Abstract
Purpose
Tinnitus is the persistent perception of a subjective sound. Tinnitus is almost universally experienced in some forms. In most cases, recovery may occur in seconds, hours, or days. How does tinnitus shift from a transient condition to a lifelong disorder? Several lines of evidence, including clinical studies and animal models, indicate that the brain, rather than the inner ear, may in some cases be the site of maintenance of tinnitus. One hypothesis is that normal electrical activity in the auditory system becomes pathologically persistent due to plasticity-like mechanisms that can lead to long-term changes in the communication between neurons. A candidate site for the expression of this so-called synaptic plasticity is a region of the brainstem called the dorsal cochlear nucleus (DCN), a site of integration of acoustic and multimodal, sensory inputs.
Conclusions
Here we review recent findings on cellular mechanisms observed in the DCN that can lead to long-term changes in the synaptic strength between different neurons in the DCN. These cellular mechanisms could provide candidate signaling pathways underlying the induction (ignition) and/or the expression (maintenance) of tinnitus.
Keywords: synaptic plasticity, dorsal cochlear nucleus, tinnitus, auditory neuroscience
Tinnitus is broadly defined as a sound perception in the absence of an acoustic event and is experienced by up to 15% of the general population (Axelsson & Ringdahl, 1989; Cooper, 1994; Quaranta, Assennato, & Sallustio, 1996). Of the 40 million individuals in the United States with tinnitus, approximately 10 million seek medical attention (Seidman & Jacobson, 1996), and 2.5 million of these are considered disabled by tinnitus due to its persistence and intensity. Despite the prevalence of tinnitus, the pathophysiology of the disorder is poorly understood. Part of this lack of understanding stems from the fact that tinnitus mechanisms involve both peripheral and central components of the auditory system (Eggermont, 2000). Although damage to the cochlea causes hearing loss and often initiates tinnitus, recent studies have established that it is the central nervous system that plays a key role in chronic tinnitus. It is interesting that ablation and stimulation of nonauditory structures modify tinnitus perception (Jeanmonod, Magnin, & Morel, 1996; Moller, Moller, & Yokota, 1992). A large number of tinnitus patients are able to modulate their tinnitus by clenching their jaw or touching the skin on their face (somatic modulation of tinnitus; Levine, 1999). These observations have led to the dorsal cochlear nucleus (DCN) disinhibition hypothesis, which can account for tinnitus due to a head or neck somatic disorder, as well as to an ear disorder (Levine, 1999). The principal cells (fusiform cells) of the DCN project directly to the central nucleus of the inferior colliculus. These cells are unique in that they serve as a convergence point for integration of auditory and somatosensory information (Shore, 2005; Shore & Zhou, 2006; Young, Nelken, & Conley, 1995).
Animal Models of Tinnitus
It is becoming accepted that animal models provide a significant opportunity for research in tinnitus. The effects of exposure to intense sound or chemical agents have been evaluated by using behavioral testing that determines whether the animals subjectively “hear” sounds in the quiet. These models require training animals to respond distinctively to the absence of an acoustic stimulus. Such behavioral tests have been devised for rats and hamsters (Bauer, Brozoski, Rojas, Boley, & Wyder, 1999; Guitton et al., 2003; Heffner & Harrington, 2002; Jastreboff, Brennan, & Sasaki, 1988; Lobarinas, Sun, Cushing, & Salvi, 2004; Prosen & May, 2005; Ruttiger, Ciuffani, Zenner, & Knipper, 2003; Turner et al., 2006). Although these models have contributed significantly to our understanding of tinnitus, they typically require complex behavioral manipulations (e.g., food or water deprivation, variable reinforcement schedules) and weeks to months of behavioral training. A recent study described a novel application of the acoustic startle reflex for rapid tinnitus screening (Turner et al., 2006). The authors showed that when a background acoustic signal is qualitatively similar to the animal’s tinnitus, poorer detection of a silent gap is performed. Food or water deprivation is not necessary, and no training, learning, memory, or motivational demands are imposed on the animal. Additionally, testing for tinnitus can be done quickly (a single 40-min session).
DCN Hyperactivity and Tinnitus
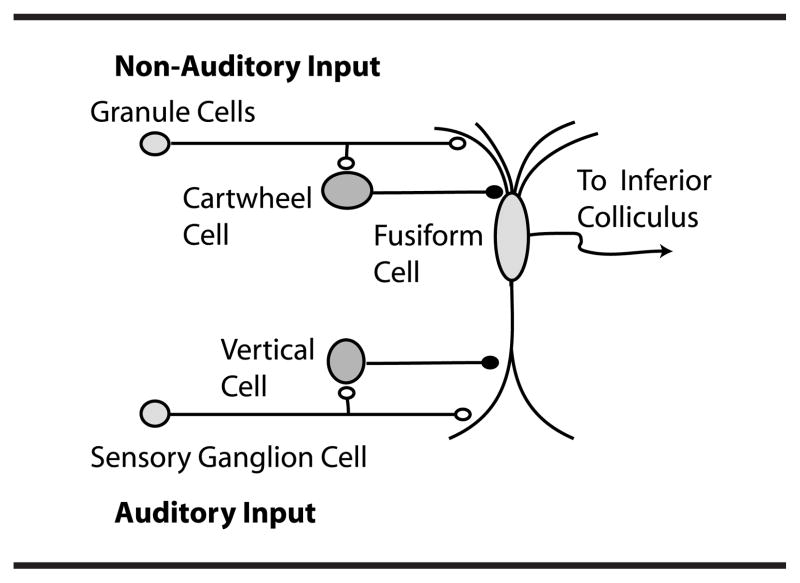
One major focus of studies in animal models of tinnitus has been on neurons in the DCN (see Figure 1). The DCN is an auditory brainstem nucleus involved at the earliest stages of sensory processing and is believed to play a key role in orientation toward sounds of interest and/or suppression of responses to expected body-generated sounds (Shore & Zhou, 2006; Sutherland, Glendenning, & Masterton, 1998; Young & Davis, 2001). DCN fusiform neurons receive input from the auditory nerve and from a system of parallel fibers carrying multimodal input, including proprioceptive signals encoding the position of the ears, as well as input from the vocal tract perhaps signaling suppression of body-generated sounds or vocal feedback (see Figure 1). DCN parallel fibers also activate inhibitory glycinergic cartwheel cells, which provide feed-forward inhibition to fusiform cells. The complex response of fusiform cells to auditory signals is shaped by feed-forward inhibition from additional cell types, the vertical cells of the deep layer (see Figure 1) and D-stellate cells of the ventral cochlear nucleus (Young & Davis, 2001). Numerous studies have shown that fusiform cells exhibit elevated levels of spontaneous electrical activity and hypersensitivity to sound in animal models of tinnitus (reviewed by Eggermont & Roberts, 2004; Kaltenbach, 2006; Kaltenbach, Zacharek, Zhang, & Frederick, 2004). In vivo single-unit recordings from DCN fusiform cells in chinchillas with behavioral evidence of tinnitus found increased spontaneous activity and evidence of fusiform cell hyperactivity (Brozoski, Bauer, & Caspary, 2002). Spontaneous activity in DCN of hamsters is increased following noise exposure using measures of electrical activity (Kaltenbach & Afman, 2000; Kaltenbach et al., 1998). This activity persists even after ablation of the cochlea (Zacharek, Kaltenbach, Mathog, & Zhang, 2002), suggesting that the inner ear is not necessary for the maintenance of tinnitus. Moreover, when behavioral and electrophysiological tests were conducted in the same hamsters, a significant correlation was found between the level of spontaneous activity in DCN and the behavioral evidence for tinnitus (Kaltenbach et al., 2004). A correlation, however, does not prove cause-and-effect relationship. It is possible that both DCN hyperactivity and tinnitus result from hearing loss, which although present in the overwhelming majority of tinnitus sufferers may be unrelated to each other. However, this explanation seems unlikely, as Brozoski et al. (2002), who exposed chinchillas to low levels of sound, succeeded in demonstrating that both hyperactivity in the DCN and behavioral evidence of tinnitus were induced even after recovery from temporary hearing loss.
Figure 1.
Dorsal cochlear nucleus (DCN) circuitry. Reprinted from Neuron, 54(2), T. Tzounopoulos, M. E. Rubio, J. E. Keen, and L. O. Trussell, “Coactivation of Pre- and Postsynaptic Signaling Mechanisms Determines Cell-Specific Spike-Timing-Dependent Plasticity,” pp. 291–301, Copyright 2007, with permission from Elsevier.
It is not yet known whether intense noise exposure causes hyperactivity in the DCN of humans. However, several studies have implicated the DCN as an important component in the modulation of tinnitus in humans. Soussi and Otto (1994) reported that tinnitus loudness could be negatively modulated by applying electrical stimuli directly to the DCN surface. In 6 out of 7 patients, stimulation of the DCN resulted in decreasing the loudness of tinnitus or eliminating it altogether.
Causes of Tinnitus Might Be Multisensory
Because the level of neuronal activity depends on the balance of excitatory and inhibitory inputs, hyperactivity in fusiform cells in animals experiencing tinnitus indicates the importance of changes in auditory and nonauditory inputs after tinnitus induction. Is there reason to think that such nonauditory signals may be important in tinnitus in humans? That nonauditory signals may contribute to tinnitus is in agreement with previously mentioned findings showing that deep brain (thalamic) stimulation or forceful contractions of head musculature can modulate the perceived loudness of tinnitus (Levine, 1999; Martin, Shi, Burchiel, & Anderson, 1999; Moller et al., 1992). It is also important to note that another cause of tinnitus (other than insults to the cochlea) is putative abnormal activity in the somatosensory system (Cacace, 2003; Levine, 1999; Shore, 2005; Shore & Zhou, 2006) resulting from head and neck injuries, whiplash, and various mandibular and dental problems (Chan & Reade, 1994). The main hypothesis of this review article is that activity-dependent mechanisms that change the balance of excitation and inhibition on fusiform cells could lead to hyperactivity of fusiform cells, via plasticity-like mechanisms recently discovered in the parallel fibers of the DCN (Tzounopoulos, Kim, Oertel, & Trussell, 2004; Tzounopoulos, Rubio, Keen, & Trussell, 2007). While earlier anatomical studies have suggested that there is a progressive reduction in the cochlear granular layer in humans and nonhuman primates (Moore, 1980; Moore & Osen, 1979), it is important to note that these studies were performed at the light microscopy level and thus were not very detailed and quantitative. However, recent studies at the electron microscopy level and with the use of different cellular and molecular markers have provided convincing evidence that the DCN granular layer of the rhesus monkey (cercopithecoid primate) has neuronal features similar to those of other nonprimate mammals (Rubio, Gudsnuk, Smith, & Ryugo, 2008).
Synaptic Plasticity in the DCN and Tinnitus
The effectiveness of specific treatments of disorders such as tinnitus can provide information about its pathophysiology. The focus of the treatment has moved from the ear and the periphery of the nervous system to the central nervous system. Treatments of tinnitus have been directed toward correcting the functional changes either by reversing the plastic changes that caused the pathological signs or by correcting the balance between excitation and inhibition using medications that enhance overall inhibition. The mechanisms that lead to plastic changes underlying the expression of tinnitus have not been identified. Elucidation of these mechanisms would lead to specific signaling pathways that could subsequently become the target of more specific and effective pharmacological treatment for tinnitus patients.
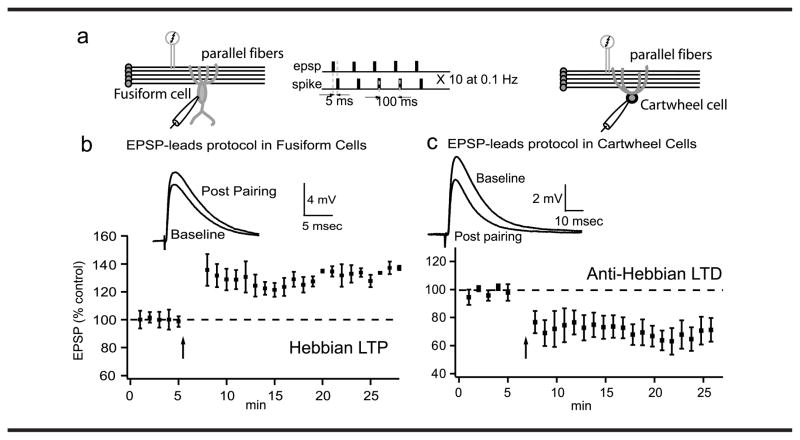
Recent studies in the DCN focused on well-established observations made in a variety of brain regions, including the parallel fiber input to DCN (Fujino & Oertel, 2003; Tzounopoulos et al., 2004, 2007), showing that transient exposure to intense electrical activity may have long-lasting effects on neural activity. It has been shown that transient exposure to intense or appropriately timed electrical stimulation may have long-lasting effects on DCN neural activity. It is likely that activity-dependent induction of a hyperactive state in the DCN may result from these mechanisms. Recent work has shown that the strength of neuronal connections in the DCN can be modified by previous activity in nerve fibers (see Figure 2), a phenomenon called synaptic plasticity (Fujino & Oertel, 2003; Tzounopoulos et al., 2004). More specifically, the parallel fiber input (the one conveying somatosensory information) to the fusiform cells can be potentiated (long-term potentiation [LTP]) or depressed (long-term depression [LTD]) depending on the specific pattern of previous synaptic activity (Tzounopoulos et al., 2004). The same protocol that leads to potentiation to fusiform cells leads to depression to cartwheel cells (see Figure 2). This is a unique finding, suggesting that the net effect of these opposing forms of plasticity would bias the circuit toward excitation of the fusiform cell. Such synapse-specific synaptic plasticity, by decreasing the activity of inhibitory interneurons (disinhibition) and simultaneously increasing excitatory input to fusiform cells, can lead to hyperactivity in fusiform cells, similar to the one observed in animal models of tinnitus.
Figure 2.
Cell-specific plasticities in the DCN. (a) Plasticity was induced by a protocol of excitatory postsynaptic potentials (EPSP)-spike pairs. (b) Representative traces of averaged EPSPs before and 15–20 min after pairing and time course of induced synaptic plasticity for fusiform cells. (c) Representative traces and time course of induced synaptic plasticity for cartwheel cells. The same protocol induces long-term potentiation (LTP) in fusiform cells and long-term depression (LTD) at cartwheel cells. These studies have demonstrated unique, opposing forms of spike timing-dependent synaptic plasticity (STDP) at parallel fiber synapses onto fusiform and cartwheel cells. STDP has emerged as a method to observe the direction of synaptic change following precise timing of pre- and postsynaptic action potentials. Presynaptic action potentials (induced by a stimulating bipolar electrode positioned in the parallel fiber area) lead to EPSPs, while postsynaptic spikes are evoked by direct current injection at the postsynaptic cell.
While it is obvious that electrical stimulation of parallel fibers can induce synaptic plasticity that could change the balance of excitation and inhibition in fusiform cells, it may not be clear how stimulation caused by noise exposure could produce similar plasticity effects. Fusiform cells are contacted by the synaptically plastic parallel fibers and by the nonplastic auditory nerve fibers (Fujino & Oertel, 2003). However, auditory nerve fiber activity can serve to induce/modulate synaptic plasticity of the parallel fiber, by analogy with the climbing fiber and parallel fiber in the cerebellum. Spikes initiated by intense auditory activity (during noise exposure) could provide the trigger to induce the types of synaptic plasticity observed in the parallel fiber inputs of fusiform cells (see Figure 2). In addition, putative abnormal activity in the somatosensory system such as from head and neck injuries could also lead to long-lasting synaptic changes via plasticity and homeostatic mechanisms.
Cellular Mechanisms of Synaptic Plasticity and Tinnitus
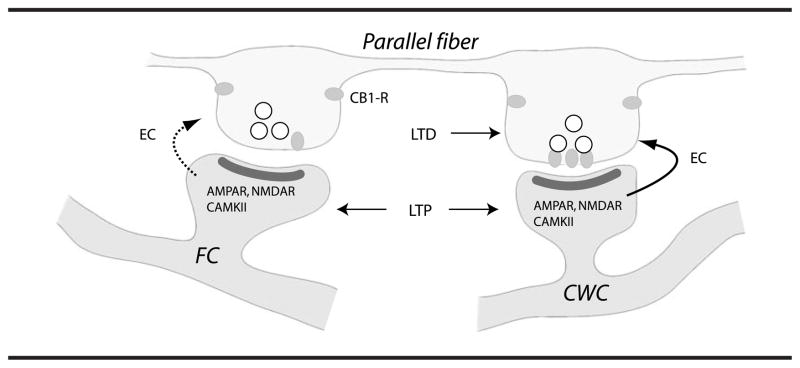
More recent studies show that these cell-specific neuroplasticities require increases in postsynaptic Ca2+ and activation of specific receptor proteins including NMDA receptors, endocannabinoid receptors, and Ca2+-dependent kinase II (CaMKII; Tzounopoulos et al., 2007). These findings show that selective engagement of retrograde endocannabinoid signaling in cartwheel cells and its interaction with the CaMKII signaling cascade mediates site-specific synaptic plasticity in the DCN. Blocking endocannabinoid signaling in cartwheel cells converted LTD to LTP, similar to the LTP observed in fusiform cells. Electrophysiological and electron microscopy experiments show that cell-specific synaptic plasticity in the DCN arises by selective presynaptic targeting of cannabinoid (CB1) receptors to terminals ending on cartwheel cells (see Figure 3). Endocannabinoids are released from postsynaptic cells and diffuse retrogradely to the presynaptic terminal where they activate cannabinoid (CB1) receptors (reviewed by Wilson & Nicoll, 2002). CB1 receptors are G protein-coupled receptors that inhibit transmitter release (Wilson & Nicoll, 2001). Identification of specific signaling pathways mediating synaptic plasticity in the DCN may provide clues for candidate cellular mechanisms involved in the induction and/or expression of tinnitus. Future studies addressing the involvement of these specific signaling pathways in the development and maintenance of tinnitus could provide leads on drugs that might be used to modify synaptic function and treat tinnitus.
Figure 3.
Mechanisms underlying cell-specific synaptic plasticity in the DCN. Cell-specific plasticity in the DCN arises by selective presynaptic targeting of CB1 receptors to terminals ending on cartwheel cells (CWC). Both CWC and fusiform cells (FC) express mechanisms for postsynaptic LTP, but only CWCs express endocannabinoid-mediated LTD. It is not clear if FC release endocannabinoids (EC) to the same level as CWC given an identical stimulus. Reprinted from Neuron, 54(2), T. Tzounopoulos, M. E. Rubio, J. E. Keen, and L. O. Trussell, “Coactivation of Pre- and Postsynaptic Signaling Mechanisms Determines Cell-Specific Spike-Timing-Dependent Plasticity,” pp. 291–301, Copyright 2007, with permission from Elsevier.
Hyperactivity in the DCN fusiform cells may result from changes in their membrane properties. The electrical discharge patterns of neurons depend critically on the type and the voltage dependence of ion channels that they express (Kanold & Manis, 1999). Therefore, activity-dependent, long-lasting changes in the biophysical characteristics of these channels may severely affect the spontaneous rate of activity of a cell and thus lead to hyperactivity.
Other Correlates of Tinnitus That Might be Mediated by Plasticity-Like Mechanisms
Recent findings suggest that once chronic tinnitus induced by noise trauma is established, removal of the DCN does not abolish this tinnitus (Brozoski & Bauer, 2005). One way to interpret these findings is that the DCN is a site where tinnitus is induced but other areas in the auditory pathways may be necessary for the maintenance. Another promising candidate as neural substrate for tinnitus is the cortex. Two possible correlates of tinnitus that have been studied by animal models are changes in cortical tonotopy and increased cortical neural synchrony (Eggermont & Roberts, 2004). Reorganization of the cortical tonotopy maps and increased neural synchrony can also be the result of a change in the balance of excitation and inhibition caused by plasticity-like mechanisms. Future studies where genetically or pharmacologically targeted circuitries can be modified in a controlled manner will determine the circuitries and the cellular mechanisms mediating the initiation and the maintenance of tinnitus.
Acknowledgments
I thank Drs. Jeremy Turner, Donald Caspary, Susan Shore, and Maria Rubio for helpful comments and discussions. This work was supported by National Institutes of Health Grant R01 DC 007905-01A1.
References
- Axelsson A, Ringdahl A. Tinnitus—a study of its prevalence and characteristics. British Journal of Audiology. 1989;23:53–62. doi: 10.3109/03005368909077819. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ, Rojas R, Boley J, Wyder M. Behavioral model of chronic tinnitus in rats. Otolaryngology—Head and Neck Surgery. 1999;121:457–462. doi: 10.1016/S0194-5998(99)70237-8. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA. The effect of dorsal cochlear nucleus ablation on tinnitus in rats. Hearing Research. 2005;206:227–236. doi: 10.1016/j.heares.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. Journal of Neuroscience. 2002;22:2383–2390. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacace AT. Expanding the biological basis of tinnitus: crossmodal origins and the role of neuroplasticity. Hearing Research. 2003;175:112–132. doi: 10.1016/s0378-5955(02)00717-7. [DOI] [PubMed] [Google Scholar]
- Chan SW, Reade PC. Tinnitus and temporoman-dibular pain-dysfunction disorder. Clinical Otolaryngology and Allied Sciences. 1994;19:370–380. doi: 10.1111/j.1365-2273.1994.tb01251.x. [DOI] [PubMed] [Google Scholar]
- Cooper JC., Jr Health and Nutrition Examination Survey of 1971–75: Part II. Tinnitus, subjective hearing loss, and well-being. Journal of the American Academy of Audiology. 1994;5:37–43. [PubMed] [Google Scholar]
- Eggermont JJ. Psychological mechanisms and neural models. In: Tyler RS, editor. Tinnitus handbook. San Diego, CA: Singular; 2000. pp. 89–122. [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends in Neuroscience. 2004;27:676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Fujino K, Oertel D. Bidirectional synaptic plasticity in the cerebellum-like mammalian dorsal cochlear nucleus. Proceedings of the National Academy of Sciences, USA. 2003;100:265–270. doi: 10.1073/pnas.0135345100. Advance online publication. Retrieved December 16, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitton MJ, Caston J, Ruel J, Johnson RM, Pujol R, Puel JL. Salicylate induces tinnitus through activation of cochlear NMDA receptors. Journal of Neuroscience. 2003;23:3944–3952. doi: 10.1523/JNEUROSCI.23-09-03944.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner HE, Harrington IA. Tinnitus in hamsters following exposure to intense sound. Hearing Research. 2002;170:83–95. doi: 10.1016/s0378-5955(02)00343-x. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Brennan JF, Sasaki CT. An animal model for tinnitus. Laryngoscope. 1988;98:280–286. doi: 10.1288/00005537-198803000-00008. [DOI] [PubMed] [Google Scholar]
- Jeanmonod D, Magnin M, Morel A. Low-threshold calcium spike bursts in the human thalamus. Common physiopathology for sensory, motor and limbic positive symptoms. Brain. 1996;119:363–375. doi: 10.1093/brain/119.2.363. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA. Summary of evidence pointing to a role of the dorsal cochlear nucleus in the etiology of tinnitus. Acta Otolaryngologica Supplementum. 2006 December;:20–26. doi: 10.1080/03655230600895309. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Afman CE. Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: A physiological model for tinnitus. Hearing Research. 2000;140:165–172. doi: 10.1016/s0378-5955(99)00197-5. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Godfrey DA, Neumann JB, McCaslin DL, Afman CE, Zhang J. Changes in spontaneous neural activity in the dorsal cochlear nucleus following exposure to intense sound: Relation to threshold shift. Hearing Research. 1998;124:78–84. doi: 10.1016/s0378-5955(98)00119-1. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zacharek MA, Zhang J, Frederick S. Activity in the dorsal cochlear nucleus of hamsters previously tested for tinnitus following intense tone exposure. Neuroscience Letters. 2004;355:121–125. doi: 10.1016/j.neulet.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Manis PB. Transient potassium currents regulate the discharge patterns of dorsal cochlear nucleus pyramidal cells. Journal of Neuroscience. 1999;19:2195–2208. doi: 10.1523/JNEUROSCI.19-06-02195.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RA. Somatic (craniocervical) tinnitus and the dorsal cochlear nucleus hypothesis. American Journal of Otolaryngology. 1999;20:351–362. doi: 10.1016/s0196-0709(99)90074-1. [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Sun W, Cushing R, Salvi R. A novel behavioral paradigm for assessing tinnitus using schedule-induced polydipsia avoidance conditioning (SIP-AC) Hearing Research. 2004;190:109–114. doi: 10.1016/S0378-5955(04)00019-X. [DOI] [PubMed] [Google Scholar]
- Martin WH, Shi Y-B, Burchiel KJ, Anderson VC. In: Hazell J, editor. Deep brain stimulation effects on hearing function and tinnitus; Proceedings of the Sixth International Tinnitus Seminar; London: The Tinnitus and Hyperacusis Centre; 1999. pp. 68–72. [Google Scholar]
- Moller AR, Moller MB, Yokota M. Some forms of tinnitus may involve the extralemniscal auditory pathway. Laryngoscope. 1992;102:1165–1171. doi: 10.1288/00005537-199210000-00012. [DOI] [PubMed] [Google Scholar]
- Moore JK. The primate cochlear nuclei: Loss of lamination as a phylogenetic process. The Journal of Comparative Neurology. 1980;193:609–629. doi: 10.1002/cne.901930303. [DOI] [PubMed] [Google Scholar]
- Moore JK, Osen KK. The cochlear nuclei in man. The American Journal of Anatomy. 1979;154:393–418. doi: 10.1002/aja.1001540306. [DOI] [PubMed] [Google Scholar]
- Prosen C, May B. In: Santi PA, editor. Behavioral and electrophysiological assessment of tinnitus in a mouse model [Abstract]; Abstracts of the Twenty-Eighth Annual Midwinter Research Meeting of the Association for Research in Otolaryngology; Mt. Royal, NJ: Association for Research in Otolarynology; 2005. p. 46. [Google Scholar]
- Quaranta A, Assennato G, Sallustio V. Epidemiology of hearing problems among adults in Italy. Scandinavian Audiology Supplementum. 1996;42:9–13. [PubMed] [Google Scholar]
- Rubio ME, Gudsnuk KA, Smith Y, Ryugo DK. Revealing the molecular layer of the primate dorsal cochlear nucleus. Neuroscience. 2008;154:99–113. doi: 10.1016/j.neuroscience.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttiger L, Ciuffani J, Zenner HP, Knipper M. A behavioral paradigm to judge acute sodium salicylate-induced sound experience in rats: A new approach for an animal model on tinnitus. Hearing Research. 2003;180:39–50. doi: 10.1016/s0378-5955(03)00075-3. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Jacobson GP. Update on tinnitus. Otolaryngology Clinics of North America. 1996;29:455–465. [PubMed] [Google Scholar]
- Shore SE. Multisensory integration in the dorsal cochlear nucleus: Unit responses to acoustic and trigeminal ganglion stimulation. European Journal of Neuroscience. 2005;21:3334–3348. doi: 10.1111/j.1460-9568.2005.04142.x. [DOI] [PubMed] [Google Scholar]
- Shore SE, Zhou J. Somatosensory influence on the cochlear nucleus and beyond. Hearing Research. 2006;216–217:90–99. doi: 10.1016/j.heares.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Soussi T, Otto SR. Effects of electrical brainstem stimulation on tinnitus. Acta Oto-laryngologica. 1994;114:135–140. doi: 10.3109/00016489409126031. [DOI] [PubMed] [Google Scholar]
- Sutherland DP, Glendenning KK, Masterton RB. Role of acoustic striae in hearing: Discrimination of sound-source elevation. Hearing Research. 1998;120:86–108. doi: 10.1016/s0378-5955(98)00056-2. [DOI] [PubMed] [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM. Gap detection deficits in rats with tinnitus: A potential novel screening tool. Behavioral Neuroscience. 2006;120:188–195. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nature Neuroscience. 2004;7:719–725. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Rubio ME, Keen JE, Trussell LO. Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity. Neuron. 2007;54:291–301. doi: 10.1016/j.neuron.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Young ED, Davis KA. Circuitry and function of the dorsal cochlear nucleus. In: Oertel D, Popper AN, Fay RR, editors. Integrative functions in the mammalian auditory pathway. New York: Springer-Verlag; 2001. pp. 166–200. [Google Scholar]
- Young ED, Nelken I, Conley RA. Somatosensory effects on neurons in dorsal cochlear nucleus. Journal of Neurophysiology. 1995;73:743–765. doi: 10.1152/jn.1995.73.2.743. [DOI] [PubMed] [Google Scholar]
- Zacharek MA, Kaltenbach JA, Mathog TA, Zhang J. Effects of cochlear ablation on noise induced hyperactivity in the hamster dorsal cochlear nucleus: Implications for the origin of noise induced tinnitus. Hearing Research. 2002;172:137–143. doi: 10.1016/s0378-5955(02)00575-0. [DOI] [PubMed] [Google Scholar]