Summary
The ectoplasmic specialization (ES) is a testis-specific, actin-based hybrid anchoring and tight junction. It is confined to the interface between Sertoli cells at the blood–testis barrier, known as the basal ES, as well as between Sertoli cells and developing spermatids designated the apical ES. The ES shares features of adherens junctions, tight junctions and focal contacts. By adopting the best features of each junction type, this hybrid nature of ES facilitates the extensive junction-restructuring events in the seminiferous epithelium during spermatogenesis. For instance, the α6β1-integrin–laminin 333 complex, which is usually limited to the cell–matrix interface in other epithelia to facilitate cell movement, is a putative apical ES constituent. Furthermore, JAM-C and CAR, two tight junction integral membrane proteins, are also components of apical ES involving in spermatid orientation. We discuss herein the mechanisms that maintain the cross-talk between ES and blood–testis barrier to facilitate cell movement and orientation in the seminiferous epithelium.
Introduction
During spermatogenesis in the seminiferous tubule of the adult testes, diploid spermatogonia (2 n) divide and differentiate into haploid spermatozoa (1 n). Only two cell types constitute the seminiferous epithelium, the Sertoli and the germ cells (Fig. 1). Somatic Sertoli cells are columnar cells that extend from the basement membrane to the seminiferous tubule lumen. The blood–testis barrier (BTB) between adjacent Sertoli cells near the basement membrane physically divides the epithelium into the basal and adluminal (or apical) compartments (Fig. 1). Each Sertoli cell in the seminiferous epithelium supports ~30 to 50 developing germ cells at different stages of their development. In order to prevent the host immune system from developing antibodies against germ cell-specific antigens, many of which appear transiently, post-meiotic germ-cell development is separated from systemic circulation by the BTB. This results in the developing germ cells obtaining their nourishment only from Sertoli cells.(1) Furthermore, the BTB also serves as a “gate-keeper” that regulates proteins and/or biomolecules that can enter the adluminal compartment from the interstitium. The BTB is therefore the tightest blood–tissue barrier in mammals (Fig. 1).(2,3)
Figure 1.
A schematic drawing to show the hybrid property of apical ES having the characteristics of AJ, FAC and TJ. The drawing illustrates the relative locations of junctions between Sertoli cells, Sertoli–germ cells and Sertoli cells–extracellular matrix (ECM) as well as different stages of germ-cell development in the seminiferous epithelium of adult rat testes (top panel). Hemidesmosomes is a cell–matrix intermediate filament based anchoring junction with α6β4-integrin as the transmembrane receptor (bottom left panel). This anchoring junction is important for Sertoli cells to anchor onto the basement membrane. However, there is no direct evidence for the presence of α6β4-integrin in the testis. The existence of focal adhesion complex between Sertoli cells and ECM in the rat testis is not well-defined. The bottom right panel is the molecular architecture of apical ES in the seminiferous epithelium. Apical ES is a hybrid AJ (e.g. cadherins/catenins, nectins/afadin), focal contact anchoring junction (e.g. integrins/laminins) and TJ (e.g. JAM-C, CAR). This model was prepared based on recent findings in the field (see text).
In addition to undergoing mitosis and meiosis during spermatogenesis, the germ cells undergo extensive changes in shape and also migrate progressively from the basal compartment toward the tubule lumen (see Fig. 1). Indeed, preleptotene spermatocytes residing outside the BTB and near the basement membrane must traverse this unique physiological barrier at stage VIII of the epithelial cycle in adult testes. This is followed by their subsequent differentiation into pachytene spermatocytes, round spermatids and finally fully developed elongate spermatids (i.e. spermatozoa) in the adluminal compartment so that they are released into the lumen at spermiation.(4) In rodent testes, such as rats and mice, the seminiferous epithelial cycle refers to the unique cellular association between developing germ cells and Sertoli cells in the seminiferous epithelium, and each cycle comprises 14 stages, I through XIV.(5,6) These stages of the epithelial cycle thus involve extensive junction restructuring at the Sertoli–Sertoli and Sertoli–germ cell interface to facilitate morphological changes of cell shape and movement.(7) If the events of junction restructuring are disrupted, spermatogenic cells cannot orientate and migrate properly, this also leads to premature germ cell release from the epithelium, resulting in infertility. Thus, a thorough understanding of the factors that regulate the cross-talk between Sertoli cells and between Sertoli and germ cells during the epithelial cycle is crucial in male reproductive physiology.
Cell–cell adhesion in all epithelia, including the seminiferous epithelium in the testis, is mediated via junction complexes constituted by tight junctions (TJ), adherens junctions (AJ), desmosomes and gap junctions.(8,9) Each of these junction types has a specific physiological role. For instance, TJ creates an impermeable barrier that prevents the leakage of ions, water and other molecules between cells, and confers cell polarity.(10) AJ links neighboring cells together by forming a continuous adhesion belt underneath the TJ, with the exception of seminiferous epithelium where AJ and TJ coexist side-by-side at the BTB.(7,11) Desmosomes facilitate intercellular anchorage via intermediate filaments such as vimentin,(12) while gap junctions facilitate cell–cell communication.(13)
Among the various types of junctions present in the seminiferous epithelium,(3,13) the ES is a testis-specific anchoring junction type. It is defined as the region between the plasma membranes of two adjacent Sertoli cells and the cisternae of the endoplasmic reticulum (ER) with hexagonally packed actin filaments. Basal ES is restricted only to the BTB and is present side-by-side with TJ, tubulobulbar complex (TBC), desmosome-like junctions (note: desmosomes found in the testis at the Sertoli–Sertoli and Sertoli–spermatocyte/round spermatid interface share features of the gap junctions and are known as desmosome-like junctions) and gap junctions (Fig. 1, for reviews, see Refs. 14,15). However, ES is also found between Sertoli cells and elongating spermatids (step 8 and beyond in rat and mouse testes) designated apical ES. Its structural features are virtually identical to the basal ES except that it is restricted only to the Sertoli cell side and TJ is absent (Fig. 1). Even though the TJ is not present at the apical ES per se, several TJ transmembrane proteins (e.g. JAM-C and CAR) have recently been found at the apical ES (see below). While most of the earlier studies on ES were largely morphological in nature (for reviews, see Refs 2,15), recent studies have placed emphasis on the identification of proteins, in particular integral membrane proteins, peripheral kinases, phosphatases and adaptors that participate in ES dynamics both in vivo(17,18,26) and in vitro.(19–21) These findings will be summarized and discussed herein; in particular, recent data regarding the cross-talk between apical and basal ES in facilitating germ cell migration.
Basal ES at the BTB, and apical ES: the biology and regulation
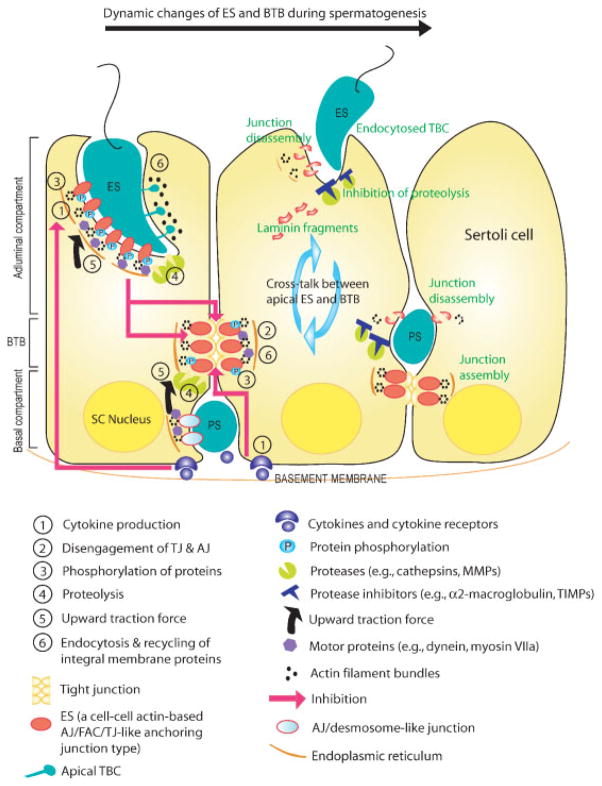
One of the crucial steps of spermatogenesis in mammals in addition to mitosis and meiosis is the migration of preleptotene spermatocytes across the BTB in the basal compartment. This occurs at stage VIII of the epithelial cycle and involves transient openings (disassembly?) of the basal ES and TJ (see Fig. 2). Interestingly, this cellular event occurs concurrently with spermiation which takes place at the apical compartment. Spermiation is a key process in spermatogenesis resulting in fully developed spermatids (i.e. spermatozoa) leaving the epithelium, entering into the tubule lumen, and eventually emptying into the epididymis for further maturation. This process involves disruption of the apical ES and apical TBC (see Fig. 2). Since the migration and spermiation occur at the opposite ends of adjacent Sertoli cells in the seminiferous epithelium, it is logical to speculate that they are coordinated via cross talk between components of the basal and apical ES (see below).
Figure 2.
A schematic drawing illustrating the mechanism of spermatid movement along Sertoli cells in the seminiferous epithelium via a cascade of biological events. Spermiation occurs at stage VIII of the epithelial cycle, which coincides with the time for the passage of preleptoene spermatocytes (PS) across the BTB. During these cellular events, the different cascades of biological events (see the numbers in the figure) happen in a highly regulated manner. We hypothesize that germ cell movement involves the continuous disassembly and reassembly of Sertoli–Sertoli and Sertoli–germ cell junctions. These require the presence of cytokines (e.g. TNFα and TGF-β3) and proteases to perturb the junctions by lowering the steady-state protein level of several integral TJ and AJ proteins. Protein kinases and protein phosphatases also mediate tyrosine phosphorylation of the AJ (cadherin/catenin/CK2/cSrc) and FAC-like (integrin/laminin/FAK/cSrc) adhesion complex or an induction of cofilin and/or gelsolin. The net result of these changes leads to a weakening of adhesion complexes at the basal and apical ES. A transient disengagement between TJ and AJ via peripheral adaptors, such as ZO-1 and catenins, is used to preserve the BTB integrity when AJ is perturbed. To assist spermatid movement, upward traction force is generated by the motor proteins, such as myosin VIIa and dynein, at both the apical and basal ES, involving GTPases. Prior to spermiation, integral membrane junction proteins such as nectin-2 and nectin-3 may be internalized by both apical and basal TBC to facilitate the depletion of elongate spermatid (ES) from the epithelium (i.e. spermiation) and the passage of PS across the BTB. Recently, it has been speculated that cleavage of laminin at the apical ES by MMPs can assist the ‘opening’ of BTB via a yet-to-be identified signaling pathway. This model was prepared based on recent findings in the field (see text). SC Nucleus, Sertoli cell nucleus.
The basal ES, a component of BTB, is known to be composed of at least two AJ transmembrane proteins: cadherins(19) and nectin-2.(22) The prevailing view is that cadherins (e.g. N- and E-cadherin) structurally interact with γ-catenin or β-catenin at a stoichiometric ratio of 1:1, which, in turn, link to the actin cytoskeleton through α-catenin, whereas nectin-2 binds to actin via afadin (for a review, see Ref. 23). These two cell adhesion proteins mediate homotypic interactions between adjacent Sertoli cells (for a review, see Ref. 23). However, recent studies seem to argue that a stable E-cadherin–β-catenin–α-catenin–actin quaternary complex indeed exists in eukaryotic cells.(24,25) For instance, while α-catenin was shown to interact with either actin filaments or the E-cadherin–β-catenin complex, α-catenin failed to interact with actin filaments and the E-cadherin-β-catenin complex simultaneously, illustrating the interaction between α-catenin and actin or α-catenin and E-cadherin/β-catenin are mutually exclusive, even in the presence of adaptors vinculin and α-actinin.(25) Furthermore, α-catenin exists as either a monomer or a dimer that binds preferentially to the E-cadherin-β-catenin complex or actin filaments, respectively.(24) In short, these findings suggest that a “stable” linkage between cadherins and actin filaments may not exist and their interactions only occur transiently and dynamically, shuffling between α-catenin–E-cadherin–β-catenin and α-catenin–actin filaments. However, it must be noted that other as yet unidentified adaptors that facilitate a stable linkage between N-cadherin–α-catenin and α-catenin–actin filament might have been destroyed or unknowingly removed in the isolated cadherin-containing membranes used by these investigators.(24,25) Furthermore, actin filaments (which exist as filament bundles) are integral components of the apical and basal ES as visualized by electron microscopy (see Fig. 1), and both E-cadherin and N-cadherin have been shown to bind to actin by co-immunoprecipitation with and without the use of cross-linkers.(19,26) Additional research, however, is needed to resolve this issue.
TJ is another junction type at the BTB at the Sertoli–Sertoli cell interface that confers barrier and cell polarity (for reviews, see Refs 13,23). There are three known transmembrane proteins at the TJ in adult rat or mouse testes: occludins, claudins and JAMs, which link to the actin-based cytoskeleton via adaptors ZO-1/ZO-2 (for reviews, see Refs 13,23). Other immunoglobulin superfamily (IgSF) type I transmembrane proteins have recently been identified in the testis. For instance, brain- and testis-specific immunoglobulin superfamily (BT-IgSF or IgSF11) was shown to be preferentially expressed in the testis and brain.(27) CLMP (CAR-linked membrane protein) was also identified in various tissues, including testes, as a new member of TJ molecules, co-localizing with occludin and ZO-1 but not E-cadherin in MDCK cells.(28) BT-IgSF and CLMP both mediate homotypic adhesion between cells.(28,29) While these TJ proteins are crucial to confer a paracellular barrier to limit the passage of substances across the cell epithelium, there are other proteins that strengthen BTB integrity at the tricellular contact interface. For instance, a newly identified TJ protein called tricellulin was found to be expressed in testes, and localized at the interface of cells in small intestine, kidney and lung.(30) It remains to be investigated if and how tricellulin regulates BTB integrity, including its cellular localization in the testis.
Since the molecular components of the BTB have been reviewed recently,(13,23) details of these protein complexes are not discussed herein. However, it is noted that TJ proteins at the BTB are localized near the basal compartment in the seminiferous epithelium, closest to, instead of farthest from, the basement membrane (a modified form of extracellular matrix in the testis). This location is in sharp contrast to other epithelia and endothelia where TJ is furthest away from the basal lamina.(9,23,31) In addition, these TJ proteins co-exist with basal ES proteins at the BTB, probably to reinforce barrier function. Indeed, basal ES and TJ proteins have recently been found to be structurally associated (or engaged) with each other under normal physiological conditions at the BTB, via adaptors, catenins and ZO-1; (32) occludin and α-catenin may share the same binding site on ZO-1 as shown in other epithelia.(33) When the ES is perturbed, such as by treatment of adult rats with a drug (e.g. adjudin), the association between AJ and TJ can be temporarily disrupted, i.e., disengaged. Furthermore, cadherin, occludin and their corresponding adaptors, catenins and ZO-1, were shown to diffuse away from the BTB.(32,34) Their association resumed when germ cells were mostly depleted from the epithelium.(32) An engagement and disengagement theory was thus proposed to describe the changes in protein–protein association between basal ES and TJ to facilitate preleptotene spermatocyte movement across the BTB at stage VIII of the epithelial cycle.(32) The transient disruption of AJ is possibly mediated via Tyr-phosphorylation of the cadherin–catenin protein complexes(34,35) while the transient surge in the levels of TJ proteins overrides the function of AJ temporarily to maintain the BTB integrity(32) (see Fig. 2). It is likely that such precise co-ordination between TJ and basal ES at the BTB facilitates preleptotene spermatocyte migration across the BTB.
Is integrin a crucial regulatory component of the basal ES?
Apart from the AJ and TJ transmembrane proteins mentioned above, integrin, a heterodimeric transmembrane receptor composed of α and β subunits, has been implicated as a component of the BTB at the basal compartment (for reviews, see Refs 23,36). In general, integrins are found at cell–matrix and cell–cell interfaces as adhesion molecules.(36) Among 18 α-subunits and 8 β-subunits present in mammals, α1, α3, α4, α5, α6, α9, β1, β2 and β3 integrins have been identified in testes.(20,36–38) For instance, α1, α3, α9, β1 subunits are localized to the basement membrane of the seminiferous epithelium, whereas α1, α4, α5, α6 and β1 subunits are found at the Sertoli cell-elongating spermatid interface (i.e. apical ES) in some stages of the epithelial cycle. It is noteworthy that in most of these earlier studies, the localization and/or cellular association of integrins in the testis was monitored by immunofluoresent microscopy or immunohistochemistry. Due to the limited resolution of the images obtained with these techniques, the precise localization of these integrins at the basal compartment is not certain. At present, it is not known whether integrins are present at the cell–matrix (FAC) or cell–cell (e.g. basal ES) interface in adult rat testes. For instance, α1β1, α3β1, α6β1 and α9β1 integrins are known receptors for a wide variety of matrix molecules such as collagens, fibronectin and laminin in other epithelia,(39,40) while α3β1 and α6β1 integrin receptors are found in the basement membrane in testes.(41,42) However, there is no concrete morphological evidence regarding the presence of focal contacts (a cell–matrix actin-based anchoring junction type) in adult rat testes. It is not even known whether it is physiologically necessary to have focal contacts to assist Sertoli cell movement on the basement membrane since Sertoli cells provide crucial structural and nutritional supports to developing germ cells in the seminiferous epithelium in vivo. It has been suggested that α6β1 integrin may be a component of the basal ES.(42,43) This possibility, however, requires further investigation, for example using immunogold EM or perhaps studying Sertoli cell-specific integrin α6 and/or β1 knockout mice. However, hemidesmosomes (a cell–matrix intermediate filament-based anchoring junction type) are found between Sertoli cells and ECM in bovine seminiferous tubules(44) and rat testes.(36) Still, little information is available for this junction type in the testis. In general, there are two known transmembrane components of hemidesmosomes, α6β4 integrin and the bullous pemphigoid (BP) antigen BP180 (also known as type XVII collagen).(45,46) α6β4 Integrin differs from other integrin receptors in that the cytoplasmic domain of β4 is much larger than other integrin β subunits.(45) In most epithelia, α6β4 integrin binds to laminin-322 to form the hemidesmosome adhesion complex, while some other laminins can also bind to α6β4 integrin.(45) Interestingly, there is no proof as yet regarding the existence of a functional α6β4 integrin in adult testes even though BP180 mRNA has been detected in the testis.(47) Studies are needed to elucidate the identity of integrins at the hemidesmosomes and/or basal ES in testes.
In addition to their adhesion function, integrins (e.g. α6 integrin) are crucial to testicular cord formation.(48,49) Testicular cords, which are the precursors of the seminiferous epithelium, comprise primordial germ cells (PGC, precursors of spermatogonia) and Sertoli cells. It has been proposed that integrins, together with ADAM (a disintegrin and metalloprotease) and tetraspanin, constitute a protein complex that facilitates the migration of PGC from the center to the periphery of the testicular cord during development.(49) The PGC may either release soluble ADAM or the disintegrin domain which, in turn, binds to integrins on Sertoli cells. This would cause detachment of PGCs from the cord which, by their own amoeboid movement, would reach the basal lamina and trigger the first step of spermatogenesis.(49) In this context, it is of interest to note that laminin also promotes the formation of cord-like structures by Sertoli cells in vitro, mimicking seminiferous cords in testes during embryonic development.(50) Furthermore, a pentapeptide of NH2-RSGIY-COOH corresponding to the cell-binding domain of laminin β1 chain was shown to possess biological activity as it inhibits Sertoli cell cord formation.(50)
The molecular architecture of apical ES
At stage VIII of the epithelial cycle in adult rat testes, fully developed elongate spermatids (i.e. spermatozoa) leave the seminiferous epithelium at spermiation, which involves extensive restructuring of the apical ES and apical TBC (Fig. 2).(6,13,16) As described above, apical ES is only visible on the Sertoli cell side by electron microscopy (EM) since the morphological structure of ES on the elongating spermatid side is not well-defined. This is not entirely unexpected since elongating spermatids are highly specialized cells composed of condensed chromatin in their heads and structurally anchored onto Sertoli cells by apical ES possessing relatively little cytoplasmic constituents. Interestingly, several AJ integral membrane proteins, adaptors and cytoskeletal proteins have recently been identified in spermatids. These include transmembrane proteins (e.g. cadherins and nectins) that anchor onto the cytoskeleton via corresponding adaptors, such as catenins and afadins. Thus, these recent findings suggest that elongating spermatids structurally contribute to the apical ES. It is also noted that the protein levels of cadherins/catenins at the apical ES are significantly lower than at the basal ES except at stage VI of the epithelial cycle.(19,51) In addition to the classical cadherins, protocadherin and Celsr cadherin are also found abundantly in testes.(52,53) Furthermore, both are found in Sertoli and germ cells, yet they apparently have features that are different from the classical cadherin–catenin protein complex. For instance, their localization is not at the site of Sertoli–germ cell adhesion junction. Protocadherinα3 (PCDHα3) is localized to the spermatid acrosomal area, intercellular bridges and flagella, while PCDHα3 was found to associate with centrosomes at the basal compartment.(52) It is believed that PCDHα3 does not function as a cell adhesion protein. Celsr 2 has been localized to Golgi complex in both Sertoli and germ cells as well as co-localized with Rab7 in germ-cell.(53) Since fragments of Celsr 2 were shown to induce germ cell depletion in an in vitro Sertoli–germ-cell coculture system,(53) it is believed that Celsr cadherin may play a role in cell adhesion that is different from the classic cadherin junctions. For the nectin–afadin protein complexes, instead of having homotypic interactions at the basal ES, they confer heterotypic adhesion between nectin-3 in germ cells and nectin-2 in Sertoli cells at the apical ES.(54) Other than these two adhesion protein complexes, the α6β1-integrin–laminin γ3 complex is the most extensively studied adhesion complex at the apical ES.(38,43,55–57)
Additionally, several TJ proteins have recently been shown to be the putative transmembrane proteins at the apical ES. For instance, a new isoform of JAM, known as JAM-C, is restricted to the apical ES but not at the BTB in mouse testes.(58) CAR is another group of TJ proteins(59,60) identified at the germ cell side of the apical ES.(61) It has been suggested that these proteins may be involved in spermatid polarization and orientation since JAM-C was found to co-localize with partitioning-defective (Par) 6, atypical protein kinase C (PKC) and Cdc42 at the apical ES of spermatids, and the Par6–PKC–Cdc42 is a known regulatory protein complex of cell polarity.(58)
The laminin-333–integrin α6β1 protein complex at the apical ES
Laminin is a heterotrimeric glycoprotein composed of one α, one β and one γ chain. Together with nidogen (also known as entactin), type IV collagen and heparan sulfate proteoglycan, these proteins are the major components of the basement membrane, which is a modified form of ECM (for a review, see Ref. 62), in mammalian testes. Laminins are crucial proteins during embryonic development. For instance, laminin β1−/−and γ1−/−mice displayed early embryonic lethality.(63) In addition to the basement membrane, laminins were also found in the interstitium.(64) When Sertoli cells were cultured in vitro, laminin and type IV collagen were detected in these cells, illustrating their de novo synthesis.(65) Subsequent studies have implicated laminins in regulating cell adhesion, migration, differentiation and morphogenesis in the basal compartment of the seminiferous epithelium. For instance, Sertoli cells in vitro mimicked their in vivo morphology only when these cells were cultured on plates coated with laminin and type IV collagen.(66,67) Besides, Sertoli cells cultured on ECM proteins are capable of forming basolateral junctions.(68) Ironically, laminin and type IV collagen are the major components of the Matrigel that was used for culturing Sertoli cells in vitro by various laboratories in recent years.(62) This also accounts for the breakthrough in studying Sertoli cell physiology using cells cultured on Matrigel-coated dishes.(68,69) Several other studies have assessed laminin functions in testes via intratesticular injection of anti-laminin serum.(70–72) This functional study was performed via multiple administration of the entire laminin complexes to the testes followed by morphological analyses. In one of the reports, up to 66% of rats showed severe sloughing of multinucleated germ cells into the lumen amidst thickening of the walls of seminiferous tubules.(71) Laminin was also found to regulate the integrity of cytoskeleton, such as actin, in Sertoli cell cultures.(73)
As reviewed herein, the study of laminin was mostly restricted to the basement membrane in the tubules, whilst its presence and/or functions in the apical compartment were poorly explored until recently. Studies in the 1980s have shown that α6β1-integrin, a transmembrane receptor for laminins at the cell–matrix interface in other epithelia, is localized to the Sertoli cell side at the apical ES, and is also the best-studied transmembrane protein at this site.(38,42,43) Subsequent studies have shown that other FAC proteins, which are usually restricted to the cell–matrix interface, such as focal adhesion kinase, integrin-linked kinase, vinculin, paxillin and α-actinin, are also associated with α6β1 integrin.(21,43,74) These unexpected observations have led several investigators to speculate on the likely presence of laminin, a ligand for α6β1 integrin, at the apical ES.(42,43,56) Indeed, the presence of laminin β1 in Drosophila germ cells(75) and the identification of a non-basement-membrane-associated laminin γ3 in mouse testes(55) have further strengthened this speculation. Besides, several laminin subunits have been found to localize outside the basal lamina of other epithelia, such as in retina(76,77) and cartilage.(78) A recent report has demonstrated a functional laminin-333 ligand composed of α3, β3 and γ3 chains is a bona fide ligand for α6β1 integrin in adult rat testes.(79) Consistent with earlier findings,(56,80) the localization of laminin α3 and γ3 chains, and α6β1-integrin at the apical ES in the seminiferous epithelium is strongest at stage VIII of the epithelial cycle where the α6β1 integrin–laminin 333 protein complex confers a strong adhesion between Sertoli cells and developing spermatids.(79) These results are also in agreement with a recent biophysical study in which the mechanical adhesion strength between Sertoli and germ cells was quantified using a micropipette pressure transducing system.(81) It was found that Sertoli cells were more tightly attached to step 8 spermatids than to spermatocytes, presumably because of the adhesion conferred by the laminin–integrin adhesion complex at the apical ES. In short, laminins are no longer restricted to the basement membrane in the testis. They are putative cell adhesion components at the apical ES. At present, five α, three β and three γ laminin chains are identified in different cell types in testes (see Table 1). Since, apical ES comprises cell–cell actin-based AJ protein complexes (e.g. cadherins/catenins and nectins/afadins), cell–matrix actin-based FAC (e.g. laminin 333-α6β1 integrin) and cell–cell TJ protein complexes (e.g. JAM-C/ZO-1, CAR), it is designated a hybrid AJ, FAC and TJ anchoring junction type (Figs. 1 and 2).
Table 1.
Laminin subunits and their cellular association/expression of adult mammalian testes
| Testicular cell types |
|||||
|---|---|---|---|---|---|
| Laminin subunits | Site of expression in the testis | SC | GC | Method of detection | References |
| α1 | BM of seminiferous tubules | + | − | IF, RT-PCR | (79,108–111) |
| α2 | BM of seminiferous tubules and microvessels in the interstitin | + | + | IF, RT-PCR | (79,108–111) |
| α3 | Apical ES | − | + | Immunogold EM, RT-PCR, IB | (79) |
| α4 | Seminiferous tubules | n.d. | n.d. | RT-PCR, IF, Northerns | (79,109–111) |
| α5 | Seminiferous tubules | n.d. | n.d. | IHC, RT-PCR, IF | (79,110–111) |
| β1 | Seminiferous tubules | + | + | IF, RT-PCR | (79) |
| β2 | Seminiferous tubules | + | + | IF, RT-PCR | (79,111) |
| β3 | Apical ES | − | + | IB, Co-IP | (79,111) |
| γ1 | Seminiferous tubules | + | − | IF, RT-PCR | (79,111) |
| γ2 | Seminiferous tubules | n.d | n.d. | RT-PCR, IF | (79,111,112) |
| γ3 | Apical ES of rat testes | + | + | RT-PCR, IF, IB, Immunogold EM | (55,56,79) |
BM, Basement membrane; IF, Immunofluorescent microscopy; IB, immunoblot; IHC, immunohistochemistry; EM, electron microscopy; Co-IP, co-immunoprecipitation; SC, Sertoli cells; GC, germ cells; RT-PCR, reverse-transcriptase-polymerase chain reaction or real-time PCR. n.d., not determined.
The hybrid nature of ES and the mechanism(s) of its regulation
It is generally believed that Sertoli cells continually alter their shape to accommodate germ cell movement while germ cells play a rather passive role in this process.(23) However, there is mounting evidence that germ cells are actively involved in their movement during spermatogenesis. For instance, many proteins that participate in cellular movement have recently been found in germ cells, as well as in Sertoli cells. These include GTPases, pFAK and ILK.(23,36,82) Furthermore, focal contacts that are mostly restricted to motile cells, such as amoebae, fibroblasts, macrophages, neutrophils and cancer cells(83,84) are apparently an integrated part of the apical ES since many proteins that constitute the FAC are found at apical ES, such as the integrin–laminin–cSrc–pFAK complex.(21,79,85,86) Cell movement based on focal contacts involves three steps: (i) cell attachment, (ii) cell movement, and (iii) detachment at the rear end.(87,88) Herein, we suggest a possible mechanism employed by Sertoli and germ cells to facilitate cell movement based on recent findings (Fig. 2) in the field.
To gain attachment or traction between Sertoli and germ cells, cell attachment is mediated through the hybrid cell–cell and cell–matrix apical ES. Laminin-333 is a ligand residing on developing spermatids (step 8 and beyond) that binds to α6β1-integrin receptor on Sertoli cell surface. Both laminin-333 and α6β1-integrin are localized to the apical ES that confers cell adhesion during the epithelial cycle, but their levels are the highest at late stage VII through early stage VIII.(56,79) It is likely that integrins at the apical ES recruit intracellular signaling molecules (e.g. pFAK, vinculin, paxillin and c-Src)(18,21) and allow cytoskeleton assembly, increasing cell adhesiveness. Recent studies have shown that, during Sertoli–germ cell anchoring junction assembly, there was an induction in the protein levels of several kinases, such as phosphatidylinositol 3-kinase (PI3K), phosphorylated protein kinase B (PKB; also known as Akt), p21-activated kinase-2 (PAK-2), and their downstream effector ERK, and many of these signaling molecules were shown to be localized to apical ES in rat testes.(86) These kinases were recruited by integrins to the apical ES and associated with actin and tubulin in the seminiferous epithelium.(18,86)
This apical ES “focal adhesion” is different from the traditional cell–matrix FAC since it is found at the Sertoli–germ cell interface. Upon cell-cell contact, traction is likely established between cells that facilitates forward movement of spermatids. Cell “contraction” is generated by motor proteins that work in concert with microtubules(87) at the apical ES. Motor proteins, such as myosin VIIa and dynein, have been identified in Sertoli and germ cells.(89–91) Myosin VIIa is associated with keap1, a cytoskeletal protein, in both the basal and apical ES.(90) Dynein light chain-1, which is a germ-cell-specific protein, is suggested to assist spermatid shaping and the final release of elongate spermatid during spermiation via serine phosphorylation by p21-activated kinase-1.(91) After this cellular contraction to facilitate spermatid movement, the apical ES-specific focal contacts are dissolved by weakening the integrin–laminin affinity. This can be achieved by receptor detachment via endocytosis or changes in tyrosine phosphorylation of integrins.(87) Indeed, in a testosterone-suppression model to study the ES dynamics by two different groups, the level of Tyr-phosphorylated FAK was found to be induced and pFAK remained associated with α6β1-integrin during spermatid depletion from the epithelium.(85) Besides, there is a concomitant increase of the p-FAK–c-Src protein complex at the apical ES,(18,21) whereas the non-phosphorylated FAK was restricted almost exclusively to the basal ES at the BTB.(21) Although it is not known whether endocytosis and/or recycling of integrin receptors actually takes place in Sertoli cells, this mechanism of integrin turnover to facilitate cell movement has been reported in other epithelia.(92,93) Indeed, internalization of integral membrane proteins by apical and basal TBC prior to spermiation has been reported.(94) In addition, laminin on the germ cell surface was shown to associate with c-Src,(79) which is a peripheral non-receptor protein kinase found in germ cells.(95) A transmembrane protein must be present in germ cells that anchors the laminin ligand and provides a “docking platform” for c-Src and other signaling molecules to regulate apical ES adhesion.(79) Much research is needed in this area regarding the role of α6β1 integrin–laminin 333 in spermatid adhesion and movement.
Regulation of BTB dynamics by apical ES proteins–cross-talk between apical and basal ES
As mentioned above, during stage VIII of the epithelial cycle, spermiation that occurs at the apical compartment coincides with preleptotene spermatocyte migration across the BTB at the basal compartment. As such, these two cellular events involving extensive junction restructuring take place at the opposite ends of the adjacent Sertoli cells in the seminiferous epithelium (see Figs. 1 and 2). Thus, it is not entirely unexpected that there is cross talk between TJ/basal ES at the BTB and the apical ES, and vice versa, since both are components located within a single columnar Sertoli cell. Indeed, recent findings have suggested that spermiation and the migration of preleptotene spermatocytes across the BTB are likely coordinated by the laminin–integrin protein complexes at the apical and basal ES.(79)
Firstly, laminin-333 is detected at the apical ES in spermatids beginning at step 8, and it becomes intensively localized to apical ES until early stage VIII prior to spermiation,(79) suggesting that its disappearance or disintegration may be crucial to spermiation. Secondly, this event coincides with the time that preleptotene spermatocytes traverse the BTB, when the levels of occludin and ZO-1 are transiently reduced. Thirdly, when the functional laminin-333 is perturbed by a blocking antibody, such as anti-laminin α3 or γ3 IgG, this treatment led to germ-cell depletion from the epithelium. Furthermore, the loss of Sertoli–spermatid adhesion induced by blocking the laminin ligand function apparently led to a transient loss of TJ proteins (e.g. occludin and ZO-1 but not JAM-A), which, in turn, perturbed the BTB integrity as manifested by a significant loss of occludin–ZO-1 protein complexes from the BTB.(79) This observation thus implicates the significance of laminin-333 in mediating the cross talk between apical ES and BTB at stage VIII of the epithelial cycle. Laminin-333 apparently serves as a “traffic cop” to co-ordinate the cross-talk between apical ES and BTB. Based on these observations, we speculate that laminin-333 at the apical ES is cleaved by a protease (e.g. MMP2) that is detected at the apical ES(56) prior to spermiation, generating the biologically active peptide(s), and this may also involve signaling function via pFAK and c-Src to mediate TJ and/or basal ES disassembly at the BTB. In other epithelia and in tumor cells that involve active cellular movement, proteolytic cleavage of laminins was shown to enhance cell migration.(96,97) Furthermore, the synthetic peptides, NH2-SDGR-COOH and NH2-RSGIY-COOH, corresponding to the sequences found in the cell-binding domain of laminin α and β1 chain, respectively, have been shown to inhibit Sertoli cell cord structures formation in vitro.(50) Additionally, a peptide of NH2-AQARSAASKVKVSMKF-COOH derived from laminin α5 chain in mice was shown to induce macrophage MMP-9 production and chemotaxis.(98) These findings thus provide evidence that peptide fragments derived from laminins can be potent biological substances that affect cell migratory behavior, organelle formation, protein production and others.
Table 2 summarizes the functional relationship between laminins and proteases in different epithelia, showing that laminins are putative substrates of different MMPs. In most cases, laminins are cleaved by MMPs into smaller fragments while, in others, the expression level of MMPs is significantly enhanced when synthetic laminins peptides were added to different cell lines.(96,99) For instance, laminin-5 is one of the best-studied laminin ligands, in which each of the three chains, laminin α3, β3 and γ2, is cleaved by different MMPs in various cell types (see Table 2) illustrating a specific enzyme (MMP) versus substrate (laminin chain) relationship. In adult rat testes, laminin γ3 chain is structurally associated with MMP2 at the apical ES, and MMP2 is also predominantly expressed at late VII through early stage VIII of the cycle.(56) Even though MMP2 is a secretory product of Sertoli, but not germ, cells, its expression is greatly enhanced when co-cultured with germ cells.(100) These results suggest that MMP2 (or other MMPs) may be involved in apical ES restructuring and that its substrates are most likely the laminin chains. It is therefore logical to speculate that the cleavage of laminin-333 that occurs at spermiation, such as by MMPs,(56) generates a biologically active fragment(s), which, in turn, sets off a cascade of events that leads to a transient loss of TJ-barrier function by lowering the levels of occludin and ZO-1.(79) These events are likely working in concert with cytokines.(34,78,101–104) For instance, it is likely that Sertoli and germ cells at the BTB produce cytokines to induce BTB opening. In a recent study, TNFα and TGF-β3 were shown to reversibly “open” the BTB by transiently reducing the level of occludin at the BTB(101) (see Fig. 2). These observations have recently been confirmed in a functional study where there was an enhanced diffusion of a small molecular dye, FITC, across the BTB following treatment of testes with TNFα, illustrating transient BTB disruption induced by TNFα treatment.(103) In short, laminins at the apical ES may mediate the cross talk between apical ES and BTB during spermatogenesis.
Table 2.
Functional relationships between laminins and proteases in multiple epithelia*
| Laminin ligand/chain | Protease partner | Cleavage/Regulation | Animal/cell types | Biological effects | Refs |
|---|---|---|---|---|---|
| α1 (laminin111) | Elastase | Cleavage of laminin α1 by elastase | RAW264.7 macrophages | Enhances the production of uPA (urokinase type plasminogen activator) and MMP-9 | (113) |
| MMP-3 | Cleavage of laminn α1 by MMP-3 | MMP-3 knock-out mice | Cleavage of laminin by MMP-3 increases the blood-brain barrier permeability | (114) | |
| α3 (laminin332) | MMP-1, MMP-9 | Induction of MMPs by LG4 domain of laminin α3 | Keratinocytes | Enhances cell migration | (96,115) |
| α5 (laminin511) | MMP-9 | Induction of MMP-9 by peptide of laminin α5, NH2-AQARSAAKVKVSMKF-COOH | Macrophages | Induces inflammatory cell chemotaxis and metalloproteinase activity | (98) |
| MT1-MMP | Proteolytic cleavage of laminin α5 | DU-145 prostate cancer type | Reduces cell adhesion and enhances cell migration | (116) | |
| β3 (laminin332) | MT1-MMP | Proteolytic cleavage of laminin β3 to 80 kDa fragment | Prostate carcinoma cells | Enhances cell migration | (117) |
| γ1 (laminin111) | MMP-9 | Induction of MMP-9 by the peptide of laminin γ1, NH2-KAFDITYVRLKF-COOH | B16-F10 melanoma cells | Promotes pulmonary metastasis | (99) |
| γ2 (laminin332) | MT1-MMP | Proteolytic cleavage of laminin γ2 | MCF10A mammary epithelial cells | Enhances cell migration | (118) |
| MMP-2 | Proteolytic cleavage of laminin γ2 | Melanoma cells, Breast epithelial cell | Induces invasive and metastatic potential of melanoma cells, enhances cell migration | (119,120) | |
| MMP-19 | Proteolytic cleavage of laminin γ2 to 105 kDa and 80 kDa fragments | HaCaT keratinocytes | Enhances cell migration | (121) | |
| Laminin-332 | MMP-10 | Proteolytic cleavage of laminin γ2 | Keratinocytes | Disorganizes cell migration, increases apoptosis in damaged keratinocytes | (122) |
| MT1-MMP | Proteolytic cleavage of domain III of laminin γ2 | Human gastric carcinoma | Expression of MT1-MMP enhances the spreading of tumor via degradation of laminin | (97) | |
| MT1-MMP | Induction of MT1-MMP by laminin-332 | Thymocytes | Promotes mature thymocyte migration via laminin-332 and MT1-MMP | (123) | |
| Laminin | MMP-9 | Proteolytic cleavage of laminin | Neuronal cells | Induces neuronal apoptosis, causing stroke | (124) |
| Kallikerin 24 | Hydrolyze laminin | Leydig cells | n.k. | (125) |
n.k., effects not known; MMP, matrix metalloprotease; MT1-MMP, membrane-type 1-MMP, a membrane associated MMP that serves as a receptor for some MMPs (e.g., pro MMP-2) and their activation, also known as MMP-14.
Only selected references are given herein because this Table is not intended to be exhaustive. Our goal is to select recent findings in the field that specific cleavage of luminin chains by their protease partners can generate biologically active fragments to regulate a wide range of physiological functions (see text for details).
Future perspectives and concluding remarks
This review summarizes recent advances in the field regarding the biology of ES in cell adhesion, orientation, polarity and movement in adult rat testes. It is increasingly clear that the events of spermiation and preleptotene spermatocyte movement across the BTB that occur at opposite ends of the Sertoli cell epithelium but concurrently at stage VIII of the epithelial cycle are coordinated by proteins at the apical and basal ES, such as laminins. Furthermore, ES uses the most-appropriate features found in anchoring and tight junctions to provide an effective yet dynamic adhesion for developing spermatids, such that spermatids can “anchor” onto Sertoli cells in the epithelium while they can orientate themselves and migrate efficiently across the epithelium during spermatogenesis. Further work, however, is needed to identify the molecules and/or mechanisms that regulate the crosstalk between the apical and basal ES in addition to laminins, presumably including cytokines, kinases (e.g. c-Src), and fragments of integrins and collagens. While ES confers the tightest adhesion between Sertoli cells and elongating spermatids versus Sertoli cells and round spermatids or spermatocytes,(81) recent findings suggest that this specialized junction type is most susceptible to toxicants or an alteration of the microenvironment in the testis. For instance, treatment of rats with either adjudin [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide formerly called AF-2364, a known Sertoli cell toxicant] or testosterone/estrogen implants (which suppress pituitary LH secretion thereby lowering the intratesticular androgen level in the tubule lumen(17,35)) was shown to selectively disrupt apical ES instead of the “weaker” AJ and desmosome-like junctions between Sertoli cells and spermatocytes.(18,21,35,80,85,105,106) These findings thus illustrate that the ES is a “friend” of spermatogenesis in good times under normal physiological conditions, however, when the testis is under assault from toxic substances, such as those from the environment (e.g. cadmium),(107) the ES turns into a “foe” of spermatogenesis, making the testis more vulnerable than other organs. The precise molecular mechanism underlying these phenomena is not known, but it must be related to the unique molecular architecture of the ES.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (NICHD, U54 HD029990 Project 3, and U01 HD045908 to CYC) and the CONRAD Program (CICCR, CIG 01-72 to CYC and CIG-01-74 to DDM), and the Hong Kong Research Grant Council (HRU7536/05M to WML).
Abbreviations
- AJ
adherens junction, a cell–cell actin-based anchoring junction type, besides AJ, anchoring junction is composed of cell–cell intermediate filament-based desmosome, cell–matrix actin-based focal contact also called focal adhesion complex (FAC) and cell–matrix intermediate filament-based hemidesmosome
- BTB
blood–testis barrier
- CAR
coxsackie and adenovirus receptor, a virus receptor that is also a tight junction protein and mediates cell–cell adhesion
- CK2
casein kinase 2
- c-Src
a member of the non-receptor protein tyrosine kinase
- DJ
desmosome-like junction, a cell–cell intermediate filament-based anchoring junction type unique to the testis having the properties of both desmosome and gap junction (GJ)
- ES
ectoplasmic specialization, a testis-specific AJ type
- FAC
focal adhesion complex
- FAK
focal adhesion kinase
- ILK
integrin-linked kinase
- JAM
junctional adhesion molecule
- MMP
metalloprotease
- TJ
tight junction
- TBC
tubulobulbar complex, a testis-specific AJ type restricted to Sertoli–Sertoli cell interface at the BTB and Sertoli-elongate spermatid interface known as the basal TBC and apical TBC, respectively, apical TBC can only be found in adult rat testes at stage VIII of the epithelial cycle prior to spermiation after the disappearance of apical ES
- TIMP
tissue inhibitor of metalloproteases
- TGF-β3
transforming growth factor-β3
- TNFα
tumor necrosis factor α
- ZO-1
zonula occludens
References
- 1.Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol. 1998;9:411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- 2.Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol. 1985;94:177–211. doi: 10.1016/s0074-7696(08)60397-6. [DOI] [PubMed] [Google Scholar]
- 3.Pelletier RM, Byers SW. The blood-testis barrier and Sertoli cell junctions: structural considerations. Microsc Res Tech. 1992;20:3–33. doi: 10.1002/jemt.1070200104. [DOI] [PubMed] [Google Scholar]
- 4.Russell LD. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat. 1977;148:313–328. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- 5.Hess RA, Schaeffer DJ, Eroschenko VP, Keen JE. Frequency of the stages in the cycle of the seminiferous epithelium in the rat. Biol Reprod. 1990;43:517–524. doi: 10.1095/biolreprod43.3.517. [DOI] [PubMed] [Google Scholar]
- 6.Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev. 1982;3:404–417. doi: 10.1210/edrv-3-4-404. [DOI] [PubMed] [Google Scholar]
- 7.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 8.Alberts B, Bray D, Lewis J, Raff M, Roberts K, et al. The Molecular Biology of the Cell. New York: Garland Publishing Inc; 1994. pp. 950–1006. [Google Scholar]
- 9.Miyoshi J, Takai Y. Molecular perspective on tight-junction assembly and epithelial polarity. Adv Drug Deliv Rev. 2005;57:815–855. doi: 10.1016/j.addr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Cereijido M, Valdes J, Shoshani L, Contreras RG. Role of tight junctions in establishing and maintaining cell polarity. Annu Rev Physiol. 1998;60:161–177. doi: 10.1146/annurev.physiol.60.1.161. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Moreno M, Jamora C, Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–548. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 12.Yin T, Green KJ. Regulation of desmosome assembly and adhesion. Semin Cell Dev Biol. 2004;15:665–677. doi: 10.1016/j.semcdb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- 14.Russell LD. Observations on rat Sertoli ectoplasmic (‘junctional’) specializations in their association with germ cells of the rat testis. Tissue Cell. 1977;9:475–498. doi: 10.1016/0040-8166(77)90007-6. [DOI] [PubMed] [Google Scholar]
- 15.Vogl AW, Pfeiffer DC, Mulholland DJ, Kimel G, Guttman J. Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch Histol Cytol. 2000;63:1–15. doi: 10.1679/aohc.63.1. [DOI] [PubMed] [Google Scholar]
- 16.Vogl AW. Distribution and function of organized concentrations of actin filaments in mammaliam spermatogenic cells and Sertoli cells. Int Rev Cytol. 1989;119:1–56. doi: 10.1016/s0074-7696(08)60648-8. [DOI] [PubMed] [Google Scholar]
- 17.O’ Donnell L, Stanton PG, Bartles JR, Robertson DM. Sertoli cell ectoplasmic specializations in the seminiferous epithelium of the testosterone-suppressed adult rat. Biol Reprod. 2000;63:99–108. doi: 10.1095/biolreprod63.1.99. [DOI] [PubMed] [Google Scholar]
- 18.Wong CH, Xia W, Lee NPY, Mruk DD, Lee WM, et al. Regulation of ectoplasmic specialization dynamics in the seminiferous epithelium by focal adhesion-associated proteins in testosterone-suppressed rat testes. Endocrinology. 2005;146:1192–1204. doi: 10.1210/en.2004-1275. [DOI] [PubMed] [Google Scholar]
- 19.Lee NPY, Mruk DD, Lee WM, Cheng CY. Is the cadherin/catenin complex a functional unit of cell-cell actin-based adherens junctions in the rat testis? Biol Reprod. 2003;68:489–508. doi: 10.1095/biolreprod.102.005793. [DOI] [PubMed] [Google Scholar]
- 20.Lui WY, Lee WM, Cheng CY. Sertoli-germ cell adherens junction dynamics in the testis are regulated by RhoB GTPase via the ROCK/LIMK signaling pathway. Biol Reprod. 2003;68:2189–2206. doi: 10.1095/biolreprod.102.011379. [DOI] [PubMed] [Google Scholar]
- 21.Siu MKY, Mruk DD, Lee WM, Cheng CY. Adhering junction dynamics in the testis are regulated by an interplay of β1-integrin and focal adhesion complex-associated proteins. Endocrinology. 2003;144:2141–2163. doi: 10.1210/en.2002-221035. [DOI] [PubMed] [Google Scholar]
- 22.Ozaki-Kuroda K, Nakanishi H, Ohta H, Tanaka H, Kurihara H, et al. Nectin couples cell-cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr Biol. 2002;12:1145–1150. doi: 10.1016/s0960-9822(02)00922-3. [DOI] [PubMed] [Google Scholar]
- 23.Wong CH, Cheng CY. The blood-testis barrier: Its biology, regulation, and physiological role in spermatogenesis. Curr Topics Dev Biol. 2005;71:263–296. doi: 10.1016/S0070-2153(05)71008-5. [DOI] [PubMed] [Google Scholar]
- 24.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. α-Catenin is a molecular switch that binds E-cadherin-β-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstruting the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee NPY, Mruk Dd, Conway AM, Cheng CY. Zyxin, axin, and Wiskott-Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl. 2004;25:200–215. doi: 10.1002/j.1939-4640.2004.tb02780.x. [DOI] [PubMed] [Google Scholar]
- 27.Suzu S, Hayashi Y, Harumi T, Nomaguchi K, Yamada M, et al. Molecular cloning of a novel immunoglobulin superfamily gene preferentially expressed by brain and testis. Biochem Biophys Res Commun. 2002;296:1215–1221. doi: 10.1016/s0006-291x(02)02025-9. [DOI] [PubMed] [Google Scholar]
- 28.Raschperger E, Engstrom U, Pettersson RF, Fuxe J. CLMP, a novel member of the CTX family and a new component of epithelial tight junctions. J Biol Chem. 2004;279:796–804. doi: 10.1074/jbc.M308249200. [DOI] [PubMed] [Google Scholar]
- 29.Harada H, Suzu S, Hayashi Y, Okada S. BT-IgSF, a novel immunoglobulin superfamily protein, functions as a cell adhesion molecule. J Cell Physiol. 2005;204:919–926. doi: 10.1002/jcp.20361. [DOI] [PubMed] [Google Scholar]
- 30.Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, et al. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–945. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol. 2004;36:1206–1237. doi: 10.1016/j.biocel.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Yan HHN, Cheng CY. Blood-testis barrier dynamics are regulated by an engagement/disengagement mechanism between tight and adherens junctions via peripheral adaptors. Proc Natl Acad Sci USA. 2005;102:11722–11727. doi: 10.1073/pnas.0503855102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller SL, Portwich M, Schmidt A, Utepbergenov DI, Huber O, et al. The tight junction protein occludin and the adherens junction protein α-catenin share a common interaction mechanism with ZO-1. J Biol Chem. 2005;280:3747–3756. doi: 10.1074/jbc.M411365200. [DOI] [PubMed] [Google Scholar]
- 34.Xia W, Cheng CY. TGF-β3 regulates anchoring junction dynamics in the seminiferous epithelium of the rat testis via the Ras/ERK signaling pathway: an in vivo study. Dev Biol. 2005;280:321–343. doi: 10.1016/j.ydbio.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Wong CH, Xia W, Mruk DD, Lee NPY, et al. Regulation of Sertoli-germ cell adherens junction dynamics via changes in protein-protein interactions of the N-cadherin-β-catenin complex which are possibly mediated by c-Src and myotubularin-related protein 2: an in vivo study using an androgen suppression model. Endocrinology. 2005;146:1268–1284. doi: 10.1210/en.2004-1194. [DOI] [PubMed] [Google Scholar]
- 36.Siu MKY, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. BioEssays. 2004;26:978–992. doi: 10.1002/bies.20099. [DOI] [PubMed] [Google Scholar]
- 37.Giebel J, Loster K, Rune GM. Localization of integrin β1, α1, α5 and α9 subunits in the rat testis. Int J Androl. 1997;20:3–9. doi: 10.1046/j.1365-2605.1997.d01-105.x. [DOI] [PubMed] [Google Scholar]
- 38.Palombi F, Salanova M, Tarone G, Parini D, Stefanini M. Distribution of β1-integrin subunit in rat seminiferous epithelium. Biol Reprod. 1992;47:1173–1182. doi: 10.1095/biolreprod47.6.1173. [DOI] [PubMed] [Google Scholar]
- 39.Mecham RP. Receptors for laminin on mammalian cells. FASEB J. 1991;5:2538–2546. doi: 10.1096/fasebj.5.11.1651264. [DOI] [PubMed] [Google Scholar]
- 40.Kreidberg JA. Functions of α3β1 integrin. Curr Opin Cell Biol. 2000;12:548–553. doi: 10.1016/s0955-0674(00)00130-7. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura Y, Handa K, Iwamoto R, Tsukamoto T, Takahasi M, et al. Immunohistochemical distribution of CD9, heparin binding epidermal growth factor-like growth factor, and integrin α3β1 in normal human tissues. J Histochem Cytochem. 2001;49:439–444. doi: 10.1177/002215540104900403. [DOI] [PubMed] [Google Scholar]
- 42.Salanova M, Stefanini M, de Curtis I, Palombi F. Integrin receptor α6β1 is localized at specific sites of cell-to-cell contact in rat seminiferous epithelium. Biol Reprod. 1995;52:79–87. doi: 10.1095/biolreprod52.1.79. [DOI] [PubMed] [Google Scholar]
- 43.Mulholland DJ, Dedhar S, Vogl AW. Rat seminiferous epithelium contains a unique junction (ectoplasmic specialization) with signaling properties both of cell/cell and cell/matrix junctions. Biol Reprod. 2001;64:396–407. doi: 10.1095/biolreprod64.1.396. [DOI] [PubMed] [Google Scholar]
- 44.Wrobel KH, Mademann R, Sinowatz F. The lamina propria of the bovine seminiferous tubule. Cell Tissue Res. 1979;202:357–377. doi: 10.1007/BF00220431. [DOI] [PubMed] [Google Scholar]
- 45.Nievers MG, Schaapveld RQJ, Sonnenberg A. Biology and function of hemidesmosomes. Matrix Biol. 1999;18:5–17. doi: 10.1016/s0945-053x(98)00003-1. [DOI] [PubMed] [Google Scholar]
- 46.Powell AM, Sakuma-Oyama Y, Oyama N, Black MM. Collagen XVII/BP180: a collagenous transmembrane protein and component of the dermoepidermal anchoring complex. Clin Exp Dermatol. 2005;30:682–687. doi: 10.1111/j.1365-2230.2005.01937.x. [DOI] [PubMed] [Google Scholar]
- 47.Aho S, Uitto J. 180-kD Bullous pemphigoid antigen/type XVII collagen: tissue-specific expression and molecular interactions with keratin 18. J Cell Biochem. 1999;72:356–367. doi: 10.1002/(sici)1097-4644(19990301)72:3<356::aid-jcb5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 48.Frojdman K, Pelliniemi LJ. Differential distribution of the α6 subunit of integrins in the development and sexual differentiation of the mouse testis. Differentiation. 1994;57:21–29. doi: 10.1046/j.1432-0436.1994.5710021.x. [DOI] [PubMed] [Google Scholar]
- 49.Tres LL, Kierszenbaum AL. The ADAM-integrin-tetraspanin complex in fetal and postnatal testicular cords. Birth Defects Res C Embryo Today. 2005;75:130–141. doi: 10.1002/bdrc.20041. [DOI] [PubMed] [Google Scholar]
- 50.Hadley MA, Weeks BS, Kleinman HK, Dym M. Laminin promotes formation of cord-like structures by Sertoli cells in vitro. Dev Biol. 1990;140:318–327. doi: 10.1016/0012-1606(90)90082-t. [DOI] [PubMed] [Google Scholar]
- 51.Johnson KJ, Boekelheide K. Dynamic testicular adhesion junctions are immunologically unique. II. Localization of classic cadherin in rat teists. Biol Reprod. 2002;66:992–1000. doi: 10.1095/biolreprod66.4.992. [DOI] [PubMed] [Google Scholar]
- 52.Johnson KJ, Zecevic A, Kwon EJ. Protocadherin α3 acts at sites distinct from classic cadherins in rat testis and sperm. Biol Reprod. 2004;70:303–312. doi: 10.1095/biolreprod.103.021758. [DOI] [PubMed] [Google Scholar]
- 53.Beall SA, Boekelheide K, Johnson KJ. Hybrid GPCR/Cadherin (Celsr) proteins in rat testis are expressed with cell type specificity and exhibit differential Sertoli cell-germ cell adhesion activity. J Androl. 2005;26:529–538. doi: 10.2164/jandrol.05003. [DOI] [PubMed] [Google Scholar]
- 54.Takai Y, Nakanishi H. Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci. 2003;116:17–27. doi: 10.1242/jcs.00167. [DOI] [PubMed] [Google Scholar]
- 55.Koch M, Olson PF, Albus A, Jin W, Hunter DD, et al. Characterization and expression of the laminin γ3 chain: a novel, non-basement membrane-associated, laminin chain. J Cell Biol. 1999;145:605–617. doi: 10.1083/jcb.145.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siu MKY, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod. 2004;70:945–964. doi: 10.1095/biolreprod.103.023606. [DOI] [PubMed] [Google Scholar]
- 57.Wine RN, Chapin RE. Adhesion and signaling proteins spatiotemporally associated with spermiation in the rat. J Androl. 1999;20:198–213. [PubMed] [Google Scholar]
- 58.Gliki G, Ebnet K, Aurrand-Lions M, Imhof BA, Adams RH. Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature. 2004;431:320–324. doi: 10.1038/nature02877. [DOI] [PubMed] [Google Scholar]
- 59.Coyne CB, Bergelson JM. CAR: a virus receptor within the tight junction. Adv Drug Deliv Rev. 2005;57:869–892. doi: 10.1016/j.addr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 60.Coyne CB, Voelker T, Pichla SL, Bergelson JM. The coxsackievirus and adenovirus receptor interacts with the multi-PDZ domain protein-1 (MUPP-1) within the tight junction. J Biol Chem. 2004;279:48079–48084. doi: 10.1074/jbc.M409061200. [DOI] [PubMed] [Google Scholar]
- 61.Mirza M, Hreinsson J, Strand M, Hovatta O, Soder O, et al. Coxsackievirus and adenovirus receptor (CAR) is expressed in male germ cells and forms a complex with the differentiation factor JAM-C in mouse testis. Exp Cell Res. 2006;312:817–830. doi: 10.1016/j.yexcr.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 62.Dym M. Basement membrane regulation of Sertoli cells. Endocr Rev. 1994;15:102–115. doi: 10.1210/edrv-15-1-102. [DOI] [PubMed] [Google Scholar]
- 63.Yurchenco PD, Wadsworth WG. Assembly and tissue functions of early embryonic laminins and netrins. Curr Opin Cell Biol. 2004;16:572–579. doi: 10.1016/j.ceb.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 64.Foidart JM, Bere EWJ, Yaar M, Rennard SI, Gullino M, et al. Distribution and immunoelectron microscopic localization of laminin, a noncollagenous basement membrane glycoprotein. Lab Invest. 1980;42:336–342. [PubMed] [Google Scholar]
- 65.Davis CM, Papadopoulos V, Sommers CL, Kleinman HK, Dym M. Differential expression of extracellular matrix components in rat Sertoli cells. Biol Reprod. 1990;43:860–869. doi: 10.1095/biolreprod43.5.860. [DOI] [PubMed] [Google Scholar]
- 66.Suarez-Quian CA, Hadley MA, Dym M. Effect of substrate on the shape of Sertoli cells in vitro. Ann N Y Acad Sci. 1984;438:417–434. doi: 10.1111/j.1749-6632.1984.tb38303.x. [DOI] [PubMed] [Google Scholar]
- 67.Tung PS, Skinner MK, Fritz IB. Cooperativity between Sertoli cells and peritubular myoid cells in the formation of the basal lamina in the seminiferous tubule. Ann N Y Acad Sci. 1984;438:435–446. doi: 10.1111/j.1749-6632.1984.tb38304.x. [DOI] [PubMed] [Google Scholar]
- 68.Tung PS, Fritz IB. Extracellular matrix promotes rat Sertoli cell histotypic expression in vitro. Biol Reprod. 1984;30:213–229. doi: 10.1095/biolreprod30.1.213. [DOI] [PubMed] [Google Scholar]
- 69.Wong CCS, Chung SSW, Grima J, Zhu LJ, Mruk DD, et al. Changes in the expression of junctional and nonjunctional complex component genes when inter-Sertoli tight junctions are formed in vitro. J Androl. 2000;21:227–237. [PubMed] [Google Scholar]
- 70.Denduchis B, Satz ML, Sztein MB, Puig RP, Doncel G, et al. Multifocal damage of the testis induced in rats by passive transfer of antibodies prepared against non-collagenous fraction of basement membrane. J Reprod Immunol. 1985;7:59–75. doi: 10.1016/0165-0378(85)90021-x. [DOI] [PubMed] [Google Scholar]
- 71.Lustig L, Doncel GF, Berensztein E, Denduchis B. Testis lesions and cellular and humoral immune responses induced in rats by immunization with laminin. Am J Reprod Immunol Microbiol. 1987;14:123–128. doi: 10.1111/j.1600-0897.1987.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 72.Lustig L, Denduchis B, Ponzio R, Lauzon M, Pelletier RM. Passive immunization with anti-laminin immunoglobulin G modifies the integrity of the seminiferous epithelium and induces arrest of spermatogenesis in the guinea pig. Biol Reprod. 2000;62:1505–1514. doi: 10.1095/biolreprod62.6.1505. [DOI] [PubMed] [Google Scholar]
- 73.Tung PS, Fritz IB. Interactions of Sertoli cells with laminin are essential to maintain integrity of the cytoskeleton and barrier functions of cells in culture in the two-chambered assembly. J Cell Physiol. 1993;156:1–11. doi: 10.1002/jcp.1041560102. [DOI] [PubMed] [Google Scholar]
- 74.Guttman JA, Janmey P, Vogl AW. Gelsolin - evidence for a role in turnover of junction-related actin filaments in Sertoli cells. J Cell Sci. 2002;115:499–505. doi: 10.1242/jcs.115.3.499. [DOI] [PubMed] [Google Scholar]
- 75.Wang F, Hanske M, Miedema K, Klein G, Ekblom P, et al. Laminin in the male germ cells of Drosophila. J Cell Biol. 1992;119:977–988. doi: 10.1083/jcb.119.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Libby RT, Hunter DD, Brunken WJ. Developmental expression of laminin β2 in rat retina. Further support for a role in rod morphogenesis. Invest Ophthalmol Vis Sci. 1996;37:1651–1661. [PubMed] [Google Scholar]
- 77.Libby RT, Champliaud MF, Claudepierre T, Xu Y, Gibbons EP, et al. Laminin expression in adult and developing retinae: evidence of two novel CNS laminins. J Neurosci. 2000;20:6517–6528. doi: 10.1523/JNEUROSCI.20-17-06517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology. 2001;142:1865–1877. doi: 10.1210/endo.142.5.8116. [DOI] [PubMed] [Google Scholar]
- 79.Yan HHN, Cheng CY. Laminin α3 forms a complex with β3 and γ3 chains that serves as the ligand for α6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem. 2006;281:17286–17303. doi: 10.1074/jbc.M513218200. [DOI] [PubMed] [Google Scholar]
- 80.Beardsley A, O’Donnell L. Characterization of normal spermiation and spermiation failure induced by hormone suppression in adult rats. Biol Reprod. 2003;68:1299–1307. doi: 10.1095/biolreprod.102.009811. [DOI] [PubMed] [Google Scholar]
- 81.Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF. Strength measurement of the Sertoli-spermatid junctional complex. J Androl. 2005;26:354–359. doi: 10.2164/jandrol.04142. [DOI] [PubMed] [Google Scholar]
- 82.Mruk DD, Lau ASN, Conway AM. Crosstalk between Rab GTPases and cell junctions. Contraception. 2005;72:280–290. doi: 10.1016/j.contraception.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 83.Preston TM, King CA. An experimental study of the interaction between the soil amoeba Naegleria gruberi and a glass substrate during amoeboid locomotion. J Cell Sci. 1978;34:145–158. doi: 10.1242/jcs.34.1.145. [DOI] [PubMed] [Google Scholar]
- 84.Kuphal S, Bauer R, Bosserhoff AK. Integrin signaling in malignant melanoma. Cancer Metastasis Rev. 2005;24:195–222. doi: 10.1007/s10555-005-1572-1. [DOI] [PubMed] [Google Scholar]
- 85.Beardsley A, Robertson DM, O’Donnell L. A complex containing α6/β1 integrin and phosphorylated FAK between Sertoli cells and elongated spermatids during spermatid release from the seminiferous epithelium. J Endocrinol. 2006;190:759–770. doi: 10.1677/joe.1.06867. [DOI] [PubMed] [Google Scholar]
- 86.Siu MKY, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–25047. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- 87.Friedl P, Brocker EB. The biology of cell locomotion within three-dimensional extracellular matrix. Cell Mol Life Sci. 2000;57:41–64. doi: 10.1007/s000180050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carragher NO, Frame MC. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 2004;14:241–249. doi: 10.1016/j.tcb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 89.Kierszenbaum AL, Rivkin E, Tres LL. The actin-based motor myosin Va is a component of the acroplaxome, an acrosome-nuclear envelope junctional plate, and of manchette-associated vesicles. Cytogenet Genome Res. 2003;103:337–344. doi: 10.1159/000076822. [DOI] [PubMed] [Google Scholar]
- 90.Velichkova M, Guttman J, Warren C, Eng L, Kline K, et al. A human homologue of Drosophila kelch associates with mysoin-VIIa in specialized adhesion junctions. Cell Motil Cytoskeleton. 2002;51:147–164. doi: 10.1002/cm.10025. [DOI] [PubMed] [Google Scholar]
- 91.Guttman JA, Kimel GH, Vogl AW. Dynein and plus-end microtubule-dependent motors are associated with specialized Sertoli cell junction plaques (ectoplasmic specializations) J Cell Sci. 2000;113:2167–2176. doi: 10.1242/jcs.113.12.2167. [DOI] [PubMed] [Google Scholar]
- 92.Caswell PT, Norman JC. Integrin trafficking and the control of cell migration. Traffic. 2006;7:14–21. doi: 10.1111/j.1600-0854.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- 93.Panicker AK, Buhusi M, Erickson A, Maness PF. Endocytosis of β1 integrins is an early event in migration promoted by the cell adhesion molecules L1. Exp Cell Res. 2006;312:299–307. doi: 10.1016/j.yexcr.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 94.Guttman JA, Takai Y, Vogl AW. Evidence that tubulobulbar complexes in the seminiferous epithelium are involved with internalization of adhesion junctions. Biol Reprod. 2004;71:548–559. doi: 10.1095/biolreprod.104.028803. [DOI] [PubMed] [Google Scholar]
- 95.Lee NPY, Cheng CY. Protein kinases and adherens junction dynamics in the seminiferous epithelium of the rat testis. J Cell Physiol. 2005;202:344–360. doi: 10.1002/jcp.20119. [DOI] [PubMed] [Google Scholar]
- 96.Utani A, Momota Y, Endo H, Kasuya Y, Beck K, et al. Laminin α3 LG4 module induces matrix metalloproteinase-1 through mitogen-activated protein kinase signaling. J Biol Chem. 2003;278:34483–34490. doi: 10.1074/jbc.M304827200. [DOI] [PubMed] [Google Scholar]
- 97.Koshikawa N, Schenk S, Moeckel G, Sharabi A, Miyazaki K, et al. Proteolytic processing of laminin-5 by MT1-MMP in tissues and its effects on epithelial cell morphology. FASEB J. 2004;18:364–366. doi: 10.1096/fj.03-0584fje. [DOI] [PubMed] [Google Scholar]
- 98.Adair-Kirk TL, Atkinson JJ, Broekelmann TJ, Doi M, Tryggvason K, et al. A site on laminin α5, AQARSAASKVKVSMKF, induces inflammatory cell production of matrix metalloproteinase-9 and chemotaxis. J Immuno. 2003;171:398–406. doi: 10.4049/jimmunol.171.1.398. [DOI] [PubMed] [Google Scholar]
- 99.Kuratomi Y, Nomizu M, Tanaka K, Ponce ML, Komiyama S, et al. Laminin γ1 chain peptide, C-16 (KAFDITYVRLKF), promotes migration, MMP-9 secretion, and pulmonary metastasis of B16-F10 mouse melanoma cells. Br J Cancer. 2002;86:1169–1173. doi: 10.1038/sj.bjc.6600187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Longin J, Guillaumot P, Chauvin M, Morera A, Le Magueresse-Battistoni B. MT1-MMP in rat testicular development and the control of Sertoli cell proMMP-2 activation. J Cell Sci. 2001;114:2125–2134. doi: 10.1242/jcs.114.11.2125. [DOI] [PubMed] [Google Scholar]
- 101.Xia W, Mruk DD, Lee WM, Cheng CY. Differential interactions between transforming growth factor-β3/TβR1, TAB1 and CD2AP selectively disrupt blood-testis barrier and Sertoli-germ cell adhesion. J Biol Chem. 2006;281:16799–16813. doi: 10.1074/jbc.M601618200. [DOI] [PubMed] [Google Scholar]
- 102.Siu MKY, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor-α, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–387. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- 103.Li MWM, Xia W, Mruk DD, Wang CQF, Yan HHN, et al. Tumor necrosis factor α reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol. 2006;190:313–329. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- 104.Lui WY, Wong CH, Mruk DD, Cheng CY. TGF-β3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology. 2003;144:1139–1142. doi: 10.1210/en.2002-0211. [DOI] [PubMed] [Google Scholar]
- 105.Chen YM, Lee NPY, Mruk DD, Lee WM, Cheng CY. Fer kinase/FerT and adherens junction dynamics in the testis: an in vitro and in vivo study. Biol Reprod. 2003;69:656–672. doi: 10.1095/biolreprod.103.016881. [DOI] [PubMed] [Google Scholar]
- 106.Wolski KM, Mruk DD, Cameron DF. The Sertoli-spermatid junction complex adhesion strength is affected in vitro by Adjudin. J Androl. 2006;27:790–794. doi: 10.2164/jandrol.106.000422. [DOI] [PubMed] [Google Scholar]
- 107.Wong CH, Mruk DD, Siu MKY, Cheng CY. Blood-testis barrier dynamics are regulated by α2-macroglobulin via the c-Jun N-terminal protein kinase pathway. Endocrinology. 2005;146:1893–1908. doi: 10.1210/en.2004-1464. [DOI] [PubMed] [Google Scholar]
- 108.Sasaki T, Giltay R, Talts U, Timpl R, Talts JF. Expression and distribution of laminin α1 and α2 chains in embryonic and adult mouse tissues: an immunochemical approach. Exp Cell Res. 2002;275:185–199. doi: 10.1006/excr.2002.5499. [DOI] [PubMed] [Google Scholar]
- 109.Iivanainen A, Kortesmaa J, Sahlberg C, Morita T, Bergmann U, et al. Primary structure, developmental expression, and immunolocalization of the murine laminin α4 chain. J Biol Chem. 1997;272:27862–27868. doi: 10.1074/jbc.272.44.27862. [DOI] [PubMed] [Google Scholar]
- 110.Frojdman K, Miner JH, Sanes JR, Pelliniemi LJ, Virtanen I. Sex-specific localization of laminin α5 chain in the differentiating rat testis and ovary. Differentiation. 1999;64:151–159. doi: 10.1046/j.1432-0436.1999.6430151.x. [DOI] [PubMed] [Google Scholar]
- 111.Hager M, Gawlik K, Nystrom A, Sasaki T, Durbeej M. Laminin α1 chain corrects male infertility caused by absence of laminin α2 chain. Am J Pathol. 2005;167:823–833. doi: 10.1016/s0002-9440(10)62054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Airenne T, Lin Y, Olsson M, Ekblom P, Vainio S, et al. Differential expression of mouse laminin γ2 and γ2* chain transcripts. Cell Tissue Res. 2000;300:129–137. doi: 10.1007/s004410000182. [DOI] [PubMed] [Google Scholar]
- 113.Faisal Khan KM, Laurie GW, McCaffrey TA, Falcone DJ. Exposure of cryptic domains in the α1-chain of laminin-1 by elastase stimulates macrophages urokinase and matrix metalloproteinase-9 expression. J Biol Chem. 2002;277:13778–13786. doi: 10.1074/jbc.M111290200. [DOI] [PubMed] [Google Scholar]
- 114.Gurney KJ, Estrada EY, Rosenberg GA. Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis. 2006;23:87–96. doi: 10.1016/j.nbd.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 115.Momota Y, Suzuki N, Kasuya Y, Kobayashi T, Mizoguchi M, et al. Laminin α3 LG4 module induces keratinocyte migration: involvement of matrix metalloproteinase-9. J Recept Signal Transduct Res. 2005;25:1–17. doi: 10.1081/rrs-200047870. [DOI] [PubMed] [Google Scholar]
- 116.Bair EL, Chen ML, McDaniel K, Sekiguchi K, Cress AE, et al. Membrane type 1 matrix metalloprotease cleaves laminin-10 and promotes prostate cancer cell migration. Neoplasia. 2005;7:380–389. doi: 10.1593/neo.04619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Udayakumar TS, Chen ML, Bair EL, von Bredow DC, Cress AE, et al. Membrane type-1-matrix metalloproteinase expressed by prostate carcinoma cells cleaves human laminin-5 β3 chain and induces cell migration. Cancer Res. 2003;63:2292–2299. [PubMed] [Google Scholar]
- 118.Gilles C, Polette M, Coraux C, Tournier JM, Meneguzzi G, et al. Contribution of MT1-MMP and of human laminin-5 γ2 chain degradation to mammary epithelial cell migration. J Cell Sci. 2001;114:2967–2976. doi: 10.1242/jcs.114.16.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Seftor REB, Seftor EA, Koshikawa N, Meltzer PS, Gardner LMG, et al. Cooperative interactions of laminin 5 γ2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res. 2001;61:6322–6327. [PubMed] [Google Scholar]
- 120.Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 121.Sadowski T, Dietrich S, Koschinsky F, Ludwig A, Proksch E, et al. Matrix metalloproteinase 19 processes the laminin 5 γ2 chain and induces epithelial cell migration. Cell Mol Life Sci. 2005;62:870–880. doi: 10.1007/s00018-005-4478-8. [DOI] [PubMed] [Google Scholar]
- 122.Krampert M, Bloch W, Sasaki T, Bugnon P, Rulicke T, et al. Activities of the matrix metalloproteinase stromelysin-2 (MMP-10) in matrix degradation and keratinocyte organization in wounded skin. Mol Biol Cell. 2004;15:5242–5254. doi: 10.1091/mbc.E04-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vivinus-Nebot M, Rousselle P, Breittmayer J, Cenciarini C, Berrih-Aknin S, et al. Mature human thymocytes migrate on laminin-5 with activation of metalloproteinase-14 and cleavage of CD44. J Immuno. 2004;172:1397–1406. doi: 10.4049/jimmunol.172.3.1397. [DOI] [PubMed] [Google Scholar]
- 124.Gu Z, Cui J, Brown S, Fridman R, Mobashery S, et al. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci. 2005;25:6401–6408. doi: 10.1523/JNEUROSCI.1563-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Matsui H, Takano N, Takahashi T. Characterization of mouse glandular kallikrein 24 expressed in testicular Leydig cells. Int J Biochem Cell Biol. 2005;37:2333–2343. doi: 10.1016/j.biocel.2005.05.011. [DOI] [PubMed] [Google Scholar]