Abstract
Throughout spermatogenesis, leptotene spermatocytes must traverse the blood-testis barrier (BTB) at stages VIII–XI to gain entry into the adluminal compartment for continued development. However, the mechanism underlying BTB restructuring remains somewhat elusive. In this study, interleukin 1 alpha (IL1A) was administered intratesticularly to adult rats in order to assess its effects on spermatogenesis. IL1A was shown to perturb Sertoli-germ cell adhesion, resulting in germ cell loss from ~50% of seminiferous tubules by 15 days posttreatment. Equally important, the functional integrity of the BTB was compromised when inulin-fluorescein isothiocyanate was detected in the adluminal compartment of the seminiferous epithelium following its administration via the jugular vein. Interestingly, IL1A did not affect the steady-state levels of proteins that confer BTB function, namely OCLN, CLDN1, F11R, TJP1, and CDH2. Instead, the localizations of OCLN, F11R, and TJP1 in the seminiferous epithelium were altered; these proteins appeared to move away from sites of cell-cell contact. Moreover, IL1A was shown to perturb the orderly arrangement of filamentous actin at the BTB and apical ectoplasmic specialization with distinct areas illustrating loss of actin filaments. Taken collectively, these results suggest that IL1A-induced BTB disruption is not mediated via the reduction of target protein levels. Instead, IL1A’s primary cellular target appears to be the Sertoli cell actin cytoskeleton. It is possible that localized production of IL1A by Sertoli and/or germ cells in vivo results in BTB restructuring, and this may facilitate the movement of leptotene spermatocytes across the BTB.
Keywords: blood-testis barrier, cytoskeleton, ectoplasmic specialization, interleukin 1alpha (IL1A), testis, tight junction
INTRODUCTION
Spermatogenesis is the process by which spermatogonia develop into spermatozoa. In order for spermatogenesis to take place, germ cells must move from the basal to the adluminal compartment of the seminiferous epithelium. This also entails that leptotene spermatocytes traverse the blood-testis barrier (BTB) at stages VIII (late)–XI of the seminiferous epithelial cycle ([1]; for review, [2]). Ultrastructurally, the BTB is composed of tight junctions, ectoplasmic specializations, desmosome-gap junctions, and tubulobulbar complexes present between adjacent Sertoli cells ([3]; for reviews [2, 4]). Their functions are supported by the Sertoli cell cytoskeleton, which in part is composed of filamentous (F-) and globular (G-) actin (for review, [5]). Basically, transmembrane junctional proteins (e.g., OCLN [formerly known as occludin], CLDN1 [formerly known as claudin-1], F11R [formerly known as junction adhesion molecule-1 (JAM-1)], CDH2 [formerly known as N-cadherin] and PVRL [formerly known as nectin]) attach to actin filaments via scaffolding proteins (e.g., TJP1 [formerly known as zonula occludens-1 (ZO-1)], CATN [formerly known as catenin], and AF6 [formerly known as afadin]), and these multiprotein complexes confer stable cell adhesion (for review, [2]). However, during germ cell movement junctions are transiently disassembled, and it appears that proteins within these complexes are rearranged, thereby leading to changes in protein-protein interactions. Thus, BTB integrity is critical not only to spermatogenesis but also to the maintenance of fertility.
Presently, there are several in vivo models that can be used to study BTB dynamics, including the cadmium chloride, glycerol, and OCLN peptide models [6–8]. For instance, i.p. injection of cadmium chloride, an environmental toxicant and endocrine disruptor, compromised the BTB, resulting in irreversible damage [7]. Likewise, intratesticular injection of glycerol adversely affected tight junction fibrils [9], and a synthetic OCLN peptide corresponding to 22-amino-acid residues in the second extracellular loop of rat OCLN had similar consequences on BTB function and spermatogenesis, except that OCLN peptide-mediated effects were shown to be reversible [8, 10]. Although these in vivo models have provided much useful information, they have a number of shortcomings. First, their mechanisms of action are not completely understood. Second, each in vivo model involves introducing a nonphysiological substance into the seminiferous epithelium.
Nevertheless, the presence of various cytokines such as TNF (tumor necrosis factor), TGFB3 (formerly known as transforming growth factor β3 [TGFβ3]), and IL1A (formerly known as interleukin-1α [IL-1α]), as well as their receptors, has been reported previously in the testis (for reviews [11–13]). For instance, TNF, a pro-inflammatory cytokine, produced by Sertoli and germ cells, is known to regulate Sertoli cell secretory function, promote germ cell survival, and function in the FASL (formerly known as Fas-L) signaling cascade (for review [11]). Likewise, both IL1A and IL1B are 17-KDa proteins and are known mediators of inflammation, although they are encoded by separate genes (for reviews [14–16]). Moreover, IL1A is secreted by Sertoli and germ cells [17, 18] and is known to inhibit Leydig cell steroidogenesis, stimulate Sertoli cell TRF (formerly known as transferrin) and IL6 (formerly known as interleukin-6 [IL-6]) production, and promote Sertoli cell proliferation [19–21]. Interestingly, recent studies have demonstrated that TNF [22] and TGFB3 [23] can reversibly disrupt the integrity of the BTB by regulating the steady-state levels of tight junction proteins. Thus, we sought to determine whether IL1A regulates BTB dynamics via a similar mechanism. In this study, we demonstrate that intratesticular administration of IL1A can adversely affect Sertoli cell structure and disrupt the actin cytoskeleton, resulting in the loss of germ cells from the seminiferous epithelium and spermatogenic arrest. This also caused tight junction proteins at the BTB to move away from sites of cell contact without significantly altering their steady-state levels and resulted in BTB disassembly.
MATERIALS AND METHODS
Animals
Sprague-Dawley rats at 90 days of age (~250–280 gm body weight [b.w.]) were purchased from Charles River Laboratories (Kingston, NY) and housed at The Rockefeller University Laboratory Animal Research Center for 24–48 h before use. During the course of experiments, animals had access to standard rat chow and water ad libitum and were exposed to 12L:12D cycles. Following experimental treatment, rats were killed by CO2 asphyxiation as directed in the 2000 Report of the American Veterinary Medical Association Panel on Euthanasia [24], and testes were removed immediately. The use of animals in this study was approved by The Rockefeller University Animal Care and Use Committee with Protocol Numbers 03017 and 06018.
Experimental Treatments of Rats
To assess the effects of IL1A on spermatogenesis, recombinant rat IL1A (three doses at 250 ng per testis with each dose administered 1 day apart in a final volume of 100 μl sterile PBS [pH 7.4]; R&D Systems, Minneapolis, MI) was injected intratesticularly via a 29-gauge syringe to adult rats (n =3 per time point for each experiment). Each injection into the testis was made randomly into the interstitium, and both testes of each animal received IL1A. This regimen of IL1A administration was selected based on results from pilot experiments that used a range of different doses to assess the status of spermatogenesis, changes in testicular morphology, and BTB integrity. Therefore, this treatment regimen was used for all the experiments described in this study unless noted otherwise. Rats were killed at different time points (0, 6, and 10 h and 1, 3, 4, 6, 8, 10, 15, 21, 30, 45, and 90 days) following treatment, and testes were removed and either snap-frozen in liquid nitrogen for lysate preparation/cryosectioning or submerged in Bouin fixative for routine histology. In this experiment, Hour 0 represents the time immediately preceding the first injection of IL1A. This indicates that rats which were killed at 6 and 10 h and 1 day received only one dose of IL1A (250 ng per testis). Control rats received an intratesticular injection of PBS only in a final volume of 100 μl. This in vivo experiment was repeated a total of three times, and statistical analyses were performed with n = 9.
Morphological Analysis
Routine histology was performed to assess the status of spermatogenesis in adult rats following IL1A treatment. In brief, Bouin fixed testes were dehydrated in ascending concentrations of ethanol, cleared, and embedded in Peel-A-Way Micro-Cut paraffin wax (Polysciences, Inc., Warrington, PA). Thereafter, 5-μm sections were cut and stained with hematoxylin and eosin. Sections were mounted with Poly-mount (Polysciences) and examined with an Olympus BX-40 microscope (Olympus Optical Co., Melville, NY). Images were captured with an Olympus DP70 12.5 MPa digital camera (Olympus) and compiled with Adobe Photoshop (Version 7.0, San Jose, CA). For statistical analysis, sections were randomly photographed at low magnification using the microscope’s 10× objective, and the images were enlarged with Adobe Photoshop and printed. At least 1000 tubules from each time point from a total of nine animals were scored either as normal or damaged. Seminiferous tubules were scored as damaged when either one of these two conditions was met: (i) five or more germ cells were present in the tubule lumen or (ii) the orderly arrangement of developing germ cells in the seminiferous epithelium was perturbed.
Lysate Preparation, SDS-PAGE, and Immunoblotting
Testis lysates were prepared in immunoprecipitation lysis buffer using a tissue:buffer ratio of 1:5, and protein concentration was determined by Coomassie blue-dye binding assay using BSA as a standard [25]. All samples within one experiment were processed simultaneously to eliminate interassay variations. Thereafter, equal amounts of lysate were resolved by SDS-polyacrylamide gels under reducing conditions [26]. This was followed by electroblotting onto nitrocellulose membranes (Whatman–Schleider and Schuell, Inc., Keene, NH). Nonspecific binding sites were blocked with 6% nonfat dry milk [w/v] in PBS-Tris buffer (10 mM Tris [pH 7.4] at 22°C containing 10 mM NaH2PO4, 0.15 M NaCl, and 0.1% Tween-20 [v/v]), and blots were incubated in primary antibody overnight at 4°C. Thereafter, blots were washed briefly and incubated in the corresponding secondary antibody (i.e., either bovine anti-mouse, bovine anti-goat, or bovine anti-rabbit IgG-conjugated to horseradish peroxidase; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and immunoreactive proteins were detected by chemiluminescence. In some cases, primary and secondary antibodies were removed from blots following incubation with stripping buffer (62.5 mM Tris-HCl [pH 6.7] at 22°C containing 100 mM 2-mercaptoethanol and 2% SDS [w/v]). Thereafter, blots were washed, blocked, and reprobed with an anti-ACTB (formerly known as actin) antibody to assess equal protein loading. Details on the primary antibodies used for immunoblotting experiments are listed in Table 1.
TABLE 1.
Antibodies used in the present study.
| Vendor | Antibody | Host species | Catalog no. | Lot no. | Usea |
|---|---|---|---|---|---|
| Cell Signaling | CLDN1 | Rabbit | 4933 | 9 | IB |
| Invitrogen | F11R | Rabbit | 36-1700 | 30979650 | IB, IF |
| Invitrogen | CDH2 | Rabbit | 33-3900 | 30778768 | IB |
| Invitrogen | OCLN | Rabbit | 71-1500 | 21074197 | IB, IF |
| Invitrogen | TJP1 | Mouse | 33-9100 | 20269234 | IB |
| Invitrogen | TJP1-FITC | Mouse | 33-9111 | 60102882 | IF |
| Santa Cruz Biotechnology | ACTB | Goat | sc-7210 | G1404 | IB |
IB, Immunoblotting; IF, immunofluorescence.
Assessing Cytoskeletal Integrity In Vivo
To assess cytoskeletal damage following IL1A treatment, sections were stained for F-actin as follows. Testis cryosections were fixed in paraformaldehyde (3.7% [w/v] in PBS [pH 7.4]), washed, and incubated briefly in Triton X-100 (0.2% [v/v] in PBS). Thereafter, sections were stained with rhodamine phalloidin (10 μg/ml in PBS containing 1% BSA [w/v]; Invitrogen–Molecular Probes, Carlsbad, CA). Sections were washed prior to mounting with ProLong Gold anti-fade reagent containing 4′,6′-diamidino-2-phenylindole (DAPI, Invitrogen-Molecular Probes) and examined under a microscope, and images were captured as described above.
Immunofluorescent Microscopy
Immunofluorescent microscopy was performed as described earlier [27] using testes that were snap-frozen in liquid nitrogen and cut at a thickness of 7 μm. Sections were mounted on poly-L-lysine-coated slides, air-dried, and fixed in Bouin fixative, and nonspecific sites were blocked with 10% nonimmune goat serum [v/v]. This was followed by immunostaining with either anti-OCLN/anti-TJP1-FITC or anti-F11R/anti-TJP1-FITC (1:100 each containing 1% nonimmune goat serum [v/v]) antibodies. After a brief washing step, sections were incubated with goat anti-rabbit IgG–Cy3 (1:100 containing 10% nonimmune goat serum [v/v]; Invitrogen, Carlsbad, CA). Immunolocalization of TJP1 did not require the use of a secondary antibody because TJP1 IgG was conjugated directly to FITC. Sections were mounted with anti-fade reagent containing DAPI and examined microscopically, and images were captured. Images were merged using Adobe Photoshop. Controls consisted of sections incubated with either PBS or a combination of rabbit and mouse IgG (1:100 each containing 1% nonimmune goat serum [v/v]) in place of primary antibodies. Details on primary antibodies used in immunofluorescence experiments are listed in Table 1.
Electron Microscopy
Control and IL1A-treated testes (15 days following treatment) were quickly excised from adult rats, decapsulated, and cut into smaller pieces while submerged in a dish containing fixative (0.2 M collidine [pH 7.4] at 22°C containing 3% glutaraldehyde [v/v]). Thereafter, testes were postfixed with osmium, washed extensively with PBS, dehydrated in ascending concentrations of ethanol and impregnated in Epon. After polymerization, ultrathin sections (80 nm) were obtained, collected on nickel grids, and stained with uranyl acetate and lead citrate. Sections were examined microscopically with a JEM 100cx transmission electron microscope (JEOL USA, Inc., Peabody, MA). Electron microscopy was performed at The Rockefeller University Bio-Imaging Resource Center.
Functional Assay to Assess BTB Integrity In Vivo
BTB integrity was assessed by a functional assay as described earlier [22]. Animals (~250–280 gm b.w.) were divided into three groups: (i) control (n =3 per time point), (ii) IL1A treatment – high dose (three doses at 250 ng per testis with each dose administered 1 day apart; n = 3 per time point), and (iii) IL1A treatment – low dose (one dose at 250 ng per testis; n =3 per time point). In this experiment, Week 0 (control) represents the time immediately preceding the first injection of IL1A. At 2, 4, and 6 wk following treatment, three animals from each group were used for functional assays to assess BTB integrity. In brief, rats were anesthetized with ketamine HCl (40–60 mg/kg b.w., i.m.) followed by administration of an analgesic (xylazine, 10–13 mg/kg b.w., i.m.). An incision of ~0.5 cm was made to expose the jugular vein, and each rat received 1 mg of inulin-fluorescein isothiocyanate (FITC; Mr ~5000; Sigma, St. Louis, MO) in a final volume of 100 μl via a 29-gauge syringe. Thereafter, the surgical area was sutured and cleansed with 70% ethanol. Rats were allowed to recover, and ~90 min after surgery, animals were killed, and testes were removed and immediately snap-frozen in liquid nitrogen. Testes were cut at a thickness of 10 μm, sections were mounted with anti-fade reagent containing DAPI, and the presence of inulin-FITC was observed microscopically. At least 100 tubules were examined from each group at 0, 2, 4, and 6 wk following treatment.
Statistical Analysis
Statistical analysis was carried out by one-way ANOVA, followed by Tukey honestly significant test using GB-STAT (Version 7.0; Dynamic Microsystems, Inc., Silver Spring, MD). Densitometric scanning of immuno-blots was performed using SigmaGel (Version 1.0; SPSS, Inc., Chicago, IL). All experiments performed in this study had triplicate data points, and each experiment was repeated three times.
RESULTS
IL1A Affects Spermatogenesis and Germ Cell Adhesion
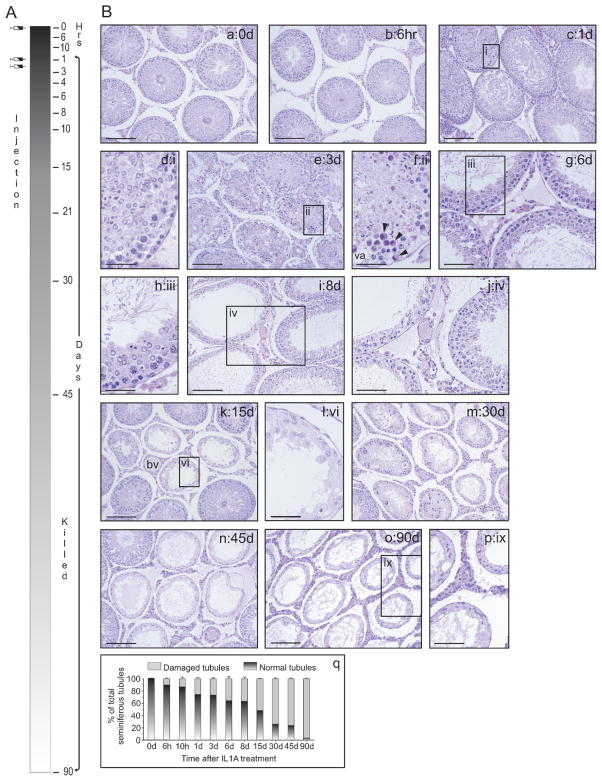
When IL1A was administered to adult rats (three doses at 250 ng per testis with each dose administered 1 day apart) and animals were killed at specified time points (0, 6, and 10 h and 1, 3, 4, 6, 8, 10, 15, 21, 30, 45, and 90 days; Fig. 1A), histological changes were noted ~1 day after the first intratesticular injection (Fig. 1B, c and d) when compared to the control (Fig. 1B, a). It should be noted that these rats received only a single dose of IL1A. Morphological changes were not detected 6 h posttreatment (Fig. 1B, b). Interestingly, the adhesion of elongated spermatids to Sertoli cells at stages VII–VIII of the epithelial cycle was shown to be affected before adhesion between Sertoli cells and other germ cell types was compromised. In other stages, however, secondary spermatocytes and round spermatids were detected in the tubule lumen, while elongated spermatids remained attached to the seminiferous epithelium. By 3 days posttreatment, ~30% of seminiferous tubules showed signs of germ cell loss (Fig. 1B, e, f, and q), with round and elongating/elongated spermatids found in the tubule lumen. Sertoli cell vacuoles were detected, and the orderly arrangement of germ cells remaining in the seminiferous epithelium was perturbed (Fig. 1B, e and f). Multinucleated germ cells were also visible (Fig. 1B, f, see arrowheads). By 6–8 days, sloughing of round and elongating/elongated spermatids, as well as pachytene spermatocytes, from the epithelium continued (Fig. 1B, g–j). By 15 days, ~50% of seminiferous tubules showed signs of damage (Fig. 1B, k, l, and q), and by 30–45 days the percentage of damaged tubules increased to ~70% (Fig. 1B, m, n, and q). By 90 days following IL1A administration, virtually all tubules were devoid of most germ cell types except spermatogonia (Fig. 1B, o, p, and q), and there were no signs of spermatogenic recovery at 90 days. Boxed areas labeled i, ii, iii, iv, vi, and ix are shown magnified in d, f, h, j, l, and p, respectively. Other signs of damage, such as an inflammatory response (e.g., infiltration of leukocytes) into the seminiferous epithelium were not apparent following careful histological examination. However, leukocytes were detected in the interstitium in a small percentage of IL1A-treated animals up to 1 day posttreatment (data not shown). This interstitial damage was not extensive and was found to surround only four to six tubules within an entire cross section of the testis when examined with the microscope’s 10× objective. All animals responded to IL1A treatment, and no animal-to-animal variations were detected in this study. Also, no morphological changes were visible in control rats that received an intratesticular injection of PBS only.
FIG. 1.
Morphological analysis of adult rat testes following intratesticular administration of IL1A. A) A time line describing the experimental treatment of animals with IL1A and their subsequent termination points. To assess the effects of IL1A on spermatogenesis, recombinant rat IL1A (three doses at 250 ng per testis with each dose administered 1 day apart) was injected intratesticularly to adult rats (n=3 per time point). In this experiment, Hour 0 represented the time immediately preceding the first injection of IL1A. Thereafter, rats were killed at 6 and 10 h and 1, 3, 4, 6, 8, 10, 15, 21, 30, 45, and 90 days posttreatment. IL1A-treated testes were processed for routine histology and hematoxylin and eosin staining. B) Histological analysis of cross sections from control (a) and IL1A-treated testes (b–p). IL1A was shown to perturb Sertoli-germ cell adhesion (c–p). q) A graph describing the percentage of normal versus damaged tubules following IL1A treatment. Boxed areas labeled i, ii, iii, iv, vi, and ix are shown magnified in d, f, h, j, l, and p, respectively. Bars=165 μm (a, b, and e), 125 μm (c), 30 μm (d, f, and h), 80 μm (i), 60 μm (g and j), 135 μm (k), 35 μm (l), 100 μm (m–o), and 50 μm (p). va, Sertoli cell vacuole; bv, blood vessel.
IL1A Does Not Affect the Steady-State Levels of Proteins That Confer BTB Function
To determine whether BTB integrity is affected by IL1A, we assessed the steady-state levels of several tight junction-and ectoplasmic specialization-associated proteins, including OCLN, CLDN1, F11R, TJP1, and CDH2, by immunoblotting. Unexpectedly, no changes in the steady-state levels of these proteins were detected up to 45 days posttreatment (Fig. 2). ACTB served as an indicator of equal protein loading onto SDS-polyacrylamide gels (data not shown).
FIG. 2.
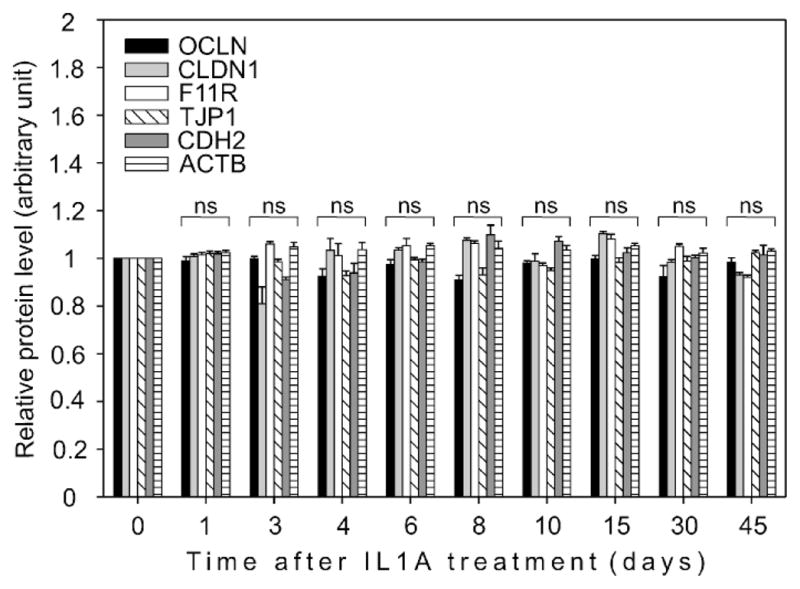
A graph representing densitometrically scanned immunoblot results. A study to examine the effects of IL1A on tight junction- and ectoplasmic specialization-associated proteins in the testis. IL1A (three doses at 250 ng per testis) was administered intratesticularly, animals were killed at different time points, and testis lysates were prepared as described. The steady-state levels of OCLN, CLDN1, F11R, TJP1, and CDH2 did not change significantly following IL1A treatment. Each data point corresponds to an average ± SD value obtained from three different experiments and normalized against the corresponding control (Day 0). The control was arbitrarily set at 1. ns, Not significantly different from control.
F-Actin Dynamics Are Perturbed Following IL1A Treatment
Actin filaments play a critical role in the maintenance of Sertoli cell shape. Equally important, they provide a cytoplasmic attachment site for transmembrane proteins and their adaptors, and this confers Sertoli-Sertoli and Sertoli-germ cell adhesion. In the control testis, F-actin localized to basal and adluminal compartments, consistent with a previously published report that described its presence at the BTB (Fig. 3a, see arrowheads) and apical ectoplasmic specialization (Fig. 3a, see asterisks) [5], respectively. F-actin also associated with peritubular myoid cells within the tunica propria (Fig. 3a, see arrows), as well as with endothelial cells that comprise microvessels found in the interstitial space (Fig. 3a). By 3 (Fig. 3c), 8 (Fig. 3e), and 30 (Fig. 3g) days after IL1A treatment, the orderly arrangement of F-actin at the BTB and the apical ectoplasmic specialization in Sertoli cells was affected severely, with areas illustrating loss of actin filaments (Fig. 3g, see arrowheads). Figure 3, b, d, f, and h, are DAPI-stained cross sections corresponding to Figure 3, a, c, e, and g, respectively.
FIG. 3.
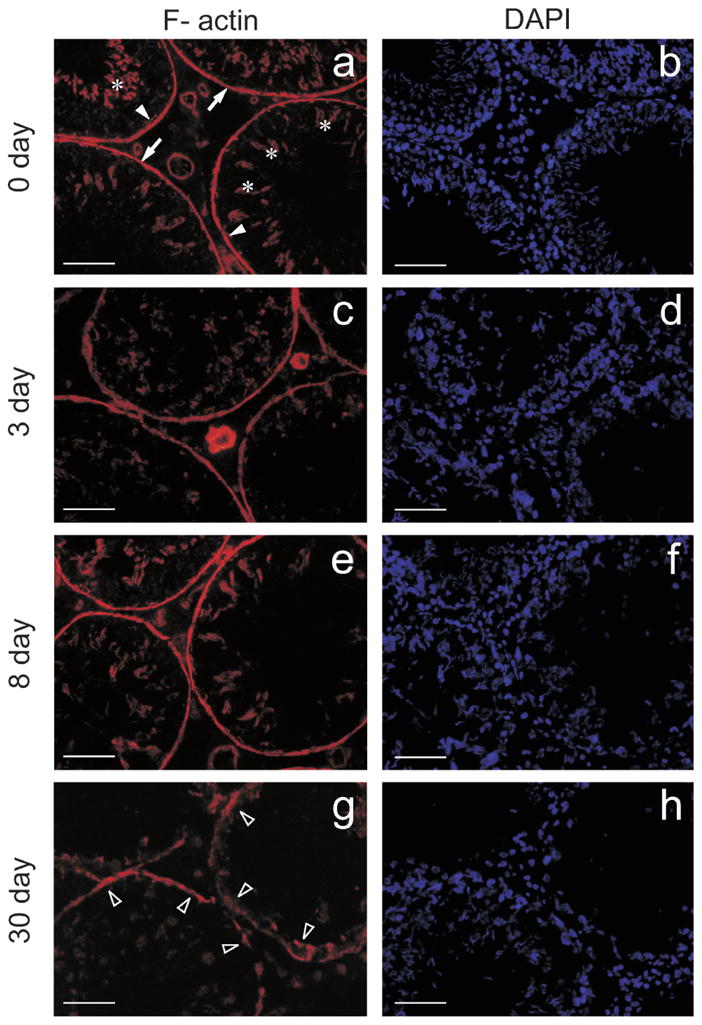
A study to assess the integrity of the F-actin cytoskeleton following administration of IL1A. IL1A (three doses at 250 ng per testis) was administered intratesticularly, animals were killed at different time points, and testes were snap-frozen in liquid nitrogen for cryosectioning. F-actin was visualized with rhodamine phalloidin. Cross sections from control (Day 0; a) and IL1A-treated testes (c, e, and g). In the control testis, F-actin localized to the BTB (a, see arrowheads) and apical ectoplasmic specialization (a, see asterisks). F-actin also associated with peritubular myoid cells within the tunica propria (a, see arrows), as well as with endothelial cells, which comprise microvessels found in the interstitial space (a). c, e, and g are cross sections from testes at 3, 8, and 30 days following IL1A administration, respectively. IL1A affected the orderly arrangement of F-actin by 3 days (c), and similar changes were detected at 8 (e) and 30 (g, see arrowheads) days posttreatment. b, d, f, and h are DAPI-stained cross sections corresponding to a, c, e, and g, respectively. Bars = 55 μm (a–f) and 40 μm (g and h).
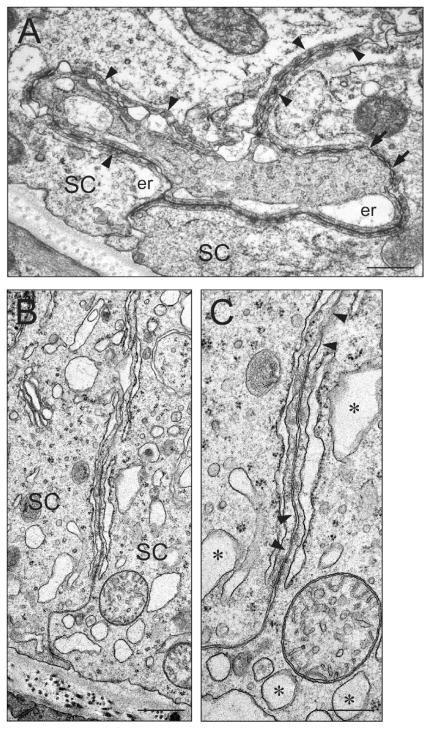
These results were corroborated by electron microscopy experiments (Fig. 4). In the control testis, the BTB was characterized by the coexistence of tight junctions (Fig. 4A; see arrows) and ectoplasmic specializations (Fig. 4A; see arrowheads) where actin filament bundles were sandwiched between the endoplasmic reticulum and the Sertoli cell plasma membrane. In the IL1A-treated testis, the orderly arrangement of F-actin at the BTB was affected with distinct areas illustrating loss of actin filaments (Fig. 4, B and C; see arrowheads in C), consistent with immunofluorescent microscopy results shown in Figure 3. Sertoli cell vacuoles (Fig. 4, B and C; see asterisks in C) were also detected in the IL1A-treated testis (also see Fig. 1).
FIG. 4.
An ultrastructural study to assess the integrity of the BTB following administration of IL1A. IL1A (three doses at 250 ng per testis) was administered intratesticularly, and animals were killed 15 days after treatment for electron microscopy. A) A micrograph of the BTB from a control testis. The BTB is characterized by the coexistence of Sertoli cell tight junctions (see arrows) and ectoplasmic specializations (see arrowheads) where actin filament bundles are sandwiched between the endoplasmic reticulum and the Sertoli cell plasma membrane. B, C) Micrographs of the BTB from an IL1A-treated testis. C is a magnified view of B. In the IL1A-treated testis, the orderly arrangement of F-actin was affected, with distinct areas illustrating loss of actin filaments (C, see arrowheads). Sertoli cell vacuoles (C, see asterisks) were also detected. Bars =0.5 μm (A and B) and 0.25 μm (C). er, Endoplasmic reticulum; SC, Sertoli cell.
Changes in the Localization of Tight Junction Proteins Following IL1A Administration
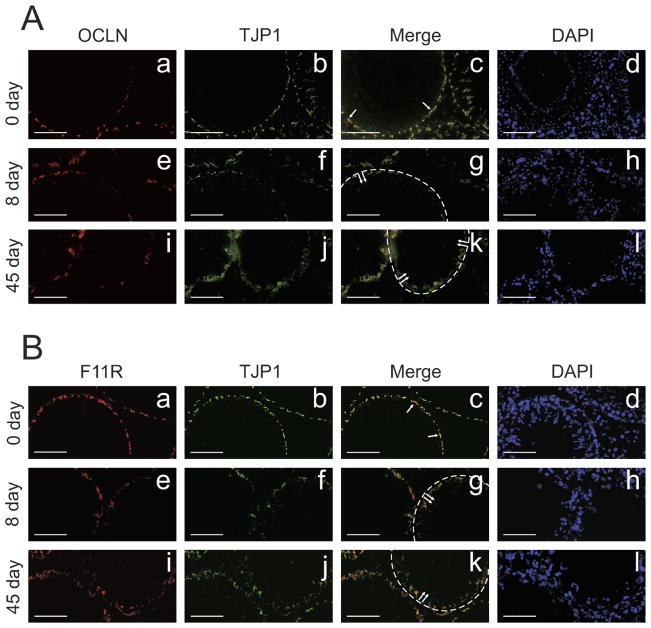
The localization of tight junction-associated proteins at the BTB was examined by immunofluorescent microscopy following IL1A treatment. In the control testis, OCLN (Fig. 5A, a) and TJP1 (Fig. 5A, b) colocalized at the basal compartment (Fig. 5A, c, see arrows), consistent with their reported localization at the BTB ([28]; for review, [2]). However, 8 (Fig. 5A, e–g) and 45 (Fig. 5A, i–k) days after IL1A treatment, OCLN and TJP1 immunostaining appeared diffuse and not well-defined (Fig. 5A, g and k, see dotted lines and arrows). These observations were consistent with a subsequent experiment in which the localization of another tight junction-associated protein, F11R, was examined by immunofluorescent microscopy. In the control testis, F11R localization was noted at the BTB (Fig. 5B, a) as reported earlier [29]. Following IL1A treatment at 8 (Fig. 5B, e) and 45 (Fig. 5B, i) days, F11R appeared to move away from the BTB (Fig. 5B, g and k, see dotted lines and arrows), and the distinct immunoreactive signal that was seen in the control testis (Fig. 5B, a) was no longer visible in IL1A-treated testes (Fig. 5B, e and i). TJP1 immunostaining (Fig. 5B, b, f, and j) was consistent with that shown in Figure 5A, b, f, and j. Figure 5A, c, g, and k, are merged images corresponding to Figure 5A, a/b, e/f, and i/j, respectively. Figure 5B, c, g, and k, are merged images corresponding to Figure 5B, a/b, e/f, and i/j, respectively. DAPI was used as an indicator of germ cell loss following IL1A treatment (Fig. 5A, d, h, and l; Fig. 5B, d, h, and l).
FIG. 5.
A study to examine the localization of tight junction-associated proteins at the BTB following administration of IL1A. IL1A (three doses at 250 ng per testis) was administered intratesticularly, animals were killed at different time points, and testes were snap-frozen in liquid nitrogen for cryosectioning. Immunofluorescent microscopy was performed as described in Materials and Methods. A) Co-localization of OCLN and TJP1 in control (a–c) and IL1A-treated testes (e–g, i–k). B) Colocalization of F11R and TJP1 in control (a–c) and IL1A-treated testes (e–g, i–k). In the control testis, OCLN (A, a), TJP1 (A, b and B, b), and F11R (B, a) colocalized at the BTB (A, c and B, c, see arrows). Following administration of IL1A at 8 and 45 days posttreatment, the localization of OCLN (A, e and i), TJP1 (A, f and j, and B, f and j), and F11R (B, e and i) appeared diffuse. In other words, it appeared as if these proteins had moved away from the BTB (A, g and k, and B, g and k, see dotted lines and double arrows). A, c, g, and k, and B, c, g, and k represent merged images. A, d, h, and l, and B, d, h, and l are DAPI-stained cross sections corresponding to A, a–c, e–g, and i–k, and B, a–c, e–g, and i–k, respectively. Bars =70 μm (A, a–h, and B, a–h) and 60 μm (A, i–l, and B, i–l).
IL1A Compromises the Functional Integrity of the BTB
To monitor the integrity of the BTB, a functional assay was used that assessed the ability of the BTB to prevent the diffusion of inulin-FITC from the systemic circulation into the adluminal compartment of the seminiferous epithelium. In this study, this was the only experiment that used two different IL1A treatment regimens: (i) low dose (one dose at 250 ng per testis) and (ii) high dose (three doses at 250 ng per testis with each dose administered 1 day apart). In the control testis, inulin-FITC surrounded the periphery of seminiferous tubules as indicated by the dotted-line circle and did not enter the adluminal compartment (Fig. 6a), illustrating the presence of a functional BTB. Moreover, when rats received a low dose of IL1A, the passage of inulin-FITC into the adluminal compartment was restricted by the BTB at 2 (Fig. 6, b–d), 4 (Fig. 6, e–g), and 6 (data not shown) wk following treatment. On the other hand, when a high dose of IL1A was used, inulin-FITC was found within the adluminal compartment at 2, 4, and 6 wk posttreatment (Fig. 6, h–p, see arrows in k and n). Dotted lines encircle the periphery of seminiferous tubules in Figure 6, k–p. Figure 6, c, f, i, l, and o are DAPI-stained cross sections corresponding to Figure 6, b, e, h, k, and n, respectively. Figure 6, d, g, j, m, and p are merged images corresponding to Figure 6, b/c, e/f, h/i, k/l, and n/o, respectively.
FIG. 6.
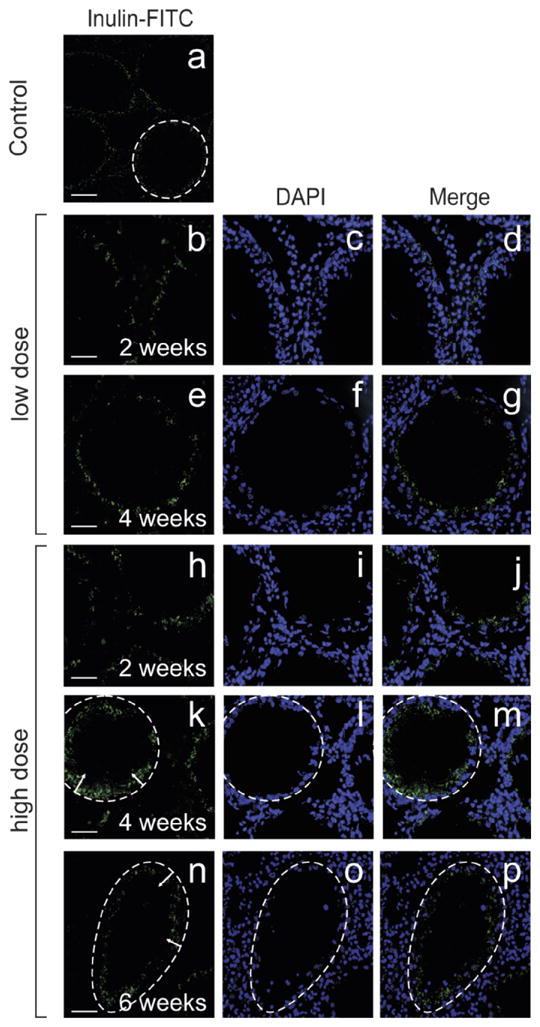
A study to assess the functional integrity of the BTB following administration of IL1A. In this experiment, two different IL1A treatment regimens were used: (i) low dose (one dose at 250 ng per testis) and (ii) high dose (three doses at 250 ng per testis with each dose administered 1 day apart). IL1A was administered intratesticularly, and animals were killed 2, 4, and 6 wk posttreatment. To assess BTB integrity, inulin-FITC was administered via the jugular vein. Animals were killed shortly thereafter, and testes were removed and snap-frozen in liquid nitrogen for cryosectioning. a) Micrograph of a control testis following an inulin-FITC experiment. b–g) Micrographs of testes 2 (b–d) and 4 (e–g) wk following administration of a low dose of IL1A. Inulin-FITC did not enter the adluminal compartment, illustrating the presence of a functional BTB. h–p) Micrographs of testes 2 (h–j), 4- (k–m), and 6- (n–p) wk following administration of a high dose of IL1A. Inulin–FITC penetrated the BTB and entered into the adluminal compartment of the seminiferous epithelium. The periphery of seminiferous tubules is outlined with dotted lines (a and k–p), and arrows indicate the relative distance that inulin-FITC had traveled from the periphery. c, f, i, l, and o are DAPI-stained cross sections corresponding to b, e, h, k, and n, respectively. d, g, j, m, and p represent merged images corresponding to b/c, e/f, h/i, k/l, and n/o, respectively. Bars = 80 μm (a), 45 μm (b and h), 35 μm (e), 40 μm (k), and 25 μm (n).
DISCUSSION
In this study, we report that Sertoli-germ cell adhesion and BTB function were compromised following intratesticular injection of IL1A. Interestingly, sloughing of elongated spermatids from the seminiferous epithelium at stages VII–VIII of the epithelial cycle was noted before adhesion between Sertoli cells and other germ cell types was significantly affected. However, at other stages, such as at stage V when elongated spermatids are embedded within Sertoli cell crypts and closest to the BTB, secondary spermatocytes and round spermatids were detected in the tubule lumen, while more developed germ cells (e.g., elongated spermatids) remained attached to the seminiferous epithelium. This seemingly suggests that IL1A affects adhesion between Sertoli cells and different types of germ cells differently and that the effects of this cytokine on spermatogenesis are stage-related. It should also be noted that affected tubules were initially restricted to sites of IL1A injection, but spermatogenic arrest quickly spread to adjacent areas. For instance, as much as ~50% of tubules examined microscopically showed signs of damage by 15 days posttreatment. With time, the number of damaged tubules increased steadily, and by 90 days virtually all tubules were devoid of germ cells. Other histological manifestations of IL1A-induced injury in the testis included Sertoli cell vacuolization, Sertoli cell cytoskeleton collapse, and the presence of multinucleated germ cells.
Recent studies have shown that intraperitoneal injection of rats with a sublethal dose of lipopolysaccharides (LPS), a constituent of the cell wall of Gram-negative bacteria and stimulator of IL1A production [20], initiated a systemic inflammatory response. This resulted in a stage-dependent loss of germ cells and spermatogenic arrest [30, 31]. For instance, 7 days after LPS treatment, spermatocytes and round spermatids accumulated in the tubule lumen [31], and the Sertoli cell has been shown to be the primary cellular target of LPS-induced damage in the testis [32]. Thus, it is not surprising that Sertoli-germ cell adhesion was affected following administration of an acute dose of IL1A. Nevertheless, testes from IL1A-treated rats did not show signs of spermatogenic recovery up to 90 days posttreatment, whereas testes from rats treated with LPS were able to recover from LPS-induced systemic inflammation and spermatogenic arrest [31].
Additionally, the effects of IL1A on spermatogenesis in vivo are different from those of TNF and TGFB. Unlike TNF and TGFB, whose effects on BTB integrity are relatively rapid and reversible [22, 23], IL1A-mediated damage in the seminiferous epithelium occurred gradually, spanning the course of several weeks. Recent studies have shown that the adverse effects of TNF and TGFB3 were mediated via a decrease in the steady-state levels of transmembrane proteins, as were their associated adaptors, at the BTB [22, 23]. On the contrary, IL1A did not significantly change the levels of tight junction- (e.g., OCLN, F11R, CLDN1, and TJP1) or ectoplasmic specialization- (e.g., CDH2) associated proteins. All of these proteins are expressed only by Sertoli cells (for review, [2]) except CDH2, whose expression was also detected in germ cells [33]. Although the level of CDH2 did not change significantly following IL1A treatment, these results may also suggest that the level of CDH2 had increased, that is, if the loss of germ cells from the seminiferous epithelium is taken into consideration. Nevertheless, the localizations of OCLN, F11R, and TJP1 in the seminiferous epithelium had changed. The immunofluorescent signals of these proteins became diffuse when compared to the corresponding control, revealing that these proteins had assumed a more cytoplasmic localization. Taken collectively, these results seemingly suggest that IL1A-induced BTB disruption is mediated via a different mechanism from those previously described for TNF and TGFB.
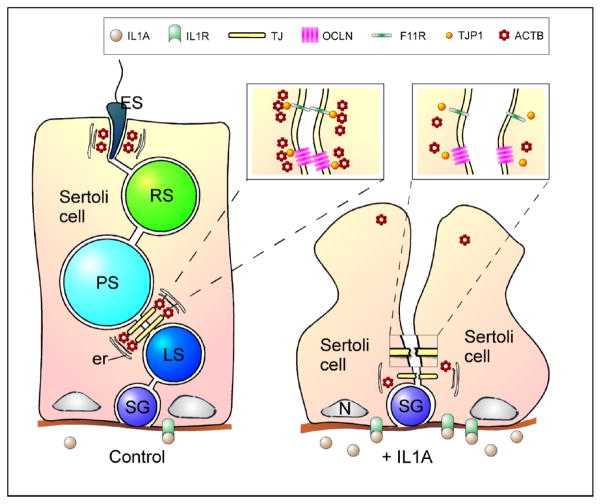
Although the mechanism underlying IL1A-mediated effects on BTB function has not yet been completely defined, we show in this study that BTB function was compromised because IL1A adversely affected the Sertoli cell actin cytoskeleton (Fig. 7) when assessed by immunofluorescent and electron microscopy. Recently, there has been increasing evidence revealing the critical role of actin in the function of cell junctions in other epithelia (for review, see [34]). For example, treatment of epithelial cells with an actin-perturbing pharmacological agent such as cytochalasin D resulted in a decrease in transepithelial electrical resistance and the disassembly of tight junctions in vitro [35]. Thus, the actin cytoskeleton is required for the function of tight junctions. Presently, it is not known whether IL1A can also affect microtubule and intermediate filament networks in Sertoli cells or whether other cytokines such as TNF and TGFB can perturb actin dynamics.
FIG. 7.
A schematic illustration describing a possible mechanism used by IL1A to regulate BTB dynamics. ACTB, actin; er, endoplasmic reticulum; ES, elongated spermatid; F11R, F11 receptor; IL1A, interleukin-1α; IL1R, interleukin-1 receptor; LS, leptotene spermatocyte; N, nucleus; OCLN, occludin; PS, pachytene spermatocyte; RS, round spermatid; SG, spermatogonium; TJ, tight junction; TJP1, tight junction protein 1.
In this context, it is interesting to note that cross-talk between IL1A, TGFB, and TNF signaling cascades downstream have been reported in other in vitro systems [36–38], and this is perhaps the primary reason behind our interest in examining the effects of IL1A, and not other cytokines, on spermatogenesis. Whether this cross-talk also applies to the testis is presently unknown, but restructuring of the BTB, especially when leptotene spermatocytes must traverse the BTB at stages VIII–XI [1], is an extremely complicated event that requires numerous players. Nevertheless, intratesticular administration of acute doses of cytokine, whether it is IL1A, TNF, or TGFB, has been shown to “open” the BTB. Taken collectively, these data suggest that IL1A facilitates the opening of the BTB by affecting the actin cytoskeleton (Fig. 7), whereas TNF and TGFB do so by lowering the steady-state levels of transmembrane proteins such as OCLN.
Footnotes
Supported in part by grants from the CONRAD Program (CICCR, CIG-01-74) to D.D.M. and from the National Institutes of Health (NICHD, U54 HD029990 and U01 HD045908) to C.Y.C. Results reported herein were part of O.S.’s graduate studies performed in the laboratory of D.D.M. and submitted to Pondicherry University for the partial fulfillment of requirements for a Doctor of Philosophy degree.
References
- 1.Russell LD. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat. 1977;148:313–328. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- 2.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 3.Russell LD. Observations on the inter-relationships of Sertoli cells at the level of the blood-testis barrier: evidence for formation and resorption of Sertoli-Sertoli tubulobulbar complexes during the spermatogenic cycle of the rat. Am J Anat. 1979;155:259–279. doi: 10.1002/aja.1001550208. [DOI] [PubMed] [Google Scholar]
- 4.Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int J Cytol. 1985;94:177–211. doi: 10.1016/s0074-7696(08)60397-6. [DOI] [PubMed] [Google Scholar]
- 5.Vogl AW, Pfeiffer DC, Redenbach DM, Grove BD. Sertoli cell cytoskeleton. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater, Florida: Cache River Press; 1993. pp. 39–86. [Google Scholar]
- 6.Wiebe JP, Barr KJ. Suppression of spermatogenesis without inhibition of steroidogenesis by 1,2,3-trihydroxypropane solution. Life Sci. 1984;34:1747–1754. doi: 10.1016/0024-3205(84)90574-5. [DOI] [PubMed] [Google Scholar]
- 7.Setchell BP, Waites GMH. Changes in the permeability of the testicular capillaries and of the “blood-testis barrier” after injection of cadmium chloride in the rat. J Endocrinol. 1970;47:81–86. doi: 10.1677/joe.0.0470081. [DOI] [PubMed] [Google Scholar]
- 8.Wong CH, Mruk DD, Lee WM, Cheng CY. Targeted and reversible disruption of the blood-testis barrier by an FSH mutant-occludin peptide conjugate. FASEB J. 2007;21:438–448. doi: 10.1096/fj.05-4144com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiebe JP, Kowalik A, Gallardi RL, Egeler O, Clubb BH. Glycerol disrupts tight junction-associated actin microfilaments, occludin, and microtubules in Sertoli cells. J Androl. 2000;21:625–635. [PubMed] [Google Scholar]
- 10.Chung NP, Mruk D, Mo MY, Lee WM, Cheng CY. A 22-amino acid synthetic peptide corresponding to the second extracellular loop of rat occludin perturbs the blood-testis barrier and disrupts spermatogenesis reversibly in vivo. Biol Reprod. 2001;65:1340–1351. doi: 10.1095/biolreprod65.5.1340. [DOI] [PubMed] [Google Scholar]
- 11.Hedger MP, Meinhardt A. Cytokines and the immune-testicular axis. J Reprod Immunol. 2003;58:1–26. doi: 10.1016/s0165-0378(02)00060-8. [DOI] [PubMed] [Google Scholar]
- 12.Pollanen P, von Euler M, Soder O. Testicular immunoregulatory factors. J Reprod Immunol. 1990;18:51–76. doi: 10.1016/0165-0378(90)90024-z. [DOI] [PubMed] [Google Scholar]
- 13.Itman C, Mendis S, Barakat B, Loveland KL. All in the family: TGF-β family action in testis development. Reproduction. 2006;132:233–246. doi: 10.1530/rep.1.01075. [DOI] [PubMed] [Google Scholar]
- 14.Dinarello CA. IL-1α. In: Durum SK, Hirano T, Vilcek J, Nicola NA, editors. Cytokine Reference, vol. 1: Ligands. New York: Academic Press; 2001. pp. 307–318. [Google Scholar]
- 15.Dinarello CA. IL-1β. In: Durum SK, Hirano T, Vilcek J, Nicola NA, editors. Cytokine Reference, vol. 1: Ligands. New York: Academic Press; 2001. pp. 351–374. [Google Scholar]
- 16.Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20(suppl 27):S1–S13. [PubMed] [Google Scholar]
- 17.Cudicini C, Lejeune H, Gomez E, Bosmans E, Ballet F, Saez J, Jegou B. Human Leydig cells and Sertoli cells are producers of interleukins-1 and -6. J Clin Endocrinol Metab. 1997;82:1426–1433. doi: 10.1210/jcem.82.5.3938. [DOI] [PubMed] [Google Scholar]
- 18.Huleihel M, Lunenfeld E, Horowitz S, Levy A, Potashnik G, Glezerman M. Production of interleukin-1-like molecules by human sperm cells. Fertil Steril. 2000;73:1132–1137. doi: 10.1016/s0015-0282(00)00499-4. [DOI] [PubMed] [Google Scholar]
- 19.Calkins JH, Guo H, Sigel MM, Lin T. Differential effects of recombinant interleukin-1a and -b on Leydig cell function. Biochem Biophys Res Commun. 1990;167:548–553. doi: 10.1016/0006-291x(90)92059-9. [DOI] [PubMed] [Google Scholar]
- 20.Syed V, Stephan JP, Gerard N, Legrand A, Parvinen M, Bardin CW, Jegou B. Residual bodies activate Sertoli cell interleukin-1α (IL-1α) release, which triggers IL-6 production by an autocrine mechanism, through the lipoxygenase pathway. Endocrinology. 1995;136:3070–3078. doi: 10.1210/endo.136.7.7789334. [DOI] [PubMed] [Google Scholar]
- 21.Huleihel M, Lunenfeld E. Involvement of intratesticular IL-1 system in the regulation of Sertoli cell functions. Mol Cell Endocrinol. 2002;22:125–132. doi: 10.1016/s0303-7207(01)00690-6. [DOI] [PubMed] [Google Scholar]
- 22.Li MW, Xia W, Mruk DD, Wang CQ, Yan HH, Siu MK, Lui WY, Lee WM, Cheng CY. Tumor necrosis factor-a reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol. 2006;190:313–329. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- 23.Xia W, Mruk DD, Lee WM, Cheng CY. Differential interactions between transforming growth factor-β3/TβR1, TAB1, and CD2AP disrupt blood-testis barrier and Sertoli-germ cell adhesion. J Biol Chem. 2006;281:6799–6813. doi: 10.1074/jbc.M601618200. [DOI] [PubMed] [Google Scholar]
- 24.Beaver BV, Reed W, Leary S, McKiernan B, Bain F, Schultz R, Bennett BT, Pascoe P, Shull E, Cork LC, Francis-Floyd R, Amass KD, et al. 2000 report of the American Veterinary Medical Association Panel in Euthanasia. J Am Vet Med Assoc. 2001;218:669–696. [Google Scholar]
- 25.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Sarkar O, Xia W, Mruk DD. Adjudin-mediated junction restructuring in the seminiferous epithelium leads to displacement of soluble guanylate cyclase from adherens junctions. J Cell Physiol. 2006;208:175–187. doi: 10.1002/jcp.20651. [DOI] [PubMed] [Google Scholar]
- 28.Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Inazawa J, Fujimoto K, Tsukita S. Mammalian occludin in epithelial cells: its expression and subcellular distribution. Eur J Cell Biol. 1997;73:222–231. [PubMed] [Google Scholar]
- 29.Xia W, Wong CH, Lee NP, Lee WM, Cheng CY. Disruption of Sertoli-germ cell adhesion function in the seminiferous epithelium of the rat testis can be limited to adherens junctions without affecting the blood-testis barrier integrity: an in vivo study using an androgen suppression model. J Cell Physiol. 2005;205:141–157. doi: 10.1002/jcp.20377. [DOI] [PubMed] [Google Scholar]
- 30.O’Bryan MK, Schlatt S, Phillips DJ, de Kretser DM, Hedger MP. Bacterial lipopolysaccharide-induced inflammation compromises testicular function at multiple levels in vivo. Endocrinology. 2000;141:238–246. doi: 10.1210/endo.141.1.7240. [DOI] [PubMed] [Google Scholar]
- 31.Liew SH, Meachem SJ, Hedger MP. A stereological analysis of the response of spermatogenesis to an acute inflammatory episode in adult rats. J Androl. 2007;28:176–185. doi: 10.2164/jandrol.106.000752. [DOI] [PubMed] [Google Scholar]
- 32.Syed V, Gerard N, Kaipia A, Bardin CW, Parvinen M, Jegou B. Identification, ontogeny, and regulation of an interleukin-6-like factor in the rat seminiferous tubule. Endocrinology. 1993;132:293–299. doi: 10.1210/endo.132.1.8380379. [DOI] [PubMed] [Google Scholar]
- 33.Chung SS, Mo MY, Silvestrini B, Lee WM, Cheng CY. Rat testicular N-cadherin: its complementary deoxyribonucleic acid cloning and regulation. Endocrinology. 1998;139:1853–1862. doi: 10.1210/endo.139.4.5958. [DOI] [PubMed] [Google Scholar]
- 34.Fanning AS. Organization and regulation of the tight junction by the actin-myosin cytoskeleton. In: Cereijido M, Anderson J, editors. Tight Junctions. New York: CRC Press; 2001. pp. 265–284. [Google Scholar]
- 35.Stevenson BR, Begg DA. Concentration-dependent effects of cytochalasin D on tight junctions and actin filaments in MDCK epithelial cells. J Cell Sci. 1994;107:367–375. doi: 10.1242/jcs.107.3.367. [DOI] [PubMed] [Google Scholar]
- 36.Lu T, Tian L, Han Y, Vogelbaum M, Stark GR. Dose-dependent cross-talk between the transforming growth factor-β and interleukin-1 signaling pathways. Proc Natl Acad Sci U S A. 2007;104:4365–4370. doi: 10.1073/pnas.0700118104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishida Y, Kondo T, Kimura A, Matsushima K, Mukaida N. Absence of IL-1 receptor antagonist impaired wound healing along with aberrant NF-kB activation and a reciprocal suppression of TGF-β signal pathway. J Immunol. 2006;176:5598–5606. doi: 10.4049/jimmunol.176.9.5598. [DOI] [PubMed] [Google Scholar]
- 38.Janes KA, Gaudet S, Albeck JG, Nielsen UB, Lauffenburger DA, Sorger PK. The response of human epithelial cells to TNF involves an inducible autocrine cascade. Cell. 2006;124:1225–1239. doi: 10.1016/j.cell.2006.01.041. [DOI] [PubMed] [Google Scholar]