Abstract
During spermatogenesis in the mammalian testis, preleptotene/leptotene spermatocytes differentiate from type B spermatogonia and traverse the blood-testis barrier (BTB) at stage VIII of the seminiferous epithelial cycle for further development. This timely movement of germ cells involves extensive junction restructuring at the BTB. Previous studies have shown that these events are regulated by testosterone (T) and cytokines [e.g., the transforming growth factor (TGF) -βs], which promote and disrupt the BTB assembly, respectively. However, the mechanisms underlying the “opening” of the BTB above a migrating preleptotene/leptotene spermatocyte and the “resealing” of the barrier underneath this cell remain obscure. We now report findings on a novel mechanism utilized by the testes to regulate these events. Using cell surface protein biotinylation coupled with immunoblotting and immunofluorescent microscopy, we assessed the kinetics of endocytosis and recycling of BTB-associated integral membrane proteins: occludin, JAM-A, and N-cadherin. It was shown that these proteins were continuously endocytosed and recycled back to the Sertoli cell surface via the clathrin-mediated but not the caveolin-mediated pathway. When T or TGF-β2 was added to Sertoli cell cultures with established functional BTB, both factors accelerated the kinetics of internalization of BTB proteins from the cell surface, perhaps above the migrating preleptotene spermatocyte, thereby opening the BTB. Likewise, T also enhanced the kinetics of recycling of internalized biotinylated proteins back to the cell surface, plausibly relocating these proteins beneath the migrating spermatocyte to reassemble the BTB. In contrast, TGF-β2 targeted internalized biotinylated proteins to late endosomes for degradation, destabilizing the BTB. In summary, the transient opening of the BTB that facilitates germ cell movement is mediated via the differential effects of T and cytokines on the kinetics of endocytosis and recycling of integral membrane proteins at the BTB. The net result of these interactions, in turn, determines the steady-state protein levels at the Sertoli-Sertoli cell interface at the BTB.
Keywords: preleptotene spermatocyte migration, seminiferous epithelial cycle, spermatogenesis, tight junction, basal ectoplasmic specialization
A major obstacle in male contraceptive development, in treating illnesses, and in altering the pathological changes in organs behind a blood-tissue barrier (e.g., blood-brain barrier) is to transport a contraceptive or a therapeutic drug across a barrier [e.g., the blood-testis barrier (BTB)]. Despite the fact that drugs can be conjugated to either transferrin receptors (1–4) or a follicle-stimulating hormone (FSH) mutant (5, 6) and transported to the brain or testis, the mechanism that regulates these barriers remains largely unknown. The testis is an interesting and unique model for studying blood-tissue barrier dynamics. For instance, the migration of preleptotene/leptotene spermatocytes across the BTB is a crucial cellular event that takes place during stage VIII of the seminiferous epithelial cycle of spermatogenesis (7). This event involves intermittent phases of junction disassembly and reassembly at the Sertoli-Sertoli cell interface to facilitate the transit of preleptotene and/or leptotene spermatocytes with cell sizes of 8–10 µm (8). At the same time, the immunological barrier function of the BTB cannot be compromised because postmeiotic germ cell antigens must be sequestered from the immune system at all times. Understanding the mechanisms that regulate these cellular events would provide new approaches for the management and/or treatment of different diseases in other blood-tissue barriers besides the delivery of contraceptive drugs. In a previous study, we demonstrated that the coexistence of tight junctions (TJs) and anchoring junctions (AJs) at the BTB via peripheral adaptors is used to reinforce barrier integrity at other stages of the epithelial cycle when the BTB is “closed” (9). At stage VIII of the epithelial cycle when the BTB has to “open,” TJs and AJs become disengaged such that TJs can transiently supersede the function of AJs during AJ restructuring and vice versa (9). However, results of this study could not account for the morphological observations made when a migrating preleptotene/leptotene spermatocyte (or a clone of preleptotene/leptotene spermatocytes) is “trapped” between a “destabilizing” and “newly formed” TJ barrier at the apical and basal region of the germ cell (10).
Studies in the past decade have focused on identifying the biomolecules that regulate the steady-state mRNA and/or protein levels of the junctional complexes at the BTB (8, 11). Among these, testosterone (T) was shown to promote adhesion at the Sertoli-Sertoli and Sertoli-germ cell interface. For instance, specific knockout of androgen receptors (ARs) in Sertoli and Leydig cells, but not in germ and peritubular myoid cells, led to infertility in mice (12, 13). After AR deletion in Sertoli cells, a significant loss in barrier function was detected, which was coupled with a reduction in the levels of TJ proteins such as occludin and claudins (14, 15). Moreover, withdrawal of T resulted in the detachment of developing spermatids (step 8 through 19 spermatids) from Sertoli cells in the seminiferous epithelium, demonstrating that T is important in cell adhesion (16). In contrast, cytokines such as the transforming growth factor (TGF) -βs have been shown to perturb the BTB in vitro (17) and in vivo (18, 19) by down-regulating the expression of integral membrane proteins at the BTB. Nonetheless, these studies failed to address the possible coordination between these biomolecules in regulating the opening and closing of the BTB.
Recent studies have demonstrated that endocytic recycling of junctional proteins regulates junction restructuring to facilitate cell migration (20–22). This mechanism allows rapid turnover of integral membrane proteins at the cell-cell and cell-matrix interface besides de novo protein synthesis (20). We hypothesized that the testis uses a similar mechanism to regulate junction restructuring at the Sertoli-Sertoli cell interface as endocytosis was recently shown to assist spermiation at the Sertoli cell-spermatid interface (23, 24). Furthermore, Sertoli cells were shown to establish a functional TJ barrier, mimicking the BTB in vivo (25–28). As such, this well-established in vitro system was used to compare the kinetics of protein endocytosis and recycling after treatment of cells with T or TGF-β2 vs. controls. Surprisingly, we observed that BTB dynamics are regulated by the coordination of both classes of biomolecules, which differentially affect the fate of endocytosed integral membrane proteins at the BTB. These results have helped us to provide a novel mechanism utilized by the testis that regulates the timely opening and closing of the BTB to facilitate preleptotene spermatocyte migration at stage VIII of the seminiferous epithelial cycle.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats were purchased from Charles River Laboratories (Kingston, NY, USA). The use of animals was approved by The Rockefeller University Animal Care and Use Committee (protocol numbers 00111, 03017, and 06018).
Antibodies and reagents
The following antibodies were used for studies described in this report, including immunoblotting and immunofluorescent microscopy: rabbit anti-occludin (catalog 71-1500, lot 51202542), rabbit anti-junctional adhesion molecule (JAM)-A (catalog 36-1700, lot 50393637), rabbit anti-claudin-1 (catalog 51-9000, lot 50799522), rabbit anti-claudin-3 (catalog 34-1700, lot 50393653), mouse anti-N-cadherin (catalog 33-3900, lot 50393487), and mouse anti-human transferrin receptor (catalog 13-6800, lot 60103175) from Invitrogen (Carlsbad, CA, USA; Zymed Laboratories, Burlingame, CA, USA); rabbit anti-claudin-3 (catalog ab15102-500, lot 167298), and mouse anti-Rab 9 (catalog ab2810-100, lot 252177) from Abcam Inc. (Cambridge, MA, USA); rabbit anti-N-cadherin (catalog sc-7939, lot J2105) and goat anti-actin (catalog sc-1616, lot F2106) from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA); and mouse anti-early endosomal antigen 1 (EEA-1) (catalog 610456, lot 76250), mouse anti-clathrin heavy chain (catalog 610499, lot 76274), and mouse anti-β1-integrin (catalog 610468, lot 35714) from BD Biosciences (San Jose, CA, USA). T, cyproterone acetate (CPA), phenylarsine oxide (PAO), and cholesterol oxidase (CO) (Streptomyces sp.) were obtained from Sigma-Aldrich Corp. (St. Louis, MO). Human TGF-β2 recombinant protein (catalog PF017, lot D27474-1) was obtained from Calbiochem (San Diego, CA, USA).
Immunoblotting
Rats (n=3 for each time point) at different ages were sacrificed, and testes were removed immediately, frozen in liquid nitrogen, and stored at −80°C until use. Testis lysates were prepared in IP buffer [0.125 M Tris, 2 mM EGTA, 0.15 M NaCl, 1% Nonidet P-40 (v/v), 10% glycerol (v/v), 2 mM PMSF, 1 mM sodium orthovanadate, 2 mM N-ethylmaleimide, 1 µg/ml leupeptin, and 1 µg/ml aprotinin, pH 7.4] as described (29). About 100 µg of protein from each sample was resolved by SDS-PAGE using either 7.5, 10, or 12% total acrylamide concentration (T; acrylamide+methylenebisacrylamide/100 ml) SDS-polyacrylamide gels under reducing conditions, depending on the apparent relative molecular mass of the target protein to be investigated. Immunoblotting was performed as described (30).
Isolation and cultures of Sertoli cells
Sertoli cells were isolated from testes of 20-day-old Sprague-Dawley rats as described earlier (29). Cells were plated on Matrigel-coated dishes [Matrigel diluted 1:7 with F12/Dulbecco’s modified Eagle’s medium (DMEM)] and cultured in serum-free F12/DMEM supplemented with epidermal growth factor, insulin, transferrin, and bacitracin at 35°C in a humidified atmosphere of 95% air/5% CO2. On day 2 (48 h after isolation), Sertoli cells were subjected to a hypotonic treatment (20 mM Tris, pH 7.4, at 22°C) for 2.5 min to remove residual germ cells (31). To assess the effects of T and CPA on the steady-state levels of integral membrane proteins at the BTB, Sertoli cells plated at 0.5 × 106 cells/cm2 on Matrigel-coated 24-well dishes at time 0 were incubated with T (2×10−7 M) or T + CPA (1×10−6 M) for 8 h, 1 day, and 5 days before termination for lysate preparation. Cultures in triplicates were used for each time point, and each experiment was repeated at least three times using different batches of Sertoli cells. It should be noted that these Sertoli cells formed a functional TJ permeability barrier by days 2–4 in vitro when assessed by transepithelial electrical resistance measurement across the cell epithelium as described (17). When examined by electron microscopy, these Sertoli cells also formed a functional BTB with the ultrastructures of TJ and basal ectoplasmic specialization (ES) (25).
Endocytosis assay
Endocytosis assays were performed as described earlier with minor modifications (32). In brief, Sertoli cells at a density of 0.5 × 106 cell/cm2 were cultured on Matrigel-coated six-well plates for 4 days, and functional TJs and AJs that mimicked the BTB in vivo were formed when assessed by functional TJ barrier assay and electron microscopy (25, 28). On day 5, Sertoli cell surface proteins were biotinylated with 0.5 mg/ml sulfo-NHS-SS-biotin (Pierce Chemical, Rockford, IL, USA) in PBS containing 0.9 mM CaCl2 and 0.33 mM MgCl2 (PBS/CM buffer) at 4°C for 30 min. The free biotin was quenched with 50 mM NH4Cl in PBS/CM buffer at 4°C for 15 min. At 4°C, endocytosis did not occur until these cultures were transferred to a CO2 incubator with 95% air/5% CO2 (v/v) either at 18 or 35°C where protein internalization could take place. To initiate endocytosis, Sertoli cell cultures were thus transferred from 4°C to a 35 or an 18°C CO2 incubator and incubated for the specific time points in the absence (controls) or presence of 2 × 10−7 M T, 1 × 10−6 M CPA, or 10 ng/ml TGF-β2 (treatment groups). To study the endocytic pathways mediating the internalization of TJ and AJ proteins at the BTB, cells were incubated with either an inhibitor of the clathrin-mediated pathway, 20 µM PAO, or an inhibitor of the caveolin-mediated pathway, 2 U/ml of CO, as described (22). At the time of termination for all endocytosis experiments, biotins on the uninternalized cell surface proteins were stripped with 50 mM sodium 2-mercaptoethanesulfonate (MESNA) (Sigma-Aldrich Corp.) in 100 mM Tris/HCl, 100 mM NaCl, and 2.5 mM CaCl2, pH 8.6, at 4°C for 30 min and quenched with 5 mg/ml iodoacetamide (Sigma-Aldrich Corp.) in PBS/CM buffer at 4°C for 15 min. Cell lysates were prepared in IP buffer (see above). Biotinylated proteins were pulled down with UltraLink Immobilized NeutrAvidin Plus beads (Pierce Chemical) from ~400 µg of total protein of Sertoli cell lysates from each sample and subjected to immunoblot analysis using corresponding antibodies against specific target integral membrane proteins. All samples within an experimental group were processed simultaneously to avoid interexperimental variation. All endocytosis experiments reported herein were repeated at least four times using different batches of Sertoli cells excluding two earlier pilot experiments, which determined the optimal termination time points to assess the kinetics of endocytosis. Each time point had triplicate cultures for each experiment.
Recycling assay
Recycling assays were performed as described earlier with minor modifications (32, 33). In brief, Sertoli cells cultured for 5 days were subjected to cell surface biotinylation as described above and then incubated at 35°C for 2 h to allow endocytosis to complete. The remaining biotinylated cell surface proteins were stripped with 50 mM MESNA in 100 mM Tris/HCl, 100 mM NaCl, and 2.5 mM CaCl2, pH 8.6, at 4°C, for 30 min and quenched with 5 mg/ml iodoacetamide in PBS/CM buffer at 4°C for 15 min. Cells were incubated at 35°C for various time points to allow recycling of internalized proteins back to the cell surface. The newly formed proteins that recycled back to the cell surface were again stripped with 50 mM MESNA in 100 mM Tris/HCl, 100 mM NaCl, and 2.5 mM CaCl2, pH 8.6, at 4°C for 30 min and quenched with 5 mg/ml iodoacetamide in PBS/CM buffers at 4°C for 15 min. Cell lysates were then prepared in IP buffer as described above to assess the disappearance of cytosolic biotinylated proteins as an index of protein recycling. To further validate the recycling of biotinylated and endocytosed protein, we also determined the reappearance of internalized protein back to the cell surface as follows. In some experiments, after Sertoli cells were incubated at 35°C to allow recycling of internalized proteins, cells were washed in PBS and incubated with 0.01% trypsin in PBS at room temperature for 20 min to extract the recycled cell surface biotinylated proteins. Further unwanted trypsinization was stopped by adding 1% soybean trypsin inhibitor (w/v) to each sample tube. Recycled biotinylated cell surface proteins were recovered from the supernatant by UltraLink Immobilized NeutrAvidin Plus beads, and cell lysates were subjected to immunoblot analysis. By using these approaches to quantify the kinetics of 1) the disappearance of cytosolic endocytosed biotinylated proteins and 2) the reappearance of biotinylated and endocytosed proteins on the Sertoli cell surface, an accurate assessment of protein recycling was obtained. All recycling assay experiments reported herein were repeated at least three times using different batches of Sertoli cells excluding initial pilot experiments which assessed the optimal experimental conditions, such as different termination time points.
Immunofluorescent microscopy
To demonstrate the effects of T and TGF-β2 on the localization or the redistribution of TJ and AJ proteins at the Sertoli-Sertoli cell interface, double-label immunofluorescence analysis of integral membrane proteins (e.g., occludin and N-cadherin) and markers of endocytosis-related proteins (e.g., EEA-1, clathrin, and Rab 9) was performed by immunofluorescent microscopy as described (9, 29). In brief, Sertoli cells with a cell density of 0.1– 0.15 × 106 cell/cm2 were cultured on the Lab-Tek Chamber Slide System (~1.8 cm2/well) (Nalge Nunc International, Roskilde, Denmark) coated with Matrigel (Collaborative Biochemical Products, Bedford, MA) (diluted 1:7 in F12/DMEM, v/v) for 4 days to allow junction assembly. Sertoli cells were then incubated with either T (2×10−7 M) or TGF-β2 (10 ng/ml) for 1–3 h at 35°C to allow endocytosis and recycling of cell surface proteins. Cells were fixed with 4% paraformaldehyde in PBS for 10 min, permeabilized with 0.1% Triton X-100, and then blocked with 10% goat serum (v/v) for 30 min. Rabbit anti-occludin (1:100) or anti-N-cadherin (1:100) antibody was incubated with either mouse anti-EEA1 (1:100), anti-Rab 9 (1:100), or anti-clathrin (1:150) antibody at 35°C. After overnight incubation, secondary antibodies conjugated with Cy3 and fluorescein isothiocyanate (FITC) (Invitrogen), diluted in PBS to 1:50, were incubated with slides for ~30 min. Cells were then washed and mounted with Vectashield Hard-Set with 4′,6′-diamino-2-phenylindole (DAPI) (a nuclear stain; Vector Laboratories, Burlingame, CA, USA). Fluorescent micrographs were obtained by using an Olympus BX40 microscope (Olympus Corp., Melville, NY, USA) equipped with Olympus UPlanF1 fluorescent optics and an Olympus DP70 12.5MPa digital camera. Fluorescent images were acquired as tagged image format (TIF) files using QImaging QCapture Software Suite (Quantitative Imaging Corp, Surrey, BC, Canada) and analyzed by using Adobe PhotoShop (version 7.0; Adobe Systems, Mountain View, CA, USA). Each fluorescent microscopy experiment was repeated at least four times using different batches of Sertoli cell cultures that yielded similar results, and representative results were reported herein.
Statistical analysis
Statistical analyses were performed by two-way analysis of variance (ANOVA) using the repeated measures model followed by Dunnett’s test to compare changes between treatment groups and their corresponding controls using the GB-STAT statistical analysis software package (version 7.0; Dynamic Microsystems, Silver Spring, MD, USA). Thus, with this method we took into consideration changes in protein levels (e.g., during protein internalization and/or recycling) in 1) treatment groups (e.g., cells treated with TGF-β2 or testosterone) vs. the corresponding controls (first variable) and 2) as a function of time (second variable).
Other methods
Protein estimation was performed by Bradford reagent using BSA as a standard as described earlier (34). For cell lysates from endocytosis and recycling experiments in which the protein concentration was relatively low vs. testis lysates, protein estimation was performed using a DC Protein Assay kit from Bio-Rad Laboratories (Hercules, CA, USA) and a Bio-Rad model 680 plate reader at 750 nm as described (35). The lanthanum experiment used to assess the relative location of TJ fibrils in the seminiferous epithelium during the transit of preleptotene/leptotene spermatocytes across the BTB was performed as described (36). Electron microscopy was performed at The Rockefeller University Bio-Imaging Resource Center. All immunoblots were densitometrically scanned using a Canon 9950F scanner and the resultant blot images were analyzed by Scion Image software (release 4.0.3.2; Scion Corp., Frederick, MA, USA) and/or SigmaGel software (version 1.05; SPSS Inc., Chicago, IL, USA).
RESULTS
Testosterone and integral membrane proteins at the BTB
In vivo study: changes in integral membrane proteins at the BTB during testicular maturation and/or aging
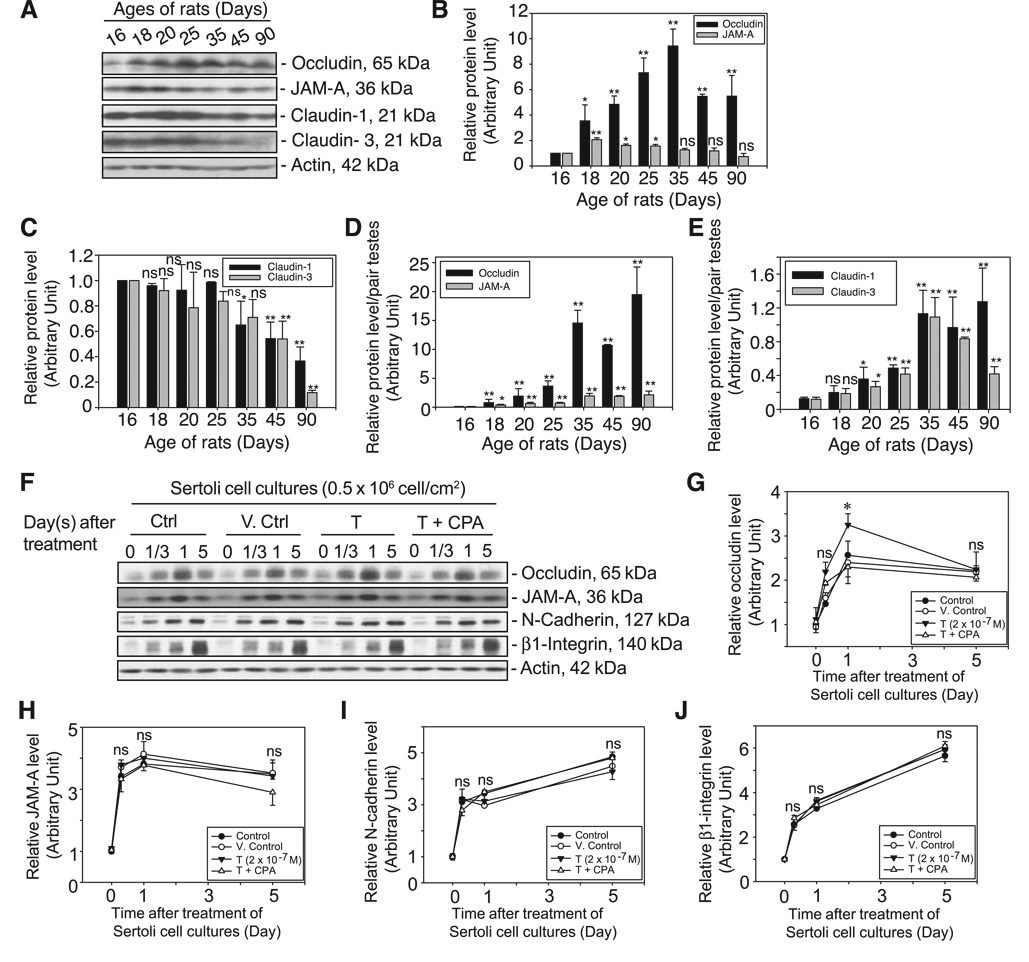
The importance of T on spermatogenesis has been illustrated in studies of cell-specific knockout of androgen receptors (ARKO mice) in Leydig and Sertoli cells, wherein these mice displayed infertility with seminiferous tubules devoid of germ cells (13, 37, 38). In this study, we examined the role of T on several integral membrane proteins at the BTB in rat testes including TJ proteins (occludin, JAM-A, and claudins 1 and 3) and basal ES protein (N-cadherin). It is of interest to note that the steady-state protein level of claudin-3 in normal adult rat testes, at least from days 45 to 90 postpartum (n=5, using antibodies from two different vendors) [note that the level of ARs is highest in adult Sertoli cells at day 90 postpartum; ref. 39), was significantly lower, by as much as 5-fold, compared with that in rats at days 16–25 postpartum (Fig. 1A–C). In contrast, JAM-A, occludin, and claudin-1 remained relatively stable during adulthood except that a transient increase in the protein levels of occludin and JAM-A was detected (but not in the levels of claudins 1 and 3) in immature rats (Fig. 1A–C). This is the time when the BTB was being formed (between days 15 and 18 postpartum), illustrating their significance in BTB function. When the changes in testis weights during development were taken into consideration and the data were expressed as protein level per pair of testes (Fig. 1D, E), it is noted that the relative organ contents of occludin and JAM-A during development peaked at day 35 postpartum and remained relatively stable thereafter until 90 days of age. This trend is also similar to the organ contents of claudin-1 and -3 except for a considerable decline in the level of claudin-3 per pair of testes by day 90 (Fig. 1E vs. D). For N-cadherin, its steady-state protein level was detected in the rat testis by day 7 after birth, peaked by day 42 with a ~4- to 5-fold increase, but declined by ~50% by day 92 (40). Thus, occludin and JAM-A, as well as N-cadherin (an integral membrane protein at the basal ES, which together with TJ constitutes the BTB) were selected for the studies reported herein. These results also illustrate that claudins and in particular claudin-3 may not be as important as other TJ integral membrane proteins in adult rat testes for BTB maintenance.
Figure 1.
In vivo and in vitro studies illustrating the significance of T on BTB dynamics in rats. Lysates from normal rat testes at different ages (days 16–90, postpartum, n=3) were prepared as described in Materials and Methods. About 100 µg of protein from each sample within an experimental group was resolved by SDS-PAGE and probed with several TJ integral membrane protein markers by immunoblotting (A). Results of this study are summarized in B–E. On the day after Sertoli cells were isolated from 20-day-old rat testes (time 0), cells were incubated immediately with 2 × 10−7 M T or T with 1 × 10−6 M CPA vs. Sertoli cells alone (Ctrl) and with a corresponding volume of ethanol, which served as the vehicle control (V. Ctrl), and the reaction was terminated after 8 h, 1 day, and 5 days (F). About 100 µg of protein from each sample was resolved by SDS-PAGE and probed with several TJ and AJ integral membrane proteins. All blots in A and F were stripped and reprobed with an anti-actin antibody to assess equal protein loading. The level of each target protein shown in A and F was densitometrically scanned and compared in B–E and G–J, respectively. Each bar is the mean ± sd of three sets of samples. The protein levels at day 16 in A and time 0 in F were arbitrarily set at 1. Statistical analysis was performed with two-way ANOVA using the repeated measures model followed by Dunnett’s test, comparing the steady-state protein levels of target proteins at other ages vs. that at day 16 as well as between samples (B, C) (n=3 rats for each time point). Data from B and C were also expressed as protein levels per pair of testes by taking into consideration changes in testis weights during maturation and shown in the corresponding bar graphs in D and E. Changes in target protein levels after treatment of Sertoli cells with T or T + CPA were compared with the corresponding control and vehicles (V. Ctrl) at each time point (G–J) by two-way ANOVA followed by Dunnett’s test. Each bar is the mean ± sd of three experiments using different batches of Sertoli cells. *P < 0.05; ns, not significantly different.
In vitro study: effects of testosterone on the steady-state levels of BTB integral membrane proteins
There are reports in the literature of in vitro models that were used to examine the role of T in junction dynamics. For instance, T was found to promote TJ and AJ assembly between Sertoli cells by inducing the steady-state mRNA and/or protein levels of occludin (41), claudin-1 and claudin-11 (42, 43), E-cadherin, and catenin (30). However, in a more recent study, it was reported that the Sertoli-Sertoli TJ and ES were stimulated by FSH only but not by T (44). To address these seemingly contrasting results, 2 × 10−7 M T was continuously supplied to Sertoli cells after their isolation for up to day 5, and it was found that T mildly and transiently induced the expression level of occludin from 8 h to day 1 after treatment, and this stimulatory effect was abolished in the presence of an antiandrogen, CPA (1×10−6M) (see T+CPA treatment group vs. T and the two controls) (Fig. 1F, G; Supplemental Fig. 1). Nonetheless, results shown in Fig. 1F–J illustrate that whereas functional experiments and AR knockout mice from earlier studies have illustrated that T promotes BTB function, it had no significant effect on the steady-state protein levels of other TJ and AJ markers, such as JAM-A or N-cadherin, at the BTB (Fig. 1F, H, I). In addition, the protein level of β1-integrin was significantly induced during TJ and AJ assembly from day 1/3 to 5 and displayed a pattern similar to those of another basal ES protein, N-cadherin (Fig. 1I vs. J) and TJ proteins, such as occludin and JAM-A (Fig. 1J vs. G, H). A CPA alone control group was not included in Fig. 1 as preliminary experiments have shown that its presence alone did not affect the steady-state levels of the BTB integral membrane proteins in these cultures (Supplemental Fig. 1).
Testosterone enhances (or accelerates) the kinetics of internalization of TJ and AJ proteins at the BTB in Sertoli cells cultured in vitro
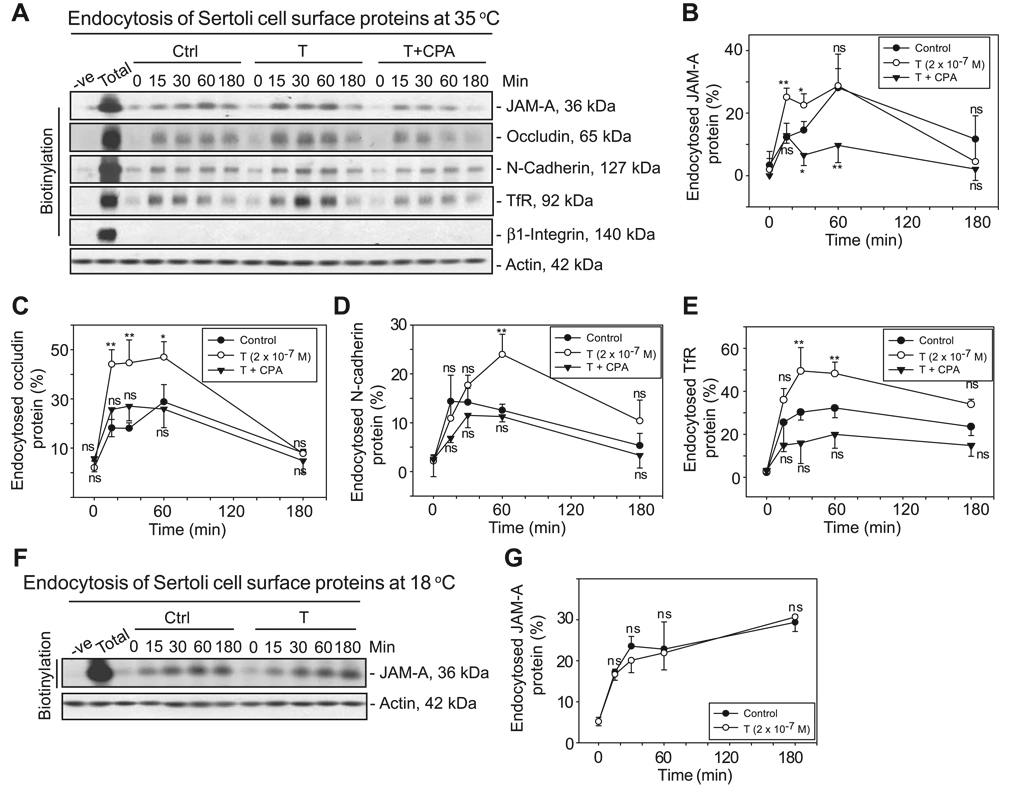
To delineate the significance of T on BTB dynamics, we investigated whether T could affect the kinetics of endocytosis of junction proteins at the BTB. By using the cell surface protein biotinylation technique, TJ and basal ES integral membrane proteins at the BTB were shown to be endocytosed time dependently under normal culture conditions when Sertoli cells were incubated at 35°C (Fig. 2A). The maximal levels of internalization of these proteins were generally detected within 60 min. Further incubation of Sertoli cells at 35°C for up to 3 h did not yield additional protein internalization; instead, a slight but consistent decline in internalized proteins was observed as recycling or degradation of endocytosed proteins might have occurred. T was anticipated to promote BTB function by “decelerating” the kinetics of protein endocytosis. Unexpectedly, the presence of 2 × 10−7 M T significantly enhanced the kinetics of internalization of TJ integral membrane proteins (JAM-A and occludin) and basal ES integral membrane protein (N-cadherin) at the BTB in Sertoli cells (Fig. 2A–E; Supplemental Fig. 2A). The unexpected effect of T that promoted protein endocytosis as depicted in Fig. 2 appeared to be specific as the presence of CPA, a progestin having antiandrogenic activity, could abolish this effect (Fig. 2). We did not include experiments wherein Sertoli cells were cultured with CPA alone as preliminary studies using CPA alone showed it to have no apparent effect on the steady-state levels of Sertoli cell BTB integral membrane proteins (see Supplemental Fig. 1). In addition, it has no apparent effect on the kinetics of internalization of occludin and transferrin receptor (TfR) as illustrated in preliminary experiments (Supplemental Fig. 1B vs. A). Interestingly, it is noted that whereas the presence of CPA could abolish the effects of T in enhancing the kinetics of internalization of occludin, N-cadherin, and TfR (Fig. 2A, C–E), its presence some-how reduced the kinetics of internalization of JAM-A vs. controls (Fig. 2A, B). The immediate explanation for this observation is not known and should be examined in future studies. It is likely that the dynamics of JAM-A endocytosis require a very low level of endogenous testosterone for its maintenance. Because primary Sertoli cells cultured in vitro were shown to exhibit 17β-hydroxysteroid dehydrogenase, 17α-hydroxylase, C17–C20 lyase, and others, and these are known to produce T (45, 46), the presence of CPA would block the endogenous T production. Surprisingly, β1-integrin was not being internalized at the Sertoli-Sertoli cell interface as shown in three separate independent experiments, at least using the cell surface protein biotinylation approach in vitro. Previous studies have shown that when cells were incubated at 18°C, this lower temperature facilitated the internalized proteins to reside in early or sorting endosomes by blocking the recycling and/or endocytic pathways (33, 47). When Sertoli cells were incubated at 18°C after biotinylation to initiate endocytosis, the levels of internalized JAM-A remained relatively steady even after 3 h of endocytosis (Fig. 2F–G), consistent with results of these earlier reports. However, we observed that when Sertoli cells were incubated at 18°C, these cells lose responsiveness to T as illustrated in studies shown in Fig. 2 and observed in three separate experiments (Fig. 2F, G vs. A–E). Therefore, all subsequent endocytosis assays were conducted at 35°C.
Figure 2.
Endocytosis assay illustrating that T at 2 × 10−7 M enhances the kinetics of the internalization of TJ and AJ integral membrane proteins at the BTB using an in vitro model. A) Sertoli cells were biotinylated for 30 min (see Materials and Methods). Thereafter, cells were incubated with either 2 × 10−7 M T alone or T with 1 × 10−6 M CPA at 35°C to allow endocytosis, and the reactions were terminated at various time points. Cell surface proteins without biotinylation served as the negative control. Total = total labeled cell surface proteins (after the 30-min biotinylation) without stripping. Biotinylated proteins were recovered by using UltraLink Immobilized NeutrAvidin Plus beads from ~400 µg of the resultant samples at each time point and were subjected to immunoblotting. Equal amounts of proteins were used at each time point as assessed by the actin blot. B–E) Kinetics of internalization of JAM-A (B), occludin (C), N-cadherin (D), and TfR (E) with T or with T + CPA. F) Sertoli cells with biotinylated cell surface proteins were incubated with or without 2 × 10−7 M T at 18°C to allow endocytosis, and the reactions were terminated at various time periods. G) Kinetics of internalization of the experiment shown in F. To assess the kinetics of internalization of integral membrane proteins, the percentage of internalized proteins vs. total biotinylated proteins was shown (y axis) and plotted against their changes against time (x axis). Each data point is the mean ± sd of three independent experiments. In each experiment, each time point had triplicate cultures. Statistical analysis was performed by two-way repeated measures ANOVA to be followed by Dunnett’s test, which compared the percentage of internalized proteins in each treatment group vs. that in its corresponding control (Ctrl) group for each time point. *P < 0.01; **P < 0.005; ns, not significantly different.
TGF-β2 perturbs the BTB by accelerating the kinetics of the internalization of integral membrane proteins
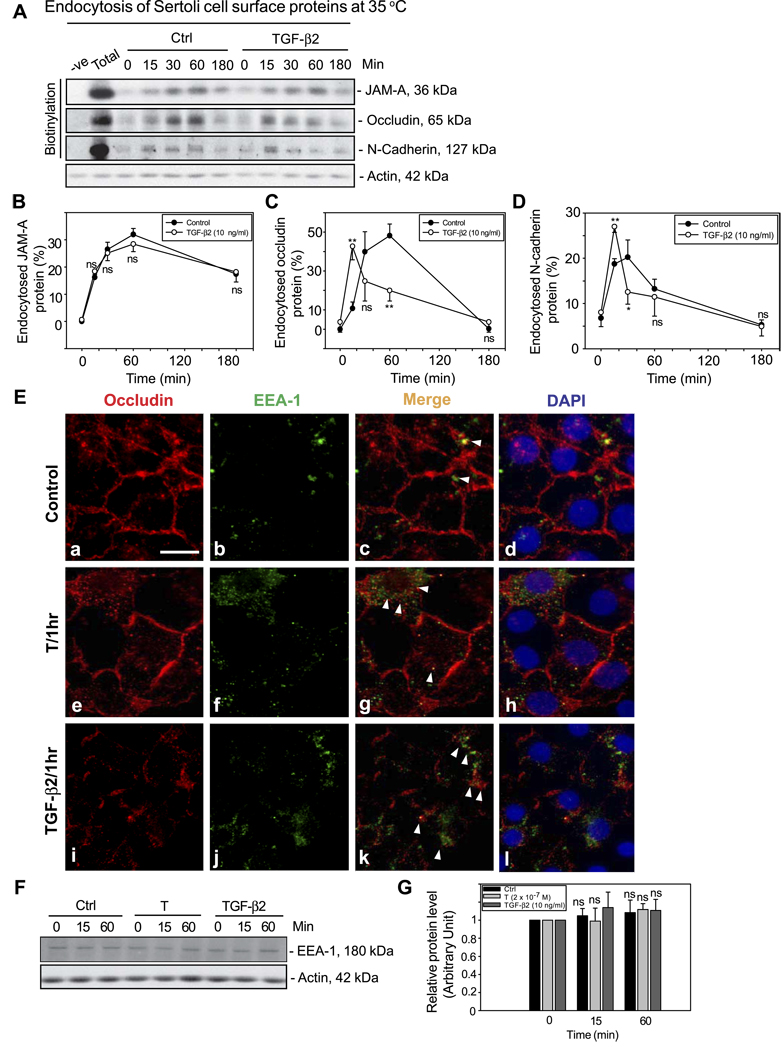
Previous studies have shown that cytokines, such as TGF-βs and tumor necrosis factor (TNF) -α, can reversibly disrupt the BTB in vivo (18, 48), by down-regulating the protein level of TJ and basal ES proteins at the BTB via a yet-to-be-defined mechanism. Herein, we demonstrate for the first time that TGF-β2 at 10 ng/ml perturbed the BTB by accelerating the kinetics of internalization of integral membrane proteins occludin and N-cadherin, but not JAM-A, at the BTB (Fig. 3A–D). It was observed that the protein levels of internalized occludin and N-cadherin after TGF-β2 treatment were ~10–30% higher than the corresponding control at 15 min (Fig. 3C, D). These results are consistent with earlier studies showing that TGF-βs and TNF-α could reduce the Sertoli cell steady-state protein levels of occludin and N-cadherin but not that of JAM-A (18, 48). Besides, it is of interest to note that the levels of internalized proteins, such as occludin, declined rapidly and statistically significantly by 30 min after exposure of these cells to TGF-β2 vs. controls and cells treated with T (Fig. 3A, C, D vs. Fig. 2A–D). These data suggest that TGF-β2 might perturb the BTB via accelerating the kinetics of internalization of integral membrane proteins, followed by the degradation of endocytosed proteins (see below). Immunofluorescent microcopy was also used to examine the effect of T and TGF-β2 on protein endocytosis (Fig. 3E; Supplemental Fig. 3). As shown in Fig. 3E, in controls, only small numbers of internalized occludin vesicles were detected as most occludin remained at the cell-cell interface (Fig. 3Ea, red fluorescence) and even fewer occludin vesicles were partially colocalized with EEA-1, an early endosome marker (Fig. 3Eb, green fluorescence) as shown in the merge image (Fig. 3Ec, yellowish orange). An increase in the number of internalized occludin vesicles was detected when cells were treated with T for 1 h and even more after treatment with TGF-β2 for 1 h (Fig. 3Ee, i), and more vesicles were also found to colocalize with EEA-1 (see white arrowheads in Fig. 3Eg, k). However, the steady-state protein levels of EEA-1 in Sertoli cells treated with either T or TGF-β2 remained relatively stable vs. those of controls (Fig. 3F, G) in the endocytosis experiments as reported in Fig. 3A.
Figure 3.
Endocytosis assay illustrating cytokines (e.g., TGF-β2) perturb the BTB by accelerating the kinetics of the internalization of BTB-integral membrane proteins using an in vitro model. A) After cell surface protein biotinylation, Sertoli cells were incubated with or without 10 ng/ml recombinant TGF-β2 at 35°C to allow endocytosis, and the reactions were terminated at various time points. About 400 µg of protein from different samples at each time point within a given experiment was used to estimate the kinetics of protein endocytosis by immunoblotting after their extraction by NeutrAvidin Plus beads. B–D) Kinetics of internalization of JAM-A, occludin, and N-cadherin with or without treatment with TGF-β2. The percentage of internalized proteins vs. total biotinylated proteins is shown on the y axis and plotted against time (x axis). Each data point is the mean ± sd of three independent experiments using different batches of Sertoli cell cultures. In each experiment, each time point had triplicate cultures. Statistical analysis was performed by two-way ANOVA followed by Dunnett’s test to compare treatment group (i.e., TGF-β2-treated Sertoli cells) against controls and over time. *P < 0.01; **P < 0.001; ns, not significantly different. E) Internalized occludin vesicles (red fluorescence, Cy3) in e and i were found to colocalize with an early endosome marker (EEA-1, green fluorescence, FITC; see f, j) as shown in merged images (g, k) when cells were treated with T (2×10−7 M) or TGF-β2 for 1 h (white arrowheads). DAPI (d, h, l) stained the cell nuclei. Scale bar = 10 µm. F) A study by immunoblot analysis using lysates from the endocytosis experiments shown in A before avidin beads extraction to assess the relative protein levels of EEA-1 in Sertoli cells in the absence (Ctrl, control) or presence of T (2×10−7 M) and TGF-β2 (10 ng/ml). Actin served as a protein loading control. G) Results shown in F were densitometrically scanned and analyzed. Each bar is the mean ± sd of results from three different experiments wherein the EEA-1 level at time 0 was arbitrarily set at 1. Statistical analysis was performed by two-way ANOVA followed by Dunnett’s test, comparing the steady-state protein levels of EEA-1 in each treatment group (T or TGF-β2) vs. its corresponding control at time 0. ns, not significantly different.
Internalization of integral membrane proteins at the BTB is mediated via the clathrin-dependent pathway
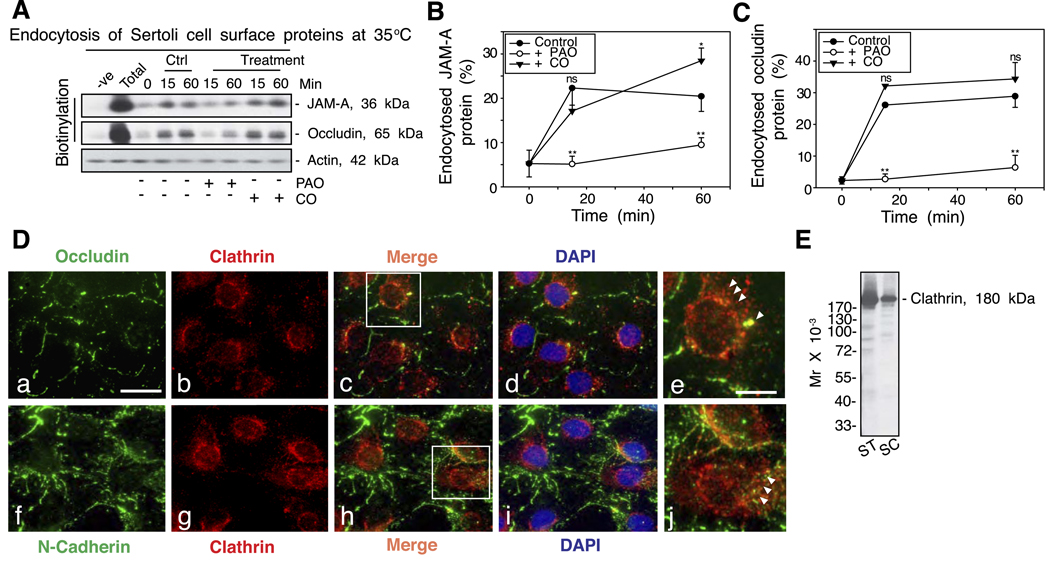
To further investigate the endocytic pathway used by the BTB proteins for internalization, specific inhibitors were used (22) as shown in Fig. 4. Incubation of Sertoli cells with 20 µM PAO for 15 min to prevent the assembly of clathrin-coated pits was shown to effectively block the endocytosis of JAM-A and occludin (Fig. 4A–C). However, the kinetics of protein endocytosis was not affected when cells were treated with 2 U/ml of CO (CO was known to deplete cholesterol in plasma membrane, thereby blocking the caveolin pathway) (Fig. 4). When Sertoli cells were immunostained with an anti-occludin or an anti-N-cadherin antibody, internalized protein vesicles were found to colocalize with clathrin (Fig. 4D). The specificity of the anti-clathrin antibody used in this study is shown in Fig. 4E as only a prominent protein band corresponding to the electrophoretic mobility of clathrin was identified in lysates of either seminiferous tubules or Sertoli cells.
Figure 4.
A study by endocytosis assay and immunofluorescent microscopy to demonstrate that endocytosis of the integral membrane proteins at the BTB is via the clathrin-mediated but not the caveolin-mediated pathway. A) After cell surface protein biotinylation, Sertoli cells were incubated with either PAO (a specific inhibitor of clathrin-mediated pathway) or CO (a specific inhibitor of caveolin-mediated pathway) at 35°C to allow endocytosis, and reactions were terminated at specified time points. About 400 µg of total proteins from samples at each time point within an experimental set was used to estimate the kinetics of protein endocytosis. For each treatment group (PAO or CO), the level of either JAM-A or occludin at time 0 was similar to the time 0 in the control (Ctrl) group (data not shown). B, C) Kinetics of internalization of JAM-A (B) and occludin (C) with treatment by either PAO or CO. The percentage of internalized proteins vs. total biotinylated proteins is shown on the y axis and plotted against time. Statistical analysis by two-way ANOVA followed by Dunnett’s test was performed by comparing the percentage of internalized proteins in treatment groups vs. the corresponding controls. Each data point is the mean ± sd of results from three separate experiments. *P < 0.05; **P < 0.005; ns, not significantly different. D). Internalized occludin and N-cadherin vesicles (green fluorescence, FITC) were found to colocalize with clathrin (red fluorescence, Cy3), as shown in merged images (white arrowheads, e and j; magnified views of the boxed areas in c and h, respectively) in these Sertoli cell cultures by day 4 after their isolation and terminated for staining with corresponding antibodies. DAPI stained for nuclei. Scale bars = 10 µm (a–d, f–i); 3 µm (e, j). E) Immunoblot analysis of lysates (~50 µg of total protein/lane) of seminiferous tubules (ST) and Sertoli cells (SC) incubated with a mouse anti-clathrin antibody to demonstrate the antibody specificity, which was used for the experiment shown in D.
Differential fate of the endocytosed BTB proteins after treatment of cells with T or TGF-β2
Results described above have shown that treatment of Sertoli cells with either T or TGF-β2 accelerated or enhanced the kinetics of integral membrane protein endocytosis, yet these biomolecules were shown to promote (14, 15) or disrupt the BTB (18, 19, 28) in earlier studies, respectively. To reconcile these seeming discrepancies, we next examined the fate of the endocytosed proteins after each treatment vs. controls by recycling assays. It was shown that internalized BTB proteins, such as occludin, JAM-A, and N-cadherin (data not shown), were continuously recycled back to the cell surface. We thus monitored the protein recycling using two different approaches by quantifying the kinetics of declining of biotinylated and endocytosed protein levels at the cytosol (Fig. 5A–C; Supplemental Fig. 4A, B) and the reappearance of internalized proteins at the cell surface (Fig. 5A, D, E; Supplemental Fig. 4C). Supplemental Fig. 4D is a schematic drawing that illustrates the technical basis of the recycling assay. The kinetics of recycling of internalized proteins was found to increase with the presence of T vs. control. For instance, there was an ~50% reduction in the protein level of cytosolic JAM-A concomitant with an increase in cell surface JAM-A protein after incubation of cells with T for 15 min vs. controls (Fig. 5A, B, D). Interestingly, when Sertoli cells were treated with TGF-β2 to study the recycling of biotinylated and endocytosed proteins, the kinetics of the disappearance of cytosolic biotinylated and endocytosed occludin, but not of JAM-A, was found to be enhanced vs. controls (Fig. 5A–C). However, it is important to note that the increase in the level of reappearance of biotinylated and endocytosed occludin on the cell surface was not detected in cells treated with TGF-β2 (Fig. 5F; Supplemental Fig. 4C). These results suggest that TGF-β2, unlike T, failed to enhance the kinetics of the recycling of BTB proteins back to the cell surface. Housekeeping target proteins (e.g., actin, tubulin, and glyceraldehyde-3-phosphate dehydrogenase) could not be retained in the samples subjected to the recycling assay when the reappearance of biotinylated and endocytosed integral membrane proteins (e.g., occludin) was monitored. This is because these samples required initial trypsinization to be followed by subsequent affinity extraction using avidin beads; as such, equal protein loading was assessed by Coomassie Blue staining in gels as shown in a representative experiment in Supplemental Fig. 5.
Figure 5.
Recycling assay illustrating the differential fate of the internalized BTB integral membrane proteins after treatment of Sertoli cells with either T or TGF-β2. A) Recycling assay was performed on day 5 after Sertoli cell isolation. After biotinylation of cell surface proteins, Sertoli cells were incubated at 35°C for 2 h to allow endocytosis. Biotin on the uninternalized cell surface proteins was stripped. Cells were then incubated with either T (2×10−7 M) or TGF-β2 (10 ng/ml) vs. controls (no treatment) at 35°C for various time points to allow recycling of internalized proteins back to the cell surface. The newly appeared biotinylated (and recycled) proteins on the cell surface were obtained by 0.01% trypsin, whereas proteins remaining in the cytosol were collected by IP buffer. B, C) Percentage of internalized and biotinylated proteins remaining in the cytosol over time in the recycling assay in the presence of either T or TGF-β2 vs. controls are shown. The percentage of internalized and biotinylated proteins at time 0 was arbitrarily set at 100. Each bar is the mean ± sd of three independent experiments using different batches of primary Sertoli cell cultures. In each experiment, each time point had triplicate cultures. D, E) Reappearance of internalized and biotinylated occludin and JAM-A on the Sertoli cell surface with or without T was monitored by immunoblotting in three independent experiments with triplicate cultures for each time point in each experiment. The immunoblots (insets; D, E) are results of a representative experiment, illustrating an increase in the level of proteins on cell surface vs. time 0; the presence of T accelerated the kinetics of recycling of JAM-A and occludin. Two additional experiments yielded similar results. The level of protein at time 0 was arbitrarily set at 1. F) Effects of TGF-β2 on the kinetics of recycling of BTB proteins. Reappearance of internalized and biotinylated occludin and JAM-A proteins on Sertoli cell surface was monitored by immunoblotting, illustrating an increase in protein levels on cell surface vs. time 0, but the presence of TGF-β2 significantly reduced the kinetics of recycling of occludin, but not of JAM-A, back to the Sertoli cell surface. The level of protein at time 0 was arbitrarily set at 1, against which statistical analysis was performed. Each bar is the mean ± sd of three independent experiments with triplicate cultures for each time point in each experiment. Statistical analysis was performed by two-way ANOVA followed by Dunnett’s test, comparing between a treatment group vs. its corresponding control (Ctrl) and over time. *P < 0.05; **P < 0.01; ns, not significantly different.
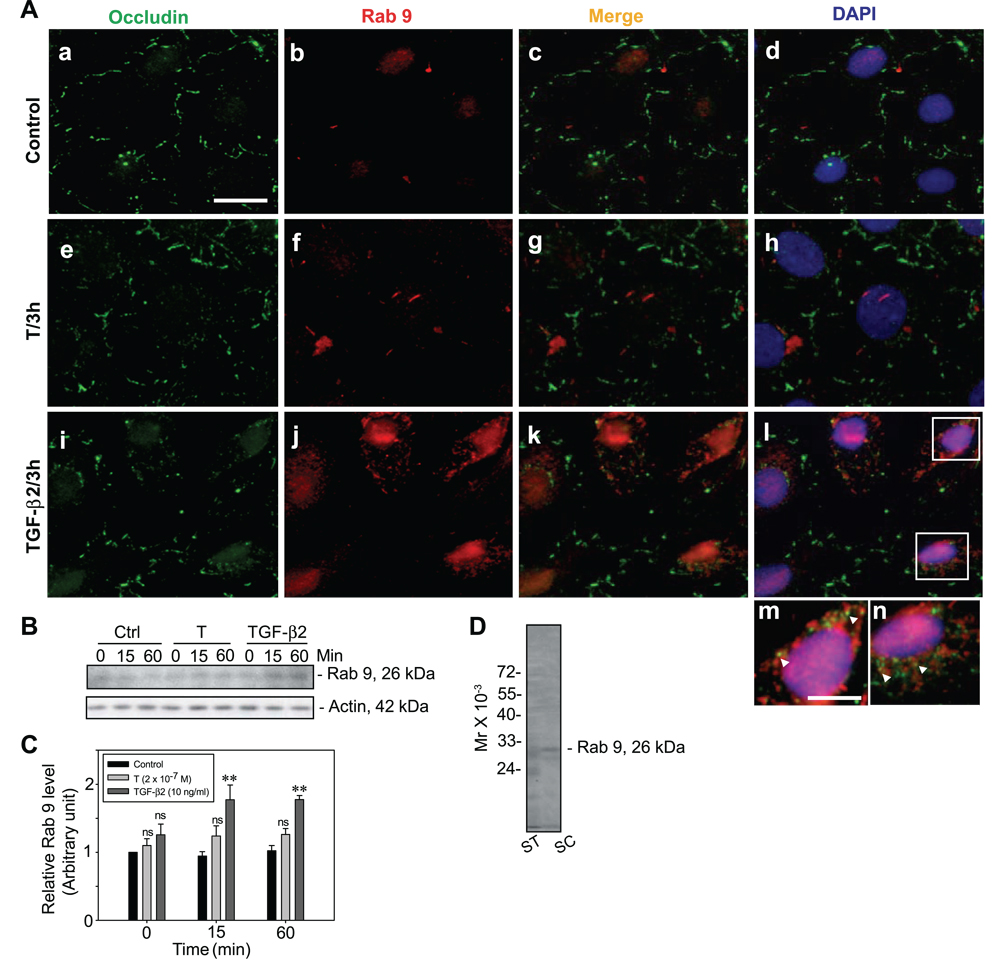
We next examined whether TGF-β2 targeted the internalized proteins to late endosomes for intracellular degradation (Fig. 6), thereby reducing the steadystate integral membrane protein levels at the Sertoli-Sertoli cell interface (Fig. 3Ei–l vs. a–d, e–h). Importantly, endocytosed occludin-containing vesicles were shown to colocalize with Rab 9, a late endosome marker (22, 49, 50), after treatment of Sertoli cells with TGF-β2 but not T (Fig. 6Ai–l vs. e– h, a–d). An increase in steady-state protein levels of Rab 9 was also noted when cells were incubated with TGF-β2, but not with T, when compared with control (Fig. 6B–C). Figure 6D illustrates the specificity of the anti-Rab 9 antibody used for studies reported herein by immunoblot analysis. In summary, these results illustrate that TGF-β2 accelerates protein internalization, but unlike T, it targets endocytosed proteins to endosome-mediated degradation instead of recycling the proteins back to the cell surface.
Figure 6.
A study by immunofluorescent microscopy and immunoblotting to demonstrate the targeting of BTB integral membrane proteins into late endosomes for degradation by TGF-β2. A) Sertoli cells were incubated with either T or TGF-β2 vs. controls (no treatments) at 35°C for 3 h to allow endocytosis and recycling of BTB proteins. Cells were then fixed and stained with anti-occludin and anti-Rab 9 (a late endosome marker) antibody to study the fate of the internalized proteins. After treatment of Sertoli cells with TGF-β2, the steady-state protein level of occludin (green fluorescence, FITC) at the cell-cell interface was much weaker (i vs. a, d; e, h), with the presence of more internalized occludin vesicles in the cytosol and an increase in Rab 9 staining (red fluorescence, Cy3) (j vs. b, f). The internalized occludin vesicles were found to colocalize with Rab 9 when cells were treated with TGF-β2 but not with T (arrowheads), shown in the magnified images in m and n from the boxed areas in l. Scale bars = 10 µm (a–l); 3 µm (m, n). B) Immunoblots illustrating the increase in the protein level of Rab 9 when cells were treated with TGF-β2 but not with T or in the control (Ctrl) cells. C) Statistical analysis was performed by comparing the protein level of Rab 9 when Sertoli cells were treated with T or TGF-β2 vs. controls. Protein at time 0 in the control group was arbitrarily set as 1, against which statistical analysis was performed. Each bar is the mean ± sd of three different experiments. Statistical analysis was performed by two-way ANOVA followed by Dunnett’s test, comparing each treatment group vs. its corresponding control group over time. *P < 0.05; **P < 0.01; ns, not significantly different. D) Immunoblot analysis of lysates (~50 µg of total protein/lane) of seminiferous tubules (ST) and Sertoli cells (SC) using the mouse anti-Rab 9 antibody.
DISCUSSION
BTB integral membrane proteins are continuously internalized and recycled back to the cell surface to maintain barrier integrity
The migration of preleptotene and leptotene spermatocytes across the BTB at stage VIII of the seminiferous epithelial cycle in the adult rat testis involves extensive restructuring of the TJ and AJ. The mechanisms used by the testis to accommodate germ cell migration across the BTB while maintaining this immunological barrier remain obscure. Herein, we provide for the first time biochemical data to illustrate that the testis uses a highly effective mechanism to induce rapid junction restructuring to permit cell migration while maintaining barrier integrity. When cell surface proteins of Sertoli cells cultured in vitro with functional TJs and AJs that mimicked the BTB in vivo were biotinylated, ~10–50% of the total biotinylated BTB proteins were continuously internalized and recycled back to the cell surface via the clathrin-mediated pathway as illustrated in this report. This finding suggests that continuous de novo synthesis of integral membrane proteins at the BTB that occurs during spermatogenesis to facilitate junction restructuring to accommodate cell movement perhaps would be augmented by protein recycling. From our study, the turnover rate of BTB integral membrane proteins is ~1–1.5 h (note that the turnover rate is estimated by doubling the time required for the integral membrane proteins at the BTB to reach the maximum level of internalization; see Fig. 2). However, stage VIII of the seminiferous epithelial cycle in the adult rat testis lasts for ~1.5 day (~30 h), which is the time when preleptotene/leptotene spermatocytes traverse the BTB. Thus, it is highly plausible that there are several waves of barrier opening to accommodate preleptotene/leptotene spermatocyte migration (Fig. 7). Furthermore, it is of interest that these germ cells traverse the BTB as “clones,” interconnected via cytoplasmic bridges (52, 53). As such, a rapid turnover rate of integral membrane proteins at the BTB proteins is needed and is physiologically necessary to achieve multiple cycles of junction disassembly and reassembly during stage VIII of the epithelial cycle to accommodate germ cell migration. In this study, occludin, instead of claudins or JAMs, was used as the TJ marker to monitor the effect of T and TGF-β2 on the kinetics internalization and recycling because this protein is consistently present at the BTB throughout adulthood in rats. Even though occludin is only present in rodent and not in human testes, the significance of occludin on spermatogenesis cannot be overlooked as occludin knockout mice were infertile (54). Furthermore, addition of exogenous occludin into occludin-deficient epithelial cells was shown to promote TJ reconstitution (55).
Figure 7.
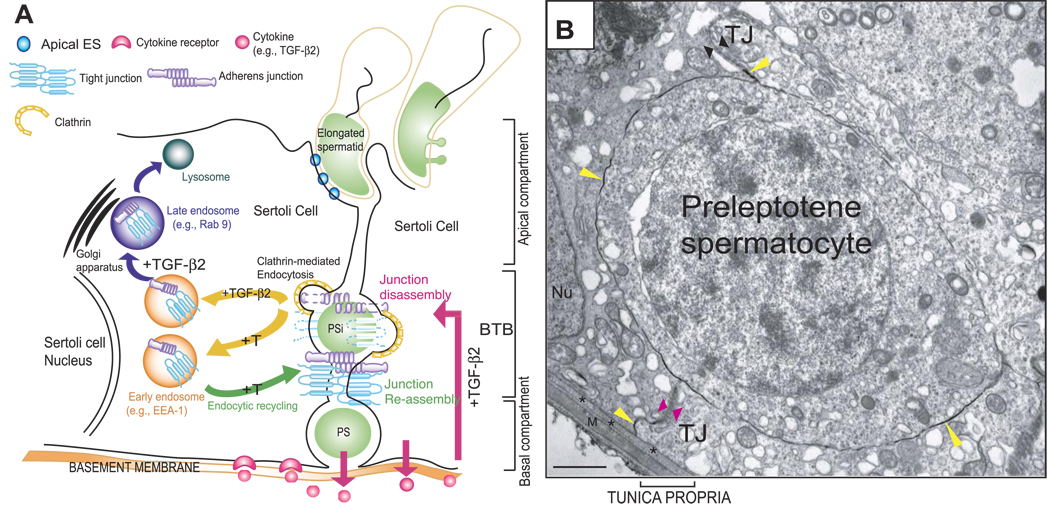
A model to illustrate that the timely opening and closing (or restructuring) of the BTB to facilitate preleptotene/leptotene spermatocyte migration across the BTB is mediated by the differential effects of T and cytokines on the endocytosis, recycling, and intracellular degradation (or transcytosis) of integral membrane proteins. A) As shown in this schematic drawing, the BTB physically divides the seminiferous epithelium into the basal and apical (or adluminal) compartments. Spermatogonia (type B) differentiate into preleptotene/leptotene spermatocytes (PS) that must traverse the BTB (PSi, preleptotene/leptotene spermatocyte in transit) at stage VIII of the seminiferous epithelial cycle of spermatogenesis. T and cytokines (e.g., TGF-β2 released by Sertoli and/or migrating spermatocytes into the microenvironment of the seminiferous epithelium) (short pink arrow) accelerate the kinetics of internalization of BTB integral membrane proteins via the clathrin-mediated endocytosis pathway (yellow arrows). This disrupts the BTB on the apical end of a migrating PSi at stage VIII of the epithelial cycle to facilitate the transit of PS across the BTB by lowering the steady-state levels of integral membrane proteins locally. Internalized proteins (e.g., occludin) are either targeted to early endosomes via their attachment with an endosome-associated marker protein (e.g., EEA-1) (purple arrow) or sorting endosomes. TGF-β2 perturbs the BTB (long pink arrow) by targeting internalized proteins (e.g., occludin) into late endosomes (e.g., Rab 9) (purple arrow), for their degradation in lysosomes, thereby lowering the steady-state integral membrane proteins at the BTB. T, in contrast, enhances the kinetics of recycling of BTB proteins back to the Sertoli cell surface, perhaps to the basal region of the migration of PS (i.e., PSi) via transcytosis to reassemble the BTB (green arrow). The net result of these interactions thus maintains the immunological barrier in the seminiferous epithelium during the transit of PS across the BTB. B) Electron micrograph of a preleptotene spermatocyte in transit corresponding to the PSi shown in A. This preleptotene spermatocyte was shown to be trapped between the TJ fibrils, as illustrated in this lanthanum study. The TJ fibrils at the apical end of this preleptotene spermatocyte appeared to become leaky (opposing black arrowheads) or opened, as lanthanum (yellow arrowheads) was able to reach the TJ fibrils (yellow arrowhead near the opposing black arrowheads) in this microenvironment to facilitate the transit of this preleptotene spermatocyte. However, new TJ fibrils were detected at the basal portion of this spermatocyte in transit (opposing pink arrowheads) that limited further entry of lanthanum (yellow arrowhead below the pink arrowhead) into the epithelium. Asterisks mark the basement membrane (also known as basal lamina), which is a modified form of extracellular matrix (51); M, myoid cell layer; Nu, Sertoli cell nucleus. Scale bar = 1 µm.
Testosterone and cytokines regulate BTB integrity via differential effects on the equilibrium between the kinetics of protein endocytosis and recycling by determining the fate of endocytosed proteins
Androgen is produced by Leydig cells residing in the interstitium. However, androgen exerts its effects via specific ARs restricted to Sertoli cells in the seminiferous epithelium. The expression level of ARs in rodents is highest in the seminiferous epithelium at stages VII–VIII of the epithelial cycle (39, 56). Mice with selective knock out of the AR in Sertoli cells are infertile, resulting from spermatogenic arrest before the first meiosis. Also, the BTB in these mice is impaired (13, 37, 57). These findings, together with in vitro studies demonstrating that T promotes the Sertoli cell-TJ barrier function (41), suggest that the BTB is a major target of androgen action, particularly at stage VIII of the epithelial cycle when the BTB opens to accommodate migrating preleptotene or leptotene spermatocytes. In this study, androgen was shown to facilitate the trafficking of integral membrane proteins between the cell surface and cytosol, as well as their recycling from the cytosol back to the cell surface instead of enhancing the expression of BTB proteins (Fig. 1), which would reinforce barrier integrity. Androgen probably exerts its effects by relocating integral membrane proteins within the BTB microenvironment, such as by reassembling (or “resealing”) the barrier beneath migrating preleptotene spermatocytes (Fig. 7A, B). Cytokines, such as interferon-γ, TGF-βs, and TNF-α, are all known to regulate junction dynamics in multiple epithelia. These cytokines were shown to perturb junctions by lowering the steady-state TJ and AJ protein levels at the intestinal barrier (58–60) and the BTB (17–19, 30, 48). Whereas cytokines promote protein endocytosis similar to T, endocytosed proteins, such as occludin, at the BTB were targeted to the endosome-mediated degradation pathway.
How does the BTB open while maintaining its immunological barrier to sequester postmeiotic germ cell antigens from the immune system during stage VIII of the epithelial cycle?
At stage VIII of the epithelial cycle during spermatogenesis, the BTB undergoes rapid restructuring to facilitate preleptotene/leptotene spermatocyte migration; yet, its integrity must be maintained to sequester postmeiotic germ cell development from the immune system. On the basis of earlier morphological studies, it was suggested that new occluding fibrils formed below the preleptotene/leptotene spermatocytes, followed by the breakdown of occluding fibrils above these spermatocytes (8, 61) as illustrated in Fig. 7. However, no biochemical and molecular evidence was available in the literature to support this postulate. The coexistence of TJ and AJ at the BTB has been proposed to supersede each other’s function transiently to maintain barrier integrity (9, 62). When the BTB opens, there is a disengagement of TJ and AJ via their peripheral adaptors so that the BTB can maintain its integrity during extensive AJ restructuring and vice versa (9). Undoubtedly, this theory does not explain the events of junction assembly and disassembly below and above the migrating preleptotene/leptotene spermatocyte when TJ fibrils are being generated and disrupted (Fig. 7B). On the basis of the results presented herein, T and cytokines (e.g., TGF-β2) work in concert via their effects on the internalization, recycling, and intracellular degradation of proteins at the BTB to regulate and coordinate these events (Fig. 7). The stage-specific expression of the AR (56) and TGF-βs (18, 28) in the seminiferous epithelium at stage VIII of adult rat testes have been reported previously, illustrating the fact that these are crucial regulators of BTB dynamics. We postulate that the increase in the kinetics of internalization and intracellular degradation of integral membrane proteins at the BTB induced by TGF-βs would contribute to lowering the steady-state levels of occluding fibrils above preleptotene/leptotene spermatocytes, thereby destabilizing the Sertoli-Sertoli cell barrier to facilitate spermatocyte migration. On the other hand, the increase in the kinetics of internalization and recycling of integral membrane proteins (e.g., occludin) at the BTB induced by T would contribute to a rapid relocation of BTB proteins from the apical to the basal region of migrating preleptotene or leptotene spermatocytes such that new occluding fibrils can form and reseal the BTB to maintain the immunological barrier (Fig. 7). Transcytosis and recycling of integral membrane proteins have been reported in migrating cells. For instance, endocytosed integrins within endocytic vesicles at the rear end of a migrating cell were shown to be transported to the leading edge for the formation of new focal contacts (63, 64). Furthermore, cytokines, such as interferon-γ, have been shown to redirect the transcytosis of β1-integrin to the basal compartment of intestine epithelial cells instead of to the leading edge, inhibiting wound closure (65). Finally, Notch (N), a transmembrane protein in Drosophila epithelial cells, was shown to relocate to AJ via dynamin- and Rab 5-dependent transcytosis, which, in turn, mediated cell-cell interactions (66). It is likely that a similar mechanism is used by Sertoli cells after androgen treatment, which reinforces the BTB integrity. This would suggest that BTB-integral membrane proteins above a preleptotene/leptotene spermatocyte are endocytosed within vesicles and transported beneath the spermatocyte via transcytosis to form new junction fibrils (Fig. 7), which should be vigorously investigated in future studies. In short, we have provided biochemical evidence to demonstrate that T and cytokines (e.g., TGF-β2) work together to differentially affect the endocytosis, recycling, endosome-mediated degradation, and possibly the transcytosis or translocation of integral membrane proteins to coordinate the timely restructuring of the BTB to facilitate the transit of preleptotene/leptotene spermatocytes across the barrier at stage VIII of the seminiferous epithelial cycle of spermatogenesis.
Supplementary Material
Acknowledgments
We thank Dr. Irving Sivin for his helpful discussion and advice on the use of two-way ANOVA and the repeated measures model followed by Dunnett’s test (experimental vs. controls) for statistical analysis of data. This work was supported in part by grants from the U.S. National Institutes of Health (National Institute of Child Health and Human Development U54 HD029990 Project 3 and U01 HD045908), the Contraceptive Research and Development (CONRAD) Program (Consortium for Industrial Collaboration in Contraceptive Research, CIG 01-72 to C.Y.C. and CIG-01-74 to D.D.M.), and the Hong Kong Research Grant Council (HKU7599/06M to W.M.L.). H.H.N.Y. was supported by a University of Hong Kong Postgraduate Research Scholar award.
REFERENCES
- 1.Smith MW, Gumbleton M. Endocytosis at the blood-brain barrier: from basic understanding to drug delivery strategies. J. Drug Target. 2006;14:191–214. doi: 10.1080/10611860600650086. [DOI] [PubMed] [Google Scholar]
- 2.Ueno M. Molecular anatomy of the brain endothelial barrier: An overview of the distributional features. Curr. Med. Chem. 2007;14:1199–1206. doi: 10.2174/092986707780597943. [DOI] [PubMed] [Google Scholar]
- 3.Spencer BJ, Verma IM. Targeted delivery of proteins across the blood-brain barrier. Proc. Natl. Acad. Sci. U. S. A. 2007;104:7594–7599. doi: 10.1073/pnas.0702170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qian ZM, Li H, Sun H, Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol. Rev. 2002;54:561–587. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- 5.Mruk DD, Wong CH, Silvestrini B, Cheng CY. A male contraceptive targeting germ cell adhesion. Nat. Med. 2006;12:1323–1328. doi: 10.1038/nm1420. [DOI] [PubMed] [Google Scholar]
- 6.Wong CH, Mruk DD, Lee WM, Cheng CY. Targeted and reversible disruption of the blood-testis barrier by an FSH mutant-occludin peptide conjugate. FASEB J. 2007;21:438–448. doi: 10.1096/fj.05-4144com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell LD. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am. J. Anat. 1977;148:313–328. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- 8.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 9.Yan HHN, Cheng CY. Blood-testis barrier dynamics are regulated by an engagement/disengagement mechanism between tight and adherens junctions via peripheral adaptors. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11722–11727. doi: 10.1073/pnas.0503855102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell LD. Form, dimensions, and cytology of mammalian Sertoli cells. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater, Florida, USA: Cache River Press; 1993. pp. 1–37. [Google Scholar]
- 11.Wong CH, Cheng CY. The blood-testis barrier: Its biology, regulation and physiological role in spermatogenesis. Curr. Top. Dev. Biol. 2005;71:263–296. doi: 10.1016/S0070-2153(05)71008-5. [DOI] [PubMed] [Google Scholar]
- 12.Tsai MY, Yeh SD, Wang RS, Yeh S, Zhang C, Lin HY, Tzeng CR, Chang C. Differential effects of spermatogenesis and fertility in mice lacking androgen receptor in individual testis cells. Proc. Natl. Acad. Sci. U. S. A. 2006;103:18975–18980. doi: 10.1073/pnas.0608565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Gendt K, Swinnen JV, Saunders PTK, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc. Natl. Acad. Sci. U. S. A. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang R, Yeh S, Chen L, Lin H, Zhang C, Ni J, Wu C, di Sant’Agnese PA, deMesy-Bentley KL, Tzeng C, Chang C. Androgen receptor in Sertoli cell is essential for germ cell nursery and junctional complex formation in mouse testes. Endocrinology. 2006;147:5624–5633. doi: 10.1210/en.2006-0138. [DOI] [PubMed] [Google Scholar]
- 15.Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc. Natl. Acad. Sci. U. S. A. 2005;102:16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Donnell L, McLachlan RI, Wreford NG, de Kretser DM, Robertson DM. Testosterone withdrawal promotes stage-specific detachment of round spermatids from the rat seminiferous epithelium. Biol. Reprod. 1996;55:895–901. doi: 10.1095/biolreprod55.4.895. [DOI] [PubMed] [Google Scholar]
- 17.Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology. 2001;142:1865–1877. doi: 10.1210/endo.142.5.8116. [DOI] [PubMed] [Google Scholar]
- 18.Xia W, Mruk DD, Lee WM, Cheng CY. Differential interactions between TGF-β3/TβR1, TAB1 and CD2AP disrupt blood-testis barrier and Sertoli-germ cell adhesion. J. Biol. Chem. 2006;281:16799–16813. doi: 10.1074/jbc.M601618200. [DOI] [PubMed] [Google Scholar]
- 19.Lui WY, Wong CH, Mruk DD, Cheng CY. TGF-β3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: An in vivo study. Endocrinology. 2003;144:1139–1142. doi: 10.1210/en.2002-0211. [DOI] [PubMed] [Google Scholar]
- 20.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of the apical junctional complex: mechanisms and possible roles in regulations of epithelial barriers. Bioessays. 2005;27:356–365. doi: 10.1002/bies.20203. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda M, Kubo A, Furuse M, Tsukita S. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J. Cell Sci. 2004;117:1247–1257. doi: 10.1242/jcs.00972. [DOI] [PubMed] [Google Scholar]
- 22.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol. Biol. Cell. 2004;15:176–188. doi: 10.1091/mbc.E03-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guttman JA, Takai Y, Vogl AW. Evidence that tubulobulbar complexes in the seminiferous epithelium are involved with internalization of adhesion junctions. Biol. Reprod. 2004;71:548–559. doi: 10.1095/biolreprod.104.028803. [DOI] [PubMed] [Google Scholar]
- 24.Vaid KS, Guttman JA, Babyak N, Deng W, McNiven MA, Mochizuki N, Finlay BB, Vogl AW. The role of dynamin 3 in the testis. J. Cell. Physiol. 2007;210:644–654. doi: 10.1002/jcp.20855. [DOI] [PubMed] [Google Scholar]
- 25.Siu M, K. Y, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J. Biol. Chem. 2005;280:25029–25047. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- 26.Byers S, Hadley MA, Djakiew D, Dym M. Growth and characterization of polarized monolayers of epididymal epithelial cells and Sertoli cells in dual environment culture chambers. J. Androl. 1986;7:59–68. doi: 10.1002/j.1939-4640.1986.tb00871.x. [DOI] [PubMed] [Google Scholar]
- 27.Janecki A, Steinberger A. Polarized Sertoli cell functions in a new two-compartment culture system. J. Androl. 1986;7:69–71. doi: 10.1002/j.1939-4640.1986.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 28.Lui WY, Lee WM, Cheng CY. Transforming growth factor β3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol. Reprod. 2003;68:1597–1612. doi: 10.1095/biolreprod.102.011387. [DOI] [PubMed] [Google Scholar]
- 29.Yan HHN, Cheng CY. Laminin α3 forms a complex with β3 and γ3 chains that serves as the ligand for α6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J. Biol. Chem. 2006;281:17286–17303. doi: 10.1074/jbc.M513218200. [DOI] [PubMed] [Google Scholar]
- 30.Siu MKY, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor-α, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–387. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- 31.Galdieri M, Ziparo E, Palombi F, Russo M, Stefanini M. Pure Sertoli cell cultures: a new model for the study of somatic-germ cell interactions. J. Androl. 1981;2:249–254. [Google Scholar]
- 32.Morimoto S, Nishimura N, Terai T, Manabe S, Yamamoto Y, Shinahara W, Miyake H, Tashiro S, Shimada M, Sasaki T. Rab 13 mediates the continuous endocytic recycling of occludin to the cell surface. J. Biol. Chem. 2005;280:2220–2228. doi: 10.1074/jbc.M406906200. [DOI] [PubMed] [Google Scholar]
- 33.Le TL, Yap AS, Stow JL. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J. Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- 34.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 35.Peterson G. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr, and Randall. Anal. Biochem. 1979;100:201–220. doi: 10.1016/0003-2697(79)90222-7. [DOI] [PubMed] [Google Scholar]
- 36.Cavicchia J, Sacerdote F. Topography of the rat blood-testis barrier after intratubular administration of intercellular tracers. Tissue Cell. 1988;20:577–586. doi: 10.1016/0040-8166(88)90059-6. [DOI] [PubMed] [Google Scholar]
- 37.Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101:6876–6881. doi: 10.1073/pnas.0307306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, Xing L, Boyce BF, Hung MC, Zhang S, Gan L, Chang C. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13498–13503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shan LX, Bardin CW, Hardy MP. Immunohistochemical analysis of androgen effects on androgen receptor expression in developing Leydig and Sertoli cells. Endocrinology. 1997;138:1259–1266. doi: 10.1210/endo.138.3.4973. [DOI] [PubMed] [Google Scholar]
- 40.Cyr D, Blaschuk OW, Robaire B. Identification and developmental regulation of cadherin messenger ribonucleic acids in the rat testis. Endocrinology. 1992;131:139–145. doi: 10.1210/endo.131.1.1611992. [DOI] [PubMed] [Google Scholar]
- 41.Chung NPY, Cheng CY. Is cadmium chloride-induced inter-Sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology. 2001;142:1878–1888. doi: 10.1210/endo.142.5.8145. [DOI] [PubMed] [Google Scholar]
- 42.Gye MC. Changes in the expression of claudins and transepithelial electrical resistance of mouse Sertoli cells by Leydig cell coculture. Int. J. Androl. 2003;26:271–278. doi: 10.1046/j.1365-2605.2003.00423.x. [DOI] [PubMed] [Google Scholar]
- 43.Florin A, Maire M, Bozec A, Hellani A, Chater S, Bars R, Chuzel F, Benahmed M. Androgens and postmeiotic germ cells regulate claudin-11 expression in rat Sertoli cells. Endocrinology. 2005;146:1532–1540. doi: 10.1210/en.2004-0834. [DOI] [PubMed] [Google Scholar]
- 44.Sluka P, O’Donnell L, Bartles JR, Stanton PG. FSH regulates the formation of adherens junctions and ectoplasmic specializations between rat Sertoli cells in vitro and in vivo. J. Endocrinol. 2006;189:381–395. doi: 10.1677/joe.1.06634. [DOI] [PubMed] [Google Scholar]
- 45.Welsh M, Wiebe J. Sertoli cell capacity to metabolize C19 steroids: Variation with age and the effect of follicle-stimulating hormone. Endocrinology. 1978;103:838–844. doi: 10.1210/endo-103-3-838. [DOI] [PubMed] [Google Scholar]
- 46.Tcholakian R, Steinberger A. In vitro metabolism of testosterone by cultured Sertoli cells and the effect of FSH. Steroids. 1979;33:495–526. doi: 10.1016/0039-128x(79)90032-1. [DOI] [PubMed] [Google Scholar]
- 47.Dunn KW, McGraw TE, Maxfield FR. Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosome. J. Cell Biol. 1989;109:3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li M, W M, Xia W, Mruk DD, Wang CQF, Yan HHN, Siu MKY, Lui WY, Lee WM, Cheng CY. Tumor necrosis factor α reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J. Endocrinol. 2006;190:313–329. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- 49.Barbero P, Bittova L, Pfeffer SR. Visualization of Rab9-mediated vesicle transport from endosomes to the trans-Golgi in living cells. J. Cell Biol. 2002;156:511–518. doi: 10.1083/jcb.200109030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Payne CK, Jones SA, Chen C, Zhuang X. Internalization and trafficking of cell surface proteoglycans and proteoglycan-binding ligands. Traffic. 2007;8:389–401. doi: 10.1111/j.1600-0854.2007.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dym M. Basement membrane regulation of Sertoli cells. Endocr. Rev. 1994;15:102–115. doi: 10.1210/edrv-15-1-102. [DOI] [PubMed] [Google Scholar]
- 52.Clermont Y, Morales C, Hermo L. Endocytic activities of Sertoli cells in the rat. Ann. N. Y. Acad. Sci. 1987;513:1–15. doi: 10.1111/j.1749-6632.1987.tb24994.x. [DOI] [PubMed] [Google Scholar]
- 53.De Kretser DM, Kerr JB. The Cytology of the Testis. Vol. 1. New York: Raven Press; 1988. [Google Scholar]
- 54.Saitou M, Furuse M, Sasaki H, Schulzke J, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li D, Mrsny RJ. Oncogenic Raf-1 disrupts epithelial tight junctions via downregulation of occludin. J. Cell Biol. 2000;148:791–800. doi: 10.1083/jcb.148.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bremner WJ, Millar MR, Sharpe RM, Saunders PT. Immunohistochemical localization of androgen receptors in the rat testis: evidence for stage-dependent expression and regulation by androgens. Endocrinology. 1994;135:1227–1234. doi: 10.1210/endo.135.3.8070367. [DOI] [PubMed] [Google Scholar]
- 57.Denolet E, de Gendt K, Allemeersch J, Engelen K, Marchal K, Van Hummelen P, Tan KAL, Sharpe RM, Saunders PTK, Swinnen JV, Verhoeven G. The effect of a Sertoli cell-selective knockout of the androgen receptor on testicular gene expression in prepubertal mice. Mol. Endocrinol. 2006;20:321–334. doi: 10.1210/me.2005-0113. [DOI] [PubMed] [Google Scholar]
- 58.Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-γ induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19:923–933. doi: 10.1096/fj.04-3260com. [DOI] [PubMed] [Google Scholar]
- 59.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-γ-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol. Biol. Cell. 2005;16:5040–5052. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD. Tumor necrosis factor-α (TNF-α) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J. Cell Sci. 1999;112:137–146. doi: 10.1242/jcs.112.1.137. [DOI] [PubMed] [Google Scholar]
- 61.Russell LD, Peterson RN. Sertoli cell junctions: Morphological and functional correlates. Int. Rev. Cytol. 1985;94:177–211. doi: 10.1016/s0074-7696(08)60397-6. [DOI] [PubMed] [Google Scholar]
- 62.Yan HHN, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: a friend or a foe of spermatogenesis? BioEssays. 2007;29:36–48. doi: 10.1002/bies.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bretscher MS. Circulating integrins: α5β1, α6β4 and Mac-1, but not α3β1, α4β1 or LFA-1. EMBO J. 1992;11:405–410. doi: 10.1002/j.1460-2075.1992.tb05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pierini LM, Lawson MA, Eddy RJ, Hendey B, Maxfield FR. Oriented endocytic recycling of α5β1 in motile neutrophils. Blood. 2000;95:2471–2480. [PubMed] [Google Scholar]
- 65.Tong Q, Vassilieva EV, Ivanov AI, Wang Z, Brown GT, Parkos CA, Nusrat A. Interferon-γ inhibits T84 epithelial cell migration by redirecting transcytosis of β1 integrin from the migrating leading edge. J. Immunol. 2005;175:4030–4038. doi: 10.4049/jimmunol.175.6.4030. [DOI] [PubMed] [Google Scholar]
- 66.Sasaki N, Sasamura T, Ishikawa HO, Kanai M, Ueda R, Saigo K, Matsuno K. Polarized exocytosis and transcytosis of Notch during its apical localization in Drosophila epithelial cells. Genes Cells. 2007;12:89–103. doi: 10.1111/j.1365-2443.2007.01037.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.