Abstract
In the retina, adenosine is released in the dark and has been shown to inhibit Ca2+ influx through voltage-gated Ca2+ channels in cones. Therefore, we tested whether adenosine can inhibit exocytosis from isolated cone photoreceptors. Simultaneous measurements of membrane exocytosis and Ca2+ were made from cones using the activity-dependent dye, Synaptored-C2, and the Ca2+ indicator dye, Fluo-4. Adenosine suppressed exocytosis in cones, indicating that transmitter release is also reduced from cone terminals, and further supports an inhibitory mechanism for modulating transmitter release onto second-order neurons. Furthermore, this raises the possibility that adenosine might be neuroprotective for photoreceptors and second-order neurons by suppressing Ca2+ levels in cones and reducing exocytosis of l-glutamate, respectively.
Keywords: activity-dependent dye, ATP, Ca2+ channel, l-glutamate, purines, Synaptored-C2
Introduction
Adenosine is an important neuromodulator and neurotransmitter in the nervous system and the retina. The actions of adenosine have generally been well correlated with inhibitory modulation of excitatory synapses [1]. In agreement with this observation, adenosine also plays an important role at the first synapse in visual processing by inhibiting voltage-dependent calcium channels that trigger l-glutamate release from both rod and cone photo-receptors [2-4]. Adenosine receptors are located on vertebrate photoreceptors [4,5], and adenosine has been shown to produce a dose-dependent inhibition of voltage-dependent Ca2+ influx into cone terminals [2]. This reduction in Ca2+ influx likely results in a decrease in fusion of synaptic vesicles at the ribbon synapse of cones, suggesting that adenosine suppresses exocytosis from cone photoreceptors. Neurotransmitter release from photoreceptors is regulated by the activity of L-type Ca2+ channels [6-8]. Given the actions of adenosine on cones, and its previously reported inhibitory effect on rod photoreceptors [3,4], and the role of L-type Ca2+ channels in the regulation of transmitter release from photoreceptors [6,8], we hypothesized that adenosine might also influence exocytosis by reducing Ca2+ influx through voltage-dependent L-type Ca2+ channels in cone photoreceptors.
To explore the presynaptic role of adenosine at the cone terminal, we tested the effects of adenosine on voltage-dependent Ca2+ influx and exocytosis from cones in the salamander retina. Simultaneous measurements of intracellular calcium ([Ca2+]i) and exocytosis were made on isolated cone photoreceptors. The present results indicate that adenosine suppresses exocytosis by inhibiting voltage-dependent Ca2+ influx into cone terminals, and that this inhibition is mediated by the activation of adenosine receptors present on cones. This study provides evidence that adenosine can inhibit transmitter release from cones, like rods, by suppressing voltage-dependent Ca2+ influx into the terminal, suggesting that adenosine functions as an inhibitory neuromodulator at the first synapse in visual processing.
Methods
Tissue preparation of retinal slices and isolated cone photoreceptors
Larval tiger salamanders (Ambystoma tigrinum; Kons, Germantown, Wisconsin, USA; or Charles Sullivan, Tennessee, USA; 7–10 inch) were cared for according to the Animal Research Committee and institutional guidelines set forth at University of California, Los Angeles. Retinal slices from larval tiger salamanders were prepared as described earlier [2]. Slices were viewed by rotating 90° under a water-immersion objective (63 × 0.95 numerical aperture) and viewed on an upright, fixed-stage microscope (Zeiss Axioskop FS2; Zeiss, Thornwood, New York, USA).
For isolated cone photoreceptors, retinas were treated as described earlier [2]. Briefly, retinas from two eyes were isolated in a chilled low Ca2+ Mg2+ -free solution (pH 7.8). The eyecup was cut into quarters and the retinal pieces were transferred to a low Ca2+ (0.5 mM) solution containing papain (10.0–12.0 U/ml) for 35 min at 25–30°C, and then washed two to three times with a chilled incubation solution containing ascorbic acid, 7.5 mg/ml bovine serum albumin, and DNase 50 U/ml (Worthington Biochemical Inc., Lakewood, New Jersey, USA). Gentle trituration of this solution resulted in a cell suspension that was allowed to settle on coverslips coated with concanavalin A (1 mg/ml).
Solutions and perfusion
Solutions were delivered to the perfusion chamber (approximately 1 .0–1.5 ml total volume) at a rate of 1 ml/min using a single-pass, gravity-feed perfusion system. Drugs were typically bath applied and stimulus time points were corrected to reflect complete solution exchange. The normal amphibian superfusate contained in mM consists of 111 NaCl, 2.5 KCl, 2 CaCl2, 0.5 MgCl2, 10 HEPES, and 5 glucose. The pH of all the solutions was adjusted to 7.8 with NaOH, and the osmolarity adjusted, if necessary, to 245 ± 5 mOsm. Solutions were continuously bubbled with 100% O2.
Simultaneous measurements of Ca2+ transients and exocytosis
Synaptored-C2 (Biotium Inc., Hayward, California, USA) is an activity-dependent dye with the same physio-chemical properties of FM 4-64 (Biotium, Hayward, California, USA) and can be used to monitor exocytosis from neurons [9]. After the removal of the eyes, as described above, and before papain enzyme treatment, the eyecups were incubated in 40 μM Synaptored-C2 for 15 min in the dark. The retinas were then washed three times in chilled calcium free (0 Ca2+) or low Ca2+ (0.5 mM) superfusate containing AVASEP-7 (500 μM; Biotium Inc.). Fluo-4/AM (Invitrogen, Carlsbad, California, USA) was prepared as a 1 mM stock solution in dimethyl sulfoxide (DMSO) and diluted in amphibian superfusate to a final concentration of 1–2 μM, and incubated for 10–15 min at 5–8°C. Images were collected with a Zeiss 510 META LSM or LSM 5 Pascal mounted to an upright microscope (Zeiss Axioplan 2 or Axioskop FS2; Zeiss) equipped with an Axoplan 63 × (0.95 numerical aperture) water-immersion objective. To activate voltage-dependent Ca2+ channels, cells were depolarized by increasing [K+]o from 2.5 to 50 mM for 1 min. Images were acquired at 10-s intervals. For analysis, a region of interest was drawn over the terminal, and the change in fluorescence was interpreted as reflecting changes in [Ca2+]i. For cones, the terminal was defined as the activity-dependent dye-labeled region located under the soma (Fig. 1b). Generally, images were acquired with a spatial resolution of 10–20 pixels per micron and an optical section thickness of approximately 1 Airy unit. Fluo-4 and Synaptored-C2 dyes were excited using the 488 nm laser line, and emitted light was collected through a 505–530 nm band-pass filter (for Fluo-4) and 650 nm long-pass filter (for Synaptored-C2), respectively. In experiments examining Synaptored-C2 fluorescence in double-loaded tissue, Fluo-4 bleed through into the 650 nm long-pass channel was assessed by imaging a sample loaded with Fluo-4 alone, and then adjusting the gain settings below the threshold for Fluo-4 fluorescence in the 650 nm low-pass channel. Fluoro-metric signals were expressed as relative fluorescence change ΔF/F=(F – Fo)/Fo, where Fo denotes the resting fluorescence level corrected for bleaching. Bleaching of fluorescent signals were corrected by fitting a single exponential curve to the resting fluorescence of each trace that was recorded before stimulation and for a 30-s interval beginning 1.5 min after the stimulation. For analysis of exocytosis, the total fluorescence lost from a given terminal was calculated as [(1 – Last3/First3) × 100)] or represented as a fraction of the initial fluorescence, where Last3 is the mean of its last three values, and First3 is the mean of its first three values. If less than 5% of the fluorescence is lost, or if τdestain is greater than 20 min, the terminal was classified as ‘nondestaining’. The kinetics of fluorescence loss is estimated as τdestain by fitting single exponentials to the data between First3 and Last3 using Prism 4.0 software (Graph Pad, San Diego, California, USA). All data are presented as mean ± SEM. Data sets are compared with unpaired Student's t-test. Significance levels are P values less than 0.05. Typically, one or two cells were analyzed on each coverslip and all experiments were performed on at least three different preparations. All drug applications occurred at least 2–3 min before stimulus treatment. Data from experiments in which there was a shifting in the x, y,or z planes were discarded.
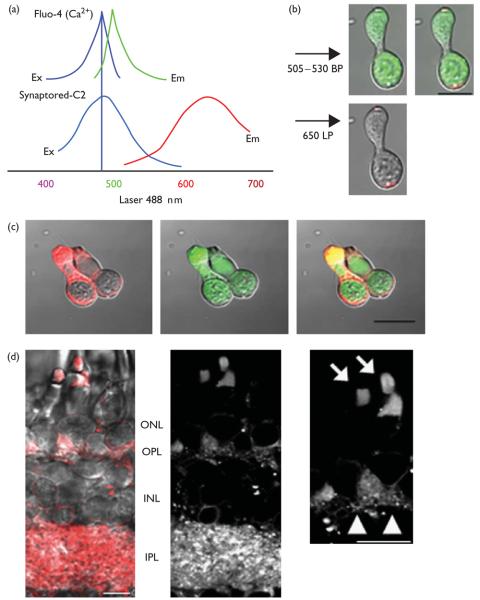
Fig. 1.
(a) Simultaneous emissions were collected from a single excitation (488 nm) for Fluo-4 and Synaptored-C2 emissions. (b) Images are shown from a small single cone using the dark loading protocol outlined in the Methods. (c) An example of a double cone that was bath loaded for 5 min with Synaptored-C2 (10 μM) in elevated [K+]o superfusate (50 mM). (d) Dark loading of the intact retinal slice with Synaptored-C2 before dissociation selectively labels both synaptic layers in the intact retina. Inset illustrates the dye loading in the synaptic terminals and outer segments of cone photoreceptors. Arrows point toward cone outer segments, and arrowheads point toward synaptic terminals with the activity-dependent dye. Scale bar is 10 μm. BP, band-pass filter; Em, emission; Ex, excitation; INL, inner nuclear layer; IPL, inner plexiform layer; LP, long-pass filter; ONL, outer nuclear layer; OPL, outer plexiform layer.
We identified some cones that either lost less than 5% of their initial fluorescence during stimulation ( < 10% of the population tested, 5/49) or had τdestain values greater than 1200 s or 20 min. A loss of less than 5% was just larger than the loss caused by photobleaching, and 1200 s was greater than 3 SDs slower than the mean τdestain, and hence, seemed likely to be nonphysiological. We considered these cones to be nondestaining and were excluded from the analysis.
Drug solutions
Adenosine was obtained from Sigma Chemical Corporation (St. Louis, Missouri, USA). Nifedipine was obtained from Calbiochem (La Jolla, California, USA). ATP was obtained from Sigma-Aldrich (St. Louis, Missouri, USA). Adenosine was prepared freshly at the final concentration used or from a 50 mM stock in DMSO. All other solutions were prepared as 100 mM stocks with either distilled H2O or DMSO. Superfusion with 0.1% DMSO alone did not affect any of the fluorescent measurements with Synaptored-C2 or Fluo-4 that we studied.
Results
L-type Ca2+ channels regulate Ca2+ influx into synaptic terminals of cone photoreceptors [6,8], and this Ca2+ entry has been shown to underlie vesicle fusion with the plasma membrane [7,10-12]. To test whether adenosine can suppress exocytosis from cones after depolarization of the synaptic terminal, we optimized a procedure to monitor intracellular Ca2+ and exocytosis simultaneously from photoreceptors, by preferentially loading Synaptored-C2, an activity-dependent dye that is equivalent to FM 4-64 [9], into synaptic vesicles of photoreceptor terminals, and used Fluo-4 to measure Ca2+ levels within the terminal. This technique was adapted from Rea et al. [12], which showed that photoreceptor terminals in the anole retina could be preferentially loaded with the activity-dependent dyes (e.g. FM 1–43) by loading intact whole retina in the dark in the presence of physiological Ca2+. Before dissociation, the intact eyecup was incubated in physiological saline in the dark with 40 μM Synaptored-C2 dye for 15 min. To prevent exocytosis, which would unload the dye, the retina was then transferred to either 0 Ca2+ or low Ca2+ 0.5 mM solution. This solution also included Advasep-7, a cyclodextran that removes excess Synaptored-C2 [13]. In eyecups that were loaded in the dark, cones (Fig. 1b) showed bright labeling that was restricted to the terminal region, and outer segment, but absent from the rest of the cell. In slices that were loaded in the dark (Fig. 1d), there was strong labeling in both the outer plexiform layer and inner plexiform layer, but almost no labeling in cone inner segments, soma, or inner nuclear layer. Rod terminals were also labeled (data not shown). When Synaptored-C2 was bath applied after retinal dissociation (Fig. 1c), the labeling was present at extrasynaptic sites, labeling most of the cell membrane. The isolated double cone showed labeling in all regions and was brightest in the inner segment. This extra-synaptic labeling also occurred in the absence of extracellular Ca2+ , as reported earlier [12]. Thus, using dark loading of intact retina, followed by a wash with Advasep-7 in low or 0 Ca2+ , we were able to obtain consistent synapse-specific loading of Synaptored-C2 in cone photoreceptor terminals.
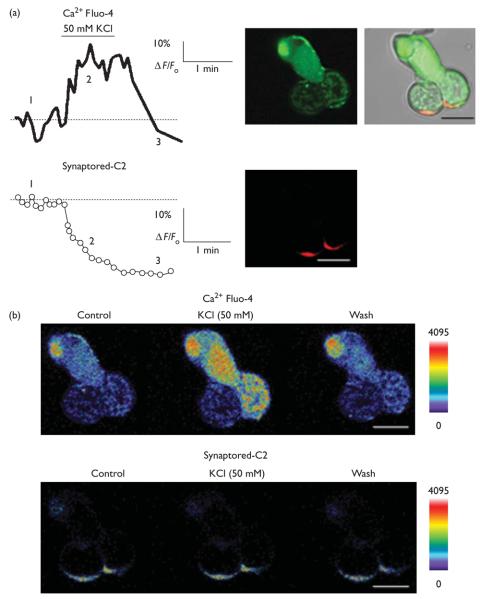
To measure the kinetics of destaining of Synaptored-C2, isolated cones were exposed to elevated K+, and an increase in [Ca2+]i levels was simultaneously measured along with the decline in Synaptored-C2 fluorescence (Fig. 2). Before stimulation, there was a minor time-dependent decline in fluorescence because of bleaching. The onset of elevated K+ caused a rapid loss of fluorescence to a steady-state level in the terminal (Fig. 2).
Fig. 2.
(a) Simultaneous imaging of Synaptored-C2 (red) destaining and [Ca2+]i (green) Fluo-4 in the cone terminal. Plots of [Ca2+]i changes in the terminal of a double cone (upper panel) and destaining (lower panel). (b) Pseudocolor images illustrating spatiotemporal [Ca2+]i and Synaptored-C2 changes within the terminal to an elevated [K+]o stimulus. Numbers in (a) correspond to time intervals of images shown in (b). Scale bar is 10 μm.
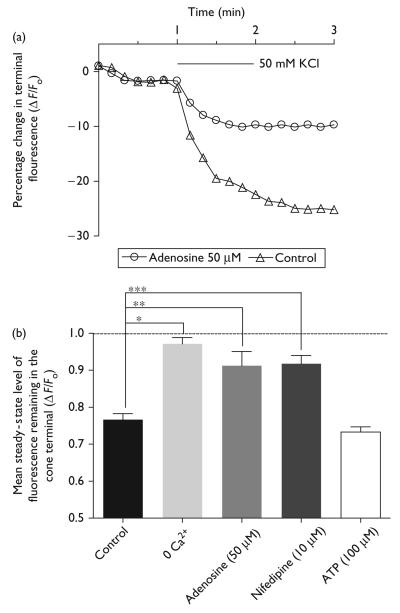
Adenosine (50 μM) prevented the Synaptored-C2 destaining after the K+-evoked depolarization in cones (Fig. 3), which coincided with a commensurate decrease in the K+-evoked Ca2+ increase in the cone terminal. The K+-evoked stimulation (50 mM) was applied for 1 min, and the mean time constant of destaining (τdestain) was 362.4 ± 17.4 s (n = 15; Fig. 3), which is similar to the destaining rate of cone terminals in anole and salamander retina [11,12]. Adenosine caused an increase in both the τdestain and the remaining normalized steady-state fluorescence, after K+-evoked depolarization (Fig. 3). The τdestain for adenosine (50 μM) was 735 ± 97.8 s, and for the remaining steady-state fluorescence was 91.2 ± 3.8% of the initial normalized fluorescence (n = 13, P = 0.0412). Adenosine caused a significant suppression of K+-evoked destaining from cones (Fig. 3; control vs. adenosine, unpaired t-test, P = 0.0014, n = 13), providing evidence that adenosine can suppress transmitter release from cones. If destaining reflects neurotransmitter release, it should depend on Ca2+ influx into the terminal. Indeed, 0 Ca2+ and nifedipine, an L-type Ca2+ channel blocker; prevented activity-dependent destaining from cone terminals when compared with elevated K+ alone (Fig. 3; 0 Ca2+, unpaired t-test, P < 0.0001, n = 6; nifedipine, unpaired t-test, P = 0.0003, n = 4). The steady-state fluorescence level remaining after an elevated [K+]o stimulation in 0 Ca2+ superfusate or nifedipine was 97.2 ± 1.6% (n = 6, P = 0.1449) and 91.8 ± 2.3% (n = 4, P = 0.0351) of the mean initial unstimulated fluorescence level, respectively. ATP receptors have been observed in mammalian photoreceptors, but are not likely associated with cones [14,15]. Therefore, to rule out any direct effect of ATP on exocytosis from cone photoreceptors, ATP (100 μM) was tested on basal [Ca2+]i levels and the K+-evoked [Ca2+] increase. ATP had no effect on basal [Ca2+i levels (data not shown), the K+-evoked [Ca2+]i increase, or exocytosis in cones (Fig. 3; 70.2 ± 1.6% (n = 5, P < 0.0001).
Fig. 3.
Kinetics of Synaptored-C2 destaining in cone photoreceptor terminals with the application of elevated [K+]o superfusate (50 mM). Plot of Synaptored-C2 destaining from a cone terminal treated with elevated [K+]o (triangles) for 1 min and elevated [K+]o for 1 min in the presence of adenosine (50 μM; circles). (b) Adenosine (50 μM) partially inhibited the destaining of Synaptored-C2 fluorescence in synaptic terminals (**P = 0.0014). 0 Ca2+ superfusate essentially prevented destaining (***P < 0.0001). Nifedipine (10 μM) partially inhibited destaining (*P = 0.0003), confirming the role of L-type channels in transmitter release from cones. ATP (100 μM) had no effect on destaining profile (for steady-state fluorescence levels in cones, see text). *P <0.05 compared with an unstimulated normalized total fluorescence value of 1.0.
It is also important to note that the kinetics of destaining in cones was faster than rods (cone τdestain = 362.4 ± 17.4 s, n = 15; rod τdestain = 431.82 ± 35.4 s, n = 4) using the same stimuli (50 mM elevated [K+]o), this is in agreement with another study showing that exocytosis in cones is faster than rods [11], which has important post-synaptic consequences [10].
Discussion
This study shows that adenosine inhibits depolarization-induced Ca2+ influx and exocytosis in cone photoreceptors. The effects of adenosine arise from the activation of adenosine receptors present on cones [2]. The potency of the effects of adenosine on depolarization-evoked Ca2+ responses (half maximal effective concentration = 15.6 μM; [2]) suggests that the observed effects of adenosine on exocytosis likely occur when cone photoreceptors are depolarized, in darkness when adenosine levels are highest [16], and transmitter release from cones is at its greatest [6,7,17].
Simultaneous imaging of Ca2+ and activity-dependent dye changes in cone terminals
Activity-dependent dyes (FM 1-43 and FM 4-64) are important tools for studying presynaptic function and membrane trafficking in neurons and other cell types [18]. Many of the difficulties associated with FM dye loading in retina have been overcome using a novel technique developed by Rea et al. [12]. In this manner, photoreceptor terminals are loaded selectively without labeling the soma and inner segment. Loading of Synaptored-C2 was restricted to both synaptic layers of the salamander retina (Fig. 1d) as reported earlier [12,19,20]. In addition, dye accumulation also occurred in the cone outer segments (Fig. 1b and d; [2,12]), which may be because of dye entering the outer segment of cones through other routes, like the cyclic GMP-gated channels. FM dyes have been shown to enter cells through other routes, in particular mechanosensitive channels, ATP-gated channels, and VR-1 channels [21]. However, entry of Synaptored-C2 into the outer segment does not preclude our ability to make measurements of exocytosis from cone terminals.
Our findings show that adenosine can suppress exocytosis from cone terminals, and as mentioned above, this is likely because of the activation of adenosine receptors on cones leading to inhibition of voltage-dependent Ca2+ influx into cone terminals (Fig. 3). As expected, destaining of Synaptored-C2 is Ca2+-dependent (Fig. 3b), experiments performed with 0 Ca2+ or the L-type Ca2+ channel blocker nifedipine prevented the destaining of the dye. Likewise, adenosine (50 μM) also reduced destaining of Synaptored-C2 suggesting that adenosine can suppress exocytosis from cones (Fig. 3a and b). ATP was also tested on isolated cones, however, no observable changes in destaining occurred when compared with control stimulations without any drug (Fig. 3b); suggesting that if P2 receptors are present they do not likely influence transmitter release from cones. In addition, we have observed that the time constant for Synaptored-C2 destaining from cones is faster than rods, suggesting that the kinetic components of release are more rapid for vesicles from cones than vesicles from rods (τcone = 362.4 ± 17.4 s, n = 5, τrod = 431.82 ± 35.4, n = 4). Support for this finding comes from earlier studies on the kinetics of vesicle release from rods and cones [10,11].
Inhibitory effects of adenosine on exocytosis combined with the light-modulated release of adenosine [4,16] in the retina could provide a novel way to regulate both the excitability and tonic release of l-glutamate from cones, which would have a profound impact on excitatory neurotransmission from cones to second-order neurons. Generally, photoreceptors are maximally depolarized in the dark, and it has been shown that endogenous adenosine is present in the dark-adapted retina [4,16]; thus, it is conceivable that the elevated adenosine levels in and around cone photoreceptors function as a tonic brake to tightly regulate l-glutamate release by influencing and shaping inputs into second-order neurons.
In addition to the potential neuromodulatory role of adenosine on cones, there could also be potential neuroprotective benefits for both photoreceptors and outer retinal neurons. Absence of L-type Ca2+ channels in rd mice have been shown to reduce the amount of photoreceptor degeneration [22], suggesting that inhibition of voltage-dependent Ca2+ influx by adenosine could inhibit Ca2+ channels and perhaps excessive release of l-glutamate, which could greatly influence apoptosis of both photoreceptors and postreceptoral neurons in the outer retina. In addition, the finding that adenosine protects retinal neurons from neurodegenerative insults, like ischemia, is consistent with a potential neuroprotective role for adenosine in cones [23]. The decrease in tonic l-glutamate release from cones would presumably accompany a decrease in the activation of voltage-dependent Ca2+ channels, which might also minimize the potential for excitotoxic damage. Finally, the present results suggest that adenosine levels or sensitivity to adenosine, for example, during ischemia or during conditions of darkness, may influence both cone neurotransmission and photoreceptor cell survival at the first synapse in visual processing.
Conclusion
Adenosine suppresses exocytosis from cone terminals, as a result of decreased vesicle fusion of the membrane that is associated with the inhibition Ca2+ influx through Ca2+ channels. The ability of adenosine to suppress exocytosis from cones is consistent with an inhibitory role of adenosine in modulating neurotransmission into second-order neurons, and raises the possibility that adenosine might be neuroprotective for photoreceptors and second-order neurons by suppressing Ca2+ levels in cones and reducing exocytosis of l-glutamate, respectively.
Acknowledgements
The authors thank Alejandro Vila and Uyenchi Huynh for providing technical support, and Arlene Hirano for critically reading earlier versions of this manuscript and providing insightful comments. This research was supported by NIH grants EY 04067, and DK 41301 and a VA Senior Career Scientist to N.C.B., Fight-For-Sight, Grant-in-Aid to S.L.S., and Fight-For-Sight Student Fellowship, Jeanette Duval Scholarship, and UCLA Undergraduate Research Scholars Program to W.D.H.
References
- 1.Latini S, Pazzagli M, Pepeu G, Pedata F. A2 adenosine receptors: their presence and neuromodulatory role in the central nervous system. Gen Pharmacol. 1996;27:925–933. doi: 10.1016/0306-3623(96)00044-4. [DOI] [PubMed] [Google Scholar]
- 2.Stella SL, Jr, Hu WD, Vila A, Brecha NC. Adenosine inhibits voltage-dependent Ca2 + influx in cone photoreceptor terminals of the tiger salamander retina. J Neurosci Res. 2007;85:1126–1137. doi: 10.1002/jnr.21210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stella SL, Jr, Bryson EJ, Thoreson WB. A2 adenosine receptors inhibit calcium influx through L-type calcium channels in rod photoreceptors of the salamander retina. J Neurophysiol. 2002;87:351–360. doi: 10.1152/jn.00010.2001. [DOI] [PubMed] [Google Scholar]
- 4.Stella SL, Jr, Bryson EJ, Cadetti L, Thoreson WB. Endogenous adenosine reduces glutamatergic output from rods through activation of A2-like adenosine receptors. J Neurophysiol. 2003;90:165–174. doi: 10.1152/jn.00671.2002. [DOI] [PubMed] [Google Scholar]
- 5.Kvanta A, Seregard S, Sejersen S, Kull B, Fredholm BB. Localization of adenosine receptor messenger RNAs in the rat eye. Exp Eye Res. 1997;65:595–602. doi: 10.1006/exer.1996.0352. [DOI] [PubMed] [Google Scholar]
- 6.Thoreson WB, Nitzan R, Miller RF. Reducing extracellular Cl- suppresses dihydropyridine-sensitive Ca2 + currents and synaptic transmission in amphibian photoreceptors. J Neurophysiol. 1997;77:2175–2190. doi: 10.1152/jn.1997.77.4.2175. [DOI] [PubMed] [Google Scholar]
- 7.Rieke F, Schwartz EA. A cGMP-gated current can control exocytosis at cone synapses. Neuron. 1994;13:863–873. doi: 10.1016/0896-6273(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson MF, Barnes S. The dihydropyridine-sensitive calcium channel subtype in cone photoreceptors. J Gen Physiol. 1996;107:621–630. doi: 10.1085/jgp.107.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brager DH, Luther PW, Erdelyi F, Szabo G, Alger BE. Regulation of exocytosis from single visualized GABAergic boutons in hippocampal slices. J Neurosci. 2003;23:10475–10486. doi: 10.1523/JNEUROSCI.23-33-10475.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabl K, Cadetti L, Thoreson WB. Kinetics of exocytosis is faster in cones than in rods. J Neurosci. 2005;25:4633–4640. doi: 10.1523/JNEUROSCI.4298-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheng Z, Choi SY, Dharia A, Li J, Sterling P, Kramer RH. Synaptic Ca2+ in darkness is lower in rods than cones, causing slower tonic release of vesicles. J Neurosci. 2007;27:5033–5042. doi: 10.1523/JNEUROSCI.5386-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rea R, Li J, Dharia A, Levitan ES, Sterling P, Kramer RH. Streamlined synaptic vesicle cycle in cone photoreceptor terminals. Neuron. 2004;41:755–766. doi: 10.1016/s0896-6273(04)00088-1. [DOI] [PubMed] [Google Scholar]
- 13.Kay AR, Alfonso A, Alford S, Cline HT, Holgado AM, Sakmann B, et al. Imaging synaptic activity in intact brain and slices with FM1-43 in C. elegans, lamprey, and rat. Neuron. 1999;24:809–817. doi: 10.1016/s0896-6273(00)81029-6. [DOI] [PubMed] [Google Scholar]
- 14.Puthussery T, Yee P, Vingrys AJ, Fletcher EL. Evidence for the involvement of purinergic P2X receptors in outer retinal processing. Eur J Neurosci. 2006;24:7–19. doi: 10.1111/j.1460-9568.2006.04895.x. [DOI] [PubMed] [Google Scholar]
- 15.Puthussery T, Fletcher EL. Neuronal expression of P2 3 purinoceptors in the rat retina. Neuroscience. 2007;146:403–414. doi: 10.1016/j.neuroscience.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 16.Ribelayga C, Mangel SC. A circadian clock and light/dark adaptation differentially regulate adenosine in the mammalian retina. J Neurosci. 2005;25:215–222. doi: 10.1523/JNEUROSCI.3138-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnes S, Kelly ME. Calcium channels at the photoreceptor synapse. Adv Exp Med Biol. 2002;514:465–476. doi: 10.1007/978-1-4615-0121-3_28. [DOI] [PubMed] [Google Scholar]
- 18.Ryan TA. Presynaptic imaging techniques. Curr Opin Neurobiol. 2001;11:544–549. doi: 10.1016/s0959-4388(00)00247-6. [DOI] [PubMed] [Google Scholar]
- 19.Miller RF, Fagerson MH, Staff NP, Wolfe R, Doerr T, Gottesman J, et al. Structure and functional connections of presynaptic terminals in the vertebrate retina revealed by activity-dependent dyes and confocal microscopy. J Comp Neurol. 2001;437:129–155. doi: 10.1002/cne.1275. [DOI] [PubMed] [Google Scholar]
- 20.Caicedo A, Espinosa-Heidmann DG, Hamasaki D, Pina Y, Cousins SW. Photoreceptor synapses degenerate early in experimental choroidal neovascularization. J Comp Neurol. 2005;483:263–277. doi: 10.1002/cne.20413. [DOI] [PubMed] [Google Scholar]
- 21.Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Read DS, McCall MA, Gregg RG. Absence of voltage-dependent calcium channels delays photoreceptor degeneration in rd mice. Exp Eye Res. 2002;75:415–420. [PubMed] [Google Scholar]
- 23.Roth S. Endogenous neuroprotection in the retina. Brain Res Bull. 2004;62:461–466. doi: 10.1016/j.brainresbull.2003.07.006. [DOI] [PubMed] [Google Scholar]