunction has also been described in healthy individuals with a strong genetic predisposition for affective disorders (Holsboer et al., 1995). Furthermore, this abnormality appears to be stable over time (Modell et al., 1998), a feature that makes measuring HPA axis function a promising marker for vulnerability to BPD.
In addition, changes in cognitive abilities due to reduced GR activity may provide a converging measure to identify deficits in some patients with BPD. Assessments of learning, memory, and executive functions have revealed that impairment of these faculties correlates with impaired GR function in BPD (Watson et al., 2006). Dysfunctional glucocorticoid processes may also be associated with deficits in circadian rhythm dysregulation, altered motivation/reward, and facial emotional labeling (Brotman et al., 2008).
Overall, the strategies outlined in this review show the potential of resiliency and susceptibility markers to help identify and treat individuals suffering from BPD. Potential targets include Bcl-2 and BAG-1, whose respective roles within apoptotic and glucocorticoid response pathways appear to be key. Therapeutics that target the GR and Bcl-2 systems may provide an effective and novel means of enhancing the resiliency of individuals who are sensitive to mania-inducing or depression-inducing environments. Future efforts are needed to identify and characterize susceptibility genes as our efforts move toward enhanced prevention, diagnosis, and treatment of BPD.
Acknowledgments
We would like to acknowledge the support of the Intramural Research Program of the National Institute of Mental Health. We would also like to acknowledge the superb editorial services of Ioline Henter.
Abbreviations
- Bcl-2
B-cell CLL/lymphoma 2
- BAG-1
Bcl-2-associated athanogene
- BPD
bipolar disorder
- BDNF
brain-derived neurotrophic factor
- ERK
extracellular signal regulated kinase
- FKBP5
51 kDa FK506-binding protein
- GR
glucocorticoid receptor
- Hsp
heat shock protein
- HPA
axis, hypothalamic–pituitary–adrenal axis
- NAA
N-acetylaspartate
- MDD
major depressive disorder
- PFC
prefrontal cortex
- rCBF
regional cerebral blood flow
- SNPs
single nucleotide polymorphisms
- sgACC
subgenual anterior cingulated cortex
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest, financial or otherwise, to disclose. This work was undertaken under the auspices of the NIMH Intramural Program; Dr. Manji is now at Johnson and Johnson Pharmaceutical Research and Development.
REFERENCES
- Alberti S, Esser C, Hohfeld J. BAG-1—a nucleotide exchange factor of Hsc70 with multiple cellular functions. Cell Stress Chaperones. 2003;8:225–231. doi: 10.1379/1466-1268(2003)008<0225:bnefoh>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angrilli A, Palomba D, Cantagallo A, Maietti A, Stegagno L. Emotional impairment after right orbitofrontal lesion in a patient without cognitive deficits. NeuroReport. 1999;10:1741–1746. doi: 10.1097/00001756-199906030-00021. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Hennen J, Wilson M, Calabrese J, Chengappa R, Keck PE, Jr, McElroy SL, Sachs G, Vieta E, Welge JA, Yatham LN, Zarate CA, Jr, Baker RW, Tohen M. Olanzapine versus placebo in acute mania: treatment responses in subgroups. J. Clin. Psychopharmacol. 2003;23:370–376. doi: 10.1097/01.jcp.0000085410.08426.9a. [DOI] [PubMed] [Google Scholar]
- Basta-Kaim A, Budziszewska B, Jaworska-Feil L, Tetich M, Kubera M, Leskiewicz M, Lason W. Mood stabilizers inhibit glucocorticoid receptor function in LMCAT cells. Eur. J. Pharmacol. 2004;495:103–110. doi: 10.1016/j.ejphar.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Beyer JL, Krishnan KR. Volumetric brain imaging findings in mood disorders. Bipolar Disord. 2002;4:89–104. doi: 10.1034/j.1399-5618.2002.01157.x. [DOI] [PubMed] [Google Scholar]
- Bian Q, Shi T, Chuang DM, Qian Y. Lithium reduces ischemia-induced hippocampal CA1 damage and behavioral deficits in gerbils. Brain Res. 2007;1184:270–276. doi: 10.1016/j.brainres.2007.09.054. [DOI] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, Papiol S, Seaman S, Lucae S, Kohli MA, Nickel T, Kunzel HE, Fuchs B, Majer M, Pfennig A, Kern N, Brunner J, Modell S, Baghai T, Deiml T, Zill P, Bondy B, Rupprecht R, Messer T, Kohnlein O, Dabitz H, Bruckl T, Muller N, Pfister H, Lieb R, Mueller JC, Lohmussaar E, Strom TM, Bettecken T, Meitinger T, Uhr M, Rein T, Holsboer F, Muller-Myhsok B. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Guyer AE, Lawson ES, Horsey SE, Rich BA, Dickstein DP, Pine DS, Leibenluft E. Facial emotion labeling deficits in children and adolescents at risk for bipolar disorder. Am. J. Psychiatry. 2008 doi: 10.1176/appi.ajp.2007.06122050. [DOI] [PubMed] [Google Scholar]
- Chen G, Zeng WZ, Yuan PX, Huang LD, Jiang YM, Zhao ZH, Manji HK. The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J. Neurochem. 1999;72:879–882. doi: 10.1046/j.1471-4159.1999.720879.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr. Opin. Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Ongur D, Price JL. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Mol. Psychiatry. 1998;3(220–6):190–191. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, Falke C, Khairova R, Zhou R, Yuan P, Machado-Vieira B, McEwen B, Manji HK. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.0812671106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Thome J. Neural plasticity to stress and antidepressant treatment. Biol. Psychiatry. 1999;46:1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- Einat H, Yuan P, Gould TD, Li J, Du J, Zhang L, Manji HK, Chen G. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J. Neurosci. 2003;23:7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat H, Yuan P, Manji HK. Increased anxiety-like behaviors and mitochondrial dysfunction in mice with targeted mutation of the Bcl-2 gene: further support for the involvement of mitochondrial function in anxiety disorders. Behav. Brain Res. 2005;165:172–180. doi: 10.1016/j.bbr.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Morinobu S, Okamoto Y, Kagaya A, Yamawaki S. Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharmacology (Berl) 2001;158:100–106. doi: 10.1007/s002130100871. [DOI] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Manic-depressive Illness: Bipolar Disorders and Recurrent Depression. Oxford; New York: Oxford University Press; 2007. Vol. [Google Scholar]
- Grafman J, Vance SC, Weingartner H, Salazar AM, Amin D. The effects of lateralized frontal lesions on mood regulation. Brain. 1986;109(Pt 6):1127–1148. doi: 10.1093/brain/109.6.1127. [DOI] [PubMed] [Google Scholar]
- Hochman A, Liang H, Offen D, Melamed E, Sternin H. Developmental changes in antioxidant enzymes and oxidative damage in kidneys, liver and brain of bcl-2 knockout mice. Cell. Mol. Biol. (Noisy-le-grand) 2000;46:41–52. [PubMed] [Google Scholar]
- Holsboer F, Lauer CJ, Schreiber W, Krieg JC. Altered hypothalamic–pituitary–adrenocortical regulation in healthy subjects at high familial risk for affective disorders. Neuroendocrinology. 1995;62:340–347. doi: 10.1159/000127023. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Workshop Summary. Washington, DC: Institute of Medicine; 2008. Neuroscience Biomarkers and Biosignatures: Converging Technologies, Emerging Partnerships. Vol. [PubMed] [Google Scholar]
- Joseph R. Frontal lobe psychopathology: mania, depression, confabulation, catatonia, perseveration, obsessive compulsions, and schizophrenia. Psychiatry. 1999;62:138–172. doi: 10.1080/00332747.1999.11024862. [DOI] [PubMed] [Google Scholar]
- Kermer P, Krajewska M, Zapata JM, Takayama S, Mai J, Krajewski S, Reed JC. Bag1 is a regulator and marker of neuronal differentiation. Cell Death Differ. 2002;9:405–413. doi: 10.1038/sj.cdd.4400972. [DOI] [PubMed] [Google Scholar]
- Kruger S, Alda M, Young LT, Goldapple K, Parikh S, Mayberg HS. Risk and resilience markers in bipolar disorder: brain responses to emotional challenge in bipolar patients and their healthy siblings. Am. J. Psychiatry. 2006;163:257–264. doi: 10.1176/appi.ajp.163.2.257. [DOI] [PubMed] [Google Scholar]
- Lai JS, Zhao C, Warsh JJ, Li PP. Cytoprotection by lithium and valproate varies between cell types and cellular stresses. Eur. J. Pharmacol. 2006;539:18–26. doi: 10.1016/j.ejphar.2006.03.076. [DOI] [PubMed] [Google Scholar]
- Lee AL, Ogle WO, Sapolsky RM. Stress and depression: possible links to neuron death in the hippocampus. Bipolar Disord. 2002;4:117–128. doi: 10.1034/j.1399-5618.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- Lekman M, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJ, Lipsky R, Wisniewski SR, Manji H, McMahon FJ, Paddock S. The FKBP5-gene in depression and treatment response—an association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Cohort. Biol. Psychiatry. 2008 doi: 10.1016/j.biopsych.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Hunsberger JG, Pearson B, Yuan P, Wang Y, Wei Y, McCammon J, Schloesser RJ, Zhou R, Du J, Chen G, McEwen B, Reed JC, Manji HK. BAG1 plays a critical role in regulating recovery from both manic-like and depression-like behavioral impairments. Proc. Natl. Acad. Sci. U. S. A. 2008;105:8766–8771. doi: 10.1073/pnas.0803736105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J. Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire TM, Thakore J, Dinan TG, Hopwood S, Breen KC. Plasma sialyltransferase levels in psychiatric disorders as a possible indicator of HPA axis function. Biol. Psychiatry. 1997;41:1131–1136. doi: 10.1016/S0006-3223(96)00223-5. [DOI] [PubMed] [Google Scholar]
- Manji HK, Moore GJ, Chen G. Lithium up-regulates the cytoprotective protein Bcl-2 in the CNS in vivo: a role for neurotrophic and neuroprotective effects in manic depressive illness. J. Clin. Psychiatry. 2000;61 Suppl. 9:82–96. [PubMed] [Google Scholar]
- Manji HK, Quiroz JA, Payne JL, Singh J, Lopes BP, Viegas JS, Zarate CA. The underlying neurobiology of bipolar disorder. World Psychiatry. 2003;2:136–146. [PMC free article] [PubMed] [Google Scholar]
- McElroy SL, Zarate CA, Cookson J, Suppes T, Huffman RF, Greene P, Ascher J. A 52-week, open-label continuation study of lamotrigine in the treatment of bipolar depression. J. Clin. Psychiatry. 2004;65:204–210. doi: 10.4088/jcp.v65n0210. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McQuade R, Leitch MM, Gartside SE, Young AH. Effect of chronic lithium treatment on glucocorticoid and 5-HT1A receptor messenger RNA in hippocampal and dorsal raphe nucleus regions of the rat brain. J. Psychopharmacol. 2004;18:496–501. doi: 10.1177/026988110401800406. [DOI] [PubMed] [Google Scholar]
- McQuillin A, Rizig M, Gurling HM. A microarray gene expression study of the molecular pharmacology of lithium carbonate on mouse brain mRNA to understand the neurobiology of mood stabilization and treatment of bipolar affective disorder. Pharmacogenet. Genomics. 2007;17:605–617. doi: 10.1097/FPC.0b013e328011b5b2. [DOI] [PubMed] [Google Scholar]
- Miller AH, Pariante CM, Pearce BD. Effects of cytokines on glucocorticoid receptor expression and function. Glucocorticoid resistance and relevance to depression. Adv. Exp. Med. Biol. 1999;461:107–116. doi: 10.1007/978-0-585-37970-8_7. [DOI] [PubMed] [Google Scholar]
- Modell S, Lauer CJ, Schreiber W, Huber J, Krieg JC, Holsboer F. Hormonal response pattern in the combined DEX-CRH test is stable over time in subjects at high familial risk for affective disorders. Neuropsychopharmacology. 1998;18:253–262. doi: 10.1016/S0893-133X(97)00144-9. [DOI] [PubMed] [Google Scholar]
- Moore GJ, Bebchuk JM, Hasanat K, Chen G, Seraji-Bozorgzad N, Wilds IB, Faulk MW, Koch S, Glitz DA, Jolkovsky L, Manji HK. Lithium increases N-acetyl-aspartate in the human brain: in vivo evidence in support of bcl-2's neurotrophic effects? Biol. Psychiatry. 2000;48:1–8. doi: 10.1016/s0006-3223(00)00252-3. [DOI] [PubMed] [Google Scholar]
- Murray F, Hutson PH. Hippocampal Bcl-2 expression is selectively increased following chronic but not acute treatment with antidepressants, 5-HT(1A) or 5-HT(2C/2B) receptor antagonists. Eur. J. Pharmacol. 2007;569:41–47. doi: 10.1016/j.ejphar.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Oral ET, Vahip S. Bipolar depression: an overview. IDrugs. 2004;7:846–850. [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol. Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Post RM, Leverich GS, Nolen WA, Kupka RW, Altshuler LL, Frye MA, Suppes T, McElroy S, Keck P, Grunze H, Walden J. A re-evaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Foundation Bipolar Network. Bipolar Disord. 2003;5:396–406. doi: 10.1046/j.1399-5618.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol. Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- Reynolds PD, Ruan Y, Smith DF, Scammell JG. Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J. Clin. Endocrinol. Metab. 1999;84:663–669. doi: 10.1210/jcem.84.2.5429. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Twardowska K. The dexamethasone/corticotropin-releasing hormone test in depression in bipolar and unipolar affective illness. J. Psychiatr. Res. 1999;33:363–370. doi: 10.1016/s0022-3956(99)00014-x. [DOI] [PubMed] [Google Scholar]
- Sajatovic M, Valenstein M, Blow FC, Ganoczy D, Ignacio RV. Treatment adherence with antipsychotic medications in bipolar disorder. Bipolar Disord. 2006;8:232–241. doi: 10.1111/j.1399-5618.2006.00314.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schneikert J, Hubner S, Martin E, Cato AC. A nuclear action of the eukaryotic cochaperone RAP46 in downregulation of glucocorticoid receptor activity. J. Cell Biol. 1999;146:929–940. doi: 10.1083/jcb.146.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltiel G, Chen G, Manji HK. Neurotrophic signaling cascades in the pathophysiology and treatment of bipolar disorder. Curr. Opin. Pharmacol. 2007;7:22–26. doi: 10.1016/j.coph.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW, Finn CT. Family, twin, and adoption studies of bipolar disorder . Am. J. Med. Genet. C. Semin. Med. Genet. 2003;123:48–58. doi: 10.1002/ajmg.c.20013. [DOI] [PubMed] [Google Scholar]
- Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Zimmerman ME, Getz GE, Mills NP, Ret J, Shear P, Adler CM. Ventricular and periventricular structural volumes in first- versus multiple-episode bipolar disorder. Am. J. Psychiatry. 2002;159:1841–1847. doi: 10.1176/appi.ajp.159.11.1841. [DOI] [PubMed] [Google Scholar]
- Takayama S, Sato T, Krajewski S, Kochel K, Irie S, Millan JA, Reed JC. Cloning and functional analysis of BAG-1: a novel Bcl-2-binding protein with anti-cell death activity. Cell. 1995;80:279–284. doi: 10.1016/0092-8674(95)90410-7. [DOI] [PubMed] [Google Scholar]
- Tohen M, Zhang F, Taylor CC, Burns P, Zarate C, Sanger T, Tollefson G. A meta-analysis of the use of typical antipsychotic agents in bipolar disorder. J. Affect. Disord. 2001;65:85–93. doi: 10.1016/s0165-0327(00)00162-2. [DOI] [PubMed] [Google Scholar]
- van Rossum EF, Binder EB, Majer M, Koper JW, Ising M, Modell S, Salyakina D, Lamberts SW, Holsboer F. Polymorphisms of the glucocorticoid receptor gene and major depression. Biol. Psychiatry. 2006;59:681–688. doi: 10.1016/j.biopsych.2006.02.007. [DOI] [PubMed] [Google Scholar]
- van West D, Van Den Eede F, Del-Favero J, Souery D, Norrback KF, Van Duijn C, Sluijs S, Adolfsson R, Mendlewicz J, Deboutte D, Van Broeckhoven C, Claes S. Glucocorticoid receptor gene-based SNP analysis in patients with recurrent major depression. Neuropsychopharmacology. 2006;31:620–627. doi: 10.1038/sj.npp.1300898. [DOI] [PubMed] [Google Scholar]
- Vermeer H, Hendriks-Stegeman BI, van der Burg B, van Buul-Offers SC, Jansen M. Glucocorticoid-induced increase in lymphocytic FKBP51 messenger ribonucleic acid expression: a potential marker for glucocorticoid sensitivity, potency, and bioavailability. J. Clin. Endocrinol. Metab. 2003;88:277–284. doi: 10.1210/jc.2002-020354. [DOI] [PubMed] [Google Scholar]
- Wassef A, Smith EM, Rose RM, Gardner R, Nguyen H, Meyer WJ. Mononuclear leukocyte glucocorticoid receptor binding characteristics and down-regulation in major depression. Psychoneuroendocrinology. 1990;15:59–68. doi: 10.1016/0306-4530(90)90047-d. [DOI] [PubMed] [Google Scholar]
- Watson S, Thompson JM, Ritchie JC, Nicol Ferrier I, Young AH. Neuropsychological impairment in bipolar disorder: the relationship with glucocorticoid receptor function. Bipolar Disord. 2006;8:85–90. doi: 10.1111/j.1399-5618.2006.00280.x. [DOI] [PubMed] [Google Scholar]
- Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S, Robinson TE, Watson SJ, Seasholtz AF, Akil H. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11851–11856. doi: 10.1073/pnas.0402208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Hebda-Bauer EK, Pletsch A, Luo J, Hoversten MT, Osetek AJ, Evans SJ, Watson SJ, Seasholtz AF, Akil H. Overexpressing the glucocorticoid receptor in forebrain causes an aging-like neuroendocrine phenotype and mild cognitive dysfunction. J. Neurosci. 2007;27:8836–8844. doi: 10.1523/JNEUROSCI.0910-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willour VL, Chen H, Toolan J, Belmonte P, Cutler DJ, Goes FS, Zandi PP, Lee RS, Mackinnon DF, Mondimore FM, Schweizer B, Depaulo JR, Jr, Gershon ES, McMahon FJ, Potash JB. Family-based association of FKBP5 in bipolar disorder. Mol. Psychiatry. 2008 doi: 10.1038/sj.mp.4002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Tohen M. Antipsychotic drug treatment in first-episode mania: a 6-month longitudinal study. J. Clin. Psychiatry. 2000;61:33–38. doi: 10.4088/jcp.v61n0109. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Quiroz JA. Combination treatment in bipolar disorder: a review of controlled trials. Bipolar Disord. 2003;5:217–225. doi: 10.1034/j.1399-5618.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Tohen M. Double-blind comparison of the continued use of antipsychotic treatment versus its discontinuation in remitted manic patients. Am. J. Psychiatry. 2004;161:169–171. doi: 10.1176/appi.ajp.161.1.169. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Tohen M, Narendran R, Tomassini EC, McDonald J, Sederer M, Madrid AR. The adverse effect profile and efficacy of divalproex sodium compared with valproic acid: a pharmacoepidemiology study. J. Clin. Psychiatry. 1999;60:232–236. doi: 10.4088/jcp.v60n0405. [DOI] [PubMed] [Google Scholar]
- Zhou R, Gray NA, Yuan P, Li X, Chen J, Chen G, Damschroder-Williams P, Du J, Zhang L, Manji HK. The anti-apoptotic, glucocorticoid receptor cochaperone protein BAG-1 is a long-term target for the actions of mood stabilizers. J. Neurosci. 2005;25:4493–4502. doi: 10.1523/JNEUROSCI.4530-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]