Abstract
Uridine-5′-diphosphoglucose (UDPG) activates the P2Y14 receptor, a neuroimmune system GPCR. P2Y14 receptor tolerates glucose substitution with small alkyl or aryl groups or its truncation to uridine-5′-diphosphate (UDP), a full agonist human P2Y14 receptor expressed in HEK-293 cells. 2-Thiouracil derivatives displayed selectivity for activation of the human P2Y14 vs. the P2Y6 receptor, such as 2-thio-UDP 4 (EC50 1.92 nM at P2Y14, 224-fold selectivity vs. P2Y6) and its β-propyloxy ester 18. EC50 of β-methyl ester of UDP and its 2-thio analogue were 2730 and 56 nM, respectively. β-t-Butyl ester of 4 was 11-fold more potent than UDPG, but β-aryloxy or larger, branched β-alkyl esters, such as cyclohexyl, were less potent. Ribose replacement of UDP with a rigid North or South methanocarba (bicyclo[3.1.0]hexane) group abolished P2Y14 receptor agonist activity. α,β-Methylene and difluoromethylene groups were well tolerated at the P2Y14 receptor and are expected to provide enhanced stability in biological systems. α,β-Methylene-2-thio-UDP 11 (EC50 0.92 nM) was 2160-fold selective versus P2Y6. Thus, these nucleotides and their congeners may serve as important pharmacological probes for the detection and characterization of the P2Y14 receptor.
Keywords: G protein-coupled receptor, nucleotides, pyrimidines, phospholipase C, carbohydrates, uracil
Introduction
Purine and pyrimidine mononucleotides and dinucleotides have a role as extracellular signaling molecules, in addition to their diverse intracellular roles.1 The P2Y family of G protein-coupled receptors (GPCRs) responds to various extracellular nucleotides to induce intracellular signaling cascades.2-4 The P2Y14 receptor was identified initially as an orphan GPCR activated by uridine-5′-diphosphoglucose (UDPG 1, Chart 1) and other endogenous UDP-sugars. This Gi-coupled receptor is expressed in the brain and in dendritic cells,5 although no functional role has yet been clearly assigned in these tissues. The P2Y14 receptor also is expressed in the placenta, adipose tissue, stomach, intestine, brain, spleen, thymus, lung, and heart.1 Thus, the P2Y14 receptor is of potential therapeutic interest for modulation of the immune system,7 as well as treatment of pain,8 diabetes,9 cystic fibrosis, and other pulmonary diseases.10,11
Chart 1.
Structures of two UDP-sugars (1, 2) that act as P2Y14 receptor agonists, UDP 3, a naturally occurring ligand of the hP2Y14 receptor, and its analogue 2-thio-UDP 4.
A current goal of our research is to identify and apply potent, selective, and stable P2Y14 receptor ligands to define the physiological role(s) of this receptor.12-14 Although the P2Y14 receptor initially was identified as a UDP-sugar-activated GPCR, we recently discovered that UDP is at least as potent as UDP-glucose and other nucleotide sugars for activation of this receptor.14 Thus, it is perhaps not surprising that our recent detailed SAR (structure activity relationship) analyses with synthetic analogues of UDPG12,13 revealed that the glucose moiety is the least restricted region of the structure for substitutions that maintain P2Y14 receptor agonist potency. This realization, together with conclusions made from our recent SAR study of UDP analogues at the P2Y6 receptor,15 suggests that β-phosphate-substitution in a new series of UDP analogues might favor activation of the P2Y14 receptor. Therefore, with the ultimate goal of identifying highly selective ligands for the P2Y14 and P2Y6 receptors, we have synthesized a new series of β-phosphate-substituted analogues of UDP and compared the potencies of these novel molecules as well as a number of previously prepared uracil nucleotide analogues at the human P2Y14 and P2Y6 receptors. Although the glucose moiety of UDPG was suggested to interact with multiple H-bonding and/or charged resides within the putative binding site of the P2Y14 receptor, its deletion or substitution with smaller phosphoester groups was tolerated at this receptor. Simple alkyl esters at this position and analogues of UDP displayed highly potent agonist activity at the P2Y14 receptor. The effects of these modifications to preserve and enhance potency at the P2Y14 receptor were additive with the previously identified potency enhancing effect of 2-thio substitution of the uracil moiety, achieving in some cases nanomolar and subnanomolar potency. Importantly, a number of these new UDP analogues exhibit high selectivity for activation of the P2Y14 receptor over the P2Y6 receptor.
Results
Chemical Synthesis
The principal objective of this study was to structurally simplify the distal end of uracil nucleotide sugars known to activate the hP2Y14 receptor and to combine potency-enhancing modifications. By removing the glucose moiety of UDPG 1, this agonist is converted into UDP 3, which we recently discovered is a highly potent agonist of the hP2Y14 receptor.14,16 The activities of analogues of 1 modified on the glucose moiety have been explored, but intermediate structures and truncated analogues related to UDP have not been systematically examined. We also were searching for leads for UDP analogues that might provide selectivity at the P2Y14 receptor over the P2Y6 receptor.
Simple ester and 5′-diphosphate analogues of the hP2Y14 receptor agonist UDPG 1 and its 2-thio derivative 2 were synthesized (Table 1). The only modifications of the nucleobase included were thiocarbonyl or methoxyamino substitution of carbonyl groups of the uracil moiety, which were studied previously in P2Y14 receptor recognition.12 Several of the analogues of UDP 3 studied here were reported in studies of SAR at the P2Y6 receptor.15,17,18 All of the nucleotide analogues were prepared in their ammonium or triethylammonium salt form according to the methods shown in Schemes 1 – 3 and tested in functional assays of the hP2Y14 and hP2Y6 receptors (Table 1).
Table 1.
In vitro pharmacological data for UDPG 1, UDP 3, and their analogues (nonsugar β-phosphoesters and other derivatives of UDP) in the inhibition of cAMP formation at recombinant hP2Y14 receptors expressed in HEK-293 cells stably transfected with the hP2Y14 and in the stimulation of PLC at recombinant hP2Y6 receptors stably expressed in 1321N1 cells. Unless noted: X, Y = O, Z = H.
 | ||||
|---|---|---|---|---|
| Compound | Modification | Structure R = | EC50, nMa | |
| UDP-sugars | hP2Y14 | hP2Y6 | ||
| 1 | UDP-[1]glucose |  |
400±90 | 16,000 |
| 2b | 2-thio-UDP-[1]glucose, |  |
11±5 | >10,000c |
| UDP analogues | hP2Y14 | hP2Y6 | ||
| 3 | UDP | HO | 160±40 | 530±60 |
| 4 | 2-thio-UDP | HO, X1 = S | 1.92±0.69 | 447±100 |
| 5 | 4-thio-UDP | HO, X2 = S | 320±130 | 2360±710 |
| 6 | Up2-β-Me-phosphonate | R = CH3 | 4580±1560 | 8000±1630 |
| 7 | N4-OCH3-CDP | HO, X2 = N-OCH3 | 3320±1620 | 70±7b |
| 8d | (N)-methanocarba UDP (pure enantiomer) | HO | NE | NE |
| 9d | (S)-methanocarba UDP (pure enantiomer) | HO | NE | 42±8b |
| 10 | Up-CH2-p (α,β-methylene UDP) | HO, Y = CH2 | 11±6 | 339±97 |
| 11 | 2-thio-Up-CH2-p (α,β-methylene-2-thio-UDP) | HO, Y = CH2, X1 = S | 0.92±0.09 | 1990±370 |
| 12b,e | Up-CF2-p (α,β-difluoromethylene UDP) | HO, Y = CF2 | 63±9 | NEb |
| 13 | 5-I-Up-CF2-p | HO, Y = CF2, Z = I | 142±74 | 127±24 |
| UDP β-esters | hP2Y14 | hP2Y6 | ||
| 14 | Up2-OMe | CH3O | 2730±680 | >10,000c |
| 15 | 2-thio-Up2-OMe | CH3O, X1 = S | 56±19 | >10,000c |
| 16 | 4-thio-Up2-OMe | CH3O, X2 = S | NE | 10,400±4200 |
| 17 | 2-thio-Up2-OEt | CH3CH2O, X1 = S | 39±20 | >10,000c |
| 18e | 2-thio-Up2-OPr | CH3(CH2)2O, X1 = S | 40±6 | 9100±1430 |
| 19 | Up2-O(CH2)2CN | O(CH2)2CN | 7460±2340 | >10,000c |
| 20 | Up2-O(CH2)2C≡CH | O(CH2)2C≡CH | 480±161 | 1520±100 |
| 21 | 2-thio-Up2-O(CH2)2C≡CH | O(CH2)2C≡CH, X1 = S | 11.0±1.4 | >10,000c |
| 22 | Up2-OCH2CHOHCH2OH | OCH2CH(OH)-CH2OH | 1600±600 | >10,000c |
| 23 | Up2-OCH(CH2OH)2 | OCH(CH2OH)2 | 167±32 | >10,000c |
| 24 | Up2-OC(CH3)3 | OC(CH3)3 | 252±93 | 10,800±1900 |
| 25 | 2-thio-Up2-OC(CH3)3 | OC(CH3)3, X1 = S | 32±1 | 2040±600 |
| 26 | Up2-O -cyclohexyl |  |
5160±1830 | >10,000c |
| 27 | Up2-OC6H5 |  |
768±304 | 7400±860 |
| 28 | Up2-OC6H4-4-NO2 | 1490±490 | 2760±810 | |
| 29 | Up2-OC6H4-3-Cl |  |
1800±860 | 7370±530 |
| 30 | Up2-OC6H4-4-OCH3 | 1900±490 | >10,000c | |
Agonist potencies reflect stimulation of phospholipase C, unless noted, and were calculated using a four-parameter logistic equation and the GraphPad software package (GraphPad, San Diego, CA). EC50 values (mean ± standard error) represent the concentration at which 50% of the maximal effect is achieved. Relative efficacies (%) were determined by comparison with the effect produced by a maximal effective concentration of reference agonist (UDP-glucose, 1) in the same experiment. If no maximal effect is given, then 100% efficacy was achieved. N = 3.
Potency at P2Y6 receptor reported in Maruoka et al.,15 Besada et al.,17 and Ko et al.18 Potency at P2Y14 receptor reported in Ko et al.12,13
<50% effect at 10 μM.
Structure is given below.
12, MRS2802; 18, MRS2907.14
NE - no effect at 10 μM. 
Scheme 1.
Synthesis of UDPG analogues including UDP β -esters. Reagents and conditions: (a) ROPO3H2, DMF, rt.
Scheme 3.
Synthesis of α,β-methylene-2-thio-UDP 11. Reagents and conditions: (a) DCC, DMF, rt.
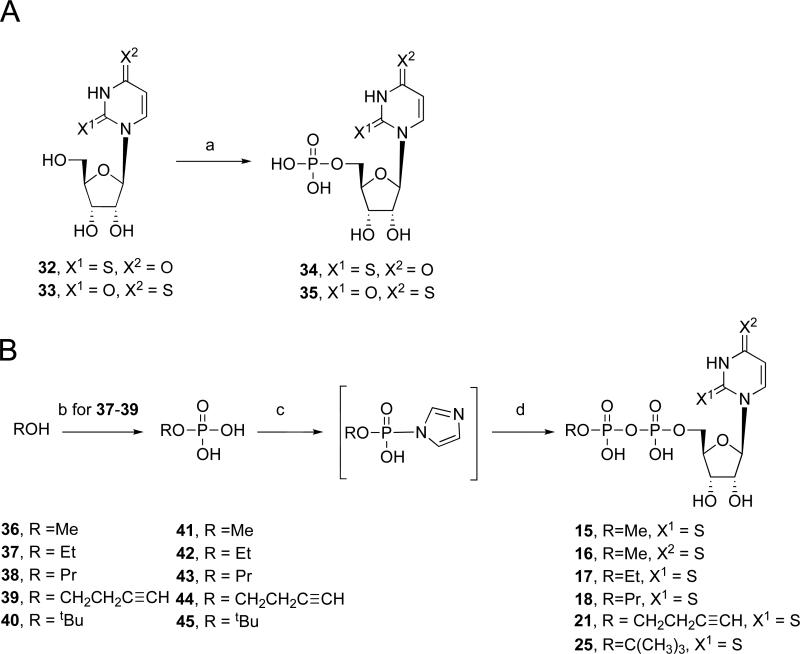
The uridine-5′-diphospho alkyl or aryl derivatives were obtained by the following methods. The corresponding alkyl or aryl monophosphate analogues were treated successively with cation-exchange resin and tributylamine. To the solution of the alkyl or aryl monophosphate tributylammonium salt in DMF, commercially available uridine 5′-monophosphate morpholidate 4-morpholine-N,N-dicyclohexylcarboxamidine salt was added to form uridine-5′-diphospho-β-ester analogues (6, 14, 19 – 20, 22 – 24, and 26 – 30) in a condensation reaction as shown in Scheme 1. 2-Thio UDP 4 and 4-thio UDP 5 were synthesized by using the previously reported procedure.17 (N)-Methanocarba-UDP 8 and (S)-methanocarba-UDP 9 were synthesized using our previously reported procedures.15,19,20 α,β-Methylene-UDP 10 and α,β-difluoromethylene-UDP 12 were also prepared using our recently published procedures or modifications thereof. 2-Thio-α,β-methylene-UDP 11 was prepared by the DCC coupling of 2-thiouridine with methylenediphosphonic acid (Scheme 3).18 Compound 7 and 13 were synthesized by using a recently developed procedure.15
For the preparation of the 2- or 4-thiocarbonyl β-alkyldiphosphate esters (15 – 18, 21 and 25), we either synthesized the alkylmonophosphate (42 – 44)21 or obtained the commercially available alkylmonophosphates (41, 45). The monophosphates were transformed to the corresponding tributylammonium salts and were then activated with 1,1′-carbonyldiimidazole in DMF for 5 h at room temperature followed by quenching of this reagent with methanol. After removing the solvent, the residue was dissolved in DMF, and 2-thio or 4-thiouridine 5′-monophosphate tributylammonium salt was added to obtain compounds 15 – 18, 21, and 25 as shown in Scheme 2. All the nucleotide analogues were characterized using HPLC, nuclear magnetic resonance (1H NMR, 31P NMR), and high-resolution mass spectrometry.
Scheme 2.
A) Synthesis of thio-UMP derivatives 34 and 35. Reagents and conditions: (a) (i) POCl3, proton sponge, PO(OMe)3, 0°C (ii) 0.2 M triethylammonium bicarbonate, rt. B) Synthesis of thio-UDP derivatives 15-18, 21, and 25. Reagents and conditions: (b) H3PO4, Pyridine, Et3N, Ac2O (c) (i) CDI, DMF, rt; (ii) 5% TEA in 1/1 MeOH/H2O; (d) 2-thiouridine-5′-monophosphate tributylammonium salt 34 or 4-thiouridine-5′-monophosphate tributylammonium salt 35, DMF, rt.
Quantification of Pharmacological Activity
Inhibition of adenylyl cyclase was quantified in HEK-293 cells stably expressing the hP2Y14 receptor. This cell line, as well as a P2Y14 receptor-expressing Chinese hamster ovary (CHO) cell line and a P2Y14 receptor-expressing C6 rat glioma cell line that we recently developed, provides a physiologically relevant test system since receptor-promoted responses mediated through a natively expressed heterotrimeric G protein (Gi) and its natively expressed effector protein (adenylyl cyclase) are quantified.14 We conclude that this system is much preferable to assay methods, employed by us and others, which utilized COS-7 cells transiently over-expressing the P2Y14 receptor with an engineered G protein, Gα-q/i protein (Gαqi5), that allows coupling of Gi-coupled receptors to activation of phospholipase C (PLC), resulting in inositol lipid hydrolysis.22-24
Although UDPG 1 is usually thought of as a specific agonist of the P2Y14 receptor, it also was found to activate the P2Y2 receptor (EC50 ~ 10 μM). 13 We now report EC50 values for 1 of 0.40 μM and 16 μ M at the P2Y14 and P2Y6 receptor, respectively (Table 1). Therefore, although selective for the P2Y14 receptor, UDPG also activates other P2Y receptors. Thus, more potent and selective agonists, preferably with a simplified chemical structure, are needed as pharmacological probes of the P2Y14 receptor.
UDP 3 and its 4-thio derivative 5 were moderately potent agonists at the hP2Y14 receptor. One of the most potent P2Y14 receptor agonists in the current set of nucleotides was the 2-thio derivative 4 of UDP, which displayed an EC50 value of 1.92 nM. Compound 4 was 83-fold more potent than 3 as a P2Y14 receptor agonist and 230-fold selective for the P2Y14 receptor in comparison to the P2Y6 receptor. Replacement of a terminal negatively charged oxygen of 3 with an uncharged methyl group in the phosphonate derivative 6 resulted in a molecule with similarly weak potencies at the two receptors. The N4-methyloxy cytidine derivative 7 was 20-fold less potent than 3 at the P2Y14 receptor and consequently displayed selectivity for the P2Y6 receptor, at which its high potency has been described.15 This finding is consistent with the reported weak P2Y14 receptor activity of the corresponding N4-methyloxy cytidine analogue of UDPG.13
Replacement of a ribose moiety of various P2Y receptor agonists with a sterically constrained bicyclic ring has been used to establish the receptor-preferred conformation. Neither of the conformationally constrained methanocarba analogues 8 (North, N) and 9 (South, S) of UDP 3 was active at the P2Y14 receptor. Consistent with these findings with analogues of 3, we have already established that the corresponding (N) and (S) methanocarba analogues of UDPG were both inactive at the P2Y14 receptor.13
The introduction of carbon-bridged substitutions of the phosphate moieties of P2 agonists has led to greater stability in biological systems due to the inability of ectonucleotidases to cleave these groups.1,27 Replacement of the bridging oxygen of the diphosphate group of UDP with a methylene 10 or difluoromethylene 12 group was well tolerated at the P2Y14 receptor, resulting in high potency with EC50 values of 11 and 63 nM, respectively. Indeed, the α,β-difluoromethylene analogue 12 was >2000-fold selective for the P2Y14 receptor in comparison to the P2Y6 receptor, at which it was inactive at the concentrations tested. Combining a carbon bridge with a known potency-enhancing uracil modification in 2-thio-α,β-methylene analogue 11 resulted in unprecedented potency at the hP2Y14 receptor with an EC50 value of 0.92 nM. The selectivity of 11 in comparison to the hP2Y6 receptor was 2160-fold. Combining the α,β-difluoromethylene and 5-iodo modifications in 13 maintained equipotency to UDP 3 at the P2Y14 receptor, which stands in contrast with the inactivity of 5-iodo-UDPG and suggests non-identical modes of uracil binding to the receptor between the two series.12 However, 13 was not selective for the P2Y14 receptor when compared to the P2Y6 receptor.
The native agonist 1 is a β-ester of the diphosphate moiety, which prompted us to explore the potency of structurally simpler β-esters at the P2Y14 receptor. Since compound 14, modified with a methyl ester group instead of glucose, weakly activated the P2Y14 receptor and was inactive at the P2Y6 receptor, this methyl ester was combined with other modifications to probe the effects on biological activity. The combination with the 2-thio modification in compound 15 increased potency by 42-fold. Thus, compound 15 was >180-fold selective for the P2Y14 receptor. The 4-thio modification in methyl ester 16 abolished and greatly reduced potency in comparison to 15 at the P2Y14 and P2Y6 receptors, respectively.
In the 2-thio series, homologation of the alkyl ester group of 15 was tolerated at the P2Y14 receptor, with the ethyl 17 and propyl 18 derivatives both displaying EC50 values of ~40 nM and >200-fold selectivity in comparison to the P2Y6 receptor. A 2-cyanoethyl group in 19 was poorly tolerated at the P2Y14 receptor. Substitution of the β-methyl ester with a t-butyl ester group in 25 produced an equipotent agonist, which was 8-fold more potent than the corresponding 2-oxo analogue 24. The β-t-butyloxy ester of 2-thio-UDP 25 was 11-fold more potent than UDPG. Introduction of a glyceryl moiety esterified through the 2-hydroxyl group in 23 did not diminish the potency of 3, but an isomeric glyceryl ester 22 was 10-fold less potent than 3.
Alkynyl ester derivatives 20 (2-oxo) or 21 (2-thio) were either 2-fold less potent or 16-fold more potent, respectively, than 3 at the P2Y14 receptor. Compound 21 was >900-fold selective in comparison to the P2Y6 receptor.
β-Aryloxy and cycloalkyloxy esters 26 – 30 of UDP were found to only weakly activate the receptor, in the order of potency phenyl > 4-nitrophenyl > 3-chloro- and 4-methoxyphenyl > cyclohexyl.
Discussion
In the present study, we have further expanded the range of potent P2Y14 receptor ligands through a systematic exploration of the SAR of structurally simple analogues at this receptor, particularly with respect to substitution of the glucose moiety with diverse alkyl moieties. We have found that the introduction of α,β-methylene and difluoromethylene groups is well tolerated at the P2Y14 receptor. These carbon-bridged nucleotides, including α,β-methylene-UDP with an EC50 value of 11 nM, are expected to display greater stability in biological systems. Methylene-bridged nucleotides tend to have increased stability by impeding breakdown by ecto-nucleotidases present on the cell surface,27-29 and thus, these carbon-bridged diphosphate analogues have good potential for in vivo applications.
The 2-thio modification, but not the 4-thio modification of the uracil ring, tended to increase potency and selectivity at the P2Y14 receptor in comparison to the P2Y6 receptor. The most selective compounds (fold selectivity for P2Y14 in comparison to P2Y6) in the present study were UDP analogues 4 (233), 11 (2160), 12 (>150), 15 (>180), 17 (>250), 18 (230), and 21 (>900). The most potent of these were 2-thio-UDP 4 and α,β-methylene-2-thio-UDP 11. Compound 4 had a high potency at the P2Y14 receptor, with an EC50 value of 1.92 nM, and the corresponding α,β-methylene analogue 11 was even twice as potent. Although it is a less potent P2Y14 receptor agonist, difluoromethylene-UDP 12 did not activate the P2Y6 receptor at the concentrations of analogue tested. Other compounds that displayed lesser selectivity for the P2Y14 receptor were (fold): 25 (64), 23 (59), 24 (43), and 10 (31). Thus, the 2-thio derivatives 4, 11, and their congeners may serve as important pharmacological probes for the detection and characterization of the P2Y14 receptor.
It is now clear that the glucose moiety of 1 is not required for activation of the P2Y14 receptor; although when it is present, there is a SAR pattern related to interaction of specific functionality of this hexose moiety with the receptor.13,26 We conclude here that UDP and its analogues are potent full agonists of the human P2Y14 receptor, consistent with our recent pharmacological studies of this receptor stably expressed in three different cell lines that utilized native Gi proteins for signal transduction.14 This finding contradicts with the original work of Fricks et al. who reported that UDP is a partial agonist/competitive antagonist of the human P2Y14 receptor, while acting as a full agonist at the rat P2Y14 receptor.16 However, the latter study quantified activity of a recombinant P2Y14 receptor transiently coexpressed with an unnatural chimeric G protein, Gaq/i that couples Gi-activating receptors to activation of PLC-β isozymes. It was suggested that this system favors “a conformation of the P2Y14-R..., which results in ligand binding selectivities and agonist activities that are not altogether consistent with activities of the receptor obtained when coupled to its cognate heterotrimeric G protein.” Thus, the efficacy of UDP appears to be a function of the G protein signaling system activated by the receptor.
In conclusion, we have identified new analogues of UDP and its simple esters that display enhanced potency and selectivity for the P2Y14 receptor and which promise to be useful as pharmacological tools to distinguish the effects of uracil nucleotides acting at P2Y14 versus P2Y6 receptors. The nanomolar potency achieved in this series is until now unprecedented for small molecular ligands of the P2Y14 receptor. It is now possible to examine these potent agonists on human cells expressing an endogenous P2Y14 receptor to aid in delineating a role for this receptor.
Experimental Section
Chemical Synthesis
1H NMR spectra were obtained with a Varian Gemini 300 spectrometer using D2O as a solvent. The chemical shifts are expressed as relative ppm from HOD (4.80 ppm). 31P NMR spectra were recorded at room temperature by use of a Varian XL 300 spectrometer (121.42 MHz); orthophosphoric acid (85%) was used as an external standard.
Purity and extent of reaction of nucleotide derivatives was checked using a Hewlett–Packard 1100 HPLC equipped with a Zorbax Eclipse 5 μm XDB-C18 analytical column (250 × 4.6 mm; Agilent Technologies Inc, Palo Alto, CA), linear gradient solvent system: 5 mM TBAP (tetrabutylammonium dihydrogenphosphate)-CH3CN from 80:20 to 40:60 in 20 min, with a flow rate of 1 mL/min (System A), or Zorbax SB-Aq 5 μm analytical column (50 × 4.6 mm; Agilent Technologies Inc, Palo Alto, CA). Mobile phase: linear gradient solvent system: 5 mM TBAP (tetrabutylammonium dihydrogenphosphate)-CH3CN from 80:20 to 40:60 in 13 min; the flow rate was 0.5 mL/min (System B). Peaks were detected by UV absorption (254 nm) with a diode array detector.
Purification of the nucleotide analogues for biological testing was carried out on (diethylamino)ethyl (DEAE)-A25 Sephadex columns with a linear gradient (0.01–0.5 M) of 0.5 M ammonium bicarbonate as the mobile phase. Some of the compounds were additionally purified by HPLC with a Luna 5-μm RP-C18(2) semipreparative column (250 × 10.0 mm; Phenomenex, Torrance, CA) and using a linear gradient solvent system of 10 mM TEAA (triethylammonium acetate)-CH3CN from 100:0 to 95:5 (or up to 99:1 to 90:10) in 30 min, with a flow rate of 2 mL/min. The tested nucleotide derivatives were confirmed by HPLC to possess a ≥ 95% purity. Compounds purified by HPLC were isolated as the triethylammonium salts. Compounds 41 and 45, other reagents, and solvents were purchased from Sigma-Aldrich (St. Louis, MO).
General Procedure for the Preparation of Uridine 5′-Diphosphoglucose Analogues
The appropriate alkyl or aryl monophosphate was first converted to its tributylammonium salt form by the treatment with ion-exchange resin (DOWEX 50WX2-200 (H)), which upon acidification of the supernatant was followed by addition of tributylamine until a basic pH was reached. The aqueous solvent was removed by lyophilization, and the residue was used without further purification. A solution in DMF (1.5 mL) of uridine 5′-monophosphate morpholidate 4-morpholine-N,N-dicyclohexylcarboxamidine salt (20 mg, 0.029 mmol) and the corresponding monophosphate (0.035 mmol, tributylammonium salt was stirred at room temperature for 2 days. After that the solvent was removed and the residue was purified by ion-exchange column chromatography using a Sephadex-DEAE A-25 resin (with a linear gradient (0.01-0.5 M) of 0.5 M ammonium bicarbonate as the mobile phase) to provide the corresponding nucleotide as the ammonium salt. Some of the uridine 5′-diphosphoglucose analogues were additionally purified by HPLC as described above.
Diphosphoric acid 1-β-methyl (C-P) 2-(uridine-5′-yl)ester, triethylammonium salt (6)
Compound 6 (4.0 mg, 23%) was obtained as a white solid following the general procedure. 1H NMR (D2O) δ 7.96 (d, J = 8.1 Hz, 1H), 6.00 (d, J = 4.8 Hz, 1H), 5.97 (d, J = 7.8 Hz, 1H), 4.38 (m, 2H), 4.29 (m, 1H), 4.21 (m, 2H), 1.46 (d, J = 16.8 Hz, 3H); 31P NMR (D2O) δ 17.82 (d, J = 23.9 Hz), −11.06 (d, J = 23.8 Hz); HRMS-EI found 401.0158 (M – H+)−. C10H15N2O11P2 requires 401.0151; purity > 98% by HPLC. (System A: 13.3 min).
Diphosphoric acid 1-β-methyl ester 2-(uridine-5′-yl)ester, ammonium salt (14)
Compound 14 (3.9 mg, 30%) was obtained as a white solid following the general procedure. 1H NMR (D2O) δ 7.97 (d, J = 8.1 Hz, 1H), 6.01 (dd, J = 3.6, 1.2 Hz, 1H), 5.97 (d, J = 8.1 Hz, 1H), 4.38 (m, 2H), 4.29 (m, 1H), 4.20 (m, 2H), 3.66 (d, J = 11.4 Hz, 3H); 31P NMR (D2O) δ −9.22 (d, J = 21.4 Hz), −11.03 (d, J = 21.4 Hz); HRMS-EI found 417.0087 (M – H+)−. C10H15N2O12P2 requires 417.0100; purity > 98% by HPLC. (System A: 11.3 min).
Diphosphoric acid 1-β-(2-cyanoethyl)ester 2-(uridine-5′-yl)ester, ammonium salt (19)
Compound 19 (3.7 mg, 26%) was obtained as a white solid following the general procedure. 1H NMR (D2O) δ 7.99 (d, J = 8.4 Hz, 1H), 5.99 (m, 2H), 4.89 (m, 2H), 4.26 (m, 3H), 4.16 (dd, J = 12.9, 6.0 Hz, 2H), 2.87 (t, J = 6.07 Hz, 2H); 31P NMR (D2O) δ −11.18 (dd, J = 42.8, 21.4 Hz); HRMS-EI found 456.0212 (M – H+)−. C12H16N3O12P2 requires 456.0209; purity > 98% by HPLC. (System A: 12.8 min).
Diphosphoric acid 1-β-(3-butynyl)ester 2-(uridine-5′-yl)ester, triethylammonium salt (20)
Compound 20 (5.31 mg, 28%) was obtained as a white solid following the general procedure. 1H NMR (D2O) δ 7.88 (d, J = 8.1 Hz, 1H), 5.98 (m, 2H), 4.41 (m, 1H), 4.21 (m, 3H), 4.08 (m, 1H), 3.83 (m, 2H), 2.53 (m, 3H),31P NMR (D2O) δ −9.61 (d, J = 16.5 Hz), −11.2 (m), HRMS-EI found 455.0257 (M – H+)−. C13H17N2 O12P2 requires 455.0265; purity > 97% by HPLC. (System B: 6.89 min).
Diphosphoric acid 1-α-glycerol ester 2-(uridine-5′-yl)ester, ammonium salt (22)
Compound 22 (2.2 mg, 15%) was obtained as a white solid following the general procedure. 1H NMR (D2O) δ 7.96 (d, J = 8.4 Hz, 1H), 5.60 (m, 2H), 4.40 (m, 2H), 4.26 (m, 3H), 3.99 (m, 3H), 3.68 (m, 2H); 31P NMR (D2O) δ −10.35 (d, J = 21.4 Hz), −10.97 (d, J = 20.8 Hz); HRMS-EI found 477.0315 (M – H+)−. C12H19N2O14P2 requires 477.0312; purity > 98% by HPLC. (System A: 13.6 min).
Diphosphoric acid 1-β-glycerol ester 2-(uridine-5′-yl)ester, triethylammonium salt (23)
Compound 23 (3.1 mg, 16%) was obtained as a white solid following the general procedure. 1H NMR (D2O) δ 7.97 (d, J = 7.8 Hz, 1H), 6.01 (m, 1H), 5.98 (d, J = 7.8 Hz, 1H), 4.39 (m, 2H), 4.30 (m, 2H), 4.24 (m, 2H), 3.77 (m, 4H); 31P NMR (D2O) δ −10.89 (dd, J = 37.9, 21.4 Hz); HRMS-EI found 477.0302 (M – H+)−. C12H19N2O14P2 requires 477.0312; purity > 98% by HPLC. (System A: 11.2 min).
Diphosphoric acid 1-β-tert-butyl ester 2-(uridine-5′-yl)ester, ammonium salt (24)
Compound 24 (4.4 mg, 31%) was obtained as a white solid following the general procedure. 1H NMR (D2O) δ 7.99 (d, J = 7.8 Hz, 1H), 5.99 (m, 2H), 4.39 (m, 2H), 4.29 (m, 1H), 4.24 (m, 2H), 1.44 (d, J = 0.3 Hz, 9H); 31P NMR (D2O) δ −11.51 (d, J = 21.4 Hz), −14.73 (d, J = 21.4 Hz); HRMS-EI found 459.0565 (M – H+)−. C13H21N2O12P2 requires 459.0570; purity > 98% by HPLC. (System A: 13.6 min).
Diphosphoric acid 1-β-cyclohexyl ester 2-(uridine-5′-yl)ester, ammonium salt (26)
Compound 26 (6.2 mg, 41%) was obtained as a white solid following the general procedure. 1H NMR (D2O) δ 8.00 (d, J = 8.4 Hz, 1H), 6.00 (m, 2H), 4.40 (m, 2H), 4.30 (m, 1H), 4.24 (m, 2H), 4.20 (m, 1H), 1.97 (m, 2H), 1.72 (m, 2H), 1.50-1.10 (m, 6H); 31P NMR (D2O) δ −11.26; HRMS-EI found 485.0719 (M – H+)−. C15H23N2O12P2 requires 485.0726; purity > 98% by HPLC. (System A: 11.8 min).
Diphosphoric acid 1-β-phenyl ester 2-(uridine-5′-yl)ester, ammonium salt (27)
Compound 27 (4.7 mg, 32%) was obtained as a white solid following the general procedure. 1H NMR (D2O) δ 7.87 (d, J = 8.4 Hz, 1H), 7.36 (m, 2H), 7.19 (m, 3H), 5.93 (d, J = 5.1 Hz, 1H), 5.81 (d, J = 8.4 Hz, 1H) 4.30 (m, 1H), 4.25 (m, 2H), 4.20 (m, 1H), 4.15 (m, 1H); 31P NMR (D2O) δ −11.11 (d, J = 21.5 Hz), −15.74 (d, J = 20.8 Hz); HRMS-EI found 479.0247 (M – H+)−. C15H17N2O12P2 requires 479.0257; purity > 98% by HPLC. (System A: 13.1 min).
Diphosphoric acid 1-β-(4-nitrophenyl)ester 2-(uridine-5′-yl)ester, ammonium salt (28)
Compound 28 (6.1 mg, 38%) was obtained as a white solid following the general procedure. 1H NMR (D2O) δ 8.21 (d, J = 9.3 Hz, 2H), δ 7.76 (d, J = 7.83 Hz, 1H), δ 7.37 (d, J = 9.0 Hz, 2H), 5.92 (m, 1H), 5.69 (d, J = 7.5 Hz, 1H), 4.25 (m, 4H), 4.14 (m, 1H); 31P NMR (D2O) δ −11.15 (d, J = 20.8 Hz), −17.12 (d, J = 20.8 Hz); HRMS-EI found 524.0110 (M – H+)−. C15H16N3O14P2 requires 524.0108; purity > 98% by HPLC. (System A: 13.7 min).
Diphosphoric acid 1-β-(3-chlorophenyl)ester 2-(uridine-5′-yl)ester, ammonium salt (29)
Compound 29 (3.7 mg, 25%) was obtained as a white solid following the general procedure. 1H NMR (D2O) δ 7.85(d, J = 8.1 Hz, 1H), 7.33 (m, 3H), 7.17 (m, 1H), 5.95 (d, J = 4.2 Hz, 1H), 5.76 (d, J = 8.1 Hz, 1H), 4.29 (m, 4H), 4.18 (m, 1H); 31P NMR (D2O) δ −11.04 (d, J = 21.3 Hz), −16.18 (d, J = 21.3 Hz); HRMS-EI found 512.9867 (M – H+)−. C15H16N2O12ClP2 requires 512.9854; purity > 98% by HPLC. (System B: 8.6 min).
Diphosphoric acid 1-β-(4-methoxyphenyl)ester 2-(uridine-5′-yl)ester, ammonium salt (30)
Compound 30 (3.3 mg, 21%) was obtained as a white solid following the general procedure. 1H NMR (D2O) δ 7.82 (d, J = 8.1 Hz, 1H), 7.18 (d, J = 8.7 Hz, 2H), δ 6.93 (d, J = 8.7 Hz, 2H), 5.95 (d, J = 4.8 Hz, 1H), δ 5.77 (d, J = 8.1 Hz, 1H), 4.29 (m, 4H), 4.16 (m, 1H); 31P NMR (D2O) δ −11.04 (d, J = 21.3 Hz), −15.37 (d, J = 21.3 Hz); HRMS-EI found 509.0362 (M – H+)−. C16H19N2O13P2 requires 509.0353; purity > 98% by HPLC. (System B: 8.84 min).
General procedure for the preparation of diphosphoric acid 1-alkyl ester 2-(thio uridine-5′-yl)ester (15-18, 21, and 25)
Monophosphates (42-44) were prepared from the corresponding alcohols (37-39) using a published procedure,21 and the spectral data were consistent with the assigned structures.
Each of the prepared monophosphates (42-44)21 or commercially available monophosphates (41, 45) was transformed to its tributylammonium salt by treatment with ion exchange resin followed by the addition of tributylamine. 1,1′-carbonyldiimidazole (10 mg, 0.06 mmol) was added to the alkyl monophosphate tributylammonium salts (0.017 mmol) (41-45) in DMF (1 mL). The reaction mixture was stirred at room temperature for 5 h. Methanol (1 mL) was added and stirring was continued at room temperature for an additional 1 h. After removal of the solvent, the residue was dried under high vacuum and dissolved in DMF (1.5 mL). 2-Thiouridine 5′-monophosphate tributylammonium salt (34)12 (5.7 mg, 0.008 mmol) for 15, 17, 18, 21, and 25 or 4-thiouridine 5′-monophosphate tributylammonium salt (35)12 (5.7 mg, 0.008 mmol) for 16 was added to the reaction mixture and it was stirred at room temperature for 2 d. After removal of the solvent, the residue was purified by the same method as general procedure using Sephadex-DEAE A-25 resin. Compounds 16 and 21 were further purified by HPLC to provide homogeneous products.
Diphosphoric acid 1-β-methyl ester 2-(2-thiouridine-5′-yl)ester, ammonium salt (15)
Compound 15 (0.82 mg, 22%) was obtained as a white solid following the general procedure. 1H NMR 1H NMR (D2O) δ 8.15 (d, J = 8.1 Hz, 1H), 6.70 (d, J = 3.6 Hz, 1H), 6.24 (d, J = 8.1 Hz, 1H), 4.43 (m, 1H), 3.34 (m, 3H), 4.24 (m, 1H), 3.66 (d, J = 11.7 Hz, 3H); 31P NMR (D2O) δ −10.68 (dd, J = 21.5, 6.6 Hz), −11.05 (d, J = 21.4 Hz); HRMS-EI found 432.9886 (M – H+)−. C10H15N2O11P2S requires 432.9872; purity > 98% by HPLC. (System A: 12.9 min).
Diphosphoric acid 1-β-methyl ester 2-(4-thiouridine-5′-yl)ester, triethyl ammonium salt (16)
Compound 16 (0.91 mg, 18%) was obtained as a white solid following the general procedure. 1H NMR 1H NMR (D2O) δ 7.9 (d, J = 7.7 Hz, 1H), 6.69 (d, J = 4.3 Hz, 1H), 5.99 (d, J = 7.5 Hz, 1H), 4.40 (m, 3H), 4.30 (m, 2H), 3.69 (m, 3H); 31P NMR (D2O) δ −10.83 (d, J = 21.3, Hz), −11.12 (d, J = 21.3 Hz); HRMS-EI found 432.9884 (M – H+)−. C10H15N2O11P2S requires 432.9872; purity > 98% by HPLC. (System B: 7.2 min in negative absorbance).
Diphosphoric acid 1-β-ethyl ester 2-(2-thiouridine-5′-yl)ester, ammonium salt (17)
Compound 17 (7.7 mg, 20%) was obtained as a white solid following the general procedure. 1H NMR 1H NMR (D2O) δ 8.22 (d, J = 8.1 Hz, 1H), 6.72 (d, J = 3.0 Hz, 1H), 6.32 (d, J = 8.4 Hz, 1H), 4.49 (m, 1H), 3.39 (m, 3H), 4.29 (m, 1H), 4.09 (m, 2H), 1.33 (m, 3H); 31P NMR (D2O) δ −10.39 (t, J = 21.3, 42.7 Hz), −11.08 (d, J = 21.3 Hz); HRMS-EI found 447.0022 (M – H+)−. C11H17N2O11P2S requires 447.0028; purity > 98% by HPLC. (System B: 7.09 min).
Diphosphoric acid 1-β-propyl ester 2-(2-thiouridine-5′-yl)ester, ammonium salt (18)
Compound 18 (0.91 mg, 23%) was obtained as a white solid following the general procedure. 1H NMR 1H NMR (D2O) δ 8.21 (d, J = 8.4 Hz, 1H), 6.69 (d, J = 3.1 Hz, 1H), 6.28 (d, J = 8.4 Hz, 1H), 4.47 (m, 1H), 3.41 (m, 3H), 4.26 (m, 1H), 3.95 (m, 2H), 1.65 (m, 2H); 0.96 (m, 3H); 31P NMR (D2O) δ −10.68 (d, J = 21.5, 4.), −11.10 (d, J = 21.3 Hz); HRMS-EI found 461.0145 (M – H+)−. C12H19N2O11P2S requires 461.0151; purity > 98% by HPLC. (System B: 7.4 min).
Diphosphoric acid 1-β-(3-butynyl) ester 2-(2-thiouridine-5′-yl)ester, triethylammonium salt (21)
Compound 21(1.18 mg, 22%) was obtained as a white solid following the general procedure. 1H NMR 1H NMR (D2O) δ 8.12 (d, J = 8.1 Hz, 1H), 6.79 (d, J = 3.1 Hz, 1H), 6.23 (d, J = 8.1 Hz, 1H), 4.43 (m, 1H), 4.35 (m, 1H), 4.10 (m, 3H), 3.89 (m, 2H), 2.54 (m, 2H); 2.41 (m, 1H); 31P NMR (D2O) δ −9.63 (d, J = 21.3), −11.12 (d, J = 21.3 Hz); HRMS-EI found 471.0022 (M – H+)−. C13H17N2O11P2S requires 471.0035; purity > 97% by HPLC. (System B: 6.9 min).
Diphosphoric acid 1-β-tert-butyl ester 2-(2-thiouridine-5′-yl)ester, ammonium salt (25)
Compound 25 (0.83 mg, 21%) was obtained as a white solid following the general procedure. 1H NMR 1H NMR (D2O) δ 8.26 (d, J = 8.4 Hz, 1H), 6.68 (d, J = 3.0 Hz, 1H), 6.26 (d, J = 8.1 Hz, 1H), 4.41 (m, 1H), 3.36 (m, 3H), 4.27 (m, 1H), 1.47 (s, 9H); 31P NMR (D2O) δ −11.00 (d, J = 21.3, Hz), −11.53 (d, J = 21.3 Hz); HRMS-EI found 475.0341 (M – H+)−. C13H21N2O11P2S requires 475.0341; purity > 98% by HPLC. (System B: 7.16 min).
Preparation of 2-thiouridine-5′-α,β-methylene-diphosphate, triethylammonium salt (11)
A solution of 2-thiouridine (13 mg, 0.05 mmol) and DCC (30mg 0.15 mmol) was stirred in DMF under a nitrogen atmosphere. Methylenediphosphonic acid (13 mg, 0.075 mmol) was added and the stirring was continued for 48 h. The solvent was removed and the product was purified by ion exchange column chromatography with a Sephadex-DEAE A-25 resin, followed by semipreparative HPLC as described above.
Compound 11(11.8 mg, 33%) was obtained as a white solid. 1H NMR 1H NMR (D2O) δ 8.22 (d, J = 7.8 Hz, 1H), 6.65 (d, J = 3.2 Hz, 1H), 6.28 (d, J = 7.8 Hz, 1H), 4.47 (m, 2H), 4.37 (m, 2H), 4.26 (m, 1H), 1.47 (t, J = 19.2 Hz, 2H); 31P NMR (D2O) δ 16.70, 13.32 (m); HRMS-EI found 416.9935 (M – H+)−. C10H15N2O10P2S requires 416.9923; purity > 98% by HPLC. (System B: 7.04 min).
Assay of P2Y6 receptor-stimulated PLC activity
Activity at the hP2Y6 receptor was quantified in 1321N1 human astrocytoma cells stably expressing this receptor. Twenty-four h after plating, the inositol lipid pool of the cells was radiolabeled by incubation in 200 μL of serum-free inositol-free Dulbecco's modified Eagle's medium, containing 0.4 μCi of myo-[3H]inositol. No changes of medium were made subsequent to the addition of [3H]inositol. Forty-eight h after transfection, cells were challenged with 50 μL of the five-fold concentrated solution of receptor agonists in 200 mM HEPES, pH 7.3, containing 50 mM LiCl for 20 min at 37 °C. Incubations were terminated by aspiration of the drug-containing medium and addition of 450 μL of ice-cold 50 mM formic acid. After 15 min at 4 °C, samples were neutralized with 150 μL of 150 mM NH4OH. [3H]Inositol phosphates were isolated by ion exchange chromatography on Dowex AG 1-X8 columns as previously described.30
Assay of P2Y14 receptor-inhibited accumlation of 3′,5′-cyclic adenosine monophosphate (cAMP)
Cell culture: Human embryonic kidney-293 cells stably expressing the hP2Y14-R (P2Y14-HEK293 cells) were generated as previously described by Fricks et al.16 P2Y14-HEK293 cells were grown in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% Genticin (Gibco), and 1% antibiotic-antimocotic (Gibco) at 37°C in a 5% CO2 environment.
cAMP accumulation: P2Y14-HEK293 cells were grown in 24-well plates and incubated with 1 microCi [3H]adenine/well in serum-free DMEM for at least 2 h prior to assay. Assays were initiated by addition of HEPES-buffered, serum-free DMEM containing 200 microCi 3-isobutyl-1-methyl-xanthine (IBMX) and 30 μM forskolin, with or without drugs, and incubation continued for 15 min at 37°C. Incubations were terminated by aspiration of the medium and addition of 450 mL ice-cold 5% trichloroacetic acid. [3H]cAMP was isolated by sequential Dowex and alumina chromatography and quantified by liquid scintillation counting as previously described by Harden et al. (53).
Data Analysis
Agonist potencies (EC50 values) were obtained from concentration-response curves by non-linear regression analysis using the GraphPad software package Prism (GraphPad, San Diego, CA). All experiments were performed in triplicate assays and repeated at least three times. The results are presented as mean ± SEM from multiple experiments or in the case of concentration effect curves from a single experiment carried out with triplicate assays that were representative of results from multiple experiments.
Supplementary Material
Figure 1.
(A) Activation of Gi-coupled P2Y14 receptor (left panel) was assessed by quantification of inhibition of forskolin-stimulated [3H]cyclic AMP accumulation in HEK293 cells stabling expressing the hP2Y14 receptor. Activation of the Gq-coupled P2Y6 receptor (right panel) was assessed by quantification of [3H]inositol phosphate accumulation in 1321N1 human astrocytoma cells stably expressing the hP2Y6 receptor by agonists Up2-OMe 14, 2-thio-Up2-OMe 15, and its O-propyl ester 18 (B) Activation of Gi-coupled P2Y14 receptor (left panel) was assessed by quantification of inhibition of forskolin-stimulated [3H]cyclic AMP accumulation in HEK293 cells stabling expressing the hP2Y14 receptor. Activation of the Gq-coupled P2Y6 receptor (right panel) was assessed by quantification of [3H]inositol phosphate accumulation in 1321N1 human astrocytoma cells stably expressing the hP2Y6 receptor by methylene-bridged agonist 10, its 2-thio analogue 11, and the difluoromethylene-bridged agonist 12.
Acknowledgments
Mass spectral measurements were carried out by Dr. John Lloyd and Dr. Noel Whittaker (NIDDK). We thank Dr. Andrei A. Ivanov and Dr. Stefano Costanzi (NIDDK) for helpful discussion and Dr. Sonia de Castro (NIDDK) for technical assistance. This research was supported in part by the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases. This work was supported by National Institutes of Health Grant GM38213 to T.K. Harden.
Abbreviations
- cAMP
3′,5′-cyclic adenosine monophosphate
- CHO
Chinese hamster ovary
- DCC
dicyclohexylcarbodiimide
- DMEM
Dulbecco's modified Eagle medium
- DMF
dimethylformamide
- HEK
human embryonic kidney
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- HPLC
high performance liquid chromatography
- PLC
phospholipase C
- SAR
structure activity relationship
- TBAP
tributylammonium phosphate
- TEAA
triethylammonium acetate
- UDP
uridine-5′-diphosphate
- UDPG
uridine-5′-diphosphoglucose
Footnotes
Supporting Information Available: NMR spectral data and HPLC traces of selected nucleotide derivatives are included. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Fumagalli M, King BF, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology. Update of the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson KA, Jarvis MF, Williams M. Perspective: Purine and pyrimidine (P2) receptors as drug targets. J. Med. Chem. 2002;45:4057–4093. doi: 10.1021/jm020046y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Kügelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacology Therapeutics. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Shaver SR. P2Y receptors: biological advances and therapeutic opportunities. Curr. Opin. Drug Disc. 2001;4:665–670. [PubMed] [Google Scholar]
- 5.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, Virchow JC, Jr., Lambrecht BN. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nature Med. 2007;13:913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 6.Gachet C. Regulation of platelet functions by P2 receptors. Ann. Rev. Pharmacol. Toxicol. 2006;46:277–300. doi: 10.1146/annurev.pharmtox.46.120604.141207. [DOI] [PubMed] [Google Scholar]
- 7.Shin A, Toy T, Rothenfusser S, Robson N, Vorac J, Dauer M, Stuplich M, Endres S, Cebon J, Maraskovsky E, Schnurr M. P2Y receptor signaling regulates phenotype and IFN-alpha secretion of human plasmacytoid dendritic cells. Blood. 2008;111:3062–3069. doi: 10.1182/blood-2007-02-071910. [DOI] [PubMed] [Google Scholar]
- 8.Malin SA, Davis BM, Koerber HR, Reynolds IJ, Albers KM, Molliver DC. Thermal nociception and TRPV1 function are attenuated in mice lacking the nucleotide receptor P2Y2. Pain. 2008;138:484–496. doi: 10.1016/j.pain.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lugo-Garcia L, Filhol R, Lajoix AD, Gross R, Petit P, Vignon J. Expression of purinergic P2Y receptor subtypes by INS-1 insulinoma beta-cells: a molecular and binding characterization. Eur. J. Pharmacol. 2007;568:54–60. doi: 10.1016/j.ejphar.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J. Biol. Chem. 2004;279:36855–36864. doi: 10.1074/jbc.M405367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller T, Bayer H, Myrtek D, Ferrari D, Sorichter S, Ziegenhagen MW, Zissel G, Virchow JC, Jr., Luttmann W, Norgauer J, Di Virgilio F, Idzko M. The P2Y14 receptor of airway epithelial cells coupling to intracellular Ca2+ and IL-8 secretion. Am. J. Respir. Cell. Mol. Biol. 2005;33:601–609. doi: 10.1165/rcmb.2005-0181OC. [DOI] [PubMed] [Google Scholar]
- 12.Ko H, Fricks I, Ivanov AA, T. Harden TK, Jacobson KA. Structure activity relationship of uridine 5′-diphosphoglucose (UDP-glucose) analogues as agonists of the human P2Y14 receptor. J. Med. Chem. 2007;50:2030–2039. doi: 10.1021/jm061222w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ko H, Das A, Carter RL, Fricks IP, Zhou Y, Ivanov AA, Melman A, Joshi BV, Kováč P, Hajduch J, Kirk KL, Harden TK, Jacobson KA. Molecular recognition in the P2Y14 receptor: Probing the structurally permissive terminal sugar moiety of uridine-5′-diphosphoglucose. Bioorg. Med. Chem. 2009;17:5298–5311. doi: 10.1016/j.bmc.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter RL, Fricks IP, Barrett MO, Burianek LE, Zhou Y, Jacobson KA, Lazarowski ER, Harden TK. Quantification of Gi-mediated inhibition of adenylyl cyclase activity reveals that UDP is a potent agonist of the human P2Y14 receptor. Mol. Pharmacol. 2009 doi: 10.1124/mol.109.058578. doi:10.1124/mol.109.058578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maruoka H, Ko H, Tosh DK, Melman A, Carter R, Costanzi S, Harden TK, Jacobson KA. Selective P2Y6 receptor agonists: Extended phosphate and (S)-methanocarba ribonucleotide analogs. 238th ACS National Meeting; Washington, DC. Aug. 2009; MEDI187. [Google Scholar]
- 16.Fricks I, Maddiletti S, Carter R, Lazarowski ER, Nicholas RA, Jacobson KA, Harden TK. UDP is a competitive antagonist at the human P2Y14 receptor and a full agonist at the rat P2Y14 receptor. J. Pharm. Exp. Therap. 2008;325:588–594. doi: 10.1124/jpet.108.136309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Besada P, Shin DH, Costanzi S, Ko H, Mathé C, Gagneron J, Gosselin G, Maddileti S, Harden TK, Jacobson KA. Structure-activity relationships of uridine 5′-diphosphate analogues at the human P2Y6 receptor. J. Med. Chem. 2006;49:5532–5543. doi: 10.1021/jm060485n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko H, Carter R, Cosyn L, Petrelli R, de Castro S, Besada P, Zhou Y, Cappellacci L, Franchetti P, Grifantini M, Van Calenbergh S, Harden TK, Jacobson KA. Synthesis and potency of novel uracil nucleotide analogues as P2Y2 and P2Y6 receptor agonists. Bioorg. Med. Chem. 2008;16:6319–6332. doi: 10.1016/j.bmc.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HS, Ravi RG, Marquez VE, Maddileti S, Wihlborg AK, Erlinge D, Malmsjö M, Boyer JL, Harden TK, Jacobson KA. Methanocarba modification of uracil and adenine nucleotides: High potency of Northern ring conformation at P2Y1, P2Y2, P2Y4 and P2Y11, but not P2Y6 receptors. J. Med. Chem. 2002;45:208–218. doi: 10.1021/jm010369e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravi RG, Kim HS, Servos J, Zimmermann H, Lee K, Maddileti S, Boyer JL, Harden TK, Jacobson KA. Adenine nucleotide analogues locked in a northern methanocarba conformation: Enhanced stability and potency as P2Y1 receptor agonists. J. Med Chem. 2002;45:2090–2100. doi: 10.1021/jm010538v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pascal R, Pirat C, Dueymes C. Facile synthesis of simple mono-alkyl phosphates from phosphoric acid and alcohols. Tetrahedron Lett. 2008;49:5300–5301. [Google Scholar]
- 22.Lazarowski ER, Shea DA, Boucher RC, Harden TK. Release of cellular UDP-glucose as a potential extracellular signaling molecule. Mol. Pharmacol. 2003;63:1190–1197. doi: 10.1124/mol.63.5.1190. [DOI] [PubMed] [Google Scholar]
- 23.Harden TK, Hawkins PT, Stephens L, Boyer JL, Downes P. Phosphoinositide hydrolysis by guanosine 5′-[gamma-thio]triphosphate-activated phospholipase C of turkey erythrocyte membranes. Biochem. J. 1988;252:583–593. doi: 10.1042/bj2520583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyer JL, Downes CP, Harden TK. Kinetics of activation of phospholipase C by P2Y purinergic receptor agonists and guanine nucleotides. J. Biol. Chem. 1989;264:884–890. [PubMed] [Google Scholar]
- 25.Jacobson KA, Ivanov AA, de Castro S, Harden TK, Ko H. Development of selective agonists and antagonists of P2Y receptors. Purinergic Signalling. 2009;5:75–89. doi: 10.1007/s11302-008-9106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivanov AA, Fricks I, Harden TK, Jacobson KA. Molecular dynamics simulation of the P2Y14 receptor. Ligand docking and identification of a putative binding site of the distal hexose moiety. Bioorg. Med. Chem. Lett. 2007;17:761–766. doi: 10.1016/j.bmcl.2006.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eliahu SE, Camden J, Lecka J, Weisman GA, Sévigny J, Gélinas S, Fischer B. Identification of hydrolytically stable and selective P2Y1 receptor agonists. Eur. J. Med. Chem. 2009;44:1525–1536. doi: 10.1016/j.ejmech.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim. Biophys. Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Gendaszewska-Darmach E, Maszewska M, Zakłos M, Koziołkiewicz M. Degradation of extracellular nucleotides and their analogs in HeLa and HUVEC cell cultures. Acta Biochim. Polonica. 2003;50:973–984. [PubMed] [Google Scholar]
- 30.Harden TK, Scheer AG, Smith MM. Differential modification of the interaction of cardiac muscarinic cholinergic and beta-adrenergic receptors with a guanine nucleotide binding component(s). Mol. Pharmacol. 1982;21:570–580. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.