Abstract
The extracellular signal-regulated kinase (ERK) pathway mediates neuronal plasticity in the CNS. The mood stabilizers lithium and valproate activate the ERK pathway in prefrontal cortex and hippocampus and potentiate ERK pathway-mediated neurite growth, neuronal survival and hippocampal neurogenesis. Here, we examined the role of the ERK pathway in behavioral plasticity related to facets of bipolar disorder. Mice with ERK1 ablation acquired reduced phosphorylation of RSK1, an ERK substrate, in prefrontal cortex and striatum, but not in hippocampus or cerebellum, indicating the ablation-induced brain region-specific ERK signaling deficits. ERK1 ablation produced a behavioral excitement profile similar to that induced by psychostimulants. The profile is characterized by hyperactivity, enhanced goal-directed activity and increased pleasure-related activity with potential harmful consequence. ERK1-ablated mice were hyperactive in multiple tests and resistant to behavioral despair in the forced swim test. These mice displayed more home-cage voluntary wheel running activities, rearings in a large arena and open-arm visits in an elevated plus maze. Treatments with valproate and olanzapine, but not lithium reduced baseline activities in ERK1-ablated mice. All three treatments attenuated amphetamine-induced hyperactivity in ablated mice. These data indicate a profound involvement of ERK1 signaling in behavioral excitement and in the behavioral action of antimanic agents. The extent to which ERK pathway perturbation contributes to the susceptibility, mood switch mechanism(s) and symptom pathophysiology of bipolar disorder requires further investigation. Whether there is a shared mechanism through which mood stabilizers produce their clinical actions on mood, thought and behavioral symptoms of mania also requires further investigation.
Keywords: ERK, neurotrophin, lithium, valproate, mania, depression
Introduction
The extracellular signal-regulated kinase (ERK) pathway is a tri-kinase cascade consisting of ERK1/2, activated by MEK1/2, downstream effectors of Raf-1 and B-raf. Importantly, the ERK pathway is at the nexus of multiple neuronal signaling cascades, and has been postulated to represent a signal network integrator underlying certain forms of synaptic plasticity (for review see Kelleher et al., Thomas and Huganir, Sweatt1–3). Activated ERK phosphorylates numerous proteins involved in a diverse array of cellular processes including epigenetic, transcriptional and translational regulation, dendritic organization, cellular excitability, long-term potentiation and long-term depression, neuronal survival and synaptogenesis, and neurotransmitter release.1–4 The ERK pathway is a major intracellular signaling cascade mediating the biological effects of neurotrophic factors such as brain-derived neurotrophic factor.5 Neurotrophic factors have increasingly been implicated as playing important roles in the pathophysiology and treatment of mood disorders.4,6–8
At the behavioral level, a number of studies have been undertaken to investigate the role of the ERK pathway in mediating cognitive function. Treatment of rodents with MEK inhibitor impairs memory consolidation and/or reconsolidation in various paradigms;1–3 however, selective ERK1 knockout (KO) mice show no such deficits.9,10 By contrast, pharmacological inhibition of MEK in ERK1 KO mice impairs reconsolidation of fear memories.11 These data demonstrate a central role for ERK2 in memory formation. Despite its widespread expression in the neocortex, the role of ERK1 in mediating cognitive and/or affective function remains to be elucidated.
The commonly used treatments for bipolar disorder, lithium and valproate, exert their effects by treating various facets of the manic phase of the illness, that is, euphoric or irritable mood, abnormal thought content and processing and behavioral excitement.12,13 Behavioral excitement in manic patients manifests as racing speech, sleep reduction, increased goal-directed behavior, excessive pursuits of hedonic activities even with painful consequences, aggression and physical hyperactivity.12,13 Understanding the mechanisms by which these agents exert their effects on behavioral excitement in the laboratory has the potential to lead to the development of improved therapeutics, and perhaps also to provide insights into the pathophysiology of the illness. However, these agents do not exert major neurotransmitter receptor effects, and the mechanisms by which these structurally simple molecules exert their profound behavioral effects have remained unclear.6,13,14 In this context, it is noteworthy that recent studies have demonstrated that these two structurally highly dissimilar antimanic agents activate the ERK signaling cascade in discrete regions of rodent brain and in neuronal cells in vitro (for details see4,15 and reference therein). Lithium and valproate also enhance cellular functions induced by ERK activation such as neurogenesis, neurite growth and neuronal survival (for details see4,15–17) and reference therein). Furthermore, upstream inhibition of the ERK pathway by biochemical or molecular strategies blocks ERK activation by valproate in vitro. 18,19 These findings suggest that the ERK pathway may play a role in mediating some behavioral facets of mood disorders. In view of the important role of the ERK cascade as a signal integrator in the CNS, a major regulator of synaptic plasticity, and a putative brain region-specific mediator of actions of mood modulators, we undertook a series of studies using genetic strategies to investigate the potential role of ERK subtypes in regulating affective behavioral modulation.
Materials and methods
Animals and treatments
All animal experiments were approved by the NIH Animal Care and Use Committee in accordance with NIH guidelines on the Care and Use of Animals. ERK1 KO mice were generated by standard methods.9 In brief, mice were generated on a 129SvIm/J × CD1 background and backcrossed to and maintained on the 129SvIm/J background. Wild-type (WT) and KO mice were maintained on a 12 h light/dark cycle with food and water ad libitum. Only male mice were used in the study to avoid the possible influence of the menstrual cycle on fluctuations in activity.
Lithium was administered orally in the feed (chow) (Bio-Serv, Frenchtown, NJ, USA) as previously described.20,21 Through an unknown molecular mechanism, lithium given IP or PO within a short period causes nausea, which is the foundation of lithium-induced conditioned taste aversion. However, we use lithium-containing chow to deliver lithium slowly and constantly to the animals. The feed is well tolerated by rodents, and over time their body weights increase as with normal chow. Our treatment chow contained 2.4 g kg−1 lithium carbonate. Treatment as described maintained blood lithium levels within the human therapeutic window (0.5–1.2 mEql−1) and did not cause body weight differences between the groups over weeks of treatment, suggesting the treated mice did not consume less food than control chow-treated mice. Chronic valproate treatment was carried out using sodium valproate-treated chow (24 g kg−1) using previously described methods.19 Olanzapine was dissolved in 1 n HCl, diluted with water, and pH adjusted with 1 n NaOH towards neutrality (about pH 6) without any precipitation. Vehicle and olanzapine solutions (2 mg kg−1) were injected IP (10 ml kg−1) 30 min before the behavioral tests in which they were used.
Western blot analysis
Mice were euthanized between 0800 and 1200 h to minimize potential circadian effects on phospho-ERK and phospho-RSK levels. Western blot analyses of brain samples were conducted as previously described.19,21 Because the commercially available phospho-RSK1 antibody we used in the study also recognizes RSK3 (www.cellsignal.com/pdf/9344.pdf), we used a commercially available phospho-RSK3 antibody to distinguish between RSK1 and RSK3 protein levels. DAT antibody was from Chemi-con International Inc, Temecula, CA, USA.
Elevated plus maze test
The test was conducted similarly to that described by Crawley et al.22 The elevated plus maze consisted of four Plexiglas arms (5 × 30cm). Each of the two opposing open arms was equipped with a 0.5cm lip. Each of the two opposing closed arms was equipped with a 15cm wall. The entire apparatus was situated 40cm above the floor. Mice were placed in the center of an elevated plus maze facing one of the open arms and permitted to explore the maze for 5 min. The number of entries into each arm and time spent in the open versus closed arms was measured.
Forced swim test
The test was conducted similarly to that described by Cryan et al.23 Mice were placed in a transparent Plexiglas cylindrical tank (36cm in height and 20cm diameter) filled to a depth of 17cm with water (22–23 °C) for 6 min. The mice were unable to escape or rest by touching the bottom of the tank. Water in the tank was changed after each session. Time spent swimming and immobile or floating in the water was measured during the last 4 min of the session.
Exploratory behavior
Exploratory behavior was measured over a 45 min period in a large open field (120 × 120 cm) made of Plexiglas similar to that previously described in the literature.21,24 Distance traveled, time spent in the center of the field and number of rearings was measured using the Ethovision tracking system.
Long-term home-cage voluntary wheel running activity
Long-term voluntary wheel running activity was measured by housing mice individually in cages equipped with a running wheel for 4 weeks and measuring the number of wheel turns per day using ClockLab software (Actimetrics, Wilmette, IL, USA).
Behavioral response to amphetamine
D-amphetamine (Sigma, St Louis, MO, USA) was dissolved in saline. Mice received a single IP injection of amphetamine (3 mg kg−1, 10ml kg−1) or saline (10 ml kg−1). The dose of amphetamine was chosen for its ability to increase locomotor activity without inducing stereotypic behavior. Amphetamine-induced locomotor activity was measured in Plexiglas activity boxes (35 × 35 cm) using the Ethovision tracking system. Drug-naive mice received either amphetamine or saline after a baseline 30 min of activity was established followed by another 30 min of monitored activity.
Monitoring the behavioral changes induced by lithium, valproate or olanzapine treatment
The effect of lithium, valproate or olanzapine treatments on baseline and amphetamine-induced locomotor activities of WT and ERK1 KO mice were measured using activity chambers (40 × 40 cm) equipped with photo beams (Opto-Varimex, Columbus Instruments, Columbus, OH, USA). Two chambers were used to monitor activities of treated and vehicle control mice of the same genotype simultaneously. Baseline activities of the mice were measured in the activity chambers for 30 min. After the baseline period, mice were injected with amphetamine and returned to the activity chamber. The activity was recorded for an additional 30 min.
Statistical analysis
Statistical analyses were performed by t-test or analysis of variance, followed by Fisher's PLSD or Scheffe's tests. P < 0.05 was considered significant. Data are expressed as the means ± s.e.
Results
ERK1 ablation produces regionally selective effects on ERK signaling
Mice were between 7–8 weeks of age at the start of experiments to approximate the age in humans when susceptible individuals are most likely to experience their first manic episode.12,13 Similar to observations reported previously,9 ERK1 KO mice were viable, fertile and developed normally without any gross anatomical differences from WT littermates (data not shown). Western blot analysis indicated a complete lack of ERK1 in the KO mice in all brain regions examined (Figure 1). Consistent with previously reported observations,9,10 total and activated phospho-ERK2 levels were not significantly changed in the four brain regions examined in ERK1 KO mice. Phospho-RSK1/3 levels of ERK1 KO mice were significantly decreased in prefrontal cortex and striatum but not in hippocampus and cerebellum. Phospho-RSK3 levels were not changed in any of the four regions examined. The lack of alterations in phospho-RSK3 in the prefrontal cortex and striatum demonstrates that the alterations in phospho-RSK1/3 are primarily due to phospho-RSK1 changes. Thus, the lack of ERK1 expression resulted in decreased ERK signaling to at least one of its downstream targets, RSK1, in the prefrontal cortex and striatum.
Figure 1.
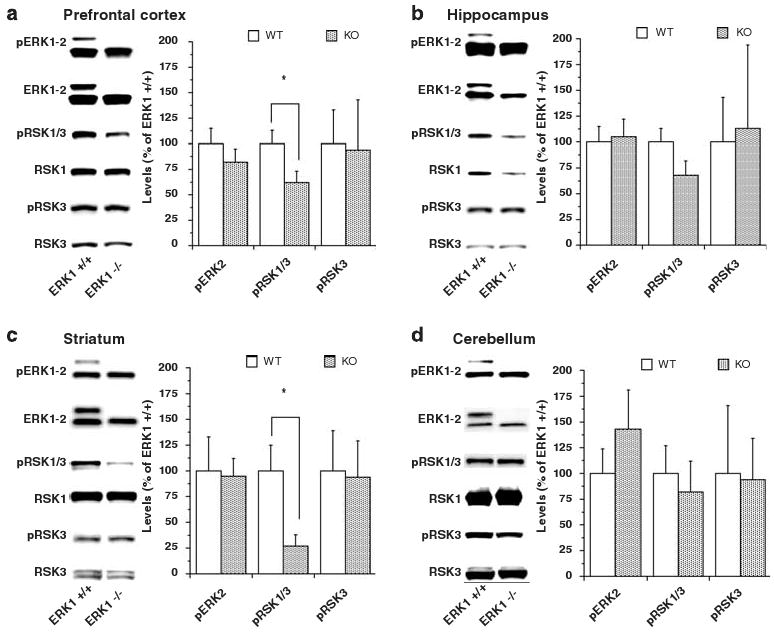
Levels of activated phospho-ERK1/2 (pERK1/2), activated phospho-RSK1/3 (pRSK1/3), activated phospho-RSK3 (pRSK3), ERK1/2, RSK1 and RSK3 in prefrontal cortex (a) hippocampus (b) striatum and (c) cerebellum (d) of wild-type (WT) and ERK1 knockout (KO) mice. Brain samples were extracted on ice, immediately frozen on dry ice and then stored at −80 °C until further analysis. The samples were prepared in a buffer containing protease inhibitor, phosphatase inhibitor I and II cocktails from Sigma. An equal amount of protein in all samples was loaded onto the gel and antibodies indicated in the figure were used according to the manufacturer's recommendations. Densitometric data are presented in the bar graphs as means ± s.e. ERK1 KO mice showed significant reductions in levels of pRSK1/3 in prefrontal cortex (t(35) = 2.07; P= 0.0455) and striatum (t(18) = 2.57; P= 0.0193). Hippocampal levels of pRSK1/3 did not significantly differ between WT and KO mice (t(36) = 1.655; P= 0.1067). *P < 0.05.
ERK1 KO mice make increased total arm entries in the elevated plus maze
Previous studies have shown that ERK1 KO mice do not differ from WT mice in performance on the rotarod, acoustic startle or prepulse inhibition tests.9 Additionally, they exhibit unimpaired learning in fear conditioning (contextual and cue) and passive avoidance tests.9 However, ERK1 KO mice on both 129Sv and C57BL/6 genetic backgrounds are hyperactive in novel environments.9,10
Because anxiety can affect locomotor activity,22 we used classical measures of anxiety to assess the possible contribution of alterations in anxiety on hyperlocomotor activity of ERK1 KO mice. ERK1 KO mice did not significantly differ from WT mice in the amounts of times spent in the closed or open arms of the elevated plus maze (Figures 2a and b). Consistent with the hyperactivity phenotype, the KO mice displayed a trend increase in the total number of arm entries (Figure 2c). The number of the open arm entries (Figure 2e) and ratio of open versus closed arm entries (Figure 2f) was also increased in the ERK KO mice. The lack of differences in times spent in the open and closed arms between the KOs and the WTs suggests that there were no differences in anxiety-associated behaviors during this experiment. This finding is consistent with the lack of differences in times spent in the center of the open field between the groups in this study (data not shown) and in previous reports.9,10 However, the increased number of open arm entries over that of closed arm entries by the KO mice suggests that the open arms were less aversive for the KOs than the WTs. Further analysis of anxiety-associated behaviors will be required to fully appreciate the role of ERK in this paradigm in these animals.
Figure 2.
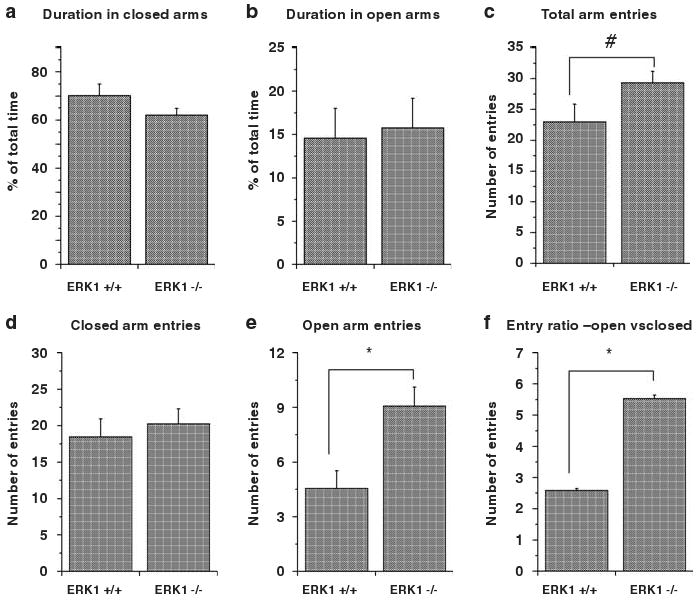
Elevated plus maze performance. Percentages of time spent in the closed arms (a) and open arms (b) did not significantly differ between WT and ERK1 KO mice (t(21) = 1.46, P = 0.159; t(21) = 0.790, P = 0.438). There was a trend increase in total number of arm entries of KO mice compared to wild type mice (c) (t(21) = 1.888, P = 0.073). The numbers of entries to the closed arms did not significantly differ between WT and ERK1 KO mice (d) (t(21) = 0.558, P = 0.523). KO mice significantly entered more times to open arms than WT mice (e) (t(21) = 3.166, P = 0.0047). The KO mice also displayed significant higher ratio of open entries versus closed arm entries (f) (t(21) = 2.115, P = 0.0465). #P < 0.10 and *P < 0.05.
ERK1 KO mice exhibit reduced immobility in the forced swim test
The forced swim test is suggested to be a useful screen for the antidepressant efficacy of a drug.23,25 Antidepressants and amphetamine reduce immobility time and increase swim time of rodents in this test.23,25–27 ERK1 KO mice spent significantly more time swimming and significantly less time immobile in the forced swim test compared to that of WT mice (Figures 3a and b), indicating that the ERK1 KO mice exhibited behaviors associated with antidepressant- and psychostimulant-induced action.
Figure 3.
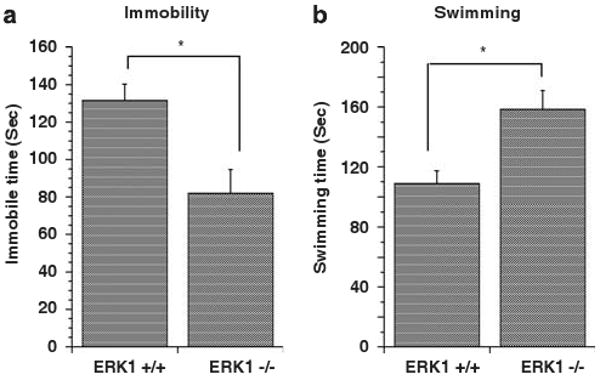
Forced swim test performance. Immobility time (a) was significantly reduced (t(20) = 3.262, P < 0.004) and time spent swimming (b) was significantly increased (t(20) = 3.279, P < 0.004) in ERK1 KO mice compared to WTs. *P < 0.05.
ERK1 KO mice exhibit hyperactivity in an open field test tailored for assessing behaviors related to curiosity or novelty seeking versus fear or self-protection
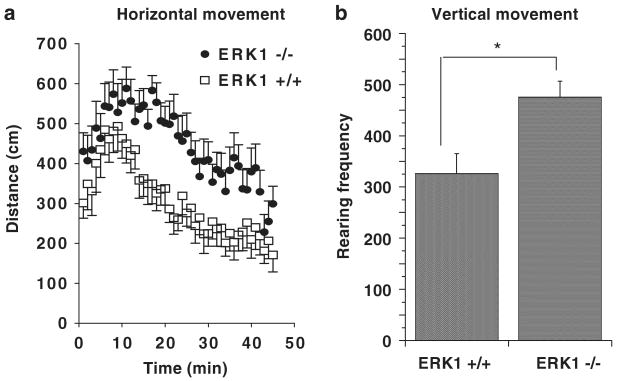
We used a large open field apparatus (120 × 120 cm) as a novel environment to monitor behaviors related to curiosity or novelty seeking versus fear or self protection as described by Decker et al.24 in ERK1 KO and WT mice. ERK1 KO mice exhibited a significant increase in locomotor activity and a significant increase in rearing behavior in the large open field compared to WTs (Figures 4a and b). Similar to the WTs, the ERK1 KOs exhibited clear habituation to the novelty of the environment as indicated by gradual movement reduction in the arena. The KO pattern of activity is consistent with an enhanced explorative behavior rather than pure locomotor unrest. That is, if the KOs were simply hyperlocomoting, they would be expected to maintain similar levels of activities throughout the experiment.
Figure 4.
Spontaneous activity in a large open field. (a) ERK1 KO mice showed increased locomotor activity in a large open field compared to WTs (F(1, 1215) = 160.8, P < 0.0001). (b) ERK1 KO mice exhibited more rearing behavior in the large open field than WTs (t(28) = 2.98, P < 0.006). *P < 0.05.
ERK1 KO mice exhibit increased home cage voluntary wheel running
Studies show that voluntary wheel running shares behavioral and molecular characteristics associated with drug-seeking behaviors28,29 and suggests that voluntary wheel running is a hedonic activity in rodents. We used running wheels attached to mouse home cages to monitor this activity. Although both WT and KO mice showed an increase in voluntary wheel running activity over time, ERK1 KO mice spent significantly more time running and ran a significantly longer distance on the wheel than WT mice during the 30-day period of testing (Figures 5a–c). These data suggest that ERK1 KO mice are more likely to engage in pleasurable activity than WT mice.
Figure 5.
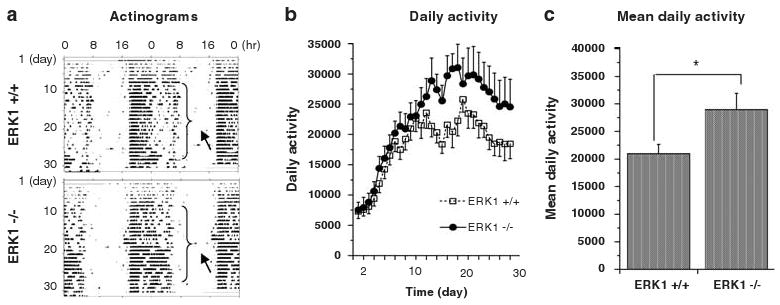
Daily voluntary wheel running activity. (a) Representative actinograms of WTand ERK1 KO mice. Arrows indicate increased daily voluntary wheel running duration in ERK1 KO mouse compared to a WT liter mate. (b) All mice showed increased voluntary wheel running activities over time (F(27, 1501) = 85.87; P < 0.001 for genotype and F(27, 501) = 11.35; P < 0.001 for day). (c) Mean daily voluntary wheel running activity in the home cage over 30 days was greater in ERK1 KO mice (t(58) = 4.125; P < 0.0001). *P < 0.05.
ERKI KO mice exhibit an enhanced response to psychostimulants
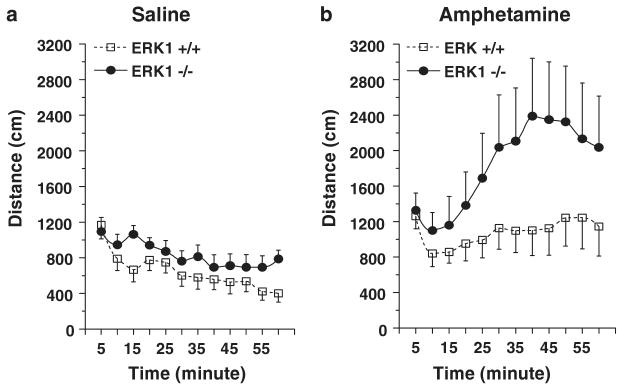
Hyperactivity is a clinical feature of several neuropsychiatric disorders such as attention deficit hyperactivity disorder (ADHD) and mania.30 However, while psychostimulants alleviate ADHD symptoms,31 they can precipitate mania or exacerbate manic symptoms in hypomanic patients.32,33 Indeed, psychostimulant-induced reduction of hyperactivity has been postulated to serve as a model of ADHD.34 To discern the nature of the hyperactivity exhibited by the ERK1 KO mice, we evaluated the locomotor response of ERK1 KO mice to amphetamine. In addition to exhibiting a significantly elevated baseline locomotor activity (Figure 6a), ERK1 KO mice also exhibited a significantly higher locomotor response to amphetamine than the WTs (Figure 6b).
Figure 6.
Effects of saline and amphetamine on locomotor activity. A smaller open field arena (compared to that used to produce data in Figure 4) was used in this set of experiments. (a) Saline-treated ERK1 KO mice were more active than saline-treated WT mice (F(1, 204) = 14.171, P < 0.0002). (b) ERK1 KO mice were more responsive to amphetamine (3 mg/kg, Intraperitoneal (i.p.)) than WT mice (F(1, 204) = 18.870; P < 0.0001).
The hypersensitivity of ERK1 KO mice to the locomotor activating effects of amphetamine suggests that the hyperactivity displayed by these mice is not consistent with that of ADHD. Rather, similar to bipolar patients, ERK1 KO mice exhibit a heightened sensitivity to amphetamine further suggesting that these mice exhibit behaviors associated with mania.
Lithium, valproate and an antipsychotic agent attenuate psychostimulant-induced hyperactivity in ERK1 KO mice
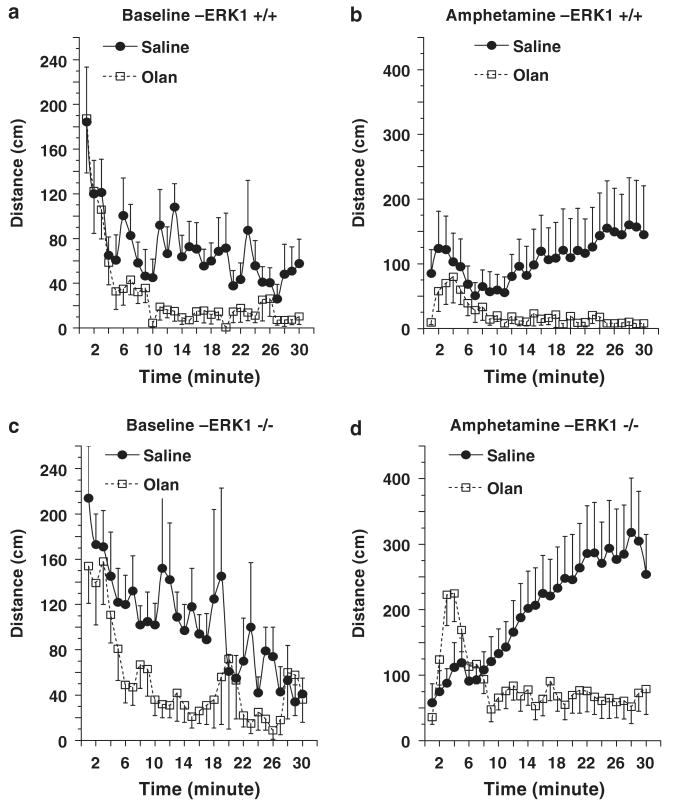
This series of experiments was conducted using computer-assisted automatic activity chambers. Treated and vehicle-control mice of the same genotype were tested simultaneously using two identical chambers. Significant differences on baseline and amphetamine-induced locomotion were detected between vehicle-control WT and KO mice (Supplementary Figure 1). These results are consistent with the data described in Figures 4 and 6, which were obtained using a different apparatus.
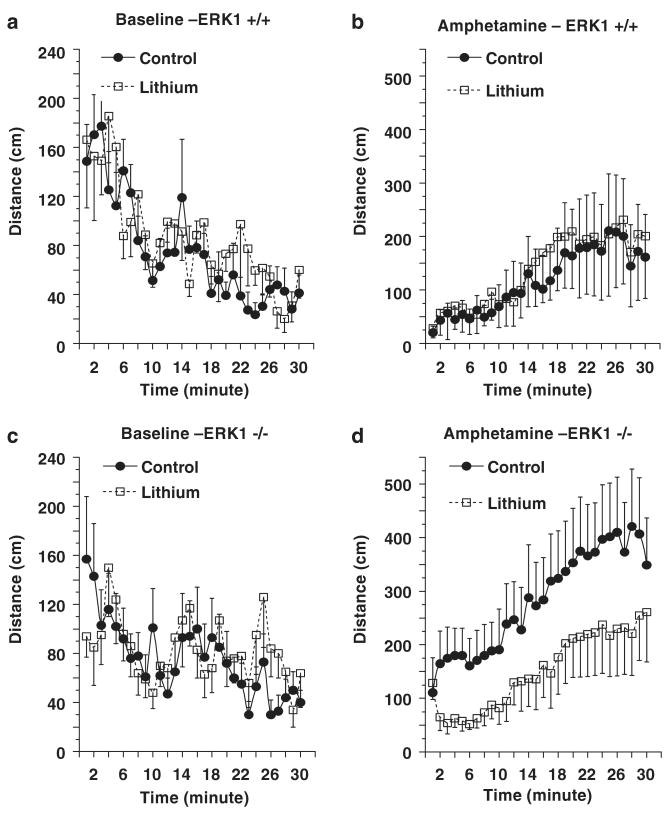
To determine if the behavioral deficits of ERK1 KO mice were responsive to lithium, the WT and KO mice were treated chronically with this agent, achieving therapeutically relevant plasma levels (WT: 0.63 ± 0.02mM; KO: 0.65 ± 0.02 mM). No significant effects of lithium treatment on baseline locomotion or amphetamine-induced hyperactivity were detected in WT mice (Figures 7a and b). These results are similar to those described earlier in mice on the same genetic background.35 Lithium treatment did not significantly alter baseline locomotion of ERK1 KO mice (Figure 7c), but did attenuate the amphetamine-induced locomotion of ERK1 KO mice (Figure 7d).
Figure 7.
Behavioral effects of chronic lithium treatment. Male WTand ERK1 KO mice were chronically treated with regular or lithium-containing chows for 5-week. Baseline activity was measured for 30 min in an activity box, after which the mice were given an IP injection of amphetamine (3 mg/kg, i.p.) and activity measured for an additional 30 min. (a and b) data from WT mice. No significant effects of chronic lithium treatment were detected in WT mice. (c and d) data from ERK1 KO mice. Lithium treatment did not alter baseline activity of ERK1 KO mice. Chronic lithium treatment significantly reduced amphetamine induced activity in ERK1 KO mice (F(1, 540) = 47.25; P < 0.0001).
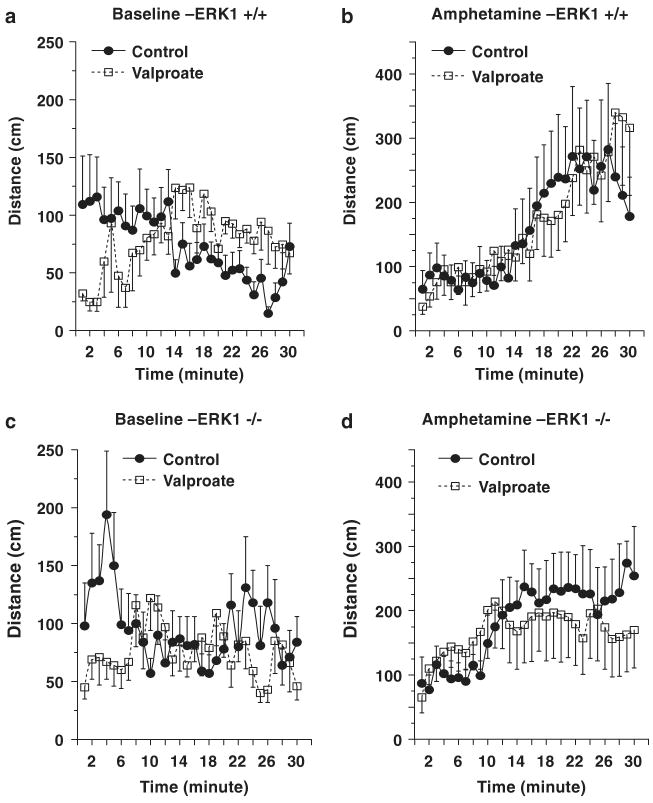
We next determined the extent to which a structurally highly dissimilar antimanic agent, valproate, exerted similar behavioral effects. In contrast to its rapid onset of effects observed in treatment of seizures, elicitation of valproate's antimanic effects require chronic administration; thus, the effects of chronic valproate were investigated in WT and ERK1 KO mice. Blood valproate levels of chronically treated mice were within the therapeutic range (WT: 90 ± 9 μg dl−1; KO: 95 ± 20 μg dl−1). Mice treated with valproate displayed delayed initiation of baseline movements (Figures 8a and c). No significant effects of valproate treatment on overall baseline and amphetamine-induced activities were detected in WT mice (Figures 8a and b). The treatment significantly lowered baseline locomotion and the late-phase (13–30 min) of amphetamine-induced locomotion in ERK1 KO mice (Figures 8c and d).
Figure 8.
Behavioral effects of chronic valproate treatment. Male WT and ERK1 KO mice were fed with regular or valproate-containing chow for 5 weeks. Baseline activity was measured for 30min after which mice received an IP amphetamine injection (3 mg/kg, i.p.) and activity was measured for an additional 30min. (a and b) data from WT mice. No significant overall effects of chronic valproate treatment were detected in WT mice. (c and d) data from ERK1 KO mice. Valproate treatment significantly reduced baseline activities of ERK1 KO mice (F(1, 360) = 7.672; P = 0.0059)(c). The treatment significantly reduced late-phase (13–30 min period) response to amphetamine (F(1, 216) = 4.567; P = 0.0337), although the early phase (1–12min period) and overall responses to amphetamine were not significantly different between the two groups (d).
Antipsychotic agents are generally considered to be broad-spectrum agents in the control of behavioral excitement and exert these effects more rapidly than lithium.36 Olanzapine is an atypical antipsychotic agent which has recently received FDA approval for the treatment of acute mania.13 Acute treatment with olanzapine attenuated baseline activities (Figures 9a and c) and blocked amphetamine-induced activities (Figures 9b and d) in WT and ERK1 KO mice.
Figure 9.
Behavioral effects of acute olanzapine treatment. Male WT and ERK1 KO mice were IP injected with saline or olanzapine (2 mg/kg) 30 min before the amphetamine response test. Baseline activity was measured for 30 min after which mice received an IP amphetamine injection (3 mg/kg, i.p.) and activity was measured for an additional 30 min. (a and b) data from WT mice. (c and d) data from ERK1 KO mice. Olanzapine treatment significantly reduced baseline activities of WT (F(1, 660) = 52.21; P < 0.001) (a) and ERK1 KO mice (F(1, 660) = 35.719; P < 0.001)(c). Olanzapine treatment also significantly reduced amphetamine-induced activities of WT (F(1, 660) = 52.21; P < 0.001) (b) and ERK1 KO mice (F(1, 660) = 90.526; P < 0.0001)(d).
ERK1 KO mice do not exhibit any alterations in dopamine transporter levels
The dopaminergic system is known to regulate various facets of behavioral excitement.12 Dopamine transporter (DAT) KO mice exhibit hyperactivity.34,37 We therefore sought to determine if ERK1 ablation was associated with any alterations in DAT levels in prefrontal cortex and striatum, regions in which ERK1 deletion resulted in ERK-signaling deficits (Figure 1). There were no significant differences in prefrontal cortical or striatal DAT levels between WT and ERK1 KO mice (Figure 10). Moreover, chronic treatment with lithium did not induce significant changes in prefrontal cortical or striatal DAT levels in either WT or ERK1 KO mice (Figure 10).
Figure 10.
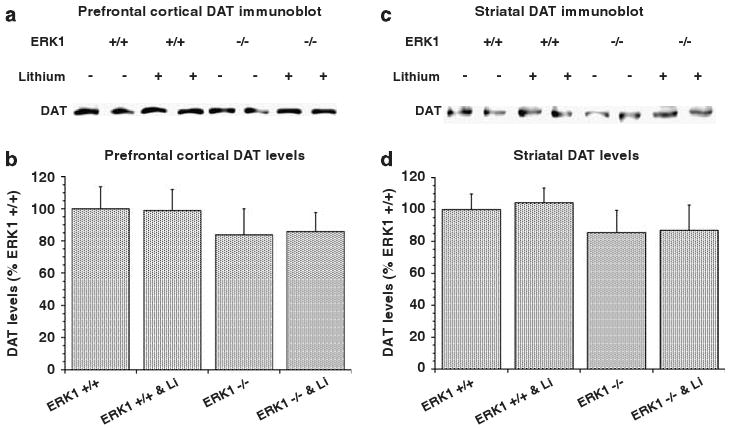
Effects of ERK1 deletion and chronic lithium treatment on DAT levels. WT and ERK1 KO mice were chronically treated with regular or lithium-containing chows as described in Figure 7. DAT levels were measured using the immunoblot method described in Figure 1 in prefrontal cortex (a and b) and striatum (c and d). DAT levels were not significantly different between the groups (c and d).
Discussion
Behavioral excitement as an activity pattern for mood disorder research
Euphoric or irritable mood, abnormalities in thought processing and content and behavioral excitement are core symptoms of mania. For animal research purposes, we define behavioral excitement as an activity pattern consisting of (1) hyperactivity in home cage and a variety of test apparati, (2) increased goal-directed activity which can not be fully explained by hyperactivity alone and (3) increased pleasure-related activity with potential harmful consequence which cannot be fully explained by hyperactivity alone.
We propose that a behavioral excitement pattern in mood disorder-related research should be assessed further in the following two areas, illness relevancy and therapeutic evaluation. Clinically, behavioral excitement, especially the hyperactivity component, could also be considered to be related to conditions such as intoxication and delirium, mental retardation, dementia, schizophrenia, anxiety disorder and ADHD, in addition to mania. Therefore, related tests should be considered for inclusion in a behavioral test battery. To further support the relevancy to mania, responsiveness of the behavioral excitement phenotype to antimanic treatments should be tested. However, because existing mood stabilizers are not always effective or only partially effective in a large portion of manic patients, lack of responsiveness to these medications should not discredit the relevance of this phenotype to mania. As well, the knocked out gene product inducing the behavioral excitement phenotype may be essential for mood stabilizer efficacy.
Psychostimulants induce a range of behavioral alterations in a number of rodent behavioral tests, which is consistent collectively with the pattern of behavioral excitement and are commonly used in rodent behavioral models of mania38,39 (Supplementary Table 1). The model is based on the fact that psychostimulants induce mania relapse in remitted subjects and worsen manic symptoms in hypomanic patients.38,39 Relevance of the model to mania is strongly supported by the fact that mood stabilizers alleviate the induced behavioral changes.38,39 Recent studies show that both the behavioral alterations induced by psychostimulants and the responses of the alterations to lithium treatment are strain/genetic-dependent.35 Psychostimulants induce psychotic symptoms in susceptible subjects.38,39 These agents, at higher dosages, relative to those used in mania and drug addiction paradigms, induce stereotypy in the open field test and deficits in the prepulse inhibition test. Both tests are considered to measure symptoms of schizophrenia.38,39
Emerging behavioral data suggest that behavioral excitement represents a molecular deficit-specific phenomenon.40–44 Furthermore, hyperlocomotion, although a component of the behavioral excitement phenotype, is not always concomitant with nor predicts excitement outcomes in other tests. For instance, NR2A NMDA receptor deletion in rodents increases total and center activity in the open field test, increases exploratory activity in the elevated plus maze test and reduces immobility in the forced swim test.40 Although deletion of NR2D NMDA receptor in rodents induces changes similar to those induced by NR2A receptor deletion in the elevated plus maze and the forced swim tests, it also induces reductions in total activity and rearing in the open field test.41 Opioid agonists produce euphoria in some human subjects. Deletion of μ-opioid receptors does not affect open field activity, but does increase open arm activity in the elevated plus test and reduces immobility in the forced swim test.42 Deletion of δ-opioid receptors induces increased activity in the open field test, decreased open arm activity in the elevated plus maze test and increased immobility in the forced swim test.42 Deletion of the GABA B(1) receptor does not change overall activity in the open field test, but does induce anxiety-like behavior in the elevated plus maze test and antidepressant-like behavior in the forced swim test.43 Deletion of GABA B(2) receptors induces behavioral changes similar to those induced by depletion of GABA B(1) receptors.44
ERK1 ablation causes brain region-specific effects on ERK signaling
Previous studies have shown that deletion of ERK1 does not result in compensatory changes in ERK2 in several brain regions.9,10 Similar biochemical results were also obtained in the present study for prefrontal cortex, hippocampus, striatum and cerebellum of ERK1 KO mice. The present study used phosphorylation of an ERK substrate, RSK, as an indicator of functional ERK signaling. The data showed that ERK1 ablation resulted in an impairment of ERK signaling in prefrontal cortex and striatum, but not in hippocampus and cerebellum (Figure 1), indicating ERK1 ablation causes an ERK signaling perturbation in these selective brain regions.
ERK1 ablation-induced brain region-specific impairment of ERK signaling is associated with a behavioral excitement phenotype
ERK1 KO mice on a 129Sv background were viable, fertile, devoid of any gross anatomical abnormalities and exhibited normal performances in the rotarod and acoustic startle tests,9 suggesting that ERK1 KO mice do not have obvious biological or neurological impairments.22 Recent studies have demonstrated that the ERK pathway is a target of antimanic agents;18,19,21 notably, these agents exert their effects in the control of behavioral excitement in humans. Thus, the ERK1 KO mice were further phenotyped using a battery of relevant behavioral tests tailored to characterizing behavioral excitement. The ERK1 KO mice exhibited a behavioral excitement profile in all paradigms tested, including the large open field test, the open field test, activity chamber, home-cage voluntary wheel running, the elevated plus maze test, the forced swim test and the amphetamine activity response test. Previous studies also show that ERK1 KO mice spend more time in the drug-paired chamber in a morphine-induced conditioned place preference test10 and exhibit enhanced cocaine-induced psychomotor sensitization.45 This profile is similar in part to that induced by psychostimulants (see Supplementary Table 1 for comparisons) and is consistent with a phenotype characterized by behavioral excitement.
ERK1 KO mice exhibited more risk-taking activities, which, to some extent, could not be fully explained by hyperlocomotion alone. Movement and rearing in a novel environment are considered to be driven by novelty-seeking tendencies that are pitted against the potential for exposure to predators.22 The elevated plus maze offers animals two strategies: exploration in walled (or closed) arms or in open arms. Rodents generally spend more time in the closed arms (Figures 2a and b). Anxiolytics induce greater open arm exploration. Consistent with a hyperactive phenotype, ERK1 KO mice entered more closed and open arms than controls (Figure 2c); however, the increase in total entries was due to increases in entries to the open arms (Figure 2e) not the closed arms (Figure 2d).
Rodents have a natural tendency to run on a wheel, and voluntary wheel running has been shown to induce conditioned place preference and neurochemical changes similar to those induced by psychostimulants.28,29 In the home cage wheel running experiment, mice were offered two choices, stay in the cage or play on the wheel. ERK1 KO mice spent more time running on the wheels (Figure 5a) and ran a longer period of time (Figures 5b and c). The conditioned place preference test monitors pleasure-seeking behavior in rodents.24,28 ERK1 KO mice spend more time in a compartment in which they have been conditioned with morphine10 or cocaine.45 These data suggest that ERK1 KO mice engage in more hedonic and goal-directed activities in addition to being hyperactive.
Psychostimulant-induced psychomotor (or behavior) sensitization is a lithium-sensitive behavioral paradigm.38 ERK1 KO mice on a C57BL/6 background exhibit elevated cocaine-induced psychomotor sensitization.45 The forced swim test is a method for evaluating behavioral effects of antidepressants. Swimming or struggling to escape the water is a type of goal-directed behavior. The ratio of floating versus swimming or struggling during the test is viewed as an index of behavioral despair.25,46,47 Psychostimulants also reduce immobility in this test. The ERK1 KO mice exhibit reduced immobility and increased swimming and struggling in the forced swim test (Figure 3). These data further suggest that ERK1 KO mice display test-specific behavioral changes comparable to those induced by psychostimulants, although hyperactivity as a sole factor for decreased immobility cannot be ruled out.
Illness relevance of ERK1 ablation-associated behavioral excitement
Previous studies have shown that ERK1 KO mice of both 129Sv and C57BJ backgrounds exhibit increased activities in a novel environment,9,10 indicating that ERK1 ablation-associated behavioral excitement is unlikely to be strain-dependent. Furthermore, treatment of adult rats with SL327, a blood–brain barrier-penetrating MEK inhibitor, also induces increases in locomotion in a novel environment and reduces immobility in the forced swim test,21 suggesting that the results obtained with the ERK1 KO mice are not solely due to developmental effects. Nevertheless, the potential contribution of developmental effects induced by ERK1 knockdown requires further investigation.
As previously reported, ERK1 KO mice appear to develop normally and do not show obvious signs of neuroanatomic or neurological abnormalities.9,10 Furthermore, the mice perform well on a variety of simple and complex behavioral tests 9,10(Figures 2–9). Additionally, these mice do not show obvious deficits in memory or prepulse inhibition tests.9 These are tests in which deficits are often observed in models of schizophrenia, mental retardation or dementia.48,49 These lines of evidence do not support the relevancy of the behavioral excitement phenotype in ERK1 KO mice to these clinical conditions.
Psychostimulants are clinically effective treatments for ADHD;34,37 these agents reduce hyperlocomotion in a DAT deletion model34,37 and a neonatal dopaminergic neuron deletion model50,51 of ADHD. In contrast, psychostimulants are known to trigger or exacerbate manic symptoms including behavioral excitement (Goodwin and Jamison, 199012). We therefore investigated the effects of amphetamine in the ERK 1 KO mice. In contrast to ADHD model animals, hyperlocomotion in ERK1 KO mice was further augmented, not attenuated, by amphetamine (Figures 6–9). Antimanic agents tested in this study reduced amphetamine-induced locomotion (Figures 7–9).
Pharmacological evaluation of ERK1 ablation-associated behavioral excitement
No significant effects of chronic lithium treatment on baseline locomotion were detected in ERK1 KO mice (Figure 7a), so the data do not confirm the clinical relevancy of the behavioral excitement phenotype of ERK1 KO mice to mania in humans. However, because lithium is not effective in all manic patients and because ERK1 may be required for lithium's full efficacy, the relevancy of this phenotype to mania cannot be ruled out.
Chronic valproate treatment52,53 and acute olanzapine treatment54,55 reduce locomotor activities in WT mice. These effects could mask or confound the selective effects of these treatments on abnormal hyperactivity. In the present study, chronic valproate treatment caused a delay in locomotor activity (Figure 8a), although the overall locomotor activity was not significantly altered in WT mice (Figure 8a). Significant effects of chronic valproate treatment on locomotion were detected in ERK1 KO mice (Figure 8c). Significant effects of acute olanzapine treatment on locomotion were detected in both WT and ERK1 KO mice (Figures 9a and c). Although a reservation must be taken in linking the behavioral excitement phenotype of ERK1 KO mice to mania in humans, the treatment data indicate that the behaviors exhibited by ERK1 KO mice are controllable by treatments of valproate and olanzapine (Figures 8c and 9c).
Again, in humans, psychostimulants exacerbate manic symptoms including behavioral excitement. However, there is a lack of sufficient published data to assess the effects of chronic mood stabilizer treatments on amphetamine-induced psychological and behavioral changes in healthy human subjects in comparison to bipolar patients. In the present study, significant effects of lithium or valproate treatment on amphetamine-induced locomotion were detected only in the ERK1 KOs (Figures 7 and 8), supporting a pharmacological link between behavioral changes exhibited by the ERK1 KO mice to features of mania.
Implications and limitations of the current study
To facilitate the discoveries of the biological underpinnings of mood disorders and of novel targets for improved mood disorder treatment, several laboratories have been focusing on elucidating the molecular and cellular actions of mood stabilizers and antidepressants in animals to delineate specific neuronal cascades that may impinge on those molecules affected by these disorders.4,6–8,16,56,57 ERK pathway stimulation is a known common action of mood stabilizers, lithium and valproate. The findings from the present study indicate that ERK1 perturbation is associated with a behavioral excitement profile. Together, the data support an effective role for the ERK pathway in behavioral regulation related to bipolar disorder.
In addition to the ERK pathway, there are several well-established direct or indirect targets of mood stabilizers, including IMPase,58 GSK-359 and HDAC.60 Indeed, possible crosstalk between the ERK pathway and the known direct (GSK-3 and HDAC) and indirect (PI3K and PKC pathways) mood stabilizer targets do exist.15 Mood stabilizers, especially valproate, also activate ERK2, another member of the ERK family. Both lithium and valproate activate RSK, a converging point of both ERK and PI3K pathways.4 The effects of mood stabilizers on these molecules may work additively or synergistically with ERK pathway activation in modulating mood stabilizer-sensitive behaviors. For instance, studies show that GSK-3 inhibitors regulate behaviors associated with bipolar disorder, including hyperactivity.61 Therefore, inhibition of GSK-3 may also contribute to a lithium-induced attenuation of amphetamine response in ERK1 KO mice (Figure 7). The potential involvements of other mood stabilizer-targeted molecules and the interactions between these molecules in the control of behavioral excitement require further investigation.
The role of ERK1 in the regulation of mood and thought processes of mania requires further investigation. Bipolar disorder is a unique illness with a spontaneous cycling tendency. The underlying process is speculated to involve mechanisms for susceptibility, mood swing triggering, symptom manifestation and self-limitation. Some of the genes encoding ERK pathway components and their direct targets are located in the chromosome loci implicated in bipolar genetic studies (Supplementary Table 2). In addition to signal transduction, some of these genes are involved in neurotransmitter release, neuronal excitement and circadian rhythm (Supplementary Table 2). However, the involvements of these component and target genes in the course of bipolar disorder need to be directly examined.
Intriguingly, a very recent paper has suggested that ERK may also play a role in mediating some of the behavioral effects of antidepressants.62 Indeed, recent data from several laboratories is beginning to demonstrate that molecules associated with synaptic plasticity (for example, brain-derived neurotrophic factor, tyrosine kinase receptor B or cAMP response element binding protein) can induce variable effects on behavior, depending on the brain region involved.63–74 Although current data support a brain region-specific functional role for ERK1 in behavioral excitement control, this issue needs to be further addressed directly.
In conclusion, the data presented here suggest an important role for the ERK1 signaling pathway in the modulation of behavioral excitement relevant to facets of the manic syndrome. The possibility of targeting this cascade to develop novel therapeutics remains an exciting prospect for the future.
Supplementary Material
Acknowledgments
This project was supported by funding from NIMH-IRP (Manji and Chen) and NARSAD (Chen). The views expressed in this article do not necessarily represent those of NIMH or NIH or the Federal Government.
References
- 1.Kelleher RJ, III, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- 3.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Chen G, Manji HK. The extracellular signal-regulated kinase pathway: an emerging promising target for mood stabilizers. Curr Opin Psychiatry. 2006;19:313–323. doi: 10.1097/01.yco.0000218604.63463.cd. [DOI] [PubMed] [Google Scholar]
- 5.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 6.Coyle JT, Duman RS. Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron. 2003;38:157–160. doi: 10.1016/s0896-6273(03)00195-8. [DOI] [PubMed] [Google Scholar]
- 7.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 8.Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- 9.Selcher JC, Nekrasova T, Paylor R, Landreth GE, Sweatt JD. Mice lacking the ERK1 isoform of MAP kinase are unimpaired in emotional learning. Learn Mem. 2001;8:11–19. doi: 10.1101/lm.37001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W, et al. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron. 2002;34:807–820. doi: 10.1016/s0896-6273(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 11.Cestari V, Costanzi M, Castellano C, Rossi-Arnaud C. A role for ERK2 in reconsolidation of fear memories in mice. Neurobiol Learn Mem. 2006;86:133–143. doi: 10.1016/j.nlm.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin FK, Jamison KR. Manic-Depressive Illness. Oxford University Press; New York: 1990. [Google Scholar]
- 13.Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351:476–486. doi: 10.1056/NEJMra035354. [DOI] [PubMed] [Google Scholar]
- 14.Phiel CJ, Klein PS. Molecular targets of lithium action. Annu Rev Pharmacol Toxicol. 2001;41:789–813. doi: 10.1146/annurev.pharmtox.41.1.789. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, Creson T, Sharon E, Hao Y, Wang G. Neurotrophic actions of mood-stabilizers: a recent research discovery and its potential clinical applications. Curr Psychiatry Rev. 2005;1:173–185. [Google Scholar]
- 16.Chuang DM. Neuroprotective and neurotrophic actions of the mood stabilizer lithium: can it be used to treat neurodegenerative diseases? Crit Rev Neurobiol. 2004;16:83–90. doi: 10.1615/critrevneurobiol.v16.i12.90. [DOI] [PubMed] [Google Scholar]
- 17.Chuang DM. The antiapoptotic actions of mood stabilizers: molecular mechanisms and therapeutic potentials. Ann NY Acad Sci. 2005;1053:195–204. doi: 10.1196/annals.1344.018. [DOI] [PubMed] [Google Scholar]
- 18.Yuan PX, Huang LD, Jiang YM, Gutkind JS, Manji HK, Chen G. The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J Biol Chem. 2001;276:31674–31683. doi: 10.1074/jbc.M104309200. [DOI] [PubMed] [Google Scholar]
- 19.Hao Y, Creson T, Zhang L, Li P, Du F, Yuan P, et al. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci. 2004;24:6590–6599. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. Enhancement of hippocampal neurogenesis by lithium. J neurochem. 2000;75:1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- 21.Einat H, Yuan P, Gould TD, Li J, Du J, Zhang L, et al. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci. 2003;23:7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawley JN. What's Wrong With My Mouse. Wiley-Liss; New York: 2000. [Google Scholar]
- 23.Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 24.Decker S, Grider G, Cobb M, Li XP, Huff MO, El-Mallakh RS, et al. Open field is more sensitive than automated activity monitor in documenting ouabain-induced hyperlocomotion in the development of an animal model for bipolar illness. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:455–462. doi: 10.1016/s0278-5846(99)00111-6. [DOI] [PubMed] [Google Scholar]
- 25.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 26.Cox C, Harrison-Read PE, Steinberg H, Tomkiewicz M. Lithium attenuates drug-induced hyperactivity in rats. Nature. 1971;232:336–338. doi: 10.1038/232336a0. [DOI] [PubMed] [Google Scholar]
- 27.Wieland S, Lucki I. Antidepressant-like activity of 5-HT1A agonists measured with the forced swim test. Psychopharmacology. 1990;101:497–504. doi: 10.1007/BF02244228. [DOI] [PubMed] [Google Scholar]
- 28.Werme M, Messer C, Olson L, Gilden L, Thoren P, Nestler EJ, et al. Delta FosB regulates wheel running. J Neurosci. 2002;22:8133–8138. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lett BT, Grant VL, Koh MT, Flynn G. Prior experience with wheel running produces cross-tolerance to the rewarding effect of morphine. Pharmacol Biochem Behav. 2002;72:101–105. doi: 10.1016/s0091-3057(01)00722-5. [DOI] [PubMed] [Google Scholar]
- 30.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. American Psychiatric Association; Washington DC: 2000. [Google Scholar]
- 31.Reeves G, Schweitzer J. Pharmacological management of attention-deficit hyperactivity disorder. Expert Opin Pharmacother. 2004;5:1313–1320. doi: 10.1517/14656566.5.6.1313. [DOI] [PubMed] [Google Scholar]
- 32.Anand A, Verhoeff P, Seneca N, Zoghbi SS, Seibyl JP, Charney DS, et al. Brain SPECT imaging of amphetamine-induced dopamine release in euthymic bipolar disorder patients. Am J Psychiatry. 2000;157:1108–1114. doi: 10.1176/appi.ajp.157.7.1108. [DOI] [PubMed] [Google Scholar]
- 33.Peet M, Peters S. Drug-induced mania. Drug Saf. 1995;12:146–153. doi: 10.2165/00002018-199512020-00007. [DOI] [PubMed] [Google Scholar]
- 34.Gainetdinov RR, Caron MG. An animal model of attention deficit hyperactivity disorder. Mol Med Today. 2000;6:43–44. doi: 10.1016/s1357-4310(99)01616-0. [DOI] [PubMed] [Google Scholar]
- 35.Gould TD, O'Donnell KC, Picchini AM, Manji HK. Strain differences in lithium attenuation of D-amphetamine-induced hyperlocomotion: a mouse model for the genetics of clinical response to lithium. Neuropsychopharmacology. 2007;32:1321–1333. doi: 10.1038/sj.npp.1301254. [DOI] [PubMed] [Google Scholar]
- 36.Tohen M, Jacobs TG, Feldman PD. Onset of action of antipsychotics in the treatment of mania. Bipolar Disord. 2000;2(Part 2):261–268. doi: 10.1034/j.1399-5618.2000.20307.x. [DOI] [PubMed] [Google Scholar]
- 37.Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- 38.Einat H. Establishment of a battery of simple models for facets of bipolar disorder: a practical approach to achieve increased validity, better screening and possible insights into endophenotypes of disease. Behav Genet. 2007;37:244–255. doi: 10.1007/s10519-006-9093-4. [DOI] [PubMed] [Google Scholar]
- 39.Einat H, Manji HK, Belmaker RH. New approaches to modeling bipolar disorder. Psychopharmacol Bull. 2003;37:47–63. [PubMed] [Google Scholar]
- 40.Boyce-Rustay JM, Holmes A. Genetic inactivation of the NMDA receptor NR2A subunit has anxiolytic- and antidepressant-like effects in mice. Neuropsychopharmacology. 2006;31:2405–2414. doi: 10.1038/sj.npp.1301039. [DOI] [PubMed] [Google Scholar]
- 41.Sakamoto T, Mishina M, Niki H. Mutation of NMDA receptor subunit epsilon 1: effects on audiogenic-like seizures induced by electrical stimulation of the inferior colliculus in mice. Brain Res Mol Brain Res. 2002;102:113–117. doi: 10.1016/s0169-328x(02)00189-4. [DOI] [PubMed] [Google Scholar]
- 42.Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, et al. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- 43.Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. Genetic and pharmacological evidence of a role for GABA(B) receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology. 2004;29:1050–1062. doi: 10.1038/sj.npp.1300413. [DOI] [PubMed] [Google Scholar]
- 44.Mombereau C, Kaupmann K, Gassmann M, Bettler B, van der Putten H, Cryan JF. Altered anxiety and depression-related behaviour in mice lacking GABAB(2) receptor subunits. Neuroreport. 2005;16:307–310. doi: 10.1097/00001756-200502280-00021. [DOI] [PubMed] [Google Scholar]
- 45.Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE. Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacology. 2006;31:2660–2668. doi: 10.1038/sj.npp.1301014. [DOI] [PubMed] [Google Scholar]
- 46.Lucki I, O'Leary OF. Distinguishing roles for norepinephrine and serotonin in the behavioral effects of antidepressant drugs. J Clin Psychiatry. 2004;65(Suppl 4):11–24. [PubMed] [Google Scholar]
- 47.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 48.Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol psychiatry. 2002;7:1039–1053. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- 49.Weeber EJ, Sweatt JD. Molecular neurobiology of human cognition. Neuron. 2002;33:845–848. doi: 10.1016/s0896-6273(02)00634-7. [DOI] [PubMed] [Google Scholar]
- 50.Avale ME, Falzone TL, Gelman DM, Low MJ, Grandy DK, Rubinstein M. The dopamine D4 receptor is essential for hyperactivity and impaired behavioral inhibition in a mouse model of attention deficit/hyperactivity disorder. Mol psychiatry. 2004;9:718–726. doi: 10.1038/sj.mp.4001474. [DOI] [PubMed] [Google Scholar]
- 51.Avale ME, Nemirovsky SI, Raisman-Vozari R, Rubinstein M. Elevated serotonin is involved in hyperactivity but not in the paradoxical effect of amphetamine in mice neonatally lesioned with 6-hydroxydopamine. J Neurosci Res. 2004;78:289–296. doi: 10.1002/jnr.20245. [DOI] [PubMed] [Google Scholar]
- 52.Abulaban FS, Dhariwal MA, al-Bekairi AM, Raza M. Antinociceptive activity of sodium valproate in mice after chronic treatment. Gen pharmacol. 1997;29:463–467. doi: 10.1016/s0306-3623(96)00471-5. [DOI] [PubMed] [Google Scholar]
- 53.Li S, Murakami Y, Wang M, Maeda K, Matsumoto K. The effects of chronic valproate and diazepam in a mouse model of posttraumatic stress disorder. Pharmacol Biochem Behav. 2006;85:324–331. doi: 10.1016/j.pbb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 54.Gleason SD, Shannon HE. Blockade of phencyclidine-induced hyperlocomotion by olanzapine, clozapine and serotonin receptor subtype selective antagonists in mice. Psychopharmacology. 1997;129:79–84. doi: 10.1007/s002130050165. [DOI] [PubMed] [Google Scholar]
- 55.Duncan GE, Moy SS, Lieberman JA, Koller BH. Typical and atypical antipsychotic drug effects on locomotor hyperactivity and deficits in sensorimotor gating in a genetic model of NMDA receptor hypofunction. Pharmacol Biochem Behav. 2006;85:481–491. doi: 10.1016/j.pbb.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gurvich N, Klein PS. Lithium and valproic acid: parallels and contrasts in diverse signaling contexts. Pharmacol Ther. 2002;96:45–66. doi: 10.1016/s0163-7258(02)00299-1. [DOI] [PubMed] [Google Scholar]
- 57.De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–1164. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- 58.Berridge MJ. The Albert Lasker medical awards. Inositol trisphosphate, calcium, lithium, and cell signaling. JAMA. 1989;262:1834–1841. [PubMed] [Google Scholar]
- 59.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- 61.Gould TD, Einat H, Bhat R, Manji HK. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol. 2004;7:387–390. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- 62.Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 63.Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zorner B, Wolfer DP, Brandis D, Kretz O, Zacher C, Madani R, et al. Forebrain-specific trkB-receptor knockout mice: behaviorally more hyperactive than ‘depressive’. Biol psychiatry. 2003;54:972–982. doi: 10.1016/s0006-3223(03)00418-9. [DOI] [PubMed] [Google Scholar]
- 65.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci USA. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 69.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 70.Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, et al. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 71.Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, et al. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Conti AC, Cryan JF, Dalvi A, Lucki I, Blendy JA. cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J Neurosci. 2002;22:3262–3268. doi: 10.1523/JNEUROSCI.22-08-03262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Conti AC, Kuo YC, Valentino RJ, Blendy JA. Inducible cAMP early repressor regulates corticosterone suppression after tricyclic antidepressant treatment. J Neurosci. 2004;24:1967–1975. doi: 10.1523/JNEUROSCI.4804-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.