Abstract
The chronology of the component processes subserving working memory (WM) and hemodynamic response lags have hindered the use of fMRI for exploring neural substrates of WM. In the present study, however, participants completed full trials that involved encoding two or six letters, maintaining the memory-set over a delay, and then deciding whether a probe was in the memory-set or not. Additionally, they completed encode only, encode and maintain, and encode and decide partial-trials intermixed with the full-trials. The inclusion of partial-trials allowed for the isolation of BOLD signal-changes to the different trial-periods. The results showed that only lateral and medial prefrontal cortex regions differentially responded to the 2- and 6-letter memory-sets over the trial-periods, showing greater activation to 6-letter sets during the encode and maintain trial-periods. Thus, the data showed the differential involvement of PFC in the encoding and maintenance of supra- and sub-capacity memory-sets and show the efficacy of using fMRI partial-trial methods to study WM component processes.
Working memory (WM) is a central construct in cognitive psychology. It is considered a fundamental part of intelligence (Engle, Tuholski, Laughlin, & Conway, 1999; Wechsler, 1997) and other higher-level cognitive processes (see Baddeley, 1986) such as reasoning (Goel & Grafman, 1995), problem-solving (Seyler, Kirk, & Ashcraft, 2003), and comprehension (Ehrlich, Brebion, & Tardieu, 1994). Neuroimaging research on humans has focused on identifying the neural substrates of WM component processes (see Rypma, 2006). In general, this research has shown that PFC activity is positively related to WM executive demand in that PFC activity increases with the amount of information to be remembered or manipulated (e.g., Rypma et al., 1999). Research attempts to identify the specific WM component processes mediated by PFC, however, have produced mixed results and spawned debate about the brain bases of WM operations occurring over WM trials and, particularly, whether PFC mediates WM maintenance or executive processes (see Feredoes & Postle, 2007; Rypma, 2006).
WM is a multi-component process that subserves the overall goal of holding information in a temporarily accessible state, often reorganizing or recoding the information, and making the information available for other cognitive operations. It is composed of component processes involved in encoding, maintaining, manipulating, searching, and retrieving memories (see Baddeley, 1986; Miyake & Shah, 1999; Repovs & Baddeley, 2006). WM also is known to be capacity limited, but estimates of WM capacity vary (e.g., Glanzer & Razel, 1974; Luck & Vogel, 1997; Miller, 1956). One estimate based on a comprehensive review of the literature puts the capacity as low as four (+/− 1) items (see Cowan, 2001; 2005). However, when the to-be-remembered information exceeds the capacity limit, WM executive processes can be brought online to optimally reorganize memory codes (see Baddeley, 1986; Miller, 1956).
One method for examining neural substrates of WM has been to vary the amount of material to be remembered in the context of item recognition tasks (IRTs) to examine the effects of on fMRI BOLD signal-change (see Rypma, 2006). IRT trials are composed of discrete encode, maintain, and decide trial-periods in which participants ideally engage in different WM component processes. During the encoding period participants view sets of stimuli that they are to remember. In the subsequent maintenance period, the stimuli disappear, and the participants are to mentally retain the information over a delay interval. Then, during the decision period, participants judge whether a probe item(s) was present in the memory-set or not.
The isolation of set-size effects to particular IRT periods has been considered central to the identification of the neural substrates of WM component processes. For example, set-size effects within PFC during the encode trial-period, in the absence of set-size effects during the maintain trial-period, have been hypothesized to reflect PFC mediation of executive organization processes that serve to consolidate to-be-remembered information when memory-demand exceeds capacity (i.e., chunking operations; Rypma, 2006). Studies aimed at isolating set-size effects in this way, however, have produced mixed results and have spawned debate about the nature of the cognitive operations elicited during each component-isolating IRT trial-period and about when the associated neural activity occurs (see Feredoes & Postle, 2007; Rypma, 2006).
Specifically, three patterns of results have been reported. Some studies have found that larger set-sizes elicited greater PFC activity during the encode and maintain periods (Cairo et al., 2004; Narayanan et al., 2005; but see Feredoes & Postle, 2007, for comparisons of group and individual differences accounts). Some studies have found that larger set-sizes elicited greater PFC activity only during the encode period (Jha & McCarthy, 2000; Postle et al., 1999; Rypma & D’Esposito, 1999; but see Rypma et al., 2002, for an individual differences account). Finally, some studies, focusing exclusively on maintenance processes, have found that larger set-sizes elicited greater PFC activity within the maintain trial-period (Leung, Gore, & Goldman-Rakic, 2002; Zarahn et al., 2005).
On the one hand, IRT event-related fMRI designs might seem ideal for investigating the brain bases of WM component processes. Event-related designs were developed specifically to examine trial-level effects (Rosen, Buckner, & Dale, 1998; Zarahn, Aguirre, & D’Esposito, 1997), and IRT event-related designs allow for some control over when participants engage different WM component processes. On the other hand, the measured BOLD signal-change for a given trial-period is not independent of hemodynamic influences from earlier adjacent trial-periods (e.g., Dale & Buckner, 1997). This colinearity of the BOLD response over the component trial-periods in IRTs then limits accuracy in estimating the unique portions of the BOLD response attributable to each period (Cairo et al., 2004; Manoach et al., 2003; Ollinger, Shulman, & Corbetta, 2001).
Several methods for examining PFC-related effects over IRT trial-periods have been used and these approaches have different strengths and weaknesses. One method has been to deconvolve the measured BOLD signal-change using canonical hemodynamic response function (HRF) regression models (e.g., Sakai, Rowe, & Passingham, 2002). Regression analyses with models based on canonical or subject derived HRFs, however, have been shown to underestimate the magnitude of signal-change and are biased toward producing stronger effects for participants and brain regions having task-related HRFs that match the chosen shape (Handwerker et al., 2004). Another method has been to vary the duration of the maintenance interval (e.g., Cairo et al., 2004; Rowe, Toni, Josephs, Frackowiak, & Passingham, 2000). Varying the duration of the maintenance interval increases the signal-change variability attributable to WM maintenance and therefore improves the validity of a detected maintenance effect, but this method does not eliminate the colinearity problem. Two other methods have been to use long maintenance intervals that exceed the ideal time needed for the encoding HRF to return to baseline (Jha & McCarthy, 2000) and to model only the middle or later parts of the maintenance response (e.g., Feredoes & Postle, 2007; Rypma & D’Esposito, 1999). These methods reduce the influence of the encoding-related BOLD responses on detection of maintenance-related effects, but they also do not entirely eliminate the colinearity problem and do not allow for the estimation of early maintenance effects.
Evidence suggests, however, that the use of “partial-trials” might effectively isolate IRT trial-period BOLD signal-changes (Ollinger, Corbetta, & Shulman, 2001; Ollinger, Shulman, et al., 2001). The partial-trial paradigm was proposed to isolate BOLD responses to distinct but adjacent processing periods within a trial by having participants engage those component processes in isolation or in combinations such that unique estimates of the BOLD responses for each processing period can be obtained. For example, Ollinger and colleagues demonstrated that unique BOLD response estimates (within the calcarine sulcus) to a low-contrast visual stimulus immediately followed by a high-contrast visual stimulus could be obtained for each stimulus event if intermittent presentations of the low-contrast stimulus alone (i.e., not followed by the high-contrast stimulus) occurred during data acquisition. In fact, the obtained estimates were relatively equal in magnitude and in shape to obtained estimates from separate runs in which the low- or high-contrast stimulus was presented alone.
The present study explored the use of this partial-trial method to isolate BOLD responses to encode, maintain, and decide IRT trial-periods for sub- and supra-capacity memory set-sizes. In the present study, participants worked through full-trials consisting of encoding sets of letters, maintaining representations of the letter-sets over a delay, and then deciding whether a probe letter was in the memory-set or not. They also worked through partial-trials in which they were exposed to only the encode period of a full trial (encode only), to only the encode and maintain periods of a full trial (encode-maintain), or to only the encode and decide periods of a full trial (encode-decide). This combination of full- and partial-trials provided unique samples necessary to account for the unknown signal-change parameter estimates for each component trial period in the full-trial (see Figure 1). Therefore, this partial-trial paradigm allowed for the examination of brain regions mediating WM IRT encode, maintain, and decide trial-periods, and the manipulation of memory set-size across full- and partial-trials allowed for the examination of the engagement of PFC during the trial-periods in response to supra- and sub-capacity memory-sets.
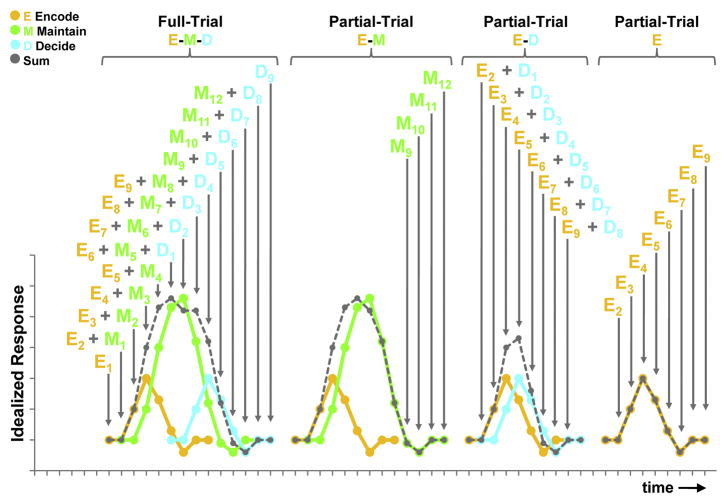
Figure 1.
Idealized hemodynamic responses for full-trial and partial-trial events illustrating combinations of trial-period effects contributing to the full-trial summed response and examples unique contributions of partial-trial trial-period effects necessary to account for the unknown signal-change parameter estimates for each component trial period in the full-trial. For the Full-Trial example, the summed response (gray with dashed lines) is the idealized total hemodynamic response over the full-trial when the encode (yellow), maintain (green), and decide (blue) idealized responses are considered together. The letters (E = Encode, M = Maintain, D = Decide) with subscripted numbers (time-point in trial-period) above identify the ideal trial-period contributors to the total hemodynamic response (gray). The letters with subscripted numbers above the partial-trial functions illustrate combinations of response contributions within partial-trials that are unique from those in the full-trial, allowing for the deconvolution of the trial-period signal-changes. In this example, there are 30 unknown parameters to estimate in a full trial (9 encode, 12 maintain, and 9 decide). The full-trial models, however, only yield 14 unique equations. The partial-trials shown illustrate examples of additional unique equations added by including partial-trials in the design, providing 21 additional unique equations to estimate the trial-period responses.
Materials and Method
Participants
Eleven right-handed participants (age M = 26, range = 19–41; 6 females) were recruited through advertisements posted on the campuses of the University of Texas at Dallas, the University of Texas Southwestern Medical Center, and the surrounding communities. Participants were prescreened for MRI contra-indicators and for medical, neurological, and psychiatric illness. The experiment was approved by the Institutional Review Boards at the University of Texas at Dallas and University of Texas Southwestern Medical Center, and the experiment was conducted according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from each participant prior to testing.
Working Memory Task
Participants completed two kinds of trials, full-trials and partial-trials. For full-trials participants were to encode two or six letters, maintain the memory-set over a delay, and then decide whether a probe-letter was in the memory-set or not. For partial-trials, participants completed encode-only, encode-maintain, and encode-decide partial-trials that were intermixed with the full trials (e.g., Figure 2). Encoding stimuli appeared on the upper half of the screen, within yellow brackets, in a field outlined in yellow, and probe stimuli appeared on the lower half of the screen, within cyan brackets, in a field outlined in cyan. The outlines remained on the screen throughout each run, and six letters appeared during both the encoding and response periods. The uppercase letters B, F, G, H, J, L, M, N, Q, R, S, W, and X served as encoding and probe stimuli. The encode, maintain, and decide periods were 2, 8, and 2 s, respectively, and a 4, 6, 8, 10, or 12 s rest period followed every trial and partial trial. A centered, colored cross was used to indicate trial (green) and rest (red) periods.
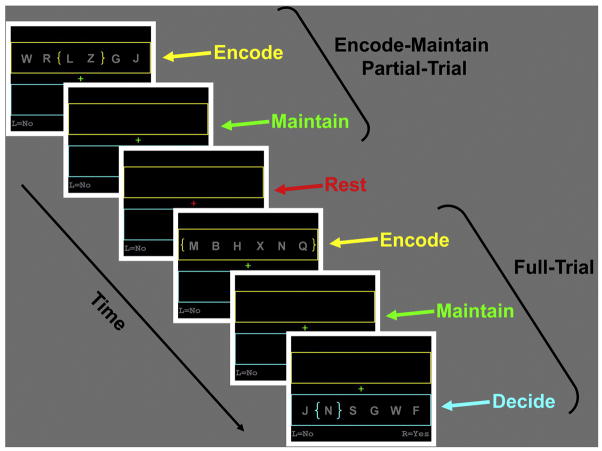
Figure 2.
Examples of Full-Trial and Partial-Trial events in the working memory task. Full-trials consisted of a 2 s period in which participants were to encode the letters appearing with the yellow brackets (either two or six letters), an 8 s period in which they were to mentally maintain the encoded letters, and a 2 s period in which they were to decide whether a probe letter appearing within the blue brackets was in the memory-set. Partial-trials consisted of encode only, encode-maintain, and encode-decide conditions. The partial-trial depicted is an encode-maintain condition in which participants would encode the letters appearing within the yellow brackets, mentally maintain the letters over the 8 s maintain period, but then “rest” when the fixation changed to red.
For the encode-decide partial-trials, a decide period immediately followed the encode period. For the encode-only and encode-maintain partial-trials, the color-change of the fixation-cross from green to red cued participants to “stop whatever they were doing mentally to try to remember the letters.” Pilot testing revealed that 4 s was adequate for participants to verbally report being able to recognize the partial-trial cue and being able to terminate their memory processes before the onset of the next trial. To further facilitate compliance, participants were told prior to testing about the analytical problem with overlapping HRFs and the purpose of the partial trials. Additionally, participants worked through practice trials prior to entering the scanner, and all reported being able to recognize the partial trial cue and to terminate their memory processes on the partial trials.
Additional steps were taken to minimize or eliminate confounds known to influence WM performance (e.g., long-term memory-based chunking and visual afterimages). Letters occurring adjacently in the English alphabet (e.g., L-M-N) did not appear adjacently in alphabetical order in the encoding sets (Eldreth et al., 2006). The letters were gray and appeared on a black background; the lowered contrast reduced the likelihood of visual afterimages.
Trials were run in a fixed random order across seven runs lasting 6 minutes each. There were 12 full-trials and 4 of each partial-trial type per run. Both set-sizes occurred equally often in each run (n = 6 full-trials and n = 6 partial-trials). The trial-type order was randomly determined for each run. For each trial-type, the target letters were randomly selected from the pool of letters, and the ordinal positions of the letters were randomly determined, except that the letters were always adjacent to each other for the 2-letter memory-sets. On trials when the probe was present, the probe was randomly selected from the memory-set. The foil and the additional five letters appearing during the decision period were randomly selected from the letters that were not used during the encoding period for that trial. The ordinal positions of the probes and foils also were randomly determined. Rest periods for each run were randomly selected without replacement from an approximately exponentially distributed set of 4, 6, 8, 10, and 12 s intervals (n = 12, 6, 3, 2, 1 per run, respectively). Two versions of the six runs were created based on these criteria, and the administration was counterbalanced across the participants.
Image Acquisition
High-resolution anatomical images using an MPRAGE sequence (1 mm isovoxel; sagittal; TE = 3.7 ms; flip angle = 12°) and functional images using an EPI sequence (voxel = 3.5 × 3.5 × 4 mm; 36 slices/volume; 180 volumes/run; TR = 2000 ms, TE = 30 ms; flip angle = 70°; matrix = 64×64; axial; inferior to superior interleaved) were collected on a Philips Achieva 3T scanner equipped with an 8-element, SENSE, receive-only head coil. Six “dummy” scans were run at the beginning of each functional run to remove T1 saturation effects.
Image Analysis
The fMRI data were analyzed using AFNI software (Cox, 1996). The data for individual participants were corrected for slice-timing offset and motion, and they were spatially filtered with a Gaussian kernel (FWHM = 8 mm). For each run, the data for each voxel were then scaled by the mean for that voxel so that the deconvolution parameter estimates would be expressed in terms of percent signal-change (i.e., 100 * yt/My).
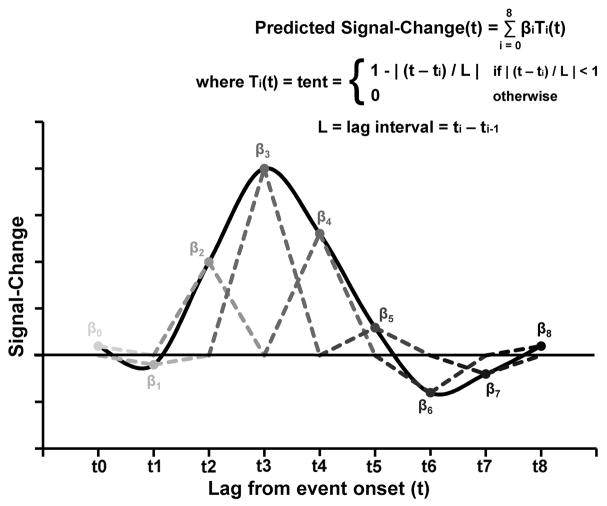
The voxel-wise time-series data for individual participants were then deconvolved using a piecewise linear B-spline regression analysis (Graybill & Iyer, 2004: Saad et al., 2006; Ward, 1998/2006). This was a basis function expansion approach, as opposed to a traditional fixed-shape regression approach. This approach allowed for the examination of working-memory component-related BOLD signal changes without assumptions regarding the exact shape of the signal-change curves. For each voxel, the BOLD response for each condition was modeled as a piecewise sum of a set of B-spline (tent) basis functions (e.g., Figure 3). Each tent spanned two time-points, except the first and last half-tents which spanned one time-point each, and each was scaled to have an initial max value of one and zeros at the base. The total number of tents per condition was equal to the number of time-points fitting the duration of the condition plus eight additional time-points, to estimate the full extent of the signal-change response per condition. Thus, the linear regression analysis of the BOLD response data per voxel on the full set of tent predictors yielded nine response amplitude parameter estimates (B) for the 2- and 6-letter encode conditions and for the 2- and 6-letter decide conditions (e.g., as in Figure 3) and 12 Bs for the 2- and 6-letter maintain conditions. Thus, for each voxel, the predicted BOLD response was equal to the sum of the three sets of scaled tent basis functions for each of the trial-periods for each of the set-size conditions, that is,
Figure 3.
An illustration of a hemodynamic response function modeled as a set of b-spline (tent) basis functions (gray dashed lines, shaded to distinguish different tents). Each tent, T(x), spans two lag intervals (L), except the first and last half-tents which span one lag each, and each initially would have been scaled to have max value of one and zeros at the base. Linear regression analysis of the signal-change data on the set of tent regressors would yield parameter estimates (βs) for scaling the tents to fit the data.
where Ti(t) = ith tent basis function (see Figure 3). Regressors for motion correction estimates and linear, quadratic, and cubic trends for each run were included in the baseline regression model.
The derived Set-Size X Trial-Period BOLD signal-change estimates were then used for the group analysis. For each participant, the area under the curve (AUC) for the BOLD response was calculated for each condition at each voxel, AUC = .5(B1) + B2 + B3 + … + .5(Bn). The AUC-maps were resampled to a 2 mm isovoxel resolution and then spatially normalized to Talairach space (Talairach & Tornoux, 1988) by transforming each participant’s 3D structural image, via a 12-parameter affine transformation, to fit it to a Talairach template (i.e., the Colin-brain template) and then equivalently transforming the AUC-maps and the tent B-maps based on structural transformation parameters.
The tent regression analysis also was calculated using Set-Size X Trial-Type (full-trial, encode-only, encode and maintenance, encode and decide) covariates to obtain BOLD signal-change estimates based on the set-size and trial-type. Nine Bs were obtained for the 2- and 6-letter encode-only conditions, 13 for the 2- and 6-letter encode-maintain conditions, 10 for the 2- and 6-letter encode-decide conditions, and 14 for the 2- and 6-letter full-trial conditions. Thus, for each voxel, the predicted BOLD response was equal to the sum of the four sets of scaled tent basis functions for each of the trial-types for each of the set-size conditions, that is,
where Ti(x) =ith tent basis function. Regressors for motion correction estimates and linear, quadratic, and cubic trends for each run were included in the baseline regression model. The resulting B-maps were resampled to a 2 mm isovoxel resolution and normalized to Talairach space.
A group-level, random-effects, 2 (Set-Size) X 3 (Trial-Period) repeated-measures ANOVA was calculated for each voxel, with the AUC estimates per participant as the dependent measure. To correct for family-wise Type I errors, the results were cluster-thresholded based on Monte-Carlo simulations (AlphaSim software; Ward, 2000) so that surviving clusters were significant with a family-wise α = .05 and a voxel-level α = .005. Clusters of 1225 μL (at 3.5 × 3.5 × 4 mm, 25 voxels; at 2 mm isovoxel, ~153 voxels) were significant with family-wise α = .05, based on the simulations (1000 iterations for a dataset having 25,579 3.5 × 3.5 × 4 mm voxels, smoothness = 8 mm FWHM, cluster = pairs of voxels having a connectivity radius < 6.37 mm).
Results
Behavioral Data
Separate 2 (Set-Size: 2 vs. 6) X 2 (Trial-Type: Full vs. Encode-Respond) repeated-measures ANOVAs were calculated for the reaction time (RT) and accuracy data. RTs for incorrect responses and outliers (RTs > |2.5| SD from a participant’s mean) were discarded. As in previous studies using the IRT (e.g., Sternberg, 1966; Rypma & D’Esposito, 1999), participants were slower and less accurate when remembering six letters (M6 = 1208 ms; M6 = 89.0%) than when remembering two letters (M2 = 1045 ms; M2 = 96.3%), for RT F(1, 10) = 43.90, p < .001, and for accuracy F(1, 10) = 9.72, p < .05. No other main effects or interactions were significant.
Cowan’s K was calculated based on performance in the 6-letter set condition to obtain estimates of WM capacity (Cowan, 2001): K = (hit rate + correct rejection rate − 1) * N; where N = set-size. The data supported the classification of six items as being above WM capacity and two items as being below capacity (M = 4.45, SD = 0.81, Range = 3.00 to 5.34)
fMRI Data
The group analysis yielded brain-wise main effects of set-size and trial-period (see Supplement Figure 1) and brain-wise Set-Size X Trial-Period interactions (Figure 4). The interaction effects were the focus of the present study, because these effects show where the set-size effects differed over the trial-periods. Whereas, the set-size main effects show the differential effects of set-size averaged over the trial-periods, and the trial-period main effects show the differential effects of the trial-periods averaged over the set-sizes.
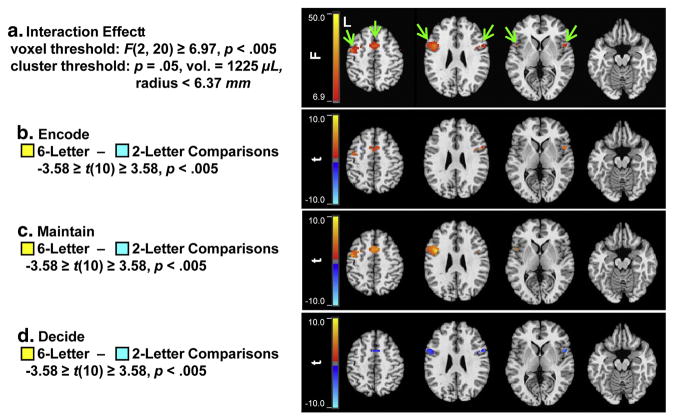
Figure 4.
Statistical parametric maps showing the interaction effects from the random-effects group ANOVA of the area under the BOLD signal-change curve estimates (cluster thresholded based on the parameters shown and color-scaled based on the F-statistics per voxel). The differences in activation between the 6- and 2-letter conditions during each trial-period (b through d) also are depicted (cluster thresholded based on the interaction effects and color scaled based on the t-statistics per voxel).
Set-Size by Trial-Period Interaction Effects
As shown in Figure 4a, three significant interaction effect clusters were found (identified by green arrows). All three were within PFC. The largest cluster was in left lateral PFC (9576 μL; tmax [10] = 5.10; Talairach coord. −29 −5 58; within middle frontal gyrus, BA6) extending from lateral superior frontal gyrus (BA6) ventrally to middle frontal gyrus (BAs 6 and 9), inferior frontal gyrus (BAs 9, 44, 46, and 47), and superior insula regions (BA 13). The right-lateralized cluster (2240 μL; tmax [10] = 3.20; Talairach coord. 57 9 30; within inferior frontal gyrus, BA9) extended ventrally across middle frontal gyrus (BAs 6 and 9) to inferior frontal gyrus (BAs 44, 46 and 47). Finally, the medial cluster (4696 μL; tmax [10] = 3.39; Talairach coord. −1 7 46; within superior frontal gyrus, BA6) extended bilaterally into superior frontal (BA6) regions and cingulate (BA32) gyri. As shown in Figures 3b–3d, the 6-letter sets were associated with greater signal-change than the 2-letter sets during the encode and maintain trial-periods, but the 2-letter sets were associated with greater signal-change during the decision trial-period. These patterns were consistent with previous fMRI findings (e.g., Rypma & D’Esposito, 1999).
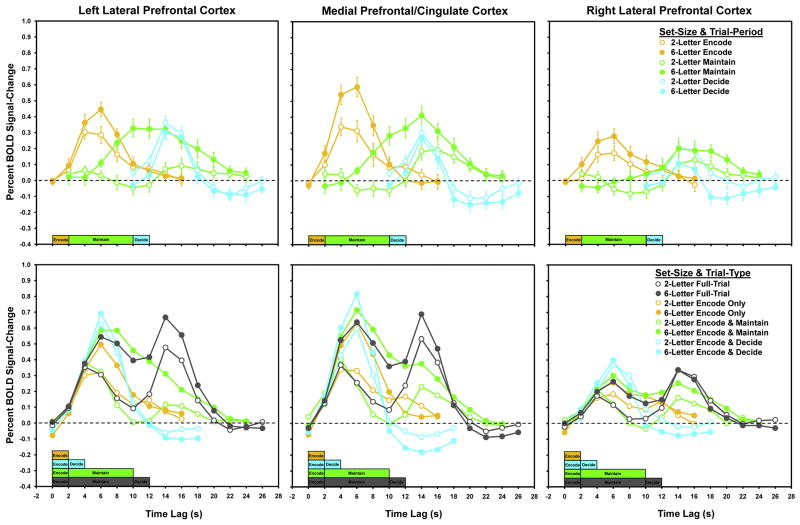
To characterize and further evaluate the trial-period by set-size BOLD signal-change time-courses, the tent parameter estimates from the B-maps for each condition were extracted from each of the clusters for each participant. The initial B-maps from which these data were extracted were the results from the regression of the BOLD data on the sets of scaled tent basis functions for each of the trial-periods (i.e., encode, maintain, and decide) for each of the set-size conditions. The average set-size based differences in the estimates were evaluated for each trial period (Figure 5 top row). The data illustrate the condition-related signal-changes identified based on the ANOVA and simple effects analyses of the AUCs, reported above.
Figure 5.
Mean percent BOLD signal-change functions obtained from lateral and medial PFC clusters showing significant Set-Size X Trial-Period interaction effects (upper row) and mean percent BOLD signal-change for Set-Size X Trial-Type conditions (lower row) within the same clusters. For both rows, closed circles depict 6-letter set effects and open circles depict 2-letter set effects. For the upper row, yellow depicts encoding period effects, green depicts maintenance period effects, and blue depicts decision period effects. For the bottom row, gray depicts full-trial effects, yellow depicts encode-only effects, green depicts encode-maintain effects, and blue depicts encode-decide effects. Color coded timelines within each graph depict corresponding trial-periods.
The data show greater involvement of the three PFC regions during both the encode and maintain periods when processing the 6-letter sets compared to the 2-letter sets. For all three regions, positive signal-change occurred for both the 6-letter and the 2-letter conditions during the encode period, but the 6-letter condition showed greater signal-change than the 2-letter condition (left PFC t[10] > 2.6, p < .025 for 6 and 8 s lags from trial onset; medial PFC t[10] > 3.4, p < .01 for lags 4–8; right PFC t[10] > 2.5, p < .025 for lags 2–8). For all three regions, positive signal-change occurred for both the 6-letter and the 2-letter conditions during the maintain period, but only for the latter lags in this period in the 2-letter condition. The 6-letter condition also was associated with greater signal-change than the 2-letter condition (left PFC t[10] > 2.6, p < .023 for lags 8–20; medial PFC t[10] > 2.96, p < .05 for lags 6–14; right PFC t[10] > 2.27, p < .05 for lags 10,12, and 16–20). Finally, for all three regions, positive signal-change occurred for both the 6-letter and the 2-letter conditions during the decision trial-period. However, the 2-letter condition was associated with greater signal-change than the 6-letter condition, particularly within right PFC (left PFC t[10] > 2.5, p < .05 for lags 10 and 26; medial PFC t[10] > 2.3, p < .05 for lags 10, 12, 24, and 26; right PFC t[10] > 2.25, p < .05 for lags 10–14, 18–20, and 24).
Set-Size by Trial-Type Effects
To examine the BOLD signal-change time-courses for full- and partial-trials, the tent parameter estimates from the B-maps for each Set-Size X Trial-Type condition for each participant were extracted from each of the three interaction clusters identified in the Set-Size X Trial-Period results. The initial B-maps from which these data were extracted were the results from the regression of the BOLD data on the sets of scaled tent basis functions for each of the trial-types (i.e., full, encode-only, encode-maintain, and encode-decide) for each of the set-size conditions. The average set-size based differences in the estimates were evaluated for each trial-types (Figure 5 bottom row).
Across all three regions the full-trial patterns for both the 6- and 2-letter sets (shown in gray) were consistent with previously published results (e.g., Curtis, Rao, & D’Esposito, 2004; Jha & McCarthy, 2000; Leung et al., 2002; Narayanan et al., 2005; Rypma & D’Esposito, 1999). Peaks were present at the beginning and the end of the full-trial function. To illustrate the linear summing of the BOLD response across the trial periods, the data in the Set-Size X Trial-Period figures (Figure 4 top row) were summed at each lag for each region and correlated with the full-trial functions for their respective regions. The summed data fit the full-trial response functions well; for both 6- and 2-letter sets, all rs (n=14) > .99, ps < .001. Additionally, across the three brain regions, the responses for the 6-letter encode-maintain condition remained elevated for a longer duration than the responses for the 6-letter encode only and the 2-letter encode-maintain conditions, thus further illustrating the maintenance-related set-size effects within these regions.
Discussion
The results from the present study revealed differential involvement of PFC in WM component processes. The partial-trial method and analysis allowed for the isolation of unique BOLD responses to the component trial-periods for the two stimulus set-sizes. Although several brain regions were involved in the IRT (e.g., encoding and decision period main effects within visual areas, see Supplement), only medial and lateral regions of PFC were differentially responsive to supra- and sub-capacity memory-sets during the encode, maintain, and decide trial-periods.
During the encode trial-period, medial and lateral PFC regions showed activation in response to both 6- and 2-letter memory sets but greater activation to the 6-letter sets. During the maintain trial-period, these regions also showed activation in response to both 6- and 2-letter memory sets and also greater activation to the 6-letter sets. Additionally, for the 2-letter sets, these PFC regions were not engaged until the latter part of the maintain period, just prior to the decide trial-period. Finally, all three PFC regions also responded differentially to the two set-sizes during the decision period, but in contrast to the encode and maintain periods, the regions showed greater activation in response to the 2-letter sets than to the 6-letter sets.
The results from the present study extend previous partial-trial findings (Manoach et al., 2003) by showing differential effects for supra- versus sub-capacity memory-sets within PFC over the different trial-periods. In the Manoach study, participants completed full-trials (i.e., including encode, maintain, and decide trial-periods) and encode-decide partial-trials. Five-letter sets were used as stimuli. Encode trial-period effects were examined by comparing mean signal-change at 4 s into the encoding period to rest; maintain trial-period effects were examined by comparing mean signal-change averaged over the estimated full-trial responses to mean signal-change estimated over the encode-decide partial-trial responses; and decide trial-period effects were examined by comparing mean signal change at probe onset to 2 s post-probe offset. The contrasts revealed decision trial-period effects within PFC but not encoding or maintenance period effects.
The present results, however, revealed encoding-related effects for both 6- and 2-letter memory-sets but greater effects and a sustained maintenance-related effect for the 6-letter set. The differences in the patterns of results between studies, at least in part, could be due to the differences in set-sizes used. The memory-set of five items was closer to the 4-item WM capacity limit (Cowan, 2001) than the six items used in the present study, possibly requiring less executive recruitment. Of course, the results of the incremental manipulation of set-size need to be examined in order to determine whether PFC recruitment increases linearly with set-size or in steps based on WM capacity, but the present study does demonstrate the efficacy of using a partial-trial method to isolate trial-period BOLD signal-changes to evaluate these hypotheses.
Results from the present study also qualify previous findings on the role of medial PFC in maintenance-related processing (Petit, Courtney, Ungerleider, & Haxby, 1998). This previous research revealed the persistent activity of pre-supplementary motor area and caudal anterior cingulate cortex during delays when remembering locations and faces. The research also suggested that this persistent activity was not mediating motor response selection or preparation, per se, but that it was mediating attentional processes or some other cognitive process facilitating a memoranda-based state of preparedness for selecting a response. The reported centroid of this previously reported sustained activity was proximal to the peak of the medial Set-Size X Trial-Period cluster found in the present study (i.e., Talairach coordinates −1 9 50 and −1 7 46, respectively). Thus, the maintenance period set-size effect observed within this region in the present study suggests that the attentional or preparedness processes depend on the memory capacity demands of the task, active when demand is high but relatively dormant when demand is low.
The present study also demonstrates the efficacy of using fMRI partial-trial methods for further disambiguating the role of PFC (and other regions) in WM component processes. The partial-trial method used in the present study allowed for the identification of the differential recruitment of PFC in IRT component processes. In previous studies, however, set-size effects within PFC have been attributed to attention allocation, manipulation processes, and storage processes (see Rympa, 2006). With respect to the encode period, the set-size effects for the present study cannot be attributed to the mere amount of material to which the participants were exposed, because the participants were shown six letters in both set-size conditions. The greater activity for the 6-letter sets resulted from the requirement to encode and remember larger versus smaller sets of letters. The set-size differences, however, could be due to the previously suggested attention allocation, manipulation processes, or storage processes or some combination of these processes. The results from the present study suggest that the systematic manipulation of the processing requirements in combination with partial-trials could allow for further disambiguation of the PFC processes mediating the encoding period.
The maintain period results in the present study do suggest that these regions mediate processes qualitatively different from WM storage, per se, because these PFC regions exhibited sustained activity over the delay for the 6-letter sets but not for the 2-letter sets. If mediating storage, then the 2-letter sets also should have been associated with sustained activity over the delay. The sustained activity over the period for 6-letter sets but not for the 2-letter sets suggests that time in the delay interval might have been exploited for the engagement of organization processes in an attempt to manipulate the supra-capacity information to fit WM capacity constraints (Rypma, Berger, & D’Esposito, 2002; Rypma & Prabhakaran, 2009). In fact, the duration of the encode period in IRTs has been hypothesized to affect the detection of maintenance-period set-size effects within PFC (Narayanan et al., 2005; Rypma, 2006). Although systematic explorations of this hypothesis have not been conducted, the 2 s encode period duration used in the present study was comparable to the 2.16 s duration used in a previous study in which maintenance-period set-size effects within PFC were found (Narayanan et al., 2005).
Finally, the set-size effects during the decide trial-period were opposite to the effects in the other two periods. This decision period pattern has been reported in previous research (Rypma & D’Esposito, 1999). The interaction pattern shows that the functions of these PFC regions change with the trial-period requirements; that is, the regions do not simply mediate encoding, storage, memory search, etc. Full understanding of the decision period PFC activation, however, also will require a better understanding of the complex relationships between neural activity and decision period component processes. For example, the engagement of encoding and maintenance processes, such as the deferral of processes for smaller memory-sets, might affect the engagement of different decision processes. The engagement of multiple PFC or PFC-controlled processes following executive deferral, for example, might produce a greater summed signal-change during the decision period than signal-change brought about by WM search processes alone. However, further experimentation is needed to verify this deferral hypothesis, but again, the results from the present study suggest that the systematic manipulation of the processing requirements in combination with partial-trials could allow for further disambiguation of the decision processes mediated by these PFC regions.
The present study was motivated by theoretical concerns regarding the role of PFC in working memory component processes, but these theoretical concerns also were linked to methodological concerns regarding optimal fMRI designs for assessing PFC function across adjacent trial-periods. The trial-type effects (i.e., full-trial versus partial-trial effects) in the present study illustrate the problem of the summing of the HRFs across IRT trial-periods, a colinearity that needs to be considered when evaluating trial-period effects. The deconvolution of the set-size effects over the trial-periods (i.e., encode, maintain, and decide period effects) in the present study, however, illustrates the power of the partial-trial method for isolating and estimating signal-changes to the adjacent trial-periods. The isolation of the signal-changes to adjacent trial-periods allowed for the examination of set-size effects over the trial-periods and for inferences about relative PFC activations to supra- and sub-capacity memory-sets.
The results from the present study also show the potential value of using the partial-trial method for examining other hypotheses regarding the role of PFC in WM, for example, executive mediation when given explicit material manipulation instructions (D’Esposito, Postle, Ballard, & Lease, 1999; Eldreth et al., 2006; Postle, Berger, & D’Esposito, 1999), when performing mental computations (Seyler et al., 2003), or when remembering information over a long delay (Jha & McCarthy, 2000). Additionally, the partial-trial method also could be used to evaluate PFC process-specific (D’Esposito et al., 2000; Curtis & D’Esposito, 2003; Petrides, 1996; Rypma, 2006) versus modality-specific hypotheses (Goldman-Rakic, 1987; Levy & Goldman-Rakic, 2000) and component process connectivity hypotheses (Woodward et al., 2006). Furthermore, the data provide evidence for the efficacy of using partial-trial methods to study other higher-level cognitive processes where assessment tasks require sequential, temporally adjacent presentations of stimuli designed to engage component processes.
Supplementary Material
Acknowledgments
The project described was supported by Grant Number AG029523 from NIH and Grant Number VA549-P-0027 from the United States Veteran’s Administration. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH or the Veteran’s Administration. The authors thank Ehsan Shokri Kojori for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baddeley AD. Working Memory. New York: Oxford University Press; 1986. [Google Scholar]
- Bor D, Duncan J, Wiseman RJ, Owen AM. Encoding strategies dissociate prefrontal activity from working memory demand. Neuron. 2003;37:361–367. doi: 10.1016/s0896-6273(02)01171-6. [DOI] [PubMed] [Google Scholar]
- Bower GH, Springston F. Pauses as recoding points in letter series. Journal of Experimental Psychology. 1970;83:421–430. [Google Scholar]
- Bower GH, Winzenz D. Group structure, coding, and memory for digit series. Experimental Psychological Monographs. 1969;80:1–17. [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. NeuroImage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Cairo TA, Liddle PF, Woodward TS, Ngan ETC. The influence of working memory load on phase specific patterns of cortical activity. Cognitive Brain Research. 2004;21:377–387. doi: 10.1016/j.cogbrainres.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. TRENDS in Cognitive Sciences. 2003;9:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Rao VY, D’Esposito M. Maintenance of spatial and motor codes during oculomotor delayed response tasks. Journal of Neuroscience. 2004;24:3944–3952. doi: 10.1523/JNEUROSCI.5640-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Haxby JV, Ungerleider LG. The role of prefrontal cortex in working memory: examining the contents of consciousness. Philosophical Transactions of the Royal Society of London. 1998;353:1819–1828. doi: 10.1098/rstb.1998.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Science. 2001;24:87–185. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Cowan N. Working Memory Capacity. New York: Psychology Press; 2005. [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dale A, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Human Brain Mapping. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Deese J, Kaufman RA. Serial effects in recall of unorganized and sequentially organized verbal material. Journal of Experimental Psychology. 1957;54:180–187. doi: 10.1037/h0040536. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: An event-related fMRI study. Brain Cognition. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Experimental Brain Research. 2000;133:3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Ehrlich MF, Brebion J, Tardieu H. Working-memory capacity and reading comprehension in yound and older adults. Journal of Psychological Research. 1994;56:110–115. doi: 10.1007/BF00419718. [DOI] [PubMed] [Google Scholar]
- Eldreth DA, Patterson MD, Porcelli AJ, Biswal BB, Rebbechi D, Rypma B. Evidence for multiple manipulation processes in prefrontal cortex. Brain Research. 2006;1123:145–156. doi: 10.1016/j.brainres.2006.07.129. [DOI] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin E, Conway ARA. Working memory, short-term memory and general fluid intelligence: A latent variable approach. Journal of Experimental Psychology: General. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Feredoes E, Postle B. Localization of load sensitivity of working memory storage: Quantitatively and qualitatively discrepant results yielded by single-subject and group-averaged approaches to fMRI group analysis. NeuroImage. 2007;35:881–903. doi: 10.1016/j.neuroimage.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, Penny WD. Statistical Parametric Mapping: The Analysis of Functional Brain Images. London: Academic Press; 2006. [Google Scholar]
- Garavan H. Serial attention within working memory. Memory & Cognition. 1998;26:263–276. doi: 10.3758/bf03201138. [DOI] [PubMed] [Google Scholar]
- Glanzer M, Razel M. The size of the unit in short-term storage. Journal of Verbal Learning and Verbal Behavior. 1974;13:114–131. [Google Scholar]
- Goel V, Grafman J. Are the frontal lobes implicated in planning functions? Interpreting data from the Tower of Hanoi. Neuropsychologia. 1995;33:623–642. doi: 10.1016/0028-3932(95)90866-p. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Plum F, Mountcastle F, editors. Handbook of Physiology. Vol. 5. Washington DC: The American Physiological Society; 1987. pp. 373–517. [Google Scholar]
- Graybill FA, Iyer HK. Regression Analysis: Concepts and Applications. Belmont, CA: Duxbury Press; 1994. [Google Scholar]
- Habeck C, Rakitin BC, Moeller J, Scarmeas N, Zarahn E, Brown T, et al. An event-related fMRI study of the neural networks underlying the encoding, maintenance, and retrieval phase in a delayed-match-tosample task. Brain Research Cognitive Brain Research. 2005;23:207–220. doi: 10.1016/j.cogbrainres.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Handwerker DA, Ollinger JM, D’Esposito M. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. NeuroImage. 2004;21:1639–1651. doi: 10.1016/j.neuroimage.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Jha AP, McCarthy G. The influence of memory load upon delay-interval activity in a working-memory task: An event-related functional MRI study. Journal of Cognitive Neuroscience. 2000;12:90–105. doi: 10.1162/089892900564091. [DOI] [PubMed] [Google Scholar]
- Leung HC, Gore JC, Goldman-Rakic PS. Sustained mnemonic response in the human middle frontal gyrus during on-line storage of spatial memoranda. Journal of Cognitive Neuroscience. 2002;14:659–671. doi: 10.1162/08989290260045882. [DOI] [PubMed] [Google Scholar]
- Lebedev MA, Messinger A, Kralik JD, Wise SP. Representation of attended versus remembered locations in prefrontal cortex. PLoS Biology. 2004;2:e365. doi: 10.1371/journal.pbio.0020365. Retrieved April 10, 2008, from http://biology.plosjournals.org/archive/1545-7885/2/11/pdf/10.1371_journal.pbio.0020365-S.pdf. [DOI] [PMC free article] [PubMed]
- Lepsien J, Friffin IC, Devlin JT, Nobre AC. Directing spatial attention in mental representations: Interactions between attentional orienting and working-memory load. NeuroImage. 2005;26:733–743. doi: 10.1016/j.neuroimage.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Experimental Brain Research. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Greve DN, Lindgren KA, Dale AM. Identifying regional activity associated with temporally separated components of working memory using event-related functional MRI. NeuroImage. 2003;20:1670–1684. doi: 10.1016/j.neuroimage.2003.08.002. [DOI] [PubMed] [Google Scholar]
- McElree B, Dosher BA. Serial position and set size in short-term memory: The time course of recognition. Journal of Experimental Psychology: General. 1989;118:346–373. [Google Scholar]
- Miller GA. The magical number seven, plus or minus two: Some limits on our capacity for processing information. Psychological Review. 1956;63:81–97. [PubMed] [Google Scholar]
- Murdock BB., Jr The serial position effect of free recall. Journal of Experimental Psychology. 1962;64:482–488. [Google Scholar]
- Narayanan NS, Prabhakaran V, Bunge SA, Christoff K, Fine EM, Gabrieli JDE. The role of prefrontal cortex in the maintenance of verbal working memory: An event-related fMRI analysis. Neuropsychology. 2005;19:223–232. doi: 10.1037/0894-4105.19.2.223. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Coull JT, Maquet P, Frith CD, Vandenberghe R, Mesulam MM. Orienting attention to locations in perceptual versus mental representations. Journal of Cognitive Neuroscience. 2004;16:363–373. doi: 10.1162/089892904322926700. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI II. Analysis. NeuroImage. 2001;13:218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI I. The method. NeuroImage. 2001;13:210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Petit L, Courtney SM, Ungerleider LG, Haxby JV. Sustained activity in the medial wall during working memory delays. The Journal of Neuroscience. 1998;18:9429–9437. doi: 10.1523/JNEUROSCI.18-22-09429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Local frontal cortical contribution to memory. Seminars in the Neurosciences. 1996;8:57–63. [Google Scholar]
- Postle BR, Berger JS, D’Esposito M. Functional neuroanatomical double dissociation of mnemoni and executive control processes contributing to working memory performance. Proceedings of the National Academy of Sciences U.S.A; 1999. pp. 12959–12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran V, Narayanan K, Zhao Z, Gabrieli JDE. Integration of diverse information in working memory with the frontal lobe. Nature Neuroscience. 2000;3:85–90. doi: 10.1038/71156. [DOI] [PubMed] [Google Scholar]
- Repovs G, Baddeley A. The multi-component model of working memory: explorations in experimental cognitive psychology. Neuroscience. 2006;139:5–21. doi: 10.1016/j.neuroscience.2005.12.061. [DOI] [PubMed] [Google Scholar]
- Rosen BR, Buckner RL, Dale AM. Event-related functional MRI: Past, present, and future. Proceedings of the National Academy of Sciences: USA. 1998;95:773–780. doi: 10.1073/pnas.95.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Rypma B. Factors controlling neural activity during delayed-response task performance: Testing a memory organization hypothesis of prefrontal function. Neuroscience. 2006;139:223–235. doi: 10.1016/j.neuroscience.2005.07.062. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, D’Esposito M. The influence of working-memory demand and subject performance on prefrontal cortical activity. Journal of Cognitive Neuroscience. 2002;14:721–731. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M. The roles of prefrontal brain regions in components of working memory: Effects of memory load and individual differences. Proceedings of the National Academy of Sciences: USA. 1999;96:6558–6563. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V. When less is more and when more is more: The mediating roles of capacity and speed in brain-behavior efficiency. Intelligence. 2009;37:207–222. doi: 10.1016/j.intell.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Chen G, Reynolds RC, Chistidis PP, Hammett KR, et al. Functional imaging analysis context (FIAC) analysis according to AFNI and SUMA. Human Brain Mapping. 2006;27:417–424. doi: 10.1002/hbm.20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nature Neuroscience. 2002;5:479–484. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Buckner RL, Koutstaal W, Dale AM, Rosen BR. Late onset of anterior prefrontal activity during true and false recognition: an event-related fMRI study. NeuroImage. 1997;6:259–269. doi: 10.1006/nimg.1997.0305. [DOI] [PubMed] [Google Scholar]
- Seyler DJ, Kirk EP, Ashcraft MH. Elementary subtraction. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:1339–1352. doi: 10.1037/0278-7393.29.6.1339. [DOI] [PubMed] [Google Scholar]
- Shah P, Miyake A. Models of working memory: An introduction. In: Miyake A, Shah P, editors. Models of working memory: Mechanisms of active maintenance and executive control. Cambridge: Cambridge University Press; 1999. pp. 1–27. [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Sternberg S. Memory-scanning: mental processes revealed by reaction-time experiements. American Scientist. 1969;57:421–457. [PubMed] [Google Scholar]
- Sternberg S. Memory scanning: New findings and current controversies. The Quarterly Journal of Experimental Psychology. 1975;27:1–32. [Google Scholar]
- Talairach J, Tornoux P. Co-planar stereotaxic atlas of the human brain. NY: Thieme Medical Publishers; 1988. [Google Scholar]
- Ward BD. Simultaneous inference for FMRI data [Computer software manual] 2000 Retrieved from http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim.
- Ward BD. Deconvolution analysis of FMRI time series data [Computer software manual] 19982006 Retrieved from http://afni.nimh.nih.gov/afni/doc/manual/3dDeconvolve.
- Wechsler D. The Wechsler Adult Intelligence Scale—. 3. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wickelgren I. Getting a grasp on working memory. Science. 1997;275:1580–1582. doi: 10.1126/science.275.5306.1580. [DOI] [PubMed] [Google Scholar]
- Woodward TS, Cairo TA, Ruff CC, Takane Y, Hunter MA, Ngan TC. Functional connectivity reveals load dependent neural systems underlying encoding and maintenance in verbal working memory. Neuroscience. 2006;139:317–325. doi: 10.1016/j.neuroscience.2005.05.043. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre GK, D’Esposito M. Temporal isolation of the neural correlates of spatial mnemonic processing with fMRI. Brain Research. 1999;7:255–268. doi: 10.1016/s0926-6410(98)00029-9. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y. Positive evidence against human hippocampal involvement in working memory maintenance of familiar stimuli. Cerebral Cortex. 2005;15:303–316. doi: 10.1093/cercor/bhh132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.