Abstract
Pineal gland tumors are rare and account for less than 1% of all primary brain tumor diagnoses. Also, they are more commonly seen in pediatric patients than in adults. We analyzed the available SEER data on pineal gland tumors that were diagnosed during the period 1973–2005. The cohort was subdivided into groups on the basis of tumor histology: germ cell tumors, pineal parenchymal tumors, gliomas, and other pineal tumors. Analyses of incidence, survival, factors influencing survival, and treatment modality are provided. Among the 633 patients with pineal tumors, male sex was predominant, i.e., sex ratio was 3:1 for the whole group and 11.8:1 for those with germ cell tumors. The 5-year overall survival (OS) for the cohort was 65% ± 2.1%. Those with germ cell tumors experienced the best survival (OS = 78.9% ± 2.3%), followed by those with gliomas (OS = 61% ± 9.3%), and those with pineal parenchymal tumors (OS = 47.2% ± 4.2%). Non–germ cell tumors, no radiotherapy, and diagnosis before 1993 were the only factors associated with a negative impact on survival. The extent of surgical tumor resection did not affect survival in any histologic subgroup. We conclude that although pineal tumors are histologically diverse, they share some similarities due to their unique location. An aggressive surgical approach should be considered with caution in this region. Further studies on different pineal tumors subtypes are needed.
Keywords: Pineal gland, germ cell tumors, pineal parenchymal tumors, pineoblastoma, incidence, SEER, glioma
INTRODUCTION
Pineal tumors account for 0.5% of all central nervous system (CNS) tumors in adults, 1% in young adults (aged 20–34 years), and 2.7% in children (aged 1–12 years) [1].
Because these cancers are so rare, it has always been difficult to collect a large number of cases to study and compare. Traditionally, descriptions of pineal tumors have been provided by institution-based data in which the number of tumors is limited or through a literature review.
Pineal tumors can be classified as germ cell tumors (GCTs), pineal parenchymal tumors (PPTs), gliomas, atypical rhabdoid/teratoid tumors (AT/RTs), or other tumors such as the most recently described entity, papillary tumors of the pineal region [2]. GCTs are the most common subtype of pineal gland tumor. In the literature, the incidence of GCTs varies from 50% to 75% of tumors in the pineal region [3–5]. These tumors arise from pluripotential germ cells, which normally do not inhabit the pineal gland. Theoretically, these germ cells mistakenly migrate to the pineal gland during embryogenesis. Per the most recent World Health Organization (WHO) [6] CNS tumor classification system, GCTs are further classified into germinomas, which is the most common subtype, and a group of nongerminomatous germ cell tumors (NGGCTs). GCTs can grow as pure forms (i.e., comprising only one cell type) or as mixed forms.
PPTs are the second most common form of pineal tumor. They represent 14% to 27% of tumors in the pineal gland [7]. In the WHO classification of CNS tumors, PPTs are further classified as pineocytoma, PPT of intermediate differentiation, including mixed pineocytoma-pineoblastoma tumors and pineoblastoma [8]. In the literature, the incidence of PPT subtypes varies greatly, i.e., the incidence of pineocytoma ranges from 14% to 60%; that of pineoblastoma is 45%; and that of PPT with intermediate differentiation is 10% [7, 8]. Other CNS tumors can arise from the supporting stroma of the pineal gland. These tumors include gliomas, fibrillary astrocytoma, anaplastic astrocytoma, glioblastoma, and pilocytic astrocytoma [7, 9].
The Surveillance, Epidemiology and End Results (SEER) database provides population-based incidence and survival data for primary malignant tumors collected from 17 registries in the United States. It collects data on patient demographics, primary tumor site, tumor histology type and grade, stage at diagnosis, first course of treatment, and follow-up for vital status and patient survival data [10]. Data generated from this registry allow for a more generalized tumor description, which is especially useful in cases of rare tumor types such as pineal gland tumors.
The aim of this study was to provide a comprehensive review of pineal gland tumors by using the SEER database. The most common tumor subgroups, epidemiologic features of patients, treatment patterns, and overall survival are described. Discussion of the most common pineal gland tumors, GCTs and PPTs and gliomas is also presented.
PATIENTS AND METHODS
Data Source and Study Population
Data of studied patients was obtained from 17 SEER registries. These data include a total of 5,306,606 tumors diagnosed during a 32-year period (January 1973–December 2005). We used the SEER*Stat 6.4.4 program to generate a matrix of all individuals with a primary tumor in the pineal gland. All patients, regardless of diagnosis, were included in this series. Radiologic findings and tumor markers were considered sufficient, in most cases, to diagnose GCTs without the need for histologic confirmation. The following patients were excluded from the analysis: two patients whose follow-up consisted of death certificate/autopsy only, three patients whose diagnosis was noted as “pinealoma," a term used nonspecifically for germinoma or PPT [11–14] in the old CNS tumor classification systems, and five patients with a diagnosis of pineocytoma. Nineteen patients with a diagnosis of primitive neuroectodermal tumor (PNET) were included with the pineoblastoma group [15].
Data Analysis
Surgical details were not available for patients diagnosed before 1983 (n=71) or for ten patients diagnosed after this time point. Cases in which patients underwent total resection (surgical removal of the entire tumor primary site) or radical surgery (surgical removal of the primary tumor site with a resection in continuity with other organs) were labeled “total excision.” We used the classification of “other surgeries” for those cases in which patients underwent tumor destruction; a term used by SEER to describe tumors destroyed by surgery, laser, or cryotherapy with no tissue sent for pathologic confirmation, excisional biopsy, simple/partial resection, debulking, or not otherwise determined.
Tumors were grouped into five main categories according to histologic type: AT/RTs, GCTs, gliomas, PPTs, and others, which include all other pineal tumor types. Tumor grades were assigned based on the fourth edition of the WHO classification for tumors of the CNS [6, 8]. For those cases in which we were not certain of the tumor’s true grade based on the available information, we used the grades provided in the database, as captured by the registries from the original pathology reports.
MedCalc for Windows, version 9.6.4.0 (MedCalc Software, Mariakerke, Belgium) was used to perform statistical calculations. Survival estimates were calculated using the Kaplan-Meier method considering all-cause mortality as an endpoint. Log-rank tests were used to compare survival estimates. The Chi-square test was used to compare categorical variables, and the unpaired t-test was used to compare continuous variables. A Cox multiple-hazards regression was used to conduct multivariate analysis with the following factors in the model: sex, age at diagnosis (≤ 18 years or >18 years), surgery, radiation treatment and year of diagnosis (before or after 1993). The year 1993 was selected as a cut-off point, because the second edition of the WHO system was published then, following the first edition published in 1979. The 1993 WHO system included a more refined classification of CNS tumors as a result of the introduction of immunohistochemistry methods into the practice of neuropathology and the introduction of new entities [16].
RESULTS
Patient Characteristics
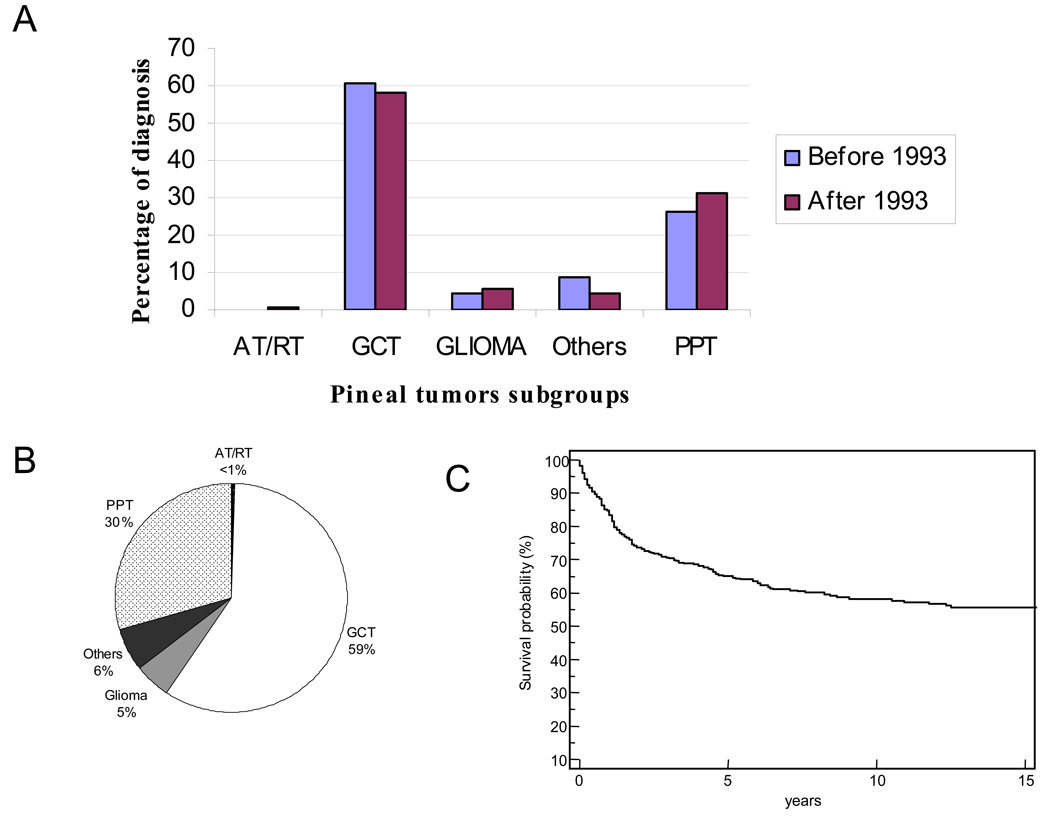
A total of 77,264 CNS tumors were registered with the SEER database between 1973 and 2005. Of those, 633 (0.8%) were pineal tumors. The cohort consisted of 477 (75%) males and 156 (25%) females (Table 1). The median age for the cohort was 17 years (range, 0–83 years); 56% of the patients were 18 years or younger at the time of diagnosis. There was no significant difference between the diagnosis and subtyping of the major tumor groups before and after 1993 (Figure 1A). However, the number of tumors categorized in the “other” group decreased after 1993, reflecting the better classification system after the introduction of immunostaining.
Table I.
Patient Characteristics
| Variable | All | (%) | AT/RT | (%) | GCT | (%) | Glioma | (%) | PPT | (%) | Othersa | (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | 633 | 3 | 373 | 32 | 187 | 38 | ||||||
| Age (years) | ||||||||||||
| Median | 17 | 1 | 16 | 38 | 21 | 37 | ||||||
| Range | 0–83 | 0–19 | 1–77 | 3–75 | 0–82 | 1–83 | ||||||
| Children | 355 | (56) | 2 | (67) | 237 | (64) | 9 | (28) | 96 | (51) | 11 | (29) |
| Adults | 278 | (44) | 1 | (33) | 136 | (36) | 23 | (72) | 91 | (49) | 27 | (71) |
| Sex | ||||||||||||
| Female | 156 | (25) | 1 | (33) | 29 | (8) | 14 | (44) | 100 | (53) | 12 | (32) |
| Male | 477 | (75) | 2 | (67) | 344 | (92) | 18 | (56) | 87 | (47) | 26 | (68) |
| Race | ||||||||||||
| Black | 76 | (12) | 0 | (0) | 28 | (8) | 2 | (6) | 42 | (22) | 4 | (11) |
| Others/Unknown | 85 | (13) | 0 | (0) | 71 | (19) | 3 | (9) | 9 | (5) | 2 | (5) |
| White | 472 | (74) | 3 | (100) | 274 | (73) | 27 | (84) | 136 | (73) | 32 | (84) |
| Grade | ||||||||||||
| I | 25 | (4) | 0 | (0) | 0 | (0) | 8 | (25) | 17 | (9) | 0 | (0) |
| II | 11 | (2) | 0 | (0) | 0 | (0) | 4 | (13) | 7 | (4) | 0 | (0) |
| III | 14 | (2) | 0 | (0) | 0 | (0) | 3 | (9) | 11 | (6) | 0 | (0) |
| IV | 547 | (86) | 3 | (100) | 373 | (100) | 9 | (28) | 152 | (81) | 10 | (26) |
| unknown | 36 | (6) | 0 | (0) | 0 | (0) | 8 | (25) | 0 | (0) | 28 | (74) |
| Radiation (n=616) | ||||||||||||
| None | 151 | (25) | 2 | (67) | 66 | (18) | 12 | (39) | 52 | (29) | 19 | (51) |
| Given | 465 | (75) | 1 | (33) | 297 | (82) | 19 | (61) | 130 | (71) | 18 | (49) |
| Surgery (n=552) | ||||||||||||
| No surgeryb | 224 | (41) | 1 | (33) | 151 | (46) | 12 | (41) | 46 | (27) | 14 | (58) |
| Total excision | 32 | (6) | 2 | (67) | 17 | (5) | 1 | (3) | 11 | (6) | 1 | (4) |
| Others/Unknown | 296 | (54) | 0 | 0 | 158 | (48) | 16 | (55) | 113 | (66) | 9 | (38) |
| Follow up | ||||||||||||
| Median | 3.5 | 1 | 4.8 | 1.5 | 2.4 | 1.1 | ||||||
| Range | 0–31.9 | 0.8–1.3 | 0–30.2 | 0–17.3 | 0–26 | 0–31.9 | ||||||
| Status | ||||||||||||
| Alive | 401 | (63) | 1 | (33) | 282 | (76) | 21 | (66) | 84 | (45) | 13 | (34) |
| Dead | 232 | (37) | 2 | (67) | 91 | (24) | 11 | (34) | 103 | (55) | 25 | (66) |
| Median Survival (years) | 19.3 | 1.0 | 27.7 | NA | 4.5 | 1.3 | ||||||
| 5-year OS (%) | 65.1 | 33.2 | 78.9 | 61.0 | 47.2 | 37.9 | ||||||
| ±SE | 2.1 | 27.2 | 2.3 | 9.3 | 4.2 | 2.1 |
The “others” group includes all other pineal tumors.
Includes cases in which biopsy only was listed as the surgical data.
NA, not applicable ; median survival is not reached yet.
Abbreviations: AT/RT, atypical rhabdoid/teratoid tumor; GCT, germ cell tumor; PNET, primitive neuroectodermal tumor; PPT, pineal parenchymal tumor
Figure 1.
(A) Histologic subtypes of pineal tumors in patients. Patients were divided into two groups on the basis of diagnosis era: those diagnosed before 1993 (blue bars) and those diagnosed after 1993 (red bars). (B) The percentages of different pineal tumor histologic subtypes. (C) Kaplan-Meier analysis showing overall survival of the entire cohort. The end point was all-cause mortality. Abbreviations: AT/RT, atypical rhabdoid/teratoid tumor; GCT, germ cell tumor; PPT, pineal parenchymal tumor.
GCTs (n=373) and PPTs (n=187) were the two most common pineal tumor subtypes, and when grouped together, they accounted for 89 % of all of the pineal tumors (Figure 1B). Histologic confirmation was available for the majority of patients 561/633 (89%). Of the 72 patients diagnosed without histologic confirmation, 46 (64%) were those with GCT. The majority (86%) of the tumors were assigned to grade IV, including GCTs diagnosed without microscopic confirmation.
Only two modalities of treatment were documented in the registry, namely surgery and radiotherapy. Surgical data were available for 552 patients: 224 patients (41.0%) had no surgery; 32 (6.0%) underwent total excision; and 296 (54.0%) had other surgeries. Most patients (75%) received radiotherapy, which was used most frequently to treat GCTs (82%).
The median follow-up period for the cohort was 3.5 years (range, 0–31.9 years). The probability of 5-year overall survival for the cohort was 65.1% ± 2.1 (Figure 1C), and the median survival time was 19.3 years.
Factors that Influenced Overall Survival
Female sex, age older than 18 years, non-GCT histologic subtype, diagnosis before 1993, and no treatment with radiotherapy were all significantly associated with worse survival (P <0.05) (Table 2). On the other hand, the extent of tumor resection did not affect survival of the whole group. The 5-year overall survival (OS) rate for those who did not undergo surgery was 70.7%, and that for patients who did was 67.0% (P = 0.76).
Table 2.
Univariate and Multivariate Analyses of Factors that Influence Survival
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Factor | HR | 95% CI | P* | HR | 95% CI | P* |
| Female sex | 1.43 | 1.06–1.94 | 0.021* | 0.75 | 0.55–1.02 | 0.065 |
| Age >18 years | 1.40 | 1.08–1.82 | 0.010* | 1.16 | 0.90–1.50 | 0.26 |
| Histology other than GCT | 2.86 | 2.21–3.69 | <0.001* | 3.36 | 2.48–4.55 | <0.001* |
| Diagnosis before 1993 | 1.53 | 1.16–2.01 | 0.0024* | 1.67 | 1.27–2.19 | <0.001* |
| No Radiotherapy | 2.09 | 1.50–2.91 | <0.001* | 1.45 | 1.09–1.92 | 0.011* |
| Surgery other than total excision | 0.90 | 0.48–1.72 | 0.76 | 0.97 | 0.49–1.91 | 0.92 |
| Grade III or IV | 0.80 | 0.37 to 1.74 | 0.57 | 1.28 | 0.85 to 1.93 | 0.25 |
P-values ≤0.05 were considered statistically significant.
The same factors were tested in a multivariate model using a Cox proportional-hazards regression: non-GCT histology, diagnosis before 1993, and no treatment with radiotherapy were the only factors that had a significantly negative impact on survival (P <0.05; Table 2).
Germ Cell Tumors
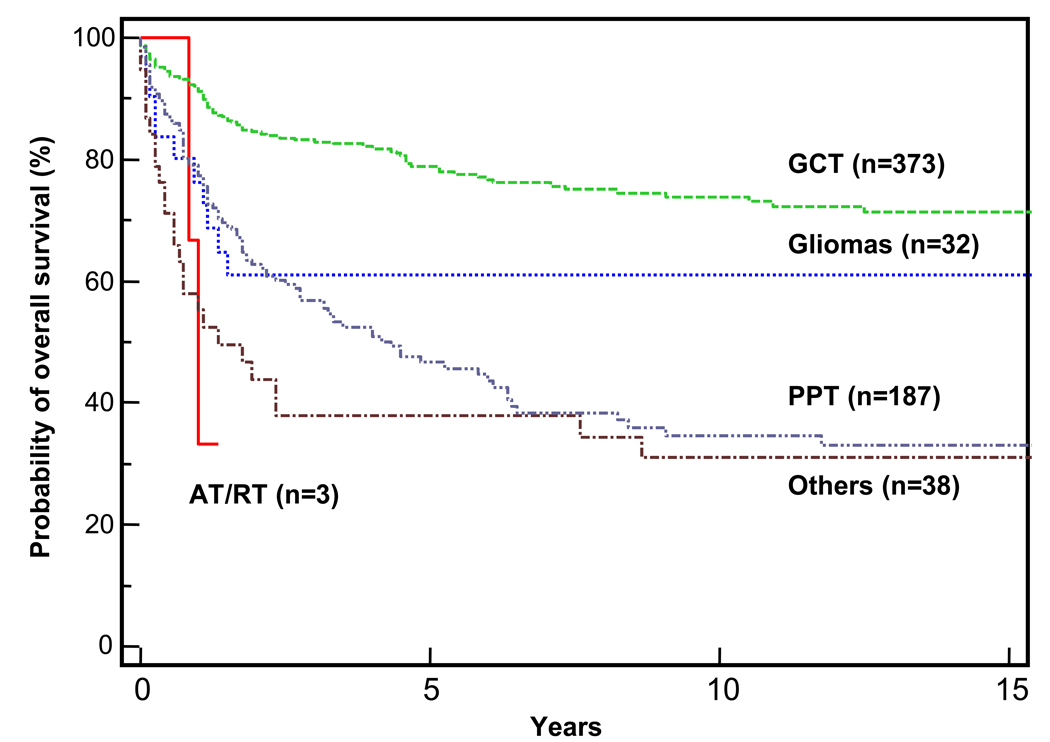
During the study period, 373 pineal GCTs were registered with the SEER database. Patients with GCTs had the best survival of all patients in this study (OS = 78.9% ± 2.3%; Figure 2 and Figure 3A). The GCT group included 285 (76%) pure germinomas, six (2%) embryonal carcinomas, five (1%) yolk sac tumors, 25 (7%) malignant teratomas, two (0.5%) choriocarcinomas, and 50 (13.5%) mixed GCTs (eight of which were teratocarcinoma).
Figure 2.
Kaplan-Meier analyses of overall survival of patients in the various pineal tumor subgroups. Abbreviations: AT/RT, atypical rhabdoid/teratoid tumor; GCT, germ cell tumor; PPT, pineal parenchymal tumor. The end point was all-cause mortality.
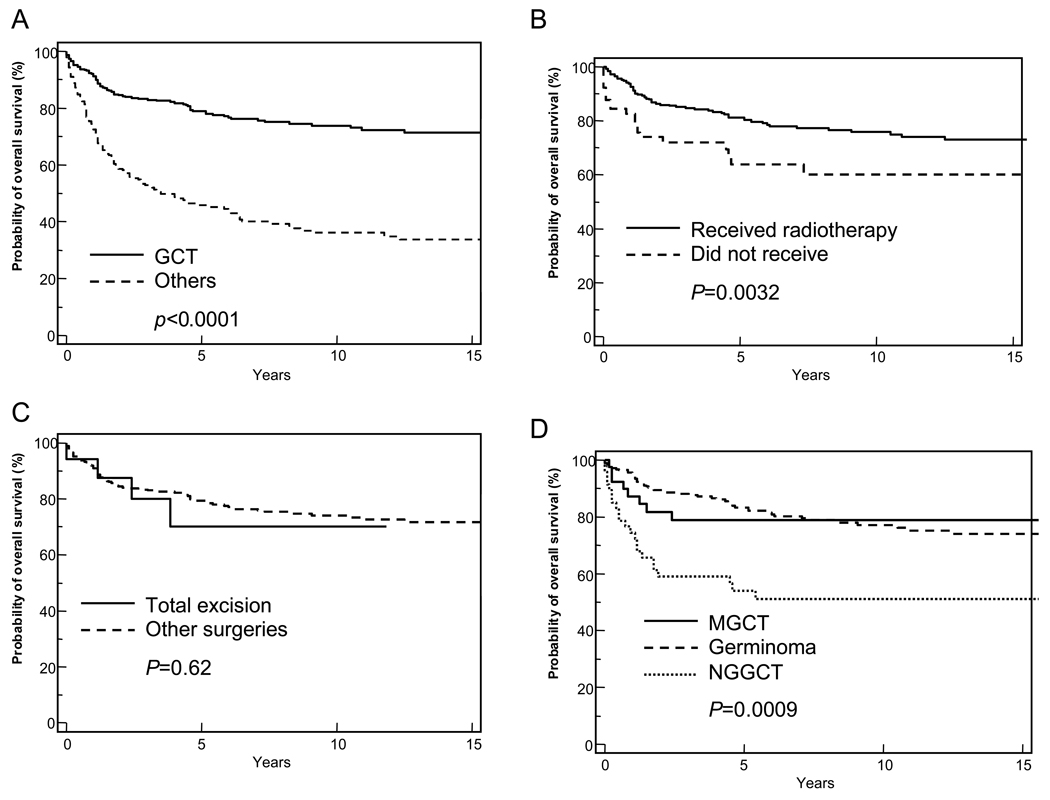
Figure 3.
Kaplan-Meier analyses of survival of patients with pineal germ cell tumors (GCTs; solid line). (A) Survival of patients with GCTs was compared with that of patients from the other histologic subgroups (dashed line). (B) Survival of patients with GCTs who received radiotherapy (solid line) was compared with that of patients with GCTs who did not (dashed line). (C) Survival of patients with GCTs who underwent total surgical excision (sold line) was compared with that of patients who underwent other, less extensive surgical procedures (dashed line). (D) Survival of patients with GCT further subdivided on the basis of GCT subtype: mixed germ cell tumors (solid line), germinomas (dashed line), or nongerminomatous germ cell tumors (dotted line). A log-rank test was used to compare survival curves, and P-values are provided. The end point for all curves was all-cause mortality.
There was a marked male predominance among patients with GCTs (male: female ratio, 11.8:1). The median age for the group was 16 years (range, 1–77 years). History on radiotherapy was available on 363 patients. Radiation treatment was administered to 297 (82%) patients with GCTs, and their OS was significantly better than that of those who did not receive radiotherapy (P = 0.0032, Figure 3B). Of the 326 patients with GCT for whom surgical data were available, 151 (46%) received no surgical procedures. For those who did receive surgery, the extent of tumor excision did not affect survival (P = 0.62; Figure 3C).
Although the sex difference was obvious among patients with pure germinoma, i.e., the male-to-female ratio was 11.4 to 1; (262 males and 23 females); however, sex was not a factor in survival (P = 0.57).
Patients with germinomas were significantly older at the time of diagnosis (mean age, 24.6 years ± 20.6 years) than were those with NGGCT (19.0 years ± 9.3 years; unpaired t-test, P <0.0001). The youngest patients were those in the teratoma group, who had a mean age of 14.0 years (range, 5–44 years) at diagnosis. The probability of 5-year OS rate was significantly better for patients with germinoma (83.3% ± 2.5%) than it was for those with NGGCT (50.8% ± 2.9%; P <0.0001; Figure 3D). More patients with germinoma received radiotherapy (P = 0.009), and a larger number of patients with NGGCT underwent surgical intervention (P <0.001).
There was no significant differences in the age at diagnosis when the NGGCT group was further subclassified into those with pure NGGCT (n=38; median age, 15 years) and those with mixed NGGCT (n=50; median age, 13 years). More importantly, the 5-year OS for patients with mixed NGGCT was significantly better (78.8% ± 6.7%) than that of patients with pure NGGCT (54.0% ± 7.5%, P = 0.016; Figure 3D).
Pineal Parenchymal Tumors
The second largest group of pineal gland tumors in the SEER was the PPTs. Although 19 of the 187 tumors categorized as PPTs in our analysis were labeled PNETs in the SEER registry, we included them here because these tumors are considered pineoblastoma [15]. The median age of patients at diagnosis of PPTs was 21 years (range, 0–82 years), and no sex difference was found. Surgical data were available for 170 (91%) patients: 113 (67%) had other surgeries; 46 (27%) had no surgery; and only 11 (6%) underwent total excision Radiotherapy was delivered to 130 (71%) patients with PPTs.
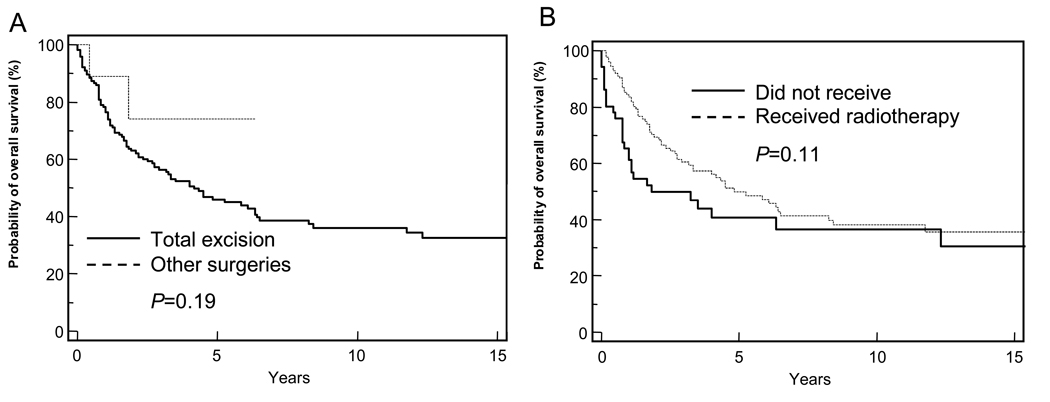
Patients with PPTs showed significantly worse survival than did patients with other pineal tumor histology (P <0.0001). The 5-year OS for the PPT group was 47.2% (Figure 2 and Table 1), and the median survival was 4.5years. There was no significant difference in survival on the basis of age, sex, surgery (Figure 4A), radiation therapy (Figure 4B), or diagnosis era.
Figure 4.
Kaplan-Meier analyses of survival of patients with pineal parenchymal tumors (PPT). (A) Survival of patients with PPT who underwent total surgical excision (sold line) was compared with that of patients who underwent less extensive procedures (dashed line). (B) Survival of patients with PPTs who received radiotherapy (solid line) was compared with that of those who did not receive radiation treatment (dashed line). A log-rank test was used to compare survival curves, and P-values are provided. The end point for all curves was all-cause mortality.
Gliomas
There were 32 cases of gliomas registered with SEER during the study period, constituting 5.0% of the patient cohort. The median age for the group was 38 years (range, 3–75 years); 72% of the cases occurred in adults. Gliomas comprise a heterogenous group of tumors, including noninfiltrative tumors (grade I) such as pilocytic astrocytoma and infiltrative, more malignant tumors such as fibrillary astrocytoma (grade II), anaplastic astrocytoma (grade III), and glioblastoma (grade IV). The probability of 5-year OS of patients with gliomas was 61% ± 9.3%; it was significantly better for patients with grade I or II tumors (n=12, P = 0.020) and in children (n= 9, P = 0.019), four of which had grade I or II tumors. Survival was not affected by radiation treatment (n=19, P = 0.070) or surgery (n=12, P = 0.49).
DISCUSSION
To our knowledge, this report is the largest analysis of pineal tumors ever conducted. GCTs were associated with better survival than other pineal tumor subtypes, indicating that histology, rather than grade, is an important factor in determining the outcome of the patient [20, 21]. Radiotherapy was also associated with better survival. PPTs showed a variety of histologic patterns, which is reflected by the degree of cellular differentiation, mitosis, and anaplasia [17]. By genetic analysis, these pineal parenchymal tumors have been proven to be of similar origin [22]. The classification of PPTs has been inconsistent over the years, in particular, the category of PPTs of intermediate differentiation, which represents 20% to 50% of PPTs [2, 23].
In 2000, Jouvet et al proposed a grading classification system for PPTs based on the architecture, mitotic figures, and imunostaining with neurofilament [24]. The use of this classification system was associated with significant differences in OS and event-free survival (EFS) though it was not fully adopted by the new 2007 CNS tumor classification system. Before 2007, the distinction between PPT subgroups was not easily or widely recognized by reporting pathologists or neuropathologists. In addition, the potentially aggressive behavior of pineoblastoma and the intermediate/mixed-group tumors and the tendency for craniospinal seeding [17] justified grouping these histologic subtypes together. This explains the absence of PPTs of intermediate differentiation from the registry.
In our data, PPT which included pineoblastoma, PNET, mixed tumors and tumors of intermediate differentiation, was seen mainly in young adults; the median age of this group was 21 years. In addition, the majority (81%, 152/187) of PPTs were grade IV at diagnosis (Table 1).
Surgical approach (i.e., total excision vs. other surgeries) did not influence survival in the cohort. In further analysis based on histologic subtypes, the extent of surgery did not influence survival of patients with GCT, PPT, or gliomas (all P-values ≥0.19). This is an important finding that may justify a safer, less aggressive surgical approach for pathologic confirmation only and avoidance of aggressive unnecessary craniotomies.
This study has clear limitations that should be taken in consideration. Information about some aspects of chemotherapy is lacking, and radiation therapy dose and treatment fields are not mentioned. In addition, no central pathology review was done, so the diagnosis of the referring institution was used as submitted, a factor that has proven to be of importance in the practice of neuropathology [25]. Although other studies have included larger data sets than ours, those studies were either dedicated to CGTs only [19, 26] or were interested in surgical description only [27, 28]. Thus, this paper is unique in that it presents analyses of survival and the impact of radiotherapy, histology, and the extent of surgical resection on survival. In addition, our study sheds light on the survival of patients with rare tumors such as pineal gliomas and pure pineal germinomas in female patients.
In conclusion, the differential diagnosis of pineal gland tumors includes different entities with different clinical features and prognoses. Although the two most common tumor subtypes, GCTs and PPTs, occurred predominantly in children, the third most common, gliomas, were more common in adults. The GCT group had the best outcome, which was further improved by radiotherapy use but not by total excision. This study, despite its many limitations, will help physicians make a differential diagnosis of pineal tumors on the basis of their patient’s age and sex. Our results also support the use of radiotherapy to improve survival. Improved outcome in the most recent period (1993–2005) may reflect improvements in surgical techniques, diagnostic correctness, delivery of radiotherapy, imaging studies, chemotherapy use, or a combination of these factors when delivered by specialized multidisciplinary teams.
Acknowledgments
This work was supported in part by grant CA21765 from the U.S. Publish Health Service; Musicians Against Childhood Cancer (MACC); The Noyes Brain Tumor Foundation; The Ryan McGhee Foundation; the American Lebanese Syrian Associated Charities (ALSAC); and the King Hussein Cancer Foundation (KHCF).
The authors thank Sharon Naron for the scientific editing of this manuscript.
REFERENCES
- 1.Cbtrus. Statistical Report: Primary Brain Tumors In The United States -Pbtcbtrotus. 2005. [Google Scholar]
- 2.Brat DJ PJ, Kleinschmidt-Demasters BK, Yachnis AT, Montine TJ, Boyer PJ, Powell SZ, Prayson RA, Mclendon RE. Neuropathology Committee, College Of American Pathologists.: Surgical Neuropathology Update. A Review Of Changes Introduced By The Who Classification Of Tumours Of The Central Nervous System. Arch Pathol Lab Med. (4th Edition.) 2008:993–1007. doi: 10.5858/2008-132-993-SNUARO. [DOI] [PubMed] [Google Scholar]
- 3.Kersh CR CW, Eisert DR, Spaulding CA, Hahn SS, Jenrette JM, 3rd, Marks RD., Jr Primary Central Nervous System Germ Cell Tumors. Effect Of Histologic Confirmation On Radiotherapy. Cancer. 1988;61:2148–2142. doi: 10.1002/1097-0142(19880601)61:11<2148::aid-cncr2820611103>3.0.co;2-q. 2152. [DOI] [PubMed] [Google Scholar]
- 4.Maria E.Echeverria Jf, Goldman Stewart. Pediatric Central Nervous System Germ Cell Tumors: A Review. The Oncologist. 2008;13:690–699. doi: 10.1634/theoncologist.2008-0037. [DOI] [PubMed] [Google Scholar]
- 5.Roger J. Packer TM, Vezina Gilbert. Central Nervous System Tumors. Pediatr Clin N Am. 2008;55:121–145. doi: 10.1016/j.pcl.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 6.David N Louis HO, Wiestler Otmar D, Cavenee Webster K. Germ Cell Tumours. Who Classification Of Tumours Of The Central Nervous System. Iarc, Lyon. :197–204. [Google Scholar]
- 7.Junko Hirato YN. Pathology Of Pineal Region Tumors. Journal Of Neuro-Oncology. 2001;54:239–249. doi: 10.1023/a:1012721723387. [DOI] [PubMed] [Google Scholar]
- 8.David N. Louis HO, Wiestler Otmar D, Cavenee Webster K. Tumours Of The Pineal Region. Who Classification Of Tumours Of The Central Nervous System. Iarc Lyon. :121–126. [Google Scholar]
- 9.Pragati Kumar MT, Sharma Ajay, Singh Daljiy. Histological Analysis Of Lesions Of The Pineal Region: A Retrospective Study Of 12 Years. Pathology Research And Practice. 2006;202:85–92. doi: 10.1016/j.prp.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 10.The Surveillance, Epidemiology, And End Results (Seer) Usa: National Cancer Institute; [Google Scholar]
- 11.Herrick MK RL. The Cytological Differentiating Potential Of Pineal Parenchymal Neoplasms (True Pinealomas). A Clinicopathological Study Of 28 Tumours. Brain. 1979;102:289–320. doi: 10.1093/brain/102.2.289. [DOI] [PubMed] [Google Scholar]
- 12.Packer RJ SL, Rosenstock JG, Rorke LB, Bilaniuk LT, Zimmerman RA, Littman PA, Bruce DA, Schut L. Pineal Region Tumors Of Childhood. Pediatr. 1984;74:97–102. [PubMed] [Google Scholar]
- 13.Hoffman HJ YM, Becker LE, Hendrick EB, Humphreys RP. Pineal Region Tumors In Childhood. Experience At The Hospital For Sick Children. Concepts Pediat Neurosurg. 1983;4:360–386. doi: 10.1159/000120821. [DOI] [PubMed] [Google Scholar]
- 14.Rubenstein LJ. Armed Force Institute Of Pathology. Washington D.C.: 1972. Tumors Of The Central Nervoous System. [Google Scholar]
- 15.Alyssa T, Reddy AJJ, Phillips Peter C, Weiss Heidi L, Packer Roger J. Outcome For Children With Supratentorial Primitive Neuroectodermal Tumors Treated With Surgery, Radiation, And Chemotherapy. Cancer. 2000;88:2189–2193. doi: 10.1002/(sici)1097-0142(20000501)88:9<2189::aid-cncr27>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 16.Kleihues P BP, Scheithauer BW. The New Who Classification Of Brain Tumours. Brain Pathol. 1993;3:255–268. doi: 10.1111/j.1750-3639.1993.tb00752.x. [DOI] [PubMed] [Google Scholar]
- 17.Schild SE, Scheithauer BW, Schomberg PJ, Hook CC, Kelly PJ, Frick L, Robinow JS, Buskirk SJ. Pineal Parenchymal Tumors. Clinical, Pathologic, And Therapeutic Aspects. Cancer. 1993;72:870–880. doi: 10.1002/1097-0142(19930801)72:3<870::aid-cncr2820720336>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 18.Kleihues P LD, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK. The Who Classification Of Tumors Of The Nervous System. J Neuropathol Exp Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. [DOI] [PubMed] [Google Scholar]
- 19.Nomura K. Epidemiology Of Germ Cell Tumors In Asia Of Pineal Region Tumor. J Neurooncol. 2001;54:211–217. doi: 10.1023/a:1012771204732. [DOI] [PubMed] [Google Scholar]
- 20.Christian H. Rickert WP. Prognosis-Related Histomorphological And Immunohistochemical Markers In Central Nervous System Tumors Of Childhood And Adolescence. Acta Neuropathol. 2005;109:69–92. doi: 10.1007/s00401-004-0959-3. [DOI] [PubMed] [Google Scholar]
- 21.Matsutani M SK, Takakura K, Fujimaki T, Nakamura O, Funata N, Seto T. Primary Intracranial Germ Cell Tumors: A Clinical Analysis Of 153 Histologically Verified Cases. J Neurosurg. 1997;86:446–455. doi: 10.3171/jns.1997.86.3.0446. [DOI] [PubMed] [Google Scholar]
- 22.Michelle FE`Vre-Montange JC, Szathmari Alexandru, Wierinckx Anne, Mottolese Carmine, Guyotat Jacques, Figarellabranger Dominique, Jouvet Anne, Lachuer Joe¨L. Microarray Analysis Reveals Differential Gene Expression Patterns In Tumors Of The Pineal Region. J Neuropathol Exp Neurol. 2006;65 doi: 10.1097/01.jnen.0000225907.90052.e3. 675 Y 684. [DOI] [PubMed] [Google Scholar]
- 23.Francois Fauchon AJ, Paquis Philippe, Ghislaine-Saint-Pierre, Mottolese Carmine, Ben Hassel Mohamed, Chauveinc Laurent, Sichez Jeanpierre, Philippon Jaques, Schlienger Michel, Bouffet Eric. Parenchymal Pineal Tumors: A Clinicopathological Study Of 76 Cases. Int J Radiation Oncology Biol Phys. 2000;46:959–968. doi: 10.1016/s0360-3016(99)00389-2. [DOI] [PubMed] [Google Scholar]
- 24.Anne Jouvet GS-P, Fauchon François, Privat Karen, Bouffet Eric, Ruchoux Marie-Magdeleine, Chauveinc Laurent, Fèvremontange Michelle. Pineal Parenchymal Tumors: A Correlation Of Histological Features With Prognosis In 66 Cases. Brain Pathology. 2000;10:49–60. doi: 10.1111/j.1750-3639.2000.tb00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilles FH TJ, Becker LE, Burger PC, Yates AJ, Pollack IF, Finlay JF. Pathologist Interobserver Variability Of Histologic Features In Childhood Brain Tumors: Results From The Ccg-945 Study. Pediatr Develop Pathol. 2008;11:108–117. doi: 10.2350/07-06-0303.1. [DOI] [PubMed] [Google Scholar]
- 26.Villano JL, Propp JM, Porter KR, Stewart AK, Valyi-Nagy T, Li X, Engelhard HH, Mccarthy BJ. Malignant Pineal Germ-Cell Tumors: An Analysis Of Cases From Three Tumor Registries. Neuro Oncol. 2008;10:121–130. doi: 10.1215/15228517-2007-054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konovalov AN PD. Principles Of Treatment Of The Pineal Region Tumors. Surg Neurol. 2003;59:250–268. doi: 10.1016/s0090-3019(03)00080-6. [DOI] [PubMed] [Google Scholar]
- 28.Regis J BP, Rouby-Volot F, Figarella-Branger D, Dufour H, Peragut JC. Pineal Region Tumors And The Role Of Stereotactic Biopsy: Review Of The Mortality, Morbidity, And Diagnostic Rates In 370 Cases. Neurosurg. 1996;39:907–912. doi: 10.1097/00006123-199611000-00003. [DOI] [PubMed] [Google Scholar]