Abstract
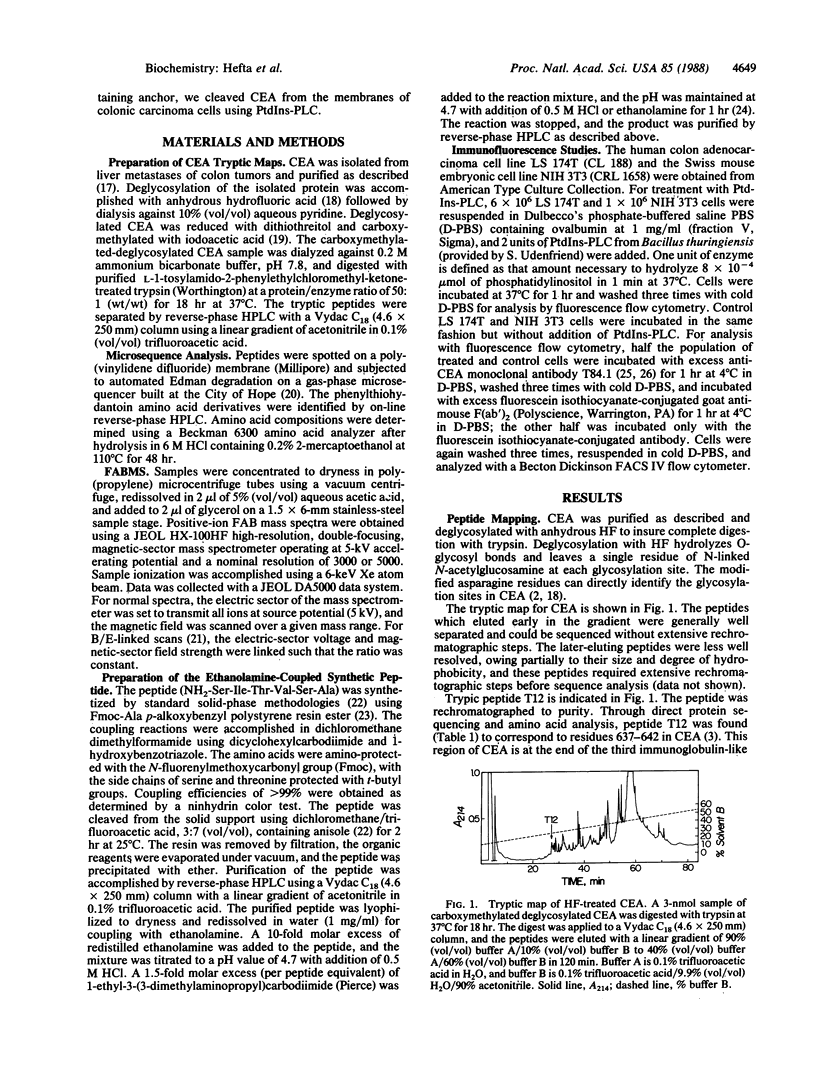
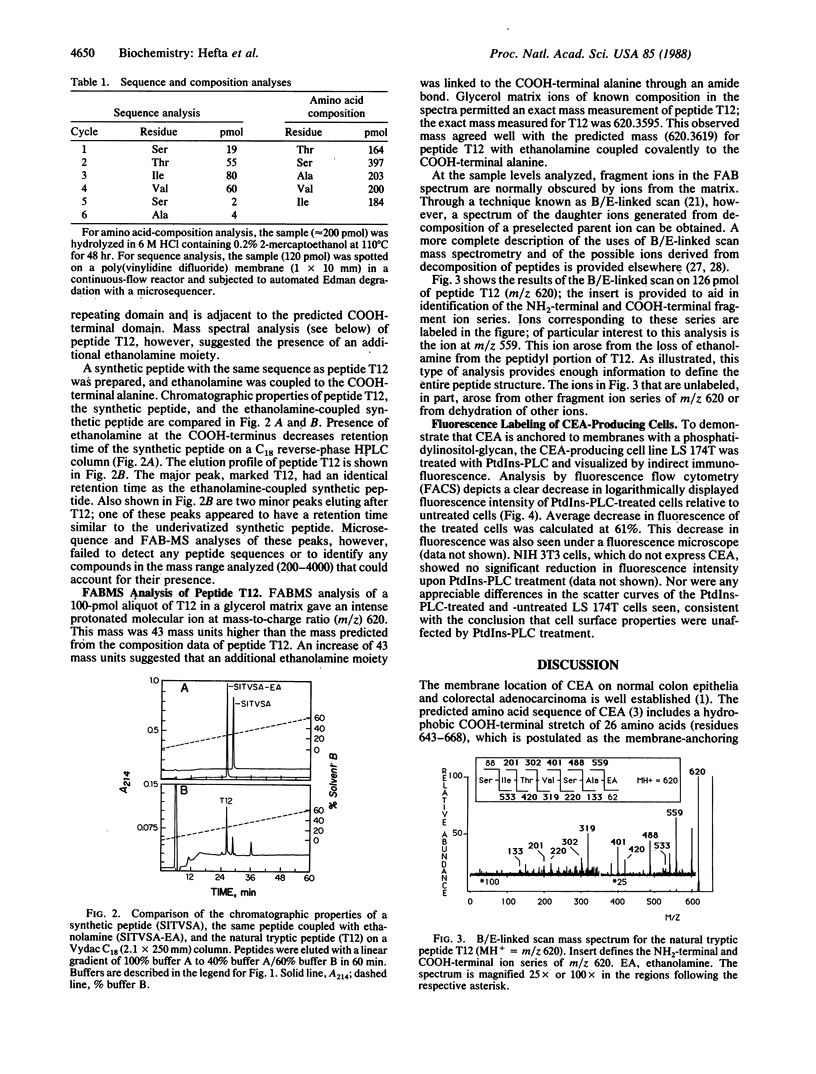
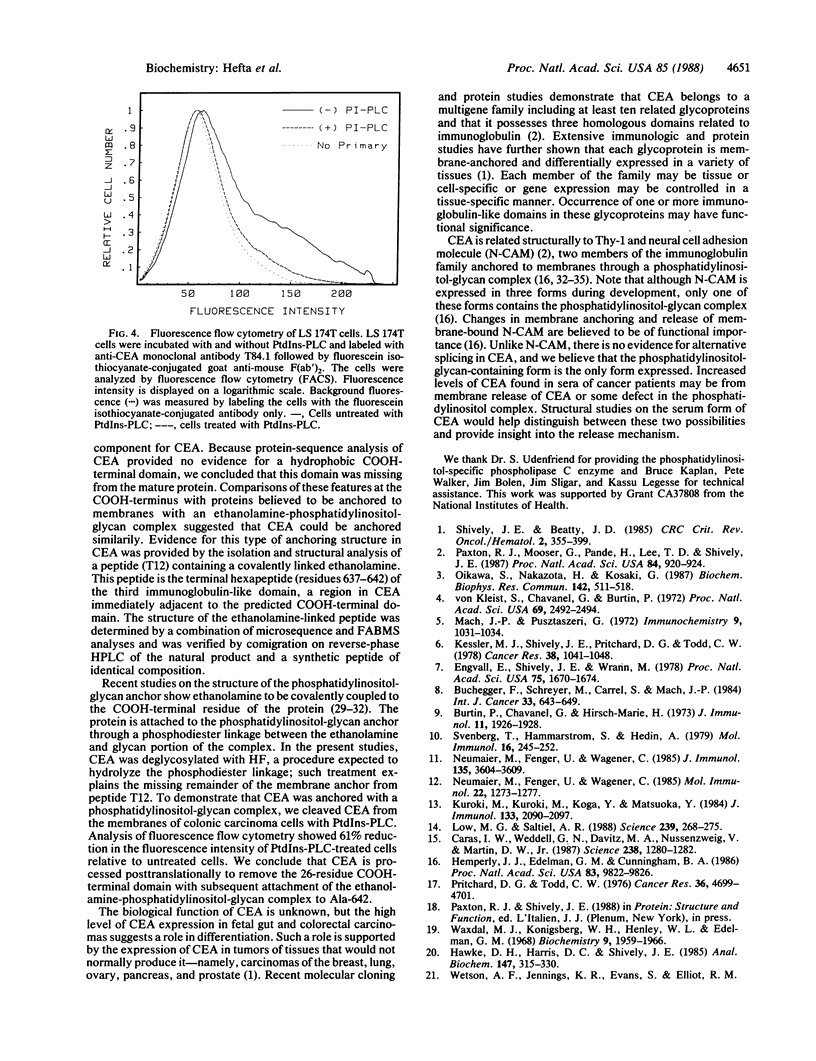
The COOH-terminal amino acid of carcinoembryonic antigen (CEA) is shown to covalently link with ethanolamine, evidence consistent with the anchorage of CEA to the plasma membrane through a phosphatidylinositol-glycan tail. Purified CEA was digested with trypsin, and the resulting peptides were isolated by reverse-phase HPLC. Tryptic hexapeptide T12, terminating atypically with alanine, corresponded in sequence (Ser-Ile-Thr-Val-Ser-Ala) with the last six residues (637-642) of the third repeating domain in the mature CEA protein. Mass determination of the hexapeptide by fast atom bombardment mass spectrometry suggested the presence of an additional ethanolamine moiety. This finding and the absence of the subsequent 26 hydrophobic residues predicted by cDNA sequence is evidence that hexapeptide T12 is the COOH-terminal peptide of mature CEA. A synthetic peptide identical to hexapeptide T12 was prepared, and ethanolamine was coupled to its COOH-terminal alanine; chromatographic properties of this synthetic ethanolamine-coupled peptide and peptide T12 were the same. B/E-linked-scan mass spectral analysis of the ethanolamine-coupled synthetic peptide and peptide T12 revealed a fragment ion series consistent with the presence of a COOH-terminal ethanolamine. Release of membrane-bound CEA from the CEA-expressing cell line LS 174T was shown by indirect immunofluorescence and flow cytometry after treatment with phosphatidylinositol-specific phospholipase C. We conclude that CEA is processed posttranslationally to remove the hydrophobic COOH-terminal residues (643-668) with subsequent addition of an ethanolamine-glycosylphosphatidylinositol moiety and that treatment of a colonic cell line with phosphatidylinositol-specific phospholipase C releases membrane-bound CEA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchegger F., Schreyer M., Carrel S., Mach J. P. Monoclonal antibodies identify a CEA crossreacting antigen of 95 kD (NCA-95) distinct in antigenicity and tissue distribution from the previously described NCA of 55 kD. Int J Cancer. 1984 May 15;33(5):643–649. doi: 10.1002/ijc.2910330515. [DOI] [PubMed] [Google Scholar]
- Burtin P., Chavanel G., Hirsch-Marie H. Characterization of a second normal antigen that cross-reacts with CEA. J Immunol. 1973 Dec;111(6):1926–1928. [PubMed] [Google Scholar]
- Caras I. W., Weddell G. N., Davitz M. A., Nussenzweig V., Martin D. W., Jr Signal for attachment of a phospholipid membrane anchor in decay accelerating factor. Science. 1987 Nov 27;238(4831):1280–1283. doi: 10.1126/science.2446389. [DOI] [PubMed] [Google Scholar]
- Engvall E., Shively J. E., Wrann M. Isolation and characterization of the normal crossreacting antigen: homology of its NH2-terminal amino acid sequence with that of carcinoembryonic antigen. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1670–1674. doi: 10.1073/pnas.75.4.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A., Homans S. W., Dwek R. A., Rademacher T. W. Glycosyl-phosphatidylinositol moiety that anchors Trypanosoma brucei variant surface glycoprotein to the membrane. Science. 1988 Feb 12;239(4841 Pt 1):753–759. doi: 10.1126/science.3340856. [DOI] [PubMed] [Google Scholar]
- Haas R., Brandt P. T., Knight J., Rosenberry T. L. Identification of amine components in a glycolipid membrane-binding domain at the C-terminus of human erythrocyte acetylcholinesterase. Biochemistry. 1986 Jun 3;25(11):3098–3105. doi: 10.1021/bi00359a005. [DOI] [PubMed] [Google Scholar]
- Hawke D. H., Harris D. C., Shively J. E. Microsequence analysis of peptides and proteins. V. Design and performance of a novel gas-liquid-solid phase instrument. Anal Biochem. 1985 Jun;147(2):315–330. doi: 10.1016/0003-2697(85)90278-7. [DOI] [PubMed] [Google Scholar]
- He H. T., Barbet J., Chaix J. C., Goridis C. Phosphatidylinositol is involved in the membrane attachment of NCAM-120, the smallest component of the neural cell adhesion molecule. EMBO J. 1986 Oct;5(10):2489–2494. doi: 10.1002/j.1460-2075.1986.tb04526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemperly J. J., Edelman G. M., Cunningham B. A. cDNA clones of the neural cell adhesion molecule (N-CAM) lacking a membrane-spanning region consistent with evidence for membrane attachment via a phosphatidylinositol intermediate. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9822–9826. doi: 10.1073/pnas.83.24.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare D. G., Koshland D. E., Jr A method for the quantitative modification and estimation of carboxylic acid groups in proteins. J Biol Chem. 1967 May 25;242(10):2447–2453. [PubMed] [Google Scholar]
- Kessler M. J., Shively J. E., Pritchard D. G., Todd C. W. Isolation, immunological characterization, and structural studies of a tumor antigen related to carcinoembryonic antigen. Cancer Res. 1978 Apr;38(4):1041–1048. [PubMed] [Google Scholar]
- Kuroki M., Kuroki M., Koga Y., Matsuoka Y. Monoclonal antibodies to carcinoembryonic antigen: a systematic analysis of antibody specificities by using related normal antigens and evidence for allotypic determinants on carcinoembryonic antigen. J Immunol. 1984 Oct;133(4):2090–2097. [PubMed] [Google Scholar]
- Low M. G., Kincade P. W. Phosphatidylinositol is the membrane-anchoring domain of the Thy-1 glycoprotein. Nature. 1985 Nov 7;318(6041):62–64. doi: 10.1038/318062a0. [DOI] [PubMed] [Google Scholar]
- Low M. G., Saltiel A. R. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- Mach J. P., Pusztaszeri G. Carcinoembryonic antigen (CEA): demonstration of a partial identity between CEA and a normal glycoprotein. Immunochemistry. 1972 Oct;9(10):1031–1034. doi: 10.1016/0019-2791(72)90113-9. [DOI] [PubMed] [Google Scholar]
- Micanovic R., Bailey C. A., Brink L., Gerber L., Pan Y. C., Hulmes J. D., Udenfriend S. Aspartic acid-484 of nascent placental alkaline phosphatase condenses with a phosphatidylinositol glycan to become the carboxyl terminus of the mature enzyme. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1398–1402. doi: 10.1073/pnas.85.5.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumaier M., Fenger U., Wagener C. Delineation of four carcinoembryonic antigen (CEA) related antigens in normal plasma by transblot studies using monoclonal anti-CEA antibodies with different epitope specificities. Mol Immunol. 1985 Nov;22(11):1273–1277. doi: 10.1016/0161-5890(85)90046-x. [DOI] [PubMed] [Google Scholar]
- Neumaier M., Fenger U., Wagener C. Monoclonal antibodies for carcinoembryonic antigen (CEA) as a model system: identification of two novel CEA-related antigens in meconium and colorectal carcinoma tissue by Western blots and differential immunoaffinity chromatography. J Immunol. 1985 Nov;135(5):3604–3609. [PubMed] [Google Scholar]
- Oikawa S., Nakazato H., Kosaki G. Primary structure of human carcinoembryonic antigen (CEA) deduced from cDNA sequence. Biochem Biophys Res Commun. 1987 Jan 30;142(2):511–518. doi: 10.1016/0006-291x(87)90304-4. [DOI] [PubMed] [Google Scholar]
- Paxton R. J., Mooser G., Pande H., Lee T. D., Shively J. E. Sequence analysis of carcinoembryonic antigen: identification of glycosylation sites and homology with the immunoglobulin supergene family. Proc Natl Acad Sci U S A. 1987 Feb;84(4):920–924. doi: 10.1073/pnas.84.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard D. G., Todd C. W. Purification of carcinoembryonic antigen by removal of contaminating mucopolysaccharides. Cancer Res. 1976 Dec;36(12):4699–4701. [PubMed] [Google Scholar]
- Sadoul K., Meyer A., Low M. G., Schachner M. Release of the 120 kDa component of the mouse neural cell adhesion molecule N-CAM from cell surfaces by phosphatidylinositol-specific phospholipase C. Neurosci Lett. 1986 Dec 23;72(3):341–346. doi: 10.1016/0304-3940(86)90538-0. [DOI] [PubMed] [Google Scholar]
- Sato K., Asada T., Ishihara M., Kunihiro F., Kammei Y., Kubota E., Costello C. E., Martin S. A., Scoble H. A., Biemann K. High-performance tandem mass spectrometry: calibration and performance of linked scans of a four-sector instrument. Anal Chem. 1987 Jul 1;59(13):1652–1659. doi: 10.1021/ac00140a016. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Beatty J. D. CEA-related antigens: molecular biology and clinical significance. Crit Rev Oncol Hematol. 1985;2(4):355–399. doi: 10.1016/s1040-8428(85)80008-1. [DOI] [PubMed] [Google Scholar]
- Svenberg T., Hammarström S., Hedin A. Purification and properties of biliary glycoprotein I (BGP I). Immunochemical relationship to carcinoembryonic antigen. Mol Immunol. 1979 Apr;16(4):245–252. doi: 10.1016/0161-5890(79)90063-4. [DOI] [PubMed] [Google Scholar]
- Tse A. G., Barclay A. N., Watts A., Williams A. F. A glycophospholipid tail at the carboxyl terminus of the Thy-1 glycoprotein of neurons and thymocytes. Science. 1985 Nov 29;230(4729):1003–1008. doi: 10.1126/science.2865810. [DOI] [PubMed] [Google Scholar]
- Wagener C., Clark B. R., Rickard K. J., Shively J. E. Monoclonal antibodies for carcinoembryonic antigen and related antigens as a model system: determination of affinities and specificities of monoclonal antibodies by using biotin-labeled antibodies and avidin as precipitating agent in a solution phase immunoassay. J Immunol. 1983 May;130(5):2302–2307. [PubMed] [Google Scholar]
- Wagener C., Yang Y. H., Crawford F. G., Shively J. E. Monoclonal antibodies for carcinoembryonic antigen and related antigens as a model system: a systematic approach for the determination of epitope specificities of monoclonal antibodies. J Immunol. 1983 May;130(5):2308–2315. [PubMed] [Google Scholar]
- Wang S. S. p-alkoxybenzyl alcohol resin and p-alkoxybenzyloxycarbonylhydrazide resin for solid phase synthesis of protected peptide fragments. J Am Chem Soc. 1973 Feb 21;95(4):1328–1333. doi: 10.1021/ja00785a602. [DOI] [PubMed] [Google Scholar]
- Waxdal M. J., Konigsberg W. H., Henley W. L., Edelman G. M. The covalent structure of a human gamma G-immunoglobulin. II. Isolation and characterization of the cyanogen bromide fragments. Biochemistry. 1968 May;7(5):1959–1966. doi: 10.1021/bi00845a046. [DOI] [PubMed] [Google Scholar]
- von Kleist S., Chavanel G., Burtin P. Identification of an antigen from normal human tissue that crossreacts with the carcinoembryonic antigen. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2492–2494. doi: 10.1073/pnas.69.9.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]



