Abstract
In this study, we tested the hypothesis that TO901317 promotes synapse plasticity and axonal regeneration after stroke. Adult male C57BL/6J mice were subjected to middle cerebral artery occlusion (MCAo) and treated with or without TO901317 starting 24 h after MCAo daily for 14 days. Axonal damage and regeneration were evaluated by immunostaining. TO901317 significantly increased synaptophysin expression and axonal regeneration, as well as decreased the expressions of amyloid betaA4 precursor protein and Nogo receptor (NgR) in the ischemic brain. To test whether TO901317 regulates the phosphorylation of phosphatidylinositol 3-kinase (p-PI3K) and Akt (p-Akt) activity in the ischemic brain, MCAo mice were treated with or without TO901317 starting 24 h after MCAo daily for 4 days and were then killed at 5 days after MCAo. TO901317 treatment significantly increased p-PI3K and p-Akt activity, but did not increase total PI3K expression in the ischemic brain. Using primary cortical neuron (PCN) culture, TO901317 significantly increased synaptophysin expression, p-PI3K activity, and decreased NgR expression compared with nontreated controls. TO901317 also significantly increased neurite outgrowth, and inhibition of the PI3K/Akt pathway by LY294002 decreased neurite outgrowth in both controls and TO901317-treated groups in cultured hypoxic PCN. These data indicate that TO901317 promotes synaptic plasticity and axonal regeneration, and that PI3K/Akt signaling activity contributes to neurite outgrowth.
Keywords: axonal regeneration, LXR agonist, Nogo receptor, phosphatidylinositol 3-kinase, stroke
Introduction
Functional recovery after acute central nervous system injury in humans, such as stroke, is exceptionally limited, leaving the affected individual with life-long neurologic deficits. The lack of functional recovery can at least in part be attributed to the restriction of axonal regeneration and neuroplasticity (Walmsley and Mir, 2007). Successful axonal outgrowth in the adult central nervous system is central to nerve regeneration and brain repair (Hou et al, 2008). Enhancement of plasticity by induction of axonal regeneration has been shown to compensate for the formerly lost function in spinal cord injury (Galtrey and Fawcett, 2007) and in stroke models (Liu et al, 2008; Papadopoulos et al, 2006).
Axon growth is a highly regulated process that requires stimulating signals from extracellular factors. Extracellular factors, such as growth factors and neurotrophins, act through receptor tyrosine kinases that stimulate axon growth. Phosphatidylinositol 3-kinase (p-PI3K) (a downstream signaling protein of receptor tyrosine kinases) regulates the local assembly of axonal cytoskeleton, especially that of microtubules (Zhou and Snider, 2006). PI3K also has an important role in nerve growth factor, insulin-like growth factor, and brain-derived neurotrophic factor-induced neurite regeneration (Bonnet et al, 2004; Laurino et al, 2005).
Liver X receptor-β (LXR-β) is a nuclear hormone receptor that regulates transcription involved in the metabolism of cholesterol and fatty acids. Activation of LXR-β induces reverse cholesterol transport and increases high-density lipoprotein cholesterol. Activation of LXR is a validated drug target for cardiovascular disease, and has been implicated in diabetes, inflammation, and neurodegenerative disease. Disrupted LXR in LXR knockout (LXR−/−) mice results in excessive lipid deposits, proliferation of astrocytes, loss of neurons and their dendrites, and disorganized myelin sheaths (Wang et al, 2002b). Liver X receptor-β is essential for the maintenance of motor neurons in the spinal cord and dopaminergic neurons in the substantia nigra (Andersson et al, 2005; Kim et al, 2008). Expression of LXR-β is essential for the formation of superficial cortical layers and for the migration of later-born neurons (Fan et al, 2008). T0901317 is a potent LXR agonist. Activation of LXRs promotes neuroprotection and decreases the expression of proinflammatory genes along with reduced nuclear factor-κB transcriptional activity in experimental stroke models (Morales et al, 2008; Sironi et al, 2008). We have shown previously that treatment of stroke in mice with an LXR agonist (TO901317) starting at 24 h after stroke significantly improves functional outcome without altering lesion volume (Chen et al, 2009). These animals were used in this study, and new data were generated to test the hypothesis that the LXR agonist promotes synaptic plasticity and axonal regeneration after stroke, restorative processes which may contribute to the improvement in neurologic outcome. In addition, the mechanisms and molecular signaling pathway of TO901317-induced axonal regeneration were investigated.
Materials and methods
Animal Middle Cerebral Artery Occlusion Model and Experimental Groups
Adult male C57BL/6J mice (age: 2 to 3 months, weight: 24 to 28 g) were purchased from Charles River (Wilmington, MA, USA). Right temporal (2 h) middle cerebral artery occlusion (MCAo) was induced by advancing a 6-0 surgical nylon suture (8.0 to 9.0 mm determined by body weight) with an expanded (heated) tip from the external carotid artery into the lumen of the internal carotid artery to block the origin of the MCA (Mao et al, 1999). Sham-operated mice underwent the same surgical procedure without suture insertion. Mice subjected to MCAo were gavaged starting 24 h after MCAo with (1) saline for controls and (2) TO901317 (30 mg/kg, Cayman Chemical, Ann Arbor, MI) daily for 14 days. Our choice of dose was guided by previous studies that have reported beneficial outcomes of TO901317 intervention in a mouse model of Alzheimer's disease (Riddell et al, 2007) and after stroke (Chen et al, 2009). Mice (n=8 per group) were killed 14 days after surgery for immunostaining. Previous studies have shown that treatment of stroke rats starting 24 h after stroke with TO901317, statin, or a increasing high-density lipoprotein agent (Niaspan, Kos Pharmaceuticals Inc., Cranbury, NJ, USA) increases angiogenic and growth factor expression beginning at 4 days after treatment (Chen et al, 2007, 2008, 2009; Zacharek et al, 2006). In addition, trophic factor expression and Akt/PI3K activity may induce axonal regeneration. Therefore, in this study, we measured Akt/PI3K and phosphorylation of PI3K at 5 days after MCAo. Another set of mice (n=6 per group) was killed at 5 days after MCAo and ischemic brain tissue from the ischemic border zone (see Figure 2A) was extracted for western blot assay. In addition, blood gases (PO2, PCO2, and pH) were measured at 5 days after MCAo (n=5 per group).
Histological and Immunohistochemical Assessment
Mice (n=8 per group) were killed 14 days after MCAo. The brains were fixed by transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde before being embedded in paraffin. As our previous study has shown that TO901317 treatment of stroke did not decrease the volume of brain infarction (Chen et al, 2009), lesion volume was not measured in this study. For immunostaining, a standard paraffin block was obtained from the center of the lesion (bregma: −1 to +1 mm). A series of 6-μm-thick sections were cut from the block. Every tenth coronal section for a total five sections was used for immunohistochemical staining. Antibody against synaptophysin (monoclonal antibody; dilution 1:1,000, Chemicon, Temecula, CA, USA), amyloid betaA4 precursor protein (APP) (dilution 1:50, Cell Signaling Technology, Danvers, MA, USA), Nogo receptor (NgR) (dilution 1:50, Santa Cruz Biotechnology, Santa Cruz, CA, USA), were used (Nourhaghighi et al, 2003; Sandhu et al, 2004). Control experiments consisted of staining brain coronal tissue sections as outlined above, but nonimmune serum was substituted for the primary antibody (Li et al, 1998). Bielschowsky silver immunostaining was used to show the axons. In brief, for Bielschowsky silver staining, slides were placed in 20% AgNO3 in the dark, and then NaOH and sodium thiosulfate were added to the slides in turn. Control experiments consisted of staining brain coronal tissue sections as outlined above, but the nonimmune serum was substituted for the primary antibody (Li et al, 1998). The immunostaining analysis was performed by an investigator who was blinded to the experimental groups.
Synaptophysin, Bielschowsky Silver, Nogo Receptor, and Amyloid BetaA4 Precursor Protein Expression Quantification
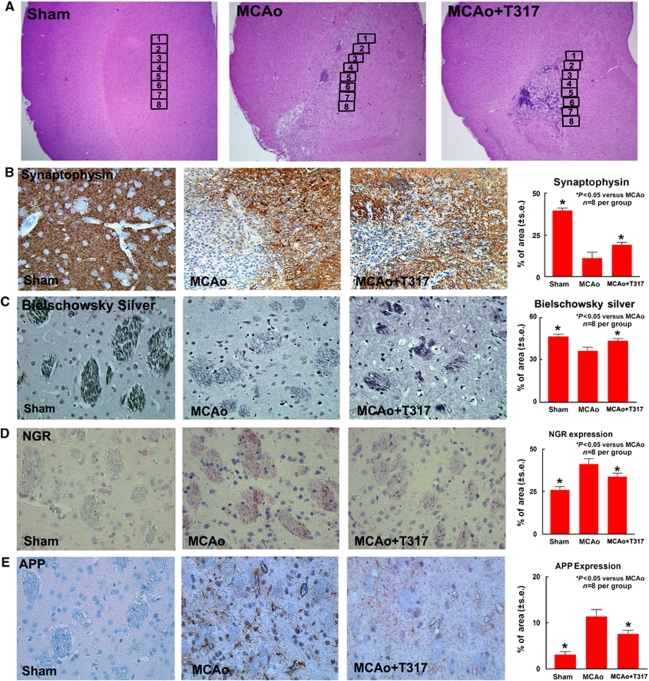
Hematoxylin and eosin staining was performed to identify the ischemic border zone (see Figure 1A). For quantitative measurements of synaptophysin, and APP, five slides from each brain sample, with each slide containing eight fields from the stratum of the ischemic border area, which is adjacent to the ischemic core, were digitized under a × 20 objective (Olympus BX40, Olympus, Tokyo, Japan) using a 3-CCD color video camera (Sony DXC-970MD, Sony, Tokyo, Japan) interfaced with an Micro Computer Imaging Device (MCID) image analysis system (Imaging Research, St Catharines, ON, Canada) (Calza et al, 2001; Chen et al, 2003a, 2003b). The positive area of Bielschowsky silver and NgR immunoreactive cells was measured in the white matter bundles of the stratum in the ischemic border. Data were analyzed in a blinded manner. The data collected from five sections and eight regions within each section were averaged to obtain a single value for one animal and were presented as the percentage of positive area for synaptophysin, NgR, APP, and Bielschowsky silver immunoreactive cells, respectively.
Figure 1.
TO901317 treatment of stroke increases synaptogenic plasticity, axonal regeneration and decreases axonal damage and NgR expression in the ischemic brain. (A) H&E staining in low magnification and eight immunostaining measurement regions in the ischemic border area. (B–E) Synaptophysin (panel B), Bielschowsky silver (panel C), NgR (panel D), and APP (panel E) expression in the ischemic border zone (IBZ) in sham-, MCAo-, and TO901317-treated MCAo mice and the quantitative data. n=8 per group.
Primary Cortical Neuron Culture and Dendrite Outgrowth Measurements
To test whether TO901317 regulates dendrite outgrowth, a primary cortical neuron (PCN) culture was used. Cultured cortical neurons were prepared from embryonic day 17 of pregnant rats and cultured in the Neurobasal medium (Invitrogen, Carlsbad, CA, USA) with B27 supplement for 5 days. To test whether TO901317 regulates PCN PI3K signaling activity, normoxic PCNs were treated with (n=6 per group) (1) controls and (2) TO901317 (1 μm) for 24 h. Western blot assay was performed.
To test whether the PI3K pathway regulates TO901317-induced dendrite outgrowth, PCNs were subjected to 1 h of oxygen and glucose deprivation, followed by 24 h of reperfusion (oxygen and glucose deprivation-reperfusion). Hypoxic PCNs were subjected to (n=6 per group) (1) nontreatment for controls, (2) TO901317 (1 μmol/L), (3) p-Akt inhibitor (LY294002, 10 μm), and (4) TO901317 (1 μmol/L) with LY294002 (10 μm) for 24 h. TUJ1 (β-tubulin III) immunostaining was performed using a monoclonal antibody, TUJ1, for dendritic outgrowth measurement. Six wells per condition (n=6 per group) were used. To trace the axonal arbors of fluorescently labeled neurons, photomicrographs were captured at 40 × magnification using a digital camera. A total of 20 neurons were captured using a digital camera in each well. The total dendrite length was measured in 20 neurons in each well using the MCID analysis system. The total length of 20 neurons was averaged in each well. The averaged length per neuron in each well is presented. Six wells were used in each group (n=6 per group).
Western Blot
Cells and brain tissue were harvested. Tissue dissection was performed according to lesion morphology. The lesion core was visible as a pale region on fresh tissue. The ischemic border zone was apparent as a light pink region surrounding the lesion core and not very sharp on 5 days after MCAo. We sampled the tissue from the ischemic border zone within this region of light pink tissue adjacent to the margin of the pale infarct area. Equal amounts of cell lysate were subjected to western blot analysis, as described previously (Chen et al, 2003a). Specific proteins were visualized using a SuperSignal West Pico Chemiluminescence Kit (Pierce, Rockford, IL, USA). The following primary antibodies were used: anti-β-actin (1:2,000; Santa Cruz Biotechnology), anti-synaptophysin (1:1,000, Chemicon), anti-NgR (1:1,000, Abcam, Cambridge, MA, USA), anti-Akt (1:1,000, Cell Signaling Technology), anti-phospho-Akt (Ser473; 1:1,000, Cell Signaling Technology), anti-PI3K (1:500, Santa Cruz Biotechnology), and anti-phospho-PI3K (1:500, Santa Cruz Biotechnology).
Statistical Analysis
Two-way ANOVA (analysis of variance) was performed for measuring data of axonal regeneration, including the percentage of positive area for synaptophysin, NgR, APP, and Bielschowsky silver in the ischemic brain. If an overall treatment group effect was detected at P<0.05, Tukey's test after post hoc test was used for multiple comparison. One-way ANOVA and least significant difference analysis after the post hoc test were performed to assess data of synaptophysin, NgR, PI3K, and p-PI3K expressions in cultured neurons and the neurite outgrowth in vitro. Independent samples t-test was used for testing the expressions of NgR, Akt, p-Akt, and p-PI3K by western blot assay in the brain tissues between the two groups. All data are presented as mean±s.e.
Results
To test whether TO901317 treatment of stroke regulates physiologic parameters, the general physiologic parameters were measured (n=5 per group). The data show that the general physiologic parameters were in the normal range and that there were no significant differences between the two groups treated with or without TO901317 (MCAo versus MCAo+TO901317-treated animals; pH: 7.44±0.02 versus 7.44±0.04; PCO2: 41.3±2.0 versus 41.7±0.9; PO2: 138.0±4.6 versus 146.7±5.8).
TO901317 Treatment of Stroke Increases Synaptophysin Expression and Axonal Regeneration, and Decreases Axonal Damage
Synaptophysin is a marker for presynaptic plasticity and synaptogenesis (Ujike et al, 2002). Bielschowsky silver is a marker for axons (Karnezis et al, 2004; Pluchino et al, 2003). To test whether TO901317 treatment of stroke induces synaptic plasticity and axonal regeneration, synaptophysin and Bielschowsky silver immunostaining were performed. Figures 1B and 1C show that synaptophysin (panel B) and Bielschowsky sliver (panel C) expressions were significantly decreased in MCAo mice compared with that in sham controls. TO901317 treatment significantly increased synaptophysin (panel B) and Bielschowsky sliver (panel C) expressions in the ischemic brain compared with control MCAo animals.
Amyloid betaA4 precursor protein is a marker for axon damage (Leclercq et al, 2001; Sherriff et al, 1994). Nogo receptor is a neuronal growth inhibitory molecule (Li et al, 2005). To test whether TO901317 treatment of stroke decreases axonal damage and inhibits neuronal growth inhibitory molecules expression, APP and NgR expressions in the ischemic brain were measured. Figures 1D and 1E show that NgR (panel D) and APP (panel E) expressions were significantly decreased in the ischemic brain in the TO901317-treated mice compared with that in control MCAo animals. These data suggest that TO901317 treatment of stroke decreases axonal damage and neuronal growth inhibitory molecule NgR expression, and promotes synaptic plasticity and axonal regeneration after stroke.
TO901317 Treatment of Stroke Promotes p-PI3K and p-Akt Activity in the Ischemic Brain
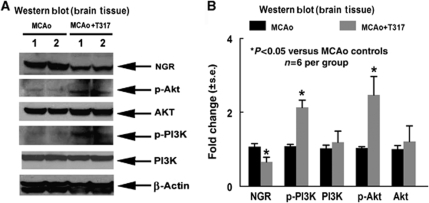
To show the mechanisms underlying TO901317-induced axonal regeneration after stroke, PI3K/Akt signaling activity was measured by western blot. Figure 2 shows that TO901317 treatment significantly increased the expressions of p-Akt, p-PI3K activity, and decreased NGR expression in the ischemic border compared with control MCAo mice (P<0.05, n=6 per group).
Figure 2.
TO901317 treatment of stroke increases p-Akt and p-PI3K activity and decreases NgR expression in the ischemic brain. (A) Western blot assay. (B) Western blot quantitation data (n=6 per group).
TO901317 Treatment Increases p-PI3K and p-Akt Activity
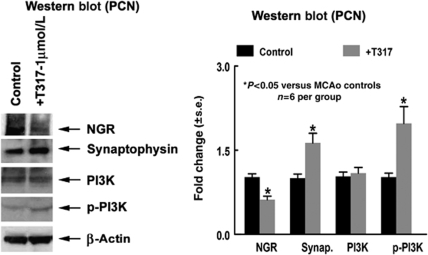
To elucidate whether TO901317 regulates axonal regeneration, an in vitro PCN culture was performed. Primary cortical neurons derived from 17-day embryonic brain were cultured in vitro and treated with or without TO901317 for 24 h. Figure 3 shows that TO901317 significantly increased synaptophysin expression, p-PI3K activity, and decreased NgR expression compared with nontreated controls (P<0.05, n=6 per group).
Figure 3.
TO901317 treatment increases p-PI3K activity and synaptophysin expression and decreases NgR expression in cultured PCN. (A) Western blot assay. (B) Western blot quantitation data (n=6 per group).
TO901317 Increased Neurite Outgrowth of Hypoxic PCN; Inhibition of the PI3K/Akt Pathway Attenuates TO901317-Induced Neurite Outgrowth
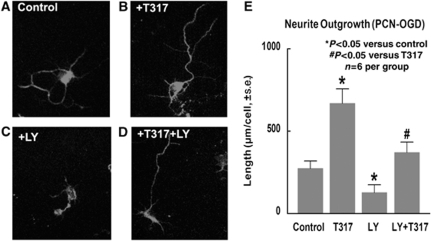
To test whether TO901317 treatment regulates neurite outgrowth, hypoxic PCNs were treated with or without TO901317. TUJ1 immunostaining was performed. TUJ1-positive cell neurite outgrowth was measured. Figure 4 shows that TO901317 significantly increased neurite outgrowth in the TO901317-treated group in hypoxic PCN (panels B and E) compared with nontreated controls (panels A and E, n=6 per group). To test whether the PI3K/Akt pathway has a role in TO901317-induced neurite outgrowth, LY294002, a PI3K inhibitor, was used. Figures 4C–4E show that the inhibition of PI3K signaling by LY294002 significantly decreases neurite outgrowth. In addition, inhibition of the PI3K/Akt pathway by LY294002 also significantly attenuate TO901317-induced neurite outgrowth in cultured hypoxic PCN (panels D and E). These data suggest that TO901317 treatment increases neuronal neurite outgrowth and that PI3K/Akt signaling activity contributes to neurite outgrowth.
Figure 4.
TO901317 increases neurite outgrowth in hypoxic PCN. Inhibition of the PI3K/Akt pathway decreased neurite outgrowth in cultured hypoxic PCNs. (A–E) TUJ1 immunostaining in cultured hypoxic PCN (1 h OGD) and treated with controls (panel A); TO901317 (1 μmol/L) (panel B); LY294002 (panel C), and TO901317 with LY294002 (panel D) and neurite outgrowth quantitative data (panel E). n=6 per group.
Discussion
We show for the first time that TO901317 treatment of stroke starting at 24 h after the onset of MCAo significantly induces axonal regeneration and synaptic plasticity as well as increases PI3K/Akt activity in the ischemic brain. The PI3K/Akt pathway contributes to axonal regeneration after stroke.
TO901317 Treatment of Stroke Promotes Synaptic Plasticity and Axonal Regeneration After Stroke
In this study, we found that TO901317 treatment of stroke significantly decreased NgR expression and increased synaptophysin expression and axonal regeneration in the ischemic brain compared with nontreated MCAo controls. After ischemia, the expressions of neuronal growth inhibitory molecules, myelin-associated glycoprotein, Nogo, and oligodendrocyte-myelin glycoprotein were redistributed around damaged axons and dendrites. Myelin-associated glycoprotein, Nogo, and oligodendrocyte-myelin glycoprotein inhibit axon growth by binding a common receptor, the Nogo66 receptor (NgR), and likely converge on a common signaling cascade (Domeniconi et al, 2002; McKerracher and Winton, 2002; Wang et al, 2007; Wang et al, 2002a). Decreased NgR expression in the ischemic brain improved axonal regeneration in the ischemic brain (Wang et al, 2007; Zhang et al, 2008). TO901317 treatment of stroke decreases NgR expression in the ischemic brain. Consistent with our in vivo finding, TO901317 also decreased NgR expression in cultured PCNs and promoted neurite outgrowth in vitro. Liver X receptor-β activators induce neuronal differentiation in rat pheochromocytoma cells and stimulate neurite outgrowth (Schmidt et al, 1999). Therefore, it is reasonable to propose that TO901317 treatment of stroke induced synaptogenesis and axonal regeneration by decreasing NgR expression.
TO901317 Treatment of Stroke Decreases Axonal Damage
Our data show that TO901317 treatment of stroke significantly decreased APP expression. Amyloid precursor protein is a transmembrane glycoprotein widely expressed in mammalian tissues and is the source of the toxic amyloid-β (Aβ) peptide associated with the pathogenesis of Alzheimer's disease. The APP is cleaved by two enzymes, β-secretase and γ-secretase, to generate the pathologic Aβ peptide. Both APP and Aβ accumulate in mitochondrial membranes, cause mitochondrial structural and functional damage, prevent neurons from functioning normally (Reddy, 2009), and induce neuronal apoptosis (McPhie et al, 2001). In addition, axonal damage, which evokes a disturbance of fast axonal transport, can occur even in the early stage of white matter lesions, and axonal damage is evaluated by analyzing the immunoreactivity of APP (Sherriff et al, 1994; Wang et al, 2007). Therefore, the decrease of APP expression by TO901317 treatment of stroke reflects the decreased axonal damage in the ischemic brain.
TO901317 Increase in PI3K/Akt Activity in the Ischemic Brain Contributes to Axonal Regeneration
Our data show that TO901317 treatment of stroke significantly increased Akt/PI3K pathway activity and promoted axonal regeneration after stroke. Inhibition of the Akt/PI3K pathway by LY294002 decreased the effects of TO901317 on axonal growth in cultured hypoxic PCNs. The PI3K is required for neural cell adhesion molecule-mediated neurite outgrowth from PC12 cells and from cerebellar and dopaminergic neurons in the primary culture (Ditlevsen et al, 2003). Activation of PI3K represents a potential mechanism for the regulation of neuronal differentiation and neurite outgrowth in SH-SY5Y cells (Pan et al, 2005). When neurite outgrowth elongation is initiated, PI3K remains essential for the maintenance of neuronal morphology (Sanchez et al, 2004). Akt also has dual roles in motoneuronal survival and nerve regeneration in vivo (Namikawa et al, 2000). Stimulating the phosphorylation of Akt may promote axonal outgrowth (Ransome and Turnley, 2008). Inhibition of the Akt/PI3K pathway by LY294002 abolishes the erythropoietin effects on Akt phosphorylation and axonal growth (Ransome and Turnley, 2008). Therefore, TO901317 promotes Akt/PI3K signaling activity, which at least partially contributes to axonal regeneration in the ischemic brain.
In summary, we show that treatment of experimental stroke with TO901317 at 24 h after stroke significantly increased synaptic plasticity and axonal regeneration, and decreased axonal damage. The Akt/PI3K pathway seems to contribute to TO901317-induced synaptic plasticity and axonal regeneration.
Acknowledgments
The authors thank Qinge Lu and Sutapa Santra for their technical assistance.
References
- Andersson S, Gustafsson N, Warner M, Gustafsson JA. Inactivation of liver X receptor beta leads to adult-onset motor neuron degeneration in male mice. Proc Natl Acad Sci USA. 2005;102:3857–3862. doi: 10.1073/pnas.0500634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet D, Garcia M, Vecino E, Lorentz JG, Sahel J, Hicks D. Brain-derived neurotrophic factor signalling in adult pig retinal ganglion cell neurite regeneration in vitro. Brain Res. 2004;1007:142–151. doi: 10.1016/j.brainres.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Calza L, Giardino L, Giuliani A, Aloe L, Levi-Montalcini R. Nerve growth factor control of neuronal expression of angiogenetic and vasoactive factors. Proc Natl Acad Sci USA. 2001;98:4160–4165. doi: 10.1073/pnas.051626998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Cui X, Zacharek A, Chopp M. Increasing Ang1/Tie2 expression by simvastatin treatment induces vascular stabilization and neuroblast migration after stroke. J Cell Mol Med. 2008;13:1348–1357. doi: 10.1111/j.1582-4934.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Cui X, Zacharek A, Jiang H, Roberts C, Zhang C, Lu M, Kapke A, Feldkamp CS, Chopp M. Niaspan increases angiogenesis and improves functional recovery after stroke. Ann Neurol. 2007;62:49–58. doi: 10.1002/ana.21160. [DOI] [PubMed] [Google Scholar]
- Chen J, Cui X, Zacharek A, Roberts C, Chopp M. eNOS mediates TO90317 treatment-induced angiogenesis and functional outcome after stroke in mice. Stroke. 2009;40:2532–2538. doi: 10.1161/STROKEAHA.108.545095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003a;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003b;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Ditlevsen DK, Kohler LB, Pedersen MV, Risell M, Kolkova K, Meyer M, Berezin V, Bock E. The role of phosphatidylinositol 3-kinase in neural cell adhesion molecule-mediated neuronal differentiation and survival. J Neurochem. 2003;84:546–556. doi: 10.1046/j.1471-4159.2003.01538.x. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y, He Z, Filbin M. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- Fan X, Kim HJ, Bouton D, Warner M, Gustafsson JA. Expression of liver X receptor beta is essential for formation of superficial cortical layers and migration of later-born neurons. Proc Natl Acad Sci USA. 2008;105:13445–13450. doi: 10.1073/pnas.0806974105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev. 2007;54:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Hou ST, Jiang SX, Smith RA. Permissive and repulsive cues and signalling pathways of axonal outgrowth and regeneration. Int Rev Cell Mol Biol. 2008;267:125–181. doi: 10.1016/S1937-6448(08)00603-5. [DOI] [PubMed] [Google Scholar]
- Karnezis T, Mandemakers W, McQualter JL, Zheng B, Ho PP, Jordan KA, Murray BM, Barres B, Tessier-Lavigne M, Bernard CC. The neurite outgrowth inhibitor Nogo A is involved in autoimmune-mediated demyelination. Nat Neurosci. 2004;7:736–744. doi: 10.1038/nn1261. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Fan X, Gabbi C, Yakimchuk K, Parini P, Warner M, Gustafsson JA. Liver X receptor beta (LXRbeta): a link between beta-sitosterol and amyotrophic lateral sclerosis-Parkinson's dementia. Proc Natl Acad Sci USA. 2008;105:2094–2099. doi: 10.1073/pnas.0711599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurino L, Wang XX, de la Houssaye BA, Sosa L, Dupraz S, Caceres A, Pfenninger KH, Quiroga S. PI3K activation by IGF-1 is essential for the regulation of membrane expansion at the nerve growth cone. J Cell Sci. 2005;118:3653–3662. doi: 10.1242/jcs.02490. [DOI] [PubMed] [Google Scholar]
- Leclercq PD, McKenzie JE, Graham DI, Gentleman SM. Axonal injury is accentuated in the caudal corpus callosum of head-injured patients. J Neurotrauma. 2001;18:1–9. doi: 10.1089/089771501750055721. [DOI] [PubMed] [Google Scholar]
- Li S, Kim JE, Budel S, Hampton TG, Strittmatter SM. Transgenic inhibition of Nogo-66 receptor function allows axonal sprouting and improved locomotion after spinal injury. Mol Cell Neurosci. 2005;29:26–39. doi: 10.1016/j.mcn.2004.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Jiang N, Powers C, Chopp M.1998Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats Stroke 291972–1980.discussion 1980–1981 [DOI] [PubMed] [Google Scholar]
- Liu Z, Li Y, Zhang X, Savant-Bhonsale S, Chopp M. Contralesional axonal remodeling of the corticospinal system in adult rats after stroke and bone marrow stromal cell treatment. Stroke. 2008;39:2571–2577. doi: 10.1161/STROKEAHA.107.511659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Yang GY, Zhou LF, Stern JD, Betz AL. Focal cerebral ischemia in the mouse: description of a model and effects of permanent and temporary occlusion. Brain Res Mol Brain Res. 1999;63:366–370. doi: 10.1016/s0169-328x(98)00271-x. [DOI] [PubMed] [Google Scholar]
- McKerracher L, Winton MJ. Nogo on the go. Neuron. 2002;36:345–348. doi: 10.1016/s0896-6273(02)01018-8. [DOI] [PubMed] [Google Scholar]
- McPhie DL, Golde T, Eckman CB, Yager D, Brant JB, Neve RL. Beta-secretase cleavage of the amyloid precursor protein mediates neuronal apoptosis caused by familial Alzheimer's disease mutations. Brain Res Mol Brain Res. 2001;97:103–113. doi: 10.1016/s0169-328x(01)00294-7. [DOI] [PubMed] [Google Scholar]
- Morales JR, Ballesteros I, Deniz JM, Hurtado O, Vivancos J, Nombela F, Lizasoain I, Castrillo A, Moro MA. Activation of liver X receptors promotes neuroprotection and reduces brain inflammation in experimental stroke. Circulation. 2008;118:1450–1459. doi: 10.1161/CIRCULATIONAHA.108.782300. [DOI] [PubMed] [Google Scholar]
- Namikawa K, Honma M, Abe K, Takeda M, Mansur K, Obata T, Miwa A, Okado H, Kiyama H. Akt/protein kinase B prevents injury-induced motoneuron death and accelerates axonal regeneration. J Neurosci. 2000;20:2875–2886. doi: 10.1523/JNEUROSCI.20-08-02875.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourhaghighi N, Teichert-Kuliszewska K, Davis J, Stewart DJ, Nag S. Altered expression of angiopoietins during blood-brain barrier breakdown and angiogenesis. Lab Invest. 2003;83:1211–1222. doi: 10.1097/01.lab.0000082383.40635.fe. [DOI] [PubMed] [Google Scholar]
- Pan J, Kao YL, Joshi S, Jeetendran S, Dipette D, Singh US. Activation of Rac1 by phosphatidylinositol 3-kinase in vivo: role in activation of mitogen-activated protein kinase (MAPK) pathways and retinoic acid-induced neuronal differentiation of SH-SY5Y cells. J Neurochem. 2005;93:571–583. doi: 10.1111/j.1471-4159.2005.03106.x. [DOI] [PubMed] [Google Scholar]
- Papadopoulos CM, Tsai SY, Cheatwood JL, Bollnow MR, Kolb BE, Schwab ME, Kartje GL. Dendritic plasticity in the adult rat following middle cerebral artery occlusion and Nogo-a neutralization. Cereb Cortex. 2006;16:529–536. doi: 10.1093/cercor/bhi132. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Ransome MI, Turnley AM. Erythropoietin promotes axonal growth in a model of neuronal polarization. Mol Cell Neurosci. 2008;38:537–547. doi: 10.1016/j.mcn.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Reddy PH. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer's disease. Exp Neurol. 2009;218:286–292. doi: 10.1016/j.expneurol.2009.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell DR, Zhou H, Comery TA, Kouranova E, Lo CF, Warwick HK, Ring RH, Kirksey Y, Aschmies S, Xu J, Kubek K, Hirst WD, Gonzales C, Chen Y, Murphy E, Leonard S, Vasylyev D, Oganesian A, Martone RL, Pangalos MN, Reinhart PH, Jacobsen JS. The LXR agonist TO901317 selectively lowers hippocampal Abeta42 and improves memory in the Tg2576 mouse model of Alzheimer's disease. Mol Cell Neurosci. 2007;34:621–628. doi: 10.1016/j.mcn.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Sanchez S, Jimenez C, Carrera AC, Diaz-Nido J, Avila J, Wandosell F. A cAMP-activated pathway, including PKA and PI3K, regulates neuronal differentiation. Neurochem Int. 2004;44:231–242. doi: 10.1016/s0197-0186(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Sandhu R, Teichert-Kuliszewska K, Nag S, Proteau G, Robb MJ, Campbell AI, Kuliszewski MA, Kutryk MJ, Stewart DJ. Reciprocal regulation of angiopoietin-1 and angiopoietin-2 following myocardial infarction in the rat. Cardiovasc Res. 2004;64:115–124. doi: 10.1016/j.cardiores.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Vogel R, Holloway MK, Rutledge SJ, Friedman O, Yang Z, Rodan GA, Friedman E. Transcription control and neuronal differentiation by agents that activate the LXR nuclear receptor family. Mol Cell Endocrinol. 1999;155:51–60. doi: 10.1016/s0303-7207(99)00115-x. [DOI] [PubMed] [Google Scholar]
- Sherriff FE, Bridges LR, Sivaloganathan S. Early detection of axonal injury after human head trauma using immunocytochemistry for beta-amyloid precursor protein. Acta Neuropathol. 1994;87:55–62. doi: 10.1007/BF00386254. [DOI] [PubMed] [Google Scholar]
- Sironi L, Mitro N, Cimino M, Gelosa P, Guerrini U, Tremoli E, Saez E. Treatment with LXR agonists after focal cerebral ischemia prevents brain damage. FEBS Lett. 2008;582:3396–3400. doi: 10.1016/j.febslet.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujike H, Takaki M, Kodama M, Kuroda S. Gene expression related to synaptogenesis, neuritogenesis, and MAP kinase in behavioral sensitization to psychostimulants. Ann NY Acad Sci. 2002;965:55–67. doi: 10.1111/j.1749-6632.2002.tb04151.x. [DOI] [PubMed] [Google Scholar]
- Walmsley AR, Mir AK. Targeting the Nogo-A signalling pathway to promote recovery following acute CNS injury. Curr Pharm Des. 2007;13:2470–2484. doi: 10.2174/138161207781368611. [DOI] [PubMed] [Google Scholar]
- Wang F, Liang Z, Hou Q, Xing S, Ling L, He M, Pei Z, Zeng J. Nogo-A is involved in secondary axonal degeneration of thalamus in hypertensive rats with focal cortical infarction. Neurosci Lett. 2007;417:255–260. doi: 10.1016/j.neulet.2007.02.080. [DOI] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002a;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- Wang L, Schuster GU, Hultenby K, Zhang Q, Andersson S, Gustafsson JA. Liver X receptors in the central nervous system: from lipid homeostasis to neuronal degeneration. Proc Natl Acad Sci USA. 2002b;99:13878–13883. doi: 10.1073/pnas.172510899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharek A, Chen J, Zhang C, Cui X, Roberts C, Jiang H, Teng H, Chopp M. Nitric oxide regulates Angiopoietin1/Tie2 expression after stroke. Neurosci Lett. 2006;404:28–32. doi: 10.1016/j.neulet.2006.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhang Q, Zhang JH, Qin X. Electro-stimulation of cerebellar fastigial nucleus (FNS) improves axonal regeneration. Front Biosci. 2008;13:6999–7007. doi: 10.2741/3205. [DOI] [PubMed] [Google Scholar]
- Zhou FQ, Snider WD. Intracellular control of developmental and regenerative axon growth. Philos Trans R Soc Lond B Biol Sci. 2006;361:1575–1592. doi: 10.1098/rstb.2006.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]