Abstract
We investigated the extent of misdirection of regenerating axons when that regeneration was enhanced using treadmill training. Retrograde fluorescent tracers were applied to the cut proximal stumps of the tibial and common fibular nerves two or four weeks after transection and surgical repair of the mouse sciatic nerve. The spatial locations of retrogradely labeled motoneurons were studied in untreated control mice and in mice receiving two weeks of treadmill training, either according to a continuous protocol (10 m/min, one hour/day, five day/week) or an interval protocol (20 m/min for two minutes, followed by a five minute rest, repeated 4 times, five days/week). More retrogradely labeled motoneurons were found in both treadmill trained groups. The magnitude of this increase was as great as or greater than that found after using other enhancement strategies. In both treadmill trained groups, the proportions of motoneurons labeled from tracer applied to the common fibular nerve that were found in spinal cord locations reserved for tibial motoneurons in intact mice was no greater than in untreated control mice and significantly less than found after electrical stimulation or chondroitinase treatment. Treadmill training in the first two weeks following peripheral nerve injury produces a marked enhancement of motor axon regeneration without increasing the propensity of those axons to choose pathways leading to functionally inappropriate targets.
Keywords: exercise, motoneurons, retrograde tracing, spinal cord
Introduction
Axons in injured peripheral nerves have a significant capacity for regeneration, yet functional recovery following peripheral nerve injury is poor (Brushart, 1998; Frostick et al., 1998). One reason for these poor functional outcomes might be the inappropriate reinnervation of muscles by regenerating axons. Muscles could be reinnervated by motoneurons that had previously innervated functional antagonists (English, 2005) or exuberant branches of the same motoneuron might reinnervate two antagonistic muscles (Brushart and Mesulam, 1980; Gordon et al., 2003). Either scenario might result in a dyskinesia and poor functional recovery.
One means of measuring the extent of misdirection of regenerating motor axons following peripheral nerve injury is to exploit the natural topographic organization of motoneurons in the spinal cord: the spatial location of the cell bodies of regenerating motor axons. In the mouse and rat, the motoneurons whose axons are found in the common fibular nerve are restricted to the rostral-most 60% of the motor nucleus of the distal sciatic nerve (English, 2005; McHanwell and Biscoe, 1981; Swett et al., 1986). In a frontal plane, common fibular motoneurons are found lateral and inferior to tibial motoneurons in the ventral horn (Brushart and Mesulam, 1980; McHanwell and Biscoe, 1981). After transection and surgical repair of the mouse sciatic nerve, cell bodies of motoneurons with axons in the common fibular nerve are no longer so restricted in their spinal locations. Approximately 20% of motoneurons that can be labeled by application of retrograde neuronal tracers to the common fibular nerve are found in the caudal-most 40% of the distal sciatic motor nucleus. In regions where both tibial and common fibular motoneurons are found, nearly one-third of the labeled common fibular motoneurons are not spatially segregated from tibial motoneurons in a frontal plane. We have termed such motoneurons topographically inappropriate (English, 2005), since their spatial localization in the spinal cord is evidence that they represent reinnervation of targets of the common fibular nerve by motoneurons that had innervated different targets prior to nerve injury.
An area of intense recent research has been the development of techniques and approaches to enhance early axon regeneration following peripheral nerve injury. A goal of this research has been to accelerate axon regeneration with the hope that this would lead to improved functional outcomes. In previous reports from this laboratory and others, enhancing axon regeneration was accomplished by reducing inhibition of early axon regeneration with bacterial enzymes that degrade proteoglycans in the distal stumps of cut nerves (Groves et al., 2005; Neubauer et al., 2007; Zuo et al., 2002) or by using brief electrical stimulation of the proximal stump of the cut nerve at the time of its surgical repair to promote axon growth (Al-Majed et al., 2000; Brushart et al., 2005; English et al., 2007; Geremia et al., 2005). In the mouse sciatic nerve model system, we showed that, although modest reinnervation of peripheral targets by motoneurons in inappropriate spinal locations occurred without enhancement, the amount of this topographically inappropriate reinnervation increased substantially if early axon regeneration was enhanced (English, 2005).
We have shown that modest treadmill exercise following nerve transection and repair results in a striking enhancement of axon regeneration (Sabatier et al., 2008). Both continuous exercise at a slow treadmill speed for one hour, five days per week, and very brief but more intense interval training resulted in enhancement of axon regeneration that was as great or greater than any method so far developed. Because we assume that treadmill exercise results in activation of axotomized motoneurons naturally, via the spinal circuitry driving locomotion, we hypothesized (English, 2005) that it might produce enhancement of axon regeneration without the increased misrouting of regenerating axons observed with other methods. The overall goal of this study was to evaluate that hypothesis.
We investigated the specificity of reinnervation of one of the two terminal branches of the mouse sciatic nerve in treadmill trained and unexercised animals by means of retrograde fluorescent labeling methods. We show here that treadmill training is a very potent enhancer of motor axon regeneration. Reinnervation of peripheral targets by motoneurons in topographically inappropriate locations is no greater in treadmill trained mice than it is in untreated controls. A preliminary report of these findings has been made (English et al., 2008).
Material and Methods
Animals and surgeries
Experiments were conducted using 19 wild type C57BL6 mice (average BW 25 Gm). In 16 mice the sciatic nerve was transected using sharp scissors just proximal to the branching of the sural nerve under ketamine (75 mg/kg) and xylazine (10 mg/kg) anesthesia. The cut stumps of the nerve were aligned as much as possible and secured in place using fibrin glue (English, 2005; MacGillivray, 2003; Menovsky and Beek, 2001). All surgical procedures were approved by the Institutional Animal Care and Use Committee of Emory University and were consistent with the Guidelines for Use of Laboratory Animals of the Society for Neuroscience. The remaining three mice were subjected to treadmill training and retrograde labeling experiments but their sciatic nerves were not cut and repaired.
Treadmill training
All experimental animals were acclimated to treadmill locomotion before being used in the study, but none had been on a treadmill for at least two weeks prior to nerve repair surgery. On the third day following nerve repair surgery, daily treadmill training was begun. Two groups of exercised animals were studied. In a Continuous Training group, mice were run on a level treadmill at 10 m/min continuously for one hour, five days per week, for two weeks. In an Interval Training group, exercise was conducted on the same treadmill, five days per week for two weeks, but at a much faster speed (20 m/min) for four intervals of two minutes duration, each followed by a five minute rest period. Both of these protocols have been shown to result in enhancement of axon regeneration in cut peripheral nerves. Mice in both groups required very little incentive to complete these exercise requirements, even after complete transection of the sciatic nerve.
Retrograde labeling
In both of the mouse groups, terminal retrograde labeling experiments were performed at either two weeks or four weeks following nerve repair. The methods used have been described in detail previously (English, 2005). Briefly, under ketamine/xylazine anesthesia, the common fibular and tibial nerves were exposed, isolated, and cut approximately 5 mm distal to the original sciatic nerve transection site. The proximal stumps were surrounded by wells of vacuum grease formed on small squares of Parafilm, and then soaked in water for five minutes. The water was then removed and replaced with crystals of dextran amines (10,000 MW, fixable) conjugated either to Alexafluor 488 or Alexafluor 594 (Invitrogen). Once in place the crystals were hydrated with a small drop of saline solution. The tracers were left in place for 90 minutes, after which they were removed and the entire surgical field was irrigated three times with normal saline solution. The distal tips of the nerve were then separated from each other and each coated with 6 μl of fibrin glue. The surgical wounds were closed in layers and the animals returned to their cages. All procedures were performed bilaterally. Animals survived five days before being euthanized.
Five days following the last application of tracer, animals were euthanized with an overdose of pentobarbital (150 mg/kg, IP) and perfused transcardially with saline followed by periodate-lysate-paraformaldehyde (PLP) fixative (McLean and Nakane, 1974). The L2-S1 segments of spinal cord were removed and stored overnight in 20% sucrose solution at 4°C for cryoprotection. Serial transverse sections were cut at 30 μm on a cryostat and mounted directly onto subbed slides. Sections were coversliped with Entellan.
Analysis
Sections were imaged at relatively low magnification (10x) using filter sets appropriate to the fluorophores employed and high resolution RGB images of each ventral horn were obtained with a low light camera using SimplePCI software (Hamamatsu, Sewickley, PA) and stored on disc for later analysis. No further processing of the obtained images was performed. Motoneurons were scored as containing one or more retrograde fluorescent label if the label filled the cell soma and extended into its proximal dendrites and also contained a visible nuclear area devoid of label (Fig. 1A) (Swett et al., 1986). Some neurons were encountered in which much weaker fluorescence was eccentrically distributed in the cell body and did not extend into the proximal dendrites. We considered these neurons to contain lipofuschin and they were not counted as labeled. Each labeled motoneuron was assigned a relative caudorostral position in the spinal cord based on the number of the serial section in which it was counted. The most caudal section containing a labeled cell was assigned a value of zero and the most rostral labeled motoneuron was assigned a position of 100. For each spinal cord studied, a frequency distribution of the relative caudorostral positions of cells was generated with a bin size of 5%. In each group of animals studied, these distributions of tibial and common fibular motor nuclei were averaged from at least four mice. The person performing this analysis was always unaware of the treatment group at the time that the analysis was performed.
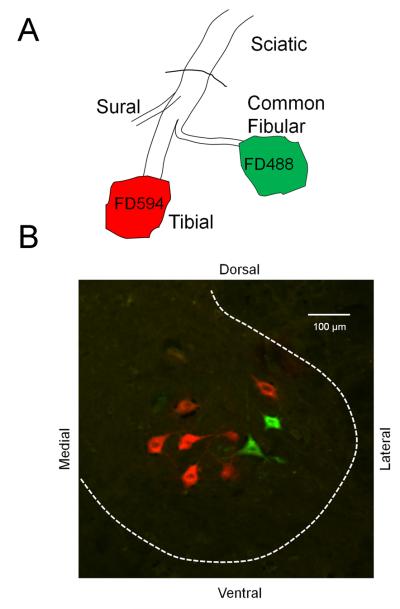
Figure 1.
Diagram of the experimental procedure used to label motoneurons. At two or four weeks after transection and end-to-end anastomosis of the sciatic nerve, its two terminal branches, the tibial and common fibular nerves, were cut ca. 5 mm distal to the original lesion site and soaked for 90 minutes in dextran amines conjugated to different fluorophores. Five days later, mice were euthanized and spinal cords were harvested for histologic analysis. B. In an image of a transverse section of the spinal cord of an intact mouse, motoneurons labeled red after application of Alexafluor 594 conjugated dextran amine to the tibial nerve and motoneurons labeled green after application of Alexafluor 488 conjugated dextran amine to the common fibular nerve are shown. This figure is also available in magenta-green format as supplementary figure 1.
We hypothesized that treadmill training would result in an enhancement of motor axon regeneration and that this enhancement would result in the retrograde labeling of more motoneurons from application of fluorescent tracers to nerves 5 mm distal to the lesion than in untreated control animals. To test this hypothesis, we compared counts of tibial and common fibular motoneurons in different treatment groups, corrected for double counting using the method of Abercrombie (Abercrombie, 1946), to those of untreated controls using analysis of variance (ANOVA) and appropriate paired, post-hoc (Fisher's Least significant differences (LSD)) testing. We compared the number of labeled motoneurons found two weeks and four weeks after sciatic nerve transection in treadmill trained groups to the numbers of labeled motoneurons found at the same survival times in untreated controls. We also compared these data to the numbers of motoneurons labeled in identical experiments when axon regeneration was enhanced, either by brief electrical stimulation of the proximal stump of the cut nerve or when chondroitinase ABC was applied to the distal stump of the cut nerve prior to repair. We have reported all of these values previously (English, 2005).
We also hypothesized that enhancing axon regeneration using treadmill exercise would do so without a loss of topographic specificity of regenerating motoneurons. Motoneurons whose axons are found in the common fibular nerve are restricted to the rostral 60% of the distal sciatic motor nucleus in mice (English, 2005). Any motoneurons whose cell bodies lie in the caudal 40% of the sciatic motor nucleus but whose axons lie in the common fibular nerve are termed topographically inappropriate (English, 2005). We determined the number of motoneurons retrogradely labeled from application of tracers to the common fibular nerve of treadmill trained animals that lie in the caudal 40% of the distribution of all labeled motoneurons. We assumed that this represents the caudorostral extent of the entire distal sciatic nerve motor nucleus, as we have shown previously (English, 2005). The mean number of topographically inappropriate motoneurons in the different treadmill trained groups, untrained animals, electrically stimulated mice, and chondroitinase ABC treated mice were compared using ANOVA and appropriate post hoc (LSD) testing. In addition, we determined the median relative caudorostral position of all of the motoneurons labeled after application of tracer to the tibial and common fibular nerves, and compared the average medians from the different treatment groups, as described above.
In the caudorostral regions of the spinal cord of normal mice in which motoneurons labeled from the two different peripheral targets coexist, those labeled from the common fibular nerve lie lateral to those marked by application of tracer to the tibial nerve (English, 2005; McHanwell and Biscoe, 1981). Following transection and surgical repair of the sciatic nerve, this segregation in a frontal plane is decreased. Fully one-third of motoneurons labeled by tracer application to the common fibular nerve lie medial to motoneurons labeled after tracer application to the tibial nerve (English, 2005). The magnitude of this decreased spatial segregation is similar when axon regeneration is enhanced, either by brief electrical stimulation or chondroitinase ABC treatment. To evaluate whether a similar loss of this frontal plane topography is found in treadmill trained mice, we evaluated the extent of segregation of the motoneurons projecting to different targets. In each of the histological sections through the spinal cord that contained different motoneurons labeled with different tracers, the number of times that each motoneuron labeled by application of tracer to the common fibular nerve was found lateral to each motoneuron labeled by application of tracer to the tibial nerve (the appropriate topography) was determined. This count was then expressed as a proportion of all of the possible mediolateral spatial interactions between the two species of motoneurons. Significance of differences in means of this laterality index between the different treatment groups was evaluated by ANOVA, with appropriate post-hoc paired testing.
Results
Application of retrograde tracers to cut common fibular and tibial nerves in mice resulted in the labeling of the cell bodies of motoneurons in the lumbar spinal cord. An example of one section of the spinal cord of an intact mouse containing labeled motoneuron somata is shown in Figure 1B. Motoneurons labeled green were marked by application of fluorescent dextran conjugated to Alexafluor 488 to the common fibular nerve. Motoneurons labeled red were marked by application of fluorescent dextran conjugated to Alexafluor 594 to the tibial nerve.
Treadmill training enhances axon regeneration in mice
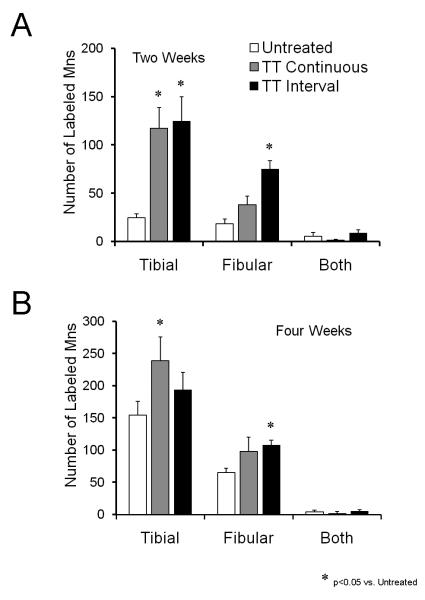
If treadmill training in the immediate post-operative period results in enhanced axon regeneration, then one might expect that more retrogradely labeled motoneurons would be found in exercised mice than in untreated controls. In Figure 2A, the average (+SEM) number of motoneurons labeled from tracer application either to the tibial or common fibular nerve 5 mm distal to a prior transection of the entire sciatic nerve is shown for two and four week survival periods. At the two week survival time, significantly (LSD, p<0.05) more motoneurons were labeled after tracer application to the tibial nerve with either continuous or interval training. The same was true after tracer application to the common fibular nerve in mice trained using an interval training strategy but not in mice using a continuous training strategy (Fig. 2A). At the four week survival time, significantly more (p<0.05) motoneurons were labeled from tracer application to the tibial nerve in the Continuous Training group and from tracer application to the common fibular nerve in the Interval Training group (Fig. 2B). The number of motoneurons labeled from application of tracer to the tibial nerve in the Interval Training group and from tracer application to the common fibular nerve in the Continuous Training group were not significantly different (p=0.33 and 0.22, respectively). There was no significant difference between the Continuous and Interval Training groups (LSD, p=0.96), nor was there any effect of treadmill training on the number of motoneurons labeled from both tibial and common fibular nerves (p=0.11).
Figure 2.
Treadmill training enhances axon regeneration in cut peripheral nerves. Mean (+SEM) numbers of motoneurons labeled by tracer application to the tibial nerve, the common fibular nerve, or both nerves are shown two weeks (A) or four weeks (B) after transection and surgical repair of the sciatic nerve. Data are shown for unexercised controls reported previously (English, 2005) and for mice trained using either a continuous or interval strategy. Each bar represents a mean of four cases.
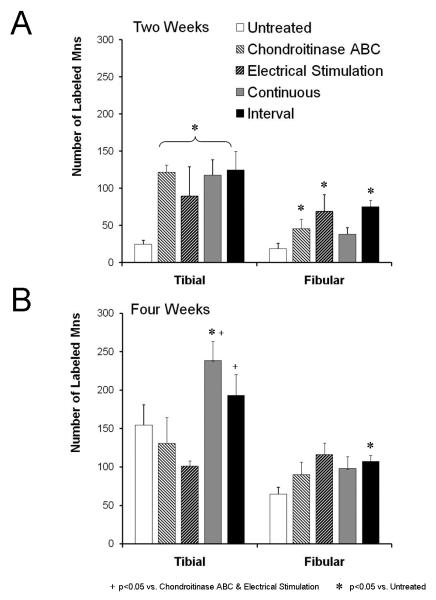
The magnitude of the increase in motoneuron counts observed in the treadmill trained mice was as great as or greater than that observed after other means of enhancing axon regeneration. As reported earlier (English, 2005), either one hour of electrical stimulation applied to the proximal stump of the cut sciatic nerve at the time of its repair or chondroitinase ABC treatment of the distal stump resulted in a significant increase in the number of labeled motoneurons over untreated controls two weeks later, but not four weeks later. When we compared these results to the results of the present study, we found no significant difference between either group of treadmill trained mice and either electrically stimulated or chondroitinase treated mice at the two week time period (Figure 3A). These similarities were the same for tibial and fibular nerves. At the four week survival time, the number of motoneurons labeled from tracer application to the tibial nerve but not to the common fibular nerve in both treadmill trained groups was significantly (p<0.05) greater than found in either the chondroitinase ABC or electrically stimulated groups (Figure 3B: +).
Figure 3.
Comparisons of the effects of treadmill training to treatments with chondroitinase ABC or electrical stimulation. The mean (+SEM) number of retrogradely labeled motoneurons counted in the different treatment groups are shown. Data for untreated and treadmill trained mice are the same as shown in Figure 2. Data for chondroitinase ABC treatment and electrical stimulation are from English (2005).
Treadmill training does not increase the number of labeled motoneurons
To evaluate whether the treadmill training itself resulted in more labeled motoneurons, we marked the motoneurons with axons in the tibial nerves of three intact mice that had been subjected to two weeks of interval training using the same methods described above. We used an interval training paradigm because it resulted in the most robust effect on axon regeneration. Differences in the mean number of retrogradely labeled motoneurons between these trained mice (236.13 ± 25.90, SEM, N=6) and untrained controls (221.87 ± 24.19, SEM, N=8) (English, 2005) were not statistically significant (unpaired t-test, p=0.93).
Topographic specificity after treadmill training
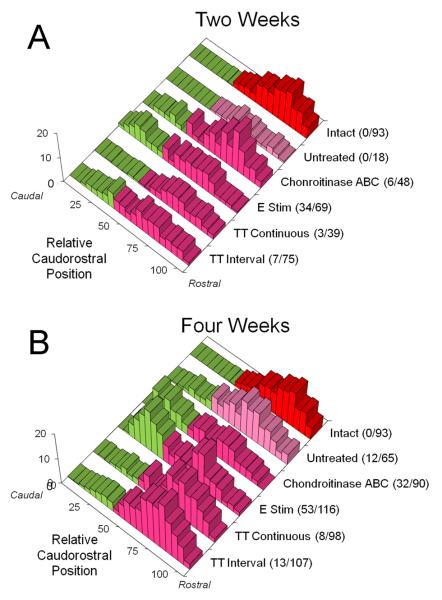
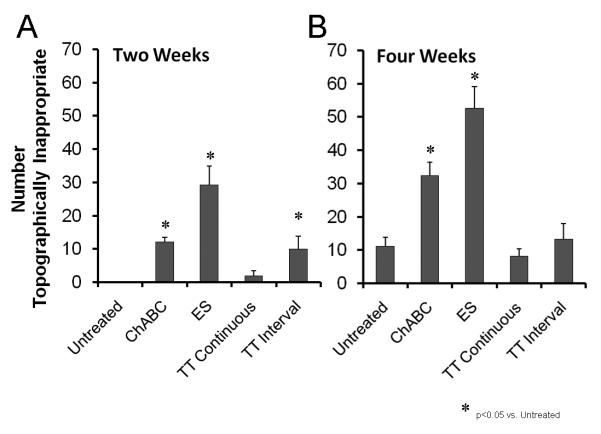
If treadmill training enhances axon regeneration without an increase in the misdirection of those axons to inappropriate targets, then the topographic specificity of target reinnervation, one measure of the extent of misdirection of the axons, might be expected to be no greater than that found without treatment. The topographic specificity of reinnervating motor axons was studied using analysis of the spatial locations of motoneurons labeled from tracer application to the common fibular nerve at two and four weeks following sciatic nerve transection. We studied the spatial locations of motoneurons in a sagittal plane by comparing the relative caudorostral distributions of labeled motoneurons in the different treatment groups. Data for chondroitinase treated and electrically stimulated mice have been published already (English, 2005). These distributions are shown in Figure 4. Data from two week (top) and four week (bottom) survival times are shown. In each of these histograms, the data from the back row are represented as frequency distributions of labeled motoneurons in intact mice. Note that labeled motoneurons are found only in the rostral most 60% of the distal sciatic motor nucleus. The regions of the graph with the bars shaded green are portions of the distal sciatic motor nucleus in which common fibular motoneurons are not found in intact mice – i.e. caudal 40%. Any motoneurons labeled by tracer application to the common fibular nerve found in this region after sciatic nerve transection and repair are considered topographically inappropriate. The different treatment groups are listed on the right edge of each graph, along with the average number of topographically inappropriate motoneurons and the average total number of motoneurons found.
Figure 4.
Distributions of retrogradely labeled motoneurons in a sagittal plane. In each graph, the relative caudorostral distribution of motoneurons labeled after application of tracer to the common fibular nerve is shown. Caudal is to the left (0%) and rostral is to the right (100%). Data from the two week survival time are shown in A. Data from the four week survival time are shown in B. The histogram shown along the back wall of each graph in red represents the distribution of labeled motoneurons in intact mice. Similar data from untreated mice, from mice in which the distal stump of the cut nerve had been treated with chondroitinase ABC, from mice in which the proximal stump was subjected to brief electrical stimulation, and mice undergoing two weeks of treadmill training (continuous or interval strategy) are shown in different rows. Each histogram represents an average of four cases. The numbers in parentheses at the right of each histogram are the mean number of labeled motoneurons found in the caudal-most 40% of the distal sciatic motor nucleus (the green region of the histogram) / the mean total number of labeled motoneurons. This figure is also available in magenta-green format as supplementary figure 2.
The mean number of labeled motoneurons in the caudal 40% of the distal sciatic motor nucleus (+SEM) for the two and four week survival periods studied is shown in Figure 5. At the two week survival time (Fig. 5 left) no motoneurons labeled from application of tracer to the common fibular nerve were found in the caudal most 40% of the sciatic motor nucleus in untreated mice (English, 2005). In the Continuous Training group, a small number of such topographically inappropriate neurons were encountered in some mice. On average this number is not significantly different from zero but it is significantly smaller than the proportion of topographically inappropriate motoneurons in interval trained mice or in mice in which axon regeneration is enhanced by brief electrical stimulation or chondroitinase ABC treatment (English, 2005). At the four week survival time, the mean proportion of topographically inappropriate reinnervating motoneurons in either group of trained mice is not significantly different when compared to similar measurements made in untreated control mice (Fig. 5 right). In both trained groups, the proportions are significantly (p<0.05) smaller than found in mice that had been treated with electrical stimulation or chondroitinase ABC.
Figure 5.
The mean (+SEM) number of motoneurons labeled from application of tracer to the common fibular nerve that is found in topographically inappropriate caudorostral positions in the distal sciatic motor nucleus is shown for the different treatment groups studied at two (A) and four week (B) survival times.
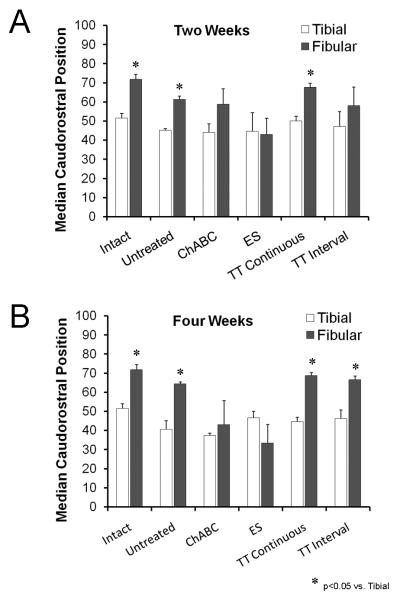
As an additional measure of sagittal topographic specificity, we determined the median caudorostral position of motoneurons labeled from tracer application to the tibial and common fibular nerves in each mouse. Average median positions (+SEM) are shown in the bar graphs in Figure 6. In intact mice, the median position of motoneurons with axons in the common fibular nerve is greater than that of motoneurons with axons in the tibial nerve, reflecting the more rostral distribution of the former, relative to the latter. If significant numbers of motoneurons labeled by tracers applied to the common fibular nerve are found in topographically inappropriate locations after sciatic nerve transection and repair, then one might expect the median position of the common fibular distribution to decrease. Such a significant decrease was found if axon regeneration was enhanced either by brief electrical stimulation or chondroitinase ABC treatment (English, 2005). In both cases, no significant difference was found between the average caudorostral medians of the tibial and fibular motor nuclei (Fig. 6). In treadmill trained mice, using either a continuous or an interval training strategy, no such decrease in average median caudorostral position was noted at either the two or four week survival times (Fig. 6). These data are additional evidence that the enhancement of axon regeneration found with treadmill exercise is accomplished with no greater loss of topographic specificity than untreated controls.
Figure 6.
The average median relative caudorostral positions (+SEM) of motoneurons labeled from tracer application to the common fibular and tibial nerves are shown for the different treatment groups at two week (left) and four week (right) survival times. Each bar represents a mean of four cases.
Mediolateral distribution of motoneurons
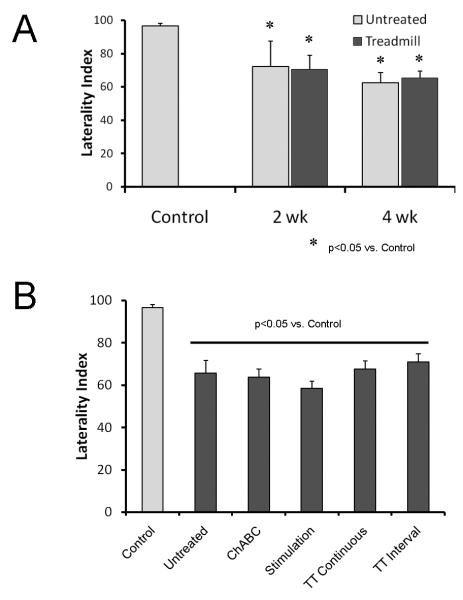
Comparing the relative caudorostral positions of labeled motoneurons is one way of evaluating the extent of misdirection of regenerating axons. In the rostral 60% of the distal sciatic motor nucleus, motoneurons innervating tibial and common fibular targets co-exist. In this region, the motoneurons with axons in the common fibular and tibial nerves differ with respect to their mediolateral locations in the sciatic motor nucleus. Those labeled from application of tracer to the common fibular nerve in intact mice are spatially grouped lateral to motoneurons labeled after application of tracer to the tibial nerve in the mouse (English, 2005; McHanwell and Biscoe, 1981) (Fig. 1B). We used the extent of this spatial segregation as an additional assay of the extent of misdirection of regenerating axons in treadmill trained mice. From histological sections containing different motoneurons marked with different tracers, a laterality index was calculated. This is the proportion of all spatial interactions between motoneurons where cells labeled from application of tracer to the common fibular nerve are found lateral to cells labeled from tracer applied to the tibial nerve (the appropriate topography). The results of this analysis for treadmill trained mice, for untreated control mice, and for mice in which axon regeneration was enhanced using either brief electrical stimulation or treatment with chondroitinase ABC are shown in Figure 7.
Figure 7.
For each section of spinal cord containing motoneurons labeled from tracer application to the common fibular nerve and motoneurons labeled from application of tracer to the tibial nerve, the proportion of all cell-cell relationships in a frontal plane in which the former were found lateral to the latter was calculated as a laterality index. A. Mean (+SEM) laterality index in intact mice, untreated mice, and treadmill trained mice are shown at two week (left) and four week (right) survival times. B. Mean values (+SEM) of laterality index are shown for different treatment groups. Data from two and four week survival times are pooled.
In the spinal cords of unoperated (control) mice, this laterality index is very high (96.65% ± 1.50%, SEM), indicating a very high degree of spatial segregation of the motoneurons with axons in the different terminal branches of the distal sciatic nerve. After transection and surgical repair of the sciatic nerve, the laterality index decreases (Fig. 7A), indicating that regenerating axons from motoneurons in this part of the spinal cord have been misdirected to different targets. By four weeks after nerve transection and repair, nearly 40% of motoneurons labeled by tracers applied to the common fibular nerve were found in such topographically inappropriate locations. In treadmill trained mice, a similar pattern of misdirection of regenerating axons to the common fibular nerve of axons that had previously innervated targets of the tibial nerve is found. Since data from Continuous and Interval Training groups did not differ significantly, they were pooled for this analysis. Differences in mean laterality index between treadmill trained mice and unoperated control mice were significant (LSD, p<0.009), but differences between trained and untreated mice were not (LSD, p=0.34).
We next compared these data on topographic relationships of motoneurons in treadmill trained mice to data from mice in which axon regeneration had been enhanced using either brief electrical stimulation of the proximal stump of the cut sciatic nerve or chondroitinase ABC treatment of the distal stump (English, 2005). Only data from the four week survival time were studied. These results are shown in Figure 7B. Differences between unoperated controls and all of these different treatment groups are statistically significant (LSD, p<0.001). Differences in spatial location in a frontal plane between the groups comprising different approaches to enhancing axon regeneration were not significant (LSD, p≥0.34). These findings are further support of the hypothesis that treadmill training results in enhanced axon regeneration with a decline of spatial segregation in the frontal plane that is no greater either than that found with no treatment at all or with other treatments designed to enhance axon regeneration after peripheral nerve injury.
Discussion
Damaged axons in cut peripheral nerves are capable of considerable regeneration, yet functional recovery following peripheral nerve injury is poor (Brushart, 1998; Frostick et al., 1998). Two different aspects of peripheral axon regeneration are most often cited as reasons for this. Axon regeneration in peripheral nerves is slow. Regenerating axons in the proximal stumps of cut nerves encounter significant inhibition in invading a regeneration pathway in the distal stump and once they enter this pathway, they encounter potent growth inhibitory molecules (Gilbert et al., 2005; Zuo et al., 1998). This inhibition delays or prevents early axon regeneration and some axons never participate in the regeneration process. In addition, regenerating axons can choose pathways in the distal stump that lead them to inappropriate targets. Such misdirection of axons could result in sensory axons reinnervating motor targets or vice-versa (loss of sensorimotor specificity), or it could lead to reinnervation of muscles by motoneurons that had previously innervated their functional antagonists. The latter could give rise to dyskinesia and poor functional recovery (de Ruiter et al., 2008).
Attempts to enhance axon regeneration, either by eliminating growth inhibitory molecules with chondroitinase ABC treatment or promoting axon elongation using electrical stimulation have had mixed effects on processes related to functional recovery. Treatment of the distal stumps of cut nerves with chondroitinase ABC resulted in longer growth of regenerating axons in the first weeks following nerve injury (Groves et al., 2005; Zuo et al., 2002). Using the femoral nerve model in the rat (Al-Majed et al., 2000) and mice (Franz et al., 2005), brief electrical stimulation both enhances axon regeneration and improves sensorimotor specificity of those regenerating axons. However, both electrical stimulation and chondroitinase treatment also result in an undesirable increase in the proportion of regenerating motor axons that were misdirected from tibial to common fibular nerves (English, 2005).
In a recent report (Sabatier et al., 2008) we have shown that modest treadmill exercise in the immediate post-operative period following transection and surgical repair of the mouse common fibular nerve results in significantly longer regenerating axons than unexercised controls. Because treadmill training produces a natural activation of motoneurons via the spinal circuits that drive locomotion, we hypothesized that it would result in an enhancement of axon regeneration without the increase in number of misdirected axons noted using either electrical stimulation or chondroitinase ABC treatment.
The main finding of this study was that either of two forms of very modest treadmill exercise resulted in an increase in the number of motoneurons whose regenerating axons had grown at least 5 mm by either two or four weeks following sciatic nerve transection. Subtle differences were found between tibial and common fibular branches of the sciatic nerve and between continuous and interval training strategies, but the overall effect of treadmill training was to enhance the regeneration of cut motor axons. These results are consistent with our earlier findings, where the lengths of regenerating axon profiles were used as an outcome measure. We interpret these findings to mean that treadmill training enhances motor axon regeneration by involving more motoneurons in the regeneration process and not simply by increasing the branching of axons already regenerating.
We used two different treadmill training paradigms to enhance axon regeneration and obtained slightly different results with each. Motoneurons whose regenerating axons entered the tibial branch of the sciatic nerve responded well to both paradigms. More retrogradely labeled motoneurons were found after tracer application to the tibial nerve than in unexercised controls and equal numbers were encountered after either continuous or interval training. Motoneurons whose regenerating axons entered the common fibular branch of the sciatic nerve responded only to the interval training strategy. We are not sure why continuous training did not result in enhancement of motor axon regeneration in the common fibular nerve but we hypothesize that it may be related to the relative activation of common fibular motoneurons in continuous vs. interval training. Muscles innervated by tibial motoneurons are active for much of each step cycle and act to support body weight and produce forward motion. Thus one might expect significant numbers of tibial motoneurons to be activated with either treadmill training protocol. In contrast, muscles innervated by common fibular motoneurons are active only for short portions of each step cycle and when they are active they act only against the weight of the foot. Thus at the slow stepping speeds used by the Continuous Training group, one might anticipate the recruitment of a smaller portion of the motoneurons pool normally innervating these muscles than at the near maximal stepping speeds used by the Interval Training group. For common fibular motoneurons, we hypothesize that the amount of activation encountered during our continuous training paradigm is simply not sufficient to enhance motor axon regeneration. We believe that this hypothesis could be tested experimentally using training paradigms that emphasize activation of muscular targets of the common fibular nerve.
An equally important finding of this study is that the enhancement of axon regeneration produced by treadmill training occurs without an increase in the proportion of misdirected motor axons to functionally inappropriate targets. We have used the two terminal branches of the distal sciatic nerve of mice as a model system. Our analysis of misdirection is based on the finding that the motoneurons whose axons are found in the common fibular nerve normally are distributed in a specific topographic manner in the spinal cord. These motoneurons are restricted to the rostral 60% of the distal sciatic motor nucleus in intact mice (English, 2005), and in regions of the spinal cord where both exist, they lie lateral to motoneurons whose axons project to the tibial branch (English, 2005; McHanwell and Biscoe, 1981). After transection and surgical repair of the sciatic nerve, some of the motoneurons reinnervating the common fibular nerve lie in these same spatial positions in the spinal cord, but others do not. We have termed this latter group topographically inappropriate (English, 2005). The presence of their axons in the common fibular nerve reflects a misdirection of regenerating axons that had been in the tibial nerve prior to nerve injury. In treadmill trained animals, motoneurons whose axons had regenerated into the common fibular nerve were found in topographically inappropriate locations in the spinal cord to the same extent as or less than found in untreated control mice. This similarity is in marked contrast to our previous results using either brief electrical stimulation or chondroitinase ABC treatment to enhance axon regeneration (English, 2005). Thus, the potential benefit of increasing the number of motoneurons involved in axon regeneration produced by treadmill training is not lessened by a significant loss of reinnervation specificity.
We have argued that topography, expressed as the spatial location of motoneurons in the spinal cord, is a powerful measure of the extent of misdirection of regenerating motor axons after transection and repair of the sciatic nerve. Certainly the spatial locations of motoneurons whose axons are found in the mouse common fibular nerve are so distinctly segregated that they are a useful model system. However, we realize that study of motor axon regeneration using topography also has its limitations. For example, we feel that we were not able to use this approach to study the specificity with which targets of the tibial nerve are reinnervated, nor were we on a solid a foundation if we wanted to study reinnervation specificity at a more detailed level, such as reinnervation of muscle groups innervating the superficial and deep peroneal nerves or even individual muscles. Study of the effects of treadmill training at these levels will have to await the development of sequential double labeling protocols, such as others have used in different model systems in rats (Brushart et al., 2005).
The reason for the preservation of topographic specificity with treadmill training and not with other means of enhancing axon regeneration is not yet clear. It is known that the topographic organization of the sciatic motor nucleus is reflected in a spatial representation of motor axons in the nerve itself (Brushart et al., 1983; Kobbert and Thanos, 2000). Axons in the common fibular nerve are found nearly exclusively in the lateral aspect of the nerve and they are distinct from axons in the tibial nerve for most of the course of the nerve. For regenerating axons in the sciatic nerve to be misdirected toward inappropriate targets, they would have to cross from one fascicle of the nerve to another at the site of the nerve injury, as noted in experiments in which the stumps of the nerve have been purposely mis-aligned (English, 2005). Some such crossing must occur, as we and others (Brushart et al., 1983) have documented misdirection of regenerating axons in carefully aligned nerve repairs. Axon regeneration in peripheral nerves also is known to be temporally staggered, as regenerating axons select endoneurial tubes in the distal site over a period of several weeks (Al-Majed et al., 2000; Brushart, 1993). Based on our previous study of axon regeneration in the mouse sciatic nerve (English, 2005) and above, we believe that axons that regenerate later are more likely to be misdirected than those regenerating earlier. The reason for this misdirection is unknown, but it is possible that a reduction in the availability of suitable targets (endoneurial tubes) in close proximity may require later regenerating axons to elongate considerably before entering a tube. Such a proposed decreased availability of proximate targets might result in crossing of fibular motor axons into tibial fascicles, and vice-versa. Brushart and colleagues (Witzel et al., 2005) have provided evidence for such neurite elongation in mice, but whether it occurs in response to limited target availability is not known.
Both treatments with chondroitinase ABC and brief electrical stimulation are known to enhance axon regeneration in peripheral nerves at least in part by decreasing the temporal staggering of the process (Al-Majed et al., 2000; English, 2005). The greater misdirection of regenerating axons found in association with these acutely applied enhancing treatments might result from this more synchronous outgrowth of neurites seeking endoneurial tubes in the distal site. Both sensory and motor axons are affected by brief electrical stimulation (Brushart et al., 2005; Geremia et al., 2005). The enhancement of axon regeneration produced by treadmill training described above also results in a reduction in temporal staggering but the treatment is applied daily over a two week period. In addition, we anticipate that the population of neurons whose axons have been stimulated to elongate is restricted to those that are activated by the spinal circuitry driving locomotion and this population is thus more selective than found after electrical stimulation or chondroitinase ABC treatment. We speculate that the decreased amount of misdirection of regenerating axons found after treadmill training is the result of a decrease in competition among regenerating axons for appropriate pathways at the surgical repair site that results from this selective enhancement.
Supplementary Material
Acknowledgements
This work was completed with support from grants NS057190 and HD032571 from the USPHS. Thanks to Saiyaka Heno, Michael Kaufman, Sam Rose, and Kylene Wood for help in exercising mice. Thanks to Michael Kaufman and Gail Schwartz for their help with some of the histological tissue processing. Thanks to Michael Kutner for his advice on the statistics.
Literature Cited
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000;20:2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM. Motor axons preferentially reinnervate motor pathways. J Neurosci. 1993;13:2730–2738. doi: 10.1523/JNEUROSCI.13-06-02730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM. Nerve repair and grafting. In: Green D, Hotchkiss R, Pederson W, editors. Green's operative hand surgery. Churchill Livingstone; New York: 1998. pp. 1381–1403. [Google Scholar]
- Brushart TM, Jari R, Verge V, Rohde C, Gordon T. Electrical stimulation restores the specificity of sensory axon regeneration. Exp Neurol. 2005;194:221–229. doi: 10.1016/j.expneurol.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Brushart TM, Mesulam MM. Alteration in connections between muscle and anterior horn motoneurons after peripheral nerve repair. Science. 1980;208:603–605. doi: 10.1126/science.7367884. [DOI] [PubMed] [Google Scholar]
- Brushart TM, Tarlov EC, Mesulam MM. Specificity of muscle reinnervation after epineurial and individual fascicular suture of the rat sciatic nerve. J Hand Surg [Am] 1983;8:248–253. doi: 10.1016/s0363-5023(83)80152-x. [DOI] [PubMed] [Google Scholar]
- de Ruiter GC, Malessy MJ, Alaid AO, Spinner RJ, Engelstad JK, Sorenson EJ, Kaufman KR, Dyck PJ, Windebank AJ. Misdirection of regenerating motor axons after nerve injury and repair in the rat sciatic nerve model. Exp Neurol. 2008;211:339–350. doi: 10.1016/j.expneurol.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. J Comp Neurol. 2005;490:427–441. doi: 10.1002/cne.20678. [DOI] [PubMed] [Google Scholar]
- English AW, Mulligan A, Cucoranu D. Treadmill training enhances axon regeneration in cut peripheral nerves without effecting topographic specificity of reinnervating motoneurons. Abstr Soc Neurosci 76.7. 2008 [Google Scholar]
- English AW, Mulligan A, Meador W, Sabatier MJ, Schwartz G. Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. Dev Neurobiol. 2007;67:158–172. doi: 10.1002/dneu.20339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz CK, Rutishauser U, Rafuse VF. Polysialylated neural cell adhesion molecule is necessary for selective targeting of regenerating motor neurons. J Neurosci. 2005;25:2081–2091. doi: 10.1523/JNEUROSCI.4880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18:397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Geremia NM, Gordon T, Al-Majed AA, Brushart TM, Verge VMK. Society for Neuroscience. 2005. Brief electrical stimulation promotes sensory neuron regeneration and intrinsic growth-associated gene expression; p. 26. Program No. 28. [DOI] [PubMed] [Google Scholar]
- Gilbert RJ, McKeon RJ, Darr A, Calabro A, Hascall VC, Bellamkonda RV. CS-4,6 is differentially upregulated in glial scar and is a potent inhibitor of neurite extension. Mol Cell Neurosci. 2005;29:545–558. doi: 10.1016/j.mcn.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gordon T, Sulaiman O, Boyd JG. Experimental strategies to promote functional recovery after peripheral nerve injuries. J Peripher Nerv Syst. 2003;8:236–250. doi: 10.1111/j.1085-9489.2003.03029.x. [DOI] [PubMed] [Google Scholar]
- Groves ML, McKeon R, Werner E, Nagarsheth M, Meador W, English AW. Axon regeneration in peripheral nerves is enhanced by proteoglycan degradation. Exp Neurol. 2005;195:278–292. doi: 10.1016/j.expneurol.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Kobbert C, Thanos S. Topographic representation of the sciatic nerve motor neurons in the spinal cord of the adult rat correlates to region-specific activation patterns of microglia. J Neurocytol. 2000;29:271–283. doi: 10.1023/a:1026523821261. [DOI] [PubMed] [Google Scholar]
- MacGillivray TE. Fibrin sealants and glues. J Card Surg. 2003;18:480–485. doi: 10.1046/j.0886-0440.2003.02073.x. [DOI] [PubMed] [Google Scholar]
- McHanwell S, Biscoe TJ. The localization of motoneurons supplying the hindlimb muscles of the mouse. Philos Trans R Soc Lond B Biol Sci. 1981;293:477–508. doi: 10.1098/rstb.1981.0082. [DOI] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysate-paraformaldehyde fixative. A new fixative for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Menovsky T, Beek JF. Laser, fibrin glue, or suture repair of peripheral nerves: a comparative, functional, and morphometric study in the rat sciatic nerve. J Neurosurg. 2001;95:694–699. doi: 10.3171/jns.2001.95.4.0694. [DOI] [PubMed] [Google Scholar]
- Neubauer D, Graham JB, Muir D. Chondroitinase treatment increases the effective length of acellular nerve grafts. Exp Neurol. 2007;207:163–170. doi: 10.1016/j.expneurol.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier M, Redmon N, Schwartz G, English A. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp Neurol. 2008;211:489–493. doi: 10.1016/j.expneurol.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swett JE, P WR, Blanks RH, Swett AL, Conley LC. Motoneurons of the rat sciatic nerve. Exp Neurol. 1986;93:227–252. doi: 10.1016/0014-4886(86)90161-5. [DOI] [PubMed] [Google Scholar]
- Witzel C, Rohde C, Brushart TM. Pathway sampling by regenerating peripheral axons. J Comp Neurol. 2005;485:183–190. doi: 10.1002/cne.20436. [DOI] [PubMed] [Google Scholar]
- Zuo J, Hernandez YJ, Muir D. Chondroitin sulfate proteoglycan with neurite-inhibiting activity is up-regulated following peripheral nerve injury. J Neurobiol. 1998;34:41–54. [PubMed] [Google Scholar]
- Zuo J, Neubauer D, Graham J, Krekoski CA, Ferguson TA, Muir D. Regeneration of axons after nerve transection repair is enhanced by degradation of chondroitin sulfate proteoglycan. Exp Neurol. 2002;176:221–228. doi: 10.1006/exnr.2002.7922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.