Mitochondria are dynamic organelles that are involved in a number of diverse processes. Most often the mitochondrion is associated with energy generation, but other important processes occur in this organelle such as fatty acid synthesis and amino acid metabolism. Although not the focus of this review, we would be remiss if we did not discuss how energy was generated via this organelle.
The mitochondrion is known as the “powerhouse” of the cell because it supplies the cell with high amounts of energy. The critical reactions that result in this high level production of energy are the Krebs Cycle (also called the Citric Acid Cycle or the Tricarboxylic Acid Cycle; TCA), and electron transport chain (ETC)/oxidative phosphorylation. Energy released from these processes is in the form of NADH (reduced nicotinamide adenine dinucleotide), FADH2 (reduced flavin adenine dinucleotide) or ATP (adenosine triphosphate).
The mitochondria have their own genome, which is comprised of 13,794 nucleotides in C. elegans. This genome only encodes 36 genes (37 in humans) [1, 2] while all of the other ~1000 genes required for normal mitochondrial function are nuclear encoded. The protein products from these nuclear encoded genes are subsequently translocated to the mitochondria and utilized in the variety of processes that occur inside the organelle. Is it possible then, that any of these nuclear encoded proteins could be translocated to other areas of the cell to participate in functions not normally attributed to the mitochondria? Are some mitochondrial proteins dual functioning or bifunctional proteins? In this review, we suggest that a subset of nuclear encoded mitochondrial proteins are involved in processes outside of the mitochondria and believe that this is a field of study that has been somewhat overlooked by the scientific community. There is growing evidence demonstrating that mitochondrial proteins not only localize to sites outside of the organelle, but also have functionality at these other sites. Although in some cases the mechanism of action of these mitochondrial proteins remains unclear, the protein localization patterns and interactions cannot be denied. We have observed that mitochondrial proteins involved in processes such as the Electron Transport Chain and the Citric Acid Cycle also play roles in meiosis, mitosis, and centrosome behavior in the early stages of C. elegans embryogenesis (see section II). In this review we will discuss published data about mitochondrial proteins that have the ability to influence progression of the cell cycle, alter chromosome morphology, localize to centrosomes, and influence centrosome number in the cell. Overall, the mitochondrion is an interesting organelle that participates in a variety of functions in the cell and is vital for the viability of an organism in numerous ways. We suggest that these essential functions are not solely due to the ability of the mitochondria to produce high amounts of energy. Throughout this discussion we attempt to demonstrate that a cell cannot live without mitochondria and study this issue from a non-energetic perspective.
In this review, we will present evidence from a number of organisms in which mutations or RNAi depletion of nuclear encoded mitochondrial genes perturbs cell cycle events, and in some cases, specifically affects centrosomal function. Given the role of mitochondria in energy production, most authors of these reports have concluded that the cellular defects are most likely due to alterations in ATP production and that processes that require high amounts of energy are deleteriously affected by abnormalities in mitochondrial function. However, in some of these reports, mitochondrial proteins are found associated with centrosomal proteins or localized to centrosomes and microtubule structures. Perhaps these mitochondrial proteins are bifunctional? In addition to their role in energy synthesis in the mitochondria, these proteins might also provide a structural or enzymatic function important for cell division and centrosome behavior. Given that nuclear encoded mitochondrial gene products must transit the cytosol to reach their mitochondrial targets, they have ample opportunity to participate in other functions in the cell outside of the mitochondria.
We will first present data showing that mitochondrial mutants can perturb cell cycle events, although some of these reports do not make a direct connection with centrosome function. We will then review mitochondrial mutants that play roles in perturbing microtubule spindle and centrosome functioning. Last, work will be presented that serves as evidence that distinct mitochondrial proteins are specifically associated with centrosome-localized proteins, suggesting that their mode of action is more likely direct and not the result of an indirect role in perturbation such as a depletion in ATP levels.
I. Mitocheckpoints: Mitochondrial Perturbations that Arrest the Cell Cycle
ATP and energy. That is what most scientists think about when they hear the word mitochondria. Without a doubt, ATP is critical for vast numbers of processes that occur in the cell. In fact, the cell is so “in tune” with cellular levels of ATP that one investigator has proposed the term “mitocheckpoint” to describe the monitoring system for mitochondrial function [3]. This checkpoint is thought to prevent replication of cells that harbor mitochondria with functional defects. If this checkpoint detects a severe mitochondrial defect then the cell is triggered to enter senescence [3]. It has been reported that mammalian HL-60 cells that have a ~15% reduction in ATP levels arrest at the G1 –S stage of the cell cycle while cells that have a ~35% ATP decrease undergo a G2-M arrest [4]. These groups suggested that the alterations in cell cycle progression were a consequence of mitochondrial defects, which cause abnormalities in genome stability triggered by defects in DNA repair activity. In some cases, alterations in normal mitochondrial function result in an increase in Reactive Oxygen Species (ROS) production. The elevated amount of ROS can interact with the base pairs of mtDNA to cause abnormal alterations in genome structure or the ROS can interact directly with proteins and alter their functionality. These factors, coupled to a decrease in ATP production, can affect subsequent processes such as transcription, replication, DNA repair, and recombination, all of which involve ATPases that might be affected by a decrease in ATP levels [3]. These studies suggest a possible link between ATP levels and cell cycle progression, but the molecular pathways by which reduced ATP levels are sensed by the cell and subsequently communicated to the cell cycle machinery has, until recently, been poorly defined. Below is an excellent example in Drosophila of how these signals are detected and transmitted to the cell cycle machinery.
Perhaps the most elegantly described mitocheckpoint- a cell cycle arrest triggered as a consequence of mitochondrial dysfunction- was elucidated by Mandal et al. The group characterized and molecularly cloned the tenured (tend) mutation in Drosophila [5]. In Drosophila, the third instar eye disc has a very well described morphogenetic furrow (MF). Within the MF, cells are arrested in G1, and immediately posterior to this furrow is a synchronous band of BrdU positive cells indicative of S phase. In their genetic screen for a “glossy” mutant adult eye phenotype, Mandal et al. were able to identify mutants in which this BrdU incorporation pattern in the third instar eye disc was aberrant. In one particular mutant, the cells posterior to the MF remained in G1, and these effects were not tissue specific as the authors also demonstrated a similar G1 arrest phenotype in wing discs. The authors characterized the tend mutation and found that it localized to a gene that encoded the small regulatory subunit Va of cytochrome oxidase (CoVa), which is one of the 13 subunits that comprise complex IV of the mitochondrial electron transport chain. This complex is important because it is responsible for a major portion of oxygen consumption in the cell; it reduces water to oxygen and translocates protons across the mitochondrial membrane into the mitochondria inner membrane space.
When the CoVa gene is mutated in Drosophila it causes deleterious effects during the progression of the cell cycle. More specifically, it induces a tight arrest at the G1 to S transition of the cell cycle. Cell cycle progression is controlled through the regulation of kinases called cyclin dependent kinases, and their associated cyclin proteins. For example, cell cycle progression from G1 to S is controlled by cyclin E and its interacting CDKs. The Mandal et al. paper attempted to demonstrate that there is an intimate link between metabolic activity and cellular sensors that are active during this transition of the cell cycle. ATP depletion in the cell causes an increase in adenosine monophosphate (AMP) levels, which activates the adenosine monophosphate activated protein kinase (AMPk). This kinase phosphorylates the cell cycle regulator p53, subsequently activating the protein and in turn allowing it to down regulate cyclin E/cdk2 and preventing progression past the G1 stage (Figure 1).
Figure 1.
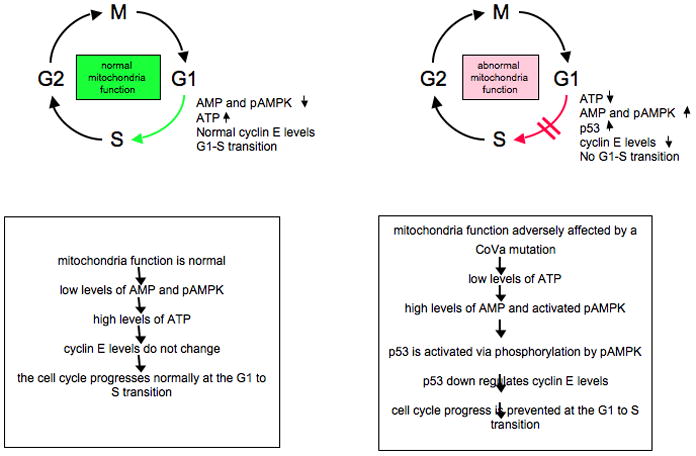
Mutations in the CoVa subunit of complex V of the electron transport chain trigger a “mitocheckpoint” that affects normal progression of the cell cycle at the G1-S transition (adapted from Mandal et al. [5]).
In the tenured mutant eye disc example, there is a decrease in cyclin E levels posterior to the morphogenetic furrow. When other factors were evaluated in these mutant cells such as the levels of other cell cycle regulators, response to developmental cues, or ability to differentiate, the only non-energy related defect detected in the cell was in cyclin E protein levels.
Do ATP levels play a role in these defects? To address this question, ATP levels were assayed in cultured Drosophila S2 cells depleted of CoVa by RNAi. The results from this assay demonstrated that CoVa depletion reduces cellular ATP levels by 60%. Since AMP can function as a sensor for energy levels in the cell, RNAi of CoVa triggered activation of AMP/AMPk via its ATP response mechanism and subsequently affected the downstream target p53 via phosphorylation. Mandal et al. demonstrated that in the double p53 and tenured mutant, the BrdU defect is rescued in the eye disc and cyclin E levels are also restored. In addition, there is a partial rescue of the glossy adult eye phenotype in the double mutant. These results strongly suggest that p53 is the downstream effector of the AMP kinase, and that p53 phosphorylation results in a subsequent reduction in cyclin E levels. Taken together, low ATP triggers high levels of AMP, which activates the AMP kinase. Activated AMP kinase phosphorylates and activates p53, which in turn inhibits cyclin E expression, likely indirectly thru the activity of a CDK inhibitor. The result of this signaling pathway, or mitocheckpoint, is a G1/S arrest.
The authors tested other mutations in genes that encode mitochondrial proteins and determined that mutations in PdsW, which functions in complex I of the electron transport chain, also gave rise to similar phenotypes to those described for CoVa. In addition, mutations in two mitochondrial ribosomal protein genes also gave rise to the glossy eye phenotype. Given that mutations in other mitochondrial genes can also perturb the cell cycle it would be interesting to test even more mitochondrial genes for their effects on cell cycle progression. Do all of the above mutations result in reduced ATP levels and does the response to low ATP levels in Drosophila always result in a G1/S arrest? Do other pathways exist where defects in mitochondrial function or proteins block the cell cycle at different stages of progression?
Interestingly, Hinchcliffe et al. used Xenopus extracts to demonstrate that Cdk2/cyclinE is required for centrosome duplication during S phase of the cell cycle [6]. When cyclin E expression is inhibited with a recombinant variant of a CDK inhibitor, centrosomes were unable to duplicate. Using immunofluorescence microscopy, they were also able to demonstrate that cyclin E localized to centrosomes in Xenopus embryos. It would be interesting to examine the effect of reduced ATP levels in Xenopus embryos. Would reduced ATP levels result in activation of AMPk? What effects would this have on downstream effectors such as p53 and cyclin E? Would a decrease in cyclin E levels lead to a defect in centrosome duplication? Taken together, we speculate that reduced ATP levels in Xenopus (possibly caused by mitochondrial dysfunction) might indirectly lead to centrosome duplication defects.
II. Mitochondrial Proteins With Dual Functions In the Cell
A. The epsilon subunit of ATP Synthase and a possible role in centrosome duplication
The isolation of additional mitochondrial mutants in Drosophila lends further support to the idea of bifunctional mitochondrial proteins. The stunted (sun) gene product was molecularly identified as a mitochondrial enzyme. A mutant was initially isolated from a zygotic lethal screen for genes that function in the cortical divisions of the developing embryos [7]. The fly embryo is an ideal stage of development to observe cell cycle progression due to the distinct phenotypical alterations that occur in each cell during cell division. Drosophila embryogenesis takes place in a syncytium, with the first 9 rounds of division occurring in the embryo interior, while divisions 10–13 occur in the cortex of the syncytial embryo. During the cortical divisions, thousands of nuclei are dividing in a confined monolayer. What is the mechanism by which collisions between neighboring spindles and nuclei are prevented? Sullivan et al. previously identified many mutations that disrupt the cortical divisions [7], eleven of which have been molecularly characterized. These mutations were in genes that encoded proteins involved in numerous functions in the cell including DNA damage repair proteins and the cell cycle. Kidd et al. demonstrated that another of these cortical division mutants was due to a defect in a key mitochondrial gene [8]! The stunted (sun) locus was molecularly identified and shown to encode the epsilon subunit of ATP synthase (complex V) of the electron transport chain. When maternal stores of this protein are depleted in embryos, a number of defects are detected during cortical divisions, such as abnormalities in the spindle, position of the centrosomes, and defects in chromosome division. The authors attribute these cellular defects to alterations in ATP levels since mitochondrial ATPase activity is reduced 6-fold in the sun mutants when compared to wildtype embryos.
When centrosome behavior was examined during cortical divisions in sun mutant embryos, most aspects were normal (duplication, separation), yet the position of the centrosome pairs between neighboring nuclei was abnormal. In wildtype embryos, spindles are equidistant from one another in an orientation that provides the most distance between centrosomes. In sun mutant embryos, the mitotic spindles are unable to repel the spindles of neighboring nuclei. This phenotype is thought to be due to abnormal spindle orientation that results in an abnormal interaction between the microtubules of neighboring nuclei. The authors suggested that the ability of microtubules to repel each other in wildtype embryos is likely due to the forces generated by motor proteins. The activity of these motor proteins may have higher energetic requirements in the developing embryo, and since ATP levels are decreased significantly in these sun mutants, perhaps they are unable to respond to this energetically demanding process. With weakened microtubule motor protein movement, the repulsive forces exerted between anti-parallel microtubules from adjacent spindles are unable to exert enough force to properly orient neighboring spindles.
The authors suggest that this cortical division phenotype is only an indirect consequence of an energy deficiency. If this phenotype was indeed solely the result of a decrease in ATP, one might anticipate that mutations in other mitochondrial genes to should have been isolated in screens for cortical division abnormalities. It remains to be determined if other mitochondrial gene mutations exist that perturb ATP levels and subsequently affect the cortical divisions in Drosophila embryos. We would like to suggest the possibility that the Sun protein might be bifunctional, and like the other mutants isolated in the screen for cortical mitotic defects, the Sun protein might have a more direct role in centrosome behavior. To determine if this is indeed the case, localization studies of Sun would be important. Any direct evidence of association with the centrosome would strengthen the suggestion that Sun might have a more direct role in centrosome behavior and spindle orientation during cortical divisions.
B. A C. elegans mitochondrial protein affects spindle behavior
In 1998, K. O’Connell performed an EMS screen in C. elegans to isolate temperature sensitive mutants defective in cell division [9]. A number of the mutants isolated from the screen were characterized and shown to have various centrosomal defects and were classified as either zyg (zygotic lethal) or spd (spindle defective) mutants. One of these mutants, spd-3, had defects as early as fertilization, when the meiotic divisions of the maternal chromosomes resume [10]. Although the meiotic spindle properly aligned perpendicular to the cortex, 23% of the embryos failed to extrude both polar bodies. Because a percentage of these embryos also had abnormally large polar bodies, the group attributed this phenotype to alignment problems of the meiotic spindle. Observations of mitotic 1-cell embryos revealed that upon pronuclear meeting the nuclei fail to rotate even though spindle astral microtubules are able to reach the cortex of the embryo. Molecular cloning of the spd-3 gene demonstrated that spd-3 encodes a novel protein with unknown function. The protein contains two transmembrane domains and several coiled coil regions, but does not have any clear homologues outside of nematodes. Given the interesting cellular phenotypes of the spd-3 mutant, it was surprising for the authors that a SPD-3::GFP fusion protein localized to the mitochondria in C. elegans. Upon further investigation it was determined that SPD-3 is a novel protein with no known homologies to mitochondrial enzymes that help produce energy. The data presented here suggests that a mitochondrial protein could also affect centrosome behavior without being localized to the centrosome.
Another surprise came when the group assayed ATP levels and found that ATP levels were not decreased in spd-3 mutants, but in fact increased by 40% [10]. This finding lead the authors to suggest that the spindle rotation abnormalities in spd-3 mutants may be due to motor proteins, such as the dynein/dynactin complex, since these proteins respond to alterations in ATP levels and function in spindle alignment in various systems. Similar spindle alignment defects have been observed with mutations in C. elegans dynactin components [11, 12]. Dinkelmann et al. observed that two dynactin components (DNC-1, a p150 glued homolog and DNC-2, a p50/dynamitin homolog) were mislocalized in spd-3 embryos. Others have also demonstrated that dynein and dynactin are required for the proper localization of organelles [13–15]. Dinkelmann et al. suggested that despite any obvious defects in localization of the mitochondria in spd-3 embryos, proper mitochondria localization and spindle alignment may be a function of the SPD-3 protein. Dinkelmann et al. also conducted further studies by disrupting various components of the electron transport chain with drug inhibitors or specific RNAi treatment, yet did not see any phenotypic alterations in development or viability consistent with dynein mutants. These observations suggests that the spindle positioning defects and abnormal meiotic progression are due to a mutant SPD-3 protein and not the result of a general defect in electron transport. This result is consistent with data from Feng et al. [16] that suggests that embryos with electron transport chain mutations are viable due to their observation that C. elegans embryos have minimal ATP requirements during the earliest stages of development. This group suggests that if there are defects in the ETC, the embryos may be able to progress successfully through development using the energy generated during other processes, such as glycolysis. Does this suggest that the observed decreases in embryonic viability in mitochondrial mutants may be due to other factors besides energy production? The Dinkelmann results suggest that mitochondrial proteins can have dramatic effects on meiosis, spindle functioning, and centrosome rotation without decreasing ATP levels. Since ATP levels actually increase in the embryo, it would be difficult to attribute these developmental defects in spd-3 mutants to energy deficits.
C. Numerous mitochondrial proteins influence centrosome behavior in C. elegans embryos
The C. elegans gonad is a terrific organ in which to study the process of mitosis and meiosis. The hermaphrodite gonad is a long U shaped tubular structure where all of the stages of the mitotic and meiotic processes are easily distinguishable by observation of chromosomal structure. The gonad is symmetric: two gonad arms connect at the center where the uterus is positioned (Figure 2). At the most distal end of each gonad arm, a pluripotent stem cell population of cells undergo mitotic divisions upon the reception of a Notch ligand signal from the distal tip cell (DTC). As these mitotic cells move towards the proximal end of the tube, and out of reach of this signal, they transition into the early stages of meiosis. More proximally, these meiotic cells enter diplotene and then differentiate first into sperm (during the L4 stage), and then into diakinetic oocytes (during the adult stage), where they remain until fertilized by sperm stored in the spermathecae.
Figure 2.

The tubular U shaped C. elegans gonad is an ideal organ to study the processes of mitosis and meiosis. The stages of each cycle are easily determined by observing the changes in chromosome structure of each germ cell. As the germ cells progress from the Distal Tip Cell (DTC) towards the proximal end of the gonad, the cells progress through mitosis then enter prophase of meiosis I. The chromosomes sequentially progress through the stages of leptotene, zygotene, pachytene, and diplotene. The oocytes then arrest in diakinesis of prophase I prior to progression through the spermatheca (sp). The entry of the sperm into the oocyte triggers resumption of meiotic progression in the early embryo. A. Cartoon of a dissected U shaped gonad from an adult hermaphrodite worm. DG, distal region of the gonad; PG, proximal region of the gonad. B. Adult dissected gonad stained with the DNA marker DAPI; PCD, region of the gonad where programmed cell death occurs (Figures courtesy of Lints, R. and Hall, D. H. Reproductive system, parts I– IV, in WormAtlas. http://www.wormatlas.org/handbook/reproductivesystem/reproductivesystem.htm and http://www.wormatlas.org/handbook/reproductivesystem/reproductivesystem2.htm).
Development of the somatic gonad and the germline starts during the second larval stage (L2) of development in C. elegans and culminates with a fully functional germline by the young adult stage of development. During this time, the somatic gonad expands from two cells to the U shaped tube described above, and the germ line expands from two primordial germ cells to about 1000 germ cells per gonad arm.
In our studies with nuclear encoded mitochondrial genes, we subject animals to continuous RNAi depletion by hatching the animals on bacteria expressing dsRNA corresponding to the gene of interest. Thus, we can deplete various mitochondrial proteins throughout development. By conducting experiments in this manner, it allows for observation of the effects of depletion of maternal stores of proteins in gametes in addition to zygotic depletion of the protein in developing embryos. When various mitochondrial proteins are depleted in L1 stage worms and onwards, the worms complete development without any clear defects in somatic or germline development. More specifically, the worms have a somatic gonad that is well populated with germ cells, and oocyte and sperm development appear normal. According to Sonnichsen et al. [17], at least 49 nuclear encoded C. elegans mitochondrial genes are essential based on reports that RNAi depletion results in embryonic lethality. We too have studied over 80 nuclear encoded mitochondrial genes and have determined that ~15 of the genes that are essential for embryonic development also have very specific defects associated with lethality. The first detectable defects in development occur once the meiotic divisions resume upon fertilization. C. elegans oocytes are in diakinesis of prophase I when they pass through the spermatheca and are fertilized. At this time, the first meiotic spindle forms as the oocyte chromosomes progress through metaphase and anaphase I and segregates the first polar body. Our RNAi studies have revealed that there are failures in polar body extrusion, often times coupled with the abnormal segregation of chromosomes into a polar body. During the first mitotic event in the treated embryo, there are also defects in chromosome segregation, chromosome morphology, and in the rotation of the spindle. These defects are so severe that they culminate in an embryonic arrest during the one or two cell stage of embryogenesis. This is a surprising result in that chromosome segregation mutants, though lethal, often do not result in an early arrest. Embryos with abnormal polar bodies and abnormal chromosome number still undergo numerous divisions despite being aneuploid and arrest (or die) as multicellular embryos with hundreds of cells.
In C. elegans there are very few examples of how maternal depletion of a specific protein causes a two cell embryonic arrest. Most often an arrest at this stage in development is associated with defects in centrosome duplication. Upon antibody staining of many of our RNAi depleted embryos, there are defects in centrosome number resulting in monopolar, tri-polar, and tetra-polar spindles (Figure 3).
Figure 3. Succinate dehydrogenase depleted embryos have an abnormal number of centrosomes in each cell.

The SPD-2 antibody labels Peri-centriolar material (PCM) and centrioles. In wildtype 1 cell embryos, there are two SPD-2 signals per cell and a very organized microtubule network. In comparison, the RNAi depleted embryos have an abnormal number of SPD-2 signals per cell and a very unorganized microtubule network. RNAi treated embryos arrested at metaphase of the first mitotic division have three or more SPD-2 signals per cell. Since SPD-2 stains PCM, this suggests that these embryos have an abnormal number of centrosomes in each cell. These abnormal staining patterns may be indicative of premature centriole disengagement. Panels A–D. A wildtype 1-cell mitotic embryo at metaphase. A. DNA stain. B. The same embryo stained with an anti-SPD-2 antibody. Two Microtubule Organizing Centers (MTOC) can be seen on both sides of the metaphase chromosomes. C. The same embryo stained with an anti-alpha tubulin antibody. A uniform arrangement of astral microtubules can be seen. D. A merge of panels A–C. Panels E–H. Two RNAi depleted metaphase embryos with the chromosomes aligned abnormally along the long axis of the embryo.
E. DNA stain. White arrows indicate the abnormally aligned chromosomes. F. Each RNAi-treated embryo reveals four SPD-2 signals flanking the metaphase chromosomes. G. Each RNAi depleted embryo has four MTOCs, which reveal an abnormal array of astral microtubules. This suggests that each PCM that is labeled with SPD-2 (panel F) is able to help construct a functional centrosome. H. A merge of panels E–G. SPD-2 antibodies were a generous gift from Kevin O’Connell.
It is often suggested that the developmental defects we have observed can easily be attributed to decreases in ATP levels since we are depleting the embryo of a mitochondrial protein. However, the defects in meiosis, mitosis and centrosome dynamics are not a result of ATP depletion. In cases where we have measured ATP levels, the treated embryos show an increase in the levels of the energetic compound. In cases where we have not measured ATP levels, it is still difficult to conclude that these defects are due to energetic deficiencies or even to altered ATP levels given that the mothers of these arrested embryos have completed their entire post-embryonic development in the presence of RNAi. The fertile adult worms appear normal and germline development seems unaffected; the first defects are only observed in their progeny. If the defects we observe are due to decreases in ATP levels one would have to argue that 1-cell embryos are the most sensitive to alterations in ATP if these phenotypes are truly linked to energy. Otherwise, our observations strongly suggests that specific mitochondrial proteins have other roles in the cell, specifically in centrosome dynamics, separate from their involvement in energy generation. Alternatively, our results could be interpreted to suggest a mitocheckpoint exists at this stage of development (see Conclusions).
D. Centrosomes and Cancer
One phenotypic hallmark of a cancer cell is an abnormal number of centrosomes, often referred to as centrosome amplification. If these extra centrosomes participate in mitotic events, multipolar spindles can arise leading to aneuploidy, which correlates with malignant behavior. However, multipolar spindles have a fitness cost to the cancerous cell and many cancer cells have mechanisms to cluster these supernumerary centrosomes into two poles, thus suppressing the formation of multipolar mitoses. By clustering these extra centrosomes, each division still appears to be the product of a bipolar spindle. Since multipolar divisions can be lethal to cancerous cells, Kwon et al. sought to identify molecules that normally promote centrosome clustering and bipolar spindle formation in cancer cells. Such molecules could thus be the targets of therapeutic treatment; this would allow one to target cancerous or pre-cancerous cells with abnormal centrosome numbers and prevent them from clustering into two poles. Such treatments could be lethal to tumor cells bearing multiple centrosomes, but spare normal cells that do not. To identify these targets, Kwon et al. [18] conducted a comprehensive screen in Drosophila S2 cells that contain extra centrosomes. The screen identified genes that normally prevent the formation of multipolar spindles by clustering their extra centrosomes during mitosis. Of the 14,000 genes that were analyzed by RNAi in this Drosophila cell line, 701 candidate genes were isolated that resulted in multipolar spindle formation when the targeted gene product was depleted. Of those genes, 292 were studied more closely and 133 genes were confirmed to have a role in centrosome clustering. These gene products were involved in diverse cellular processes, such as mitosis, cell adhesion, transcription, translation and RNA processing. Interestingly, of the 701 genes that were originally identified in their screen but were not analyzed further, fourteen encode mitochondrial proteins with human homologues ([18] and M. Kwon, personal communication). We suggest that these fourteen mitochondrial proteins may be bifunctional proteins that play a “real” role in centrosome clustering, thus affecting cancer cell survival. It would be interesting to closely examine the function of these genes in centrosome function in the near future. The genes that encode mitochondrial proteins Prohibitin and Succinate Dehydrogenase are mutated in some breast and head and neck carcinomas, respectively [19, 20]. Thus the mitochondrial proteins found in the screen, along with other mitochondrial proteins, may provide circumstantial evidence of the roles of mitochondrial proteins in centrosome organization and the formation of cancer cells.
III. Mitochondrial Proteins that Interact with Proteins Outside of the Mitochondria
A. A mitochondrial protein that associates with centrosomes
Although there are very few examples of mitochondrial proteins involved in processes outside the mitochondria, there are even fewer examples of mitochondrial proteins that directly interact with centrosomal components or influence centrosome number in the cell. In 2008, Hughes et al. conducted a screen in Drosophila to determine novel factors that associate with microtubules [21]. These Microtubule Associated Proteins (MAPs) were identified utilizing microtubule co-sedimentation assays, 1D and 2D gel electrophoresis, and mass spectrometry. Via these assays, 270 MAPs were identified from Drosophila embryo extracts and grouped into five major groups based on Gene Ontology (GO) categories. Many of these proteins were involved in diverse processes in the cell such as protein folding and degradation, DNA/RNA association, and microtubule function. The remaining proteins identified in this screen, almost half, functioned in various other processes with unknown functions in the cell. Interestingly, of the 270 MAPs that were found, 12 (4.5% of the total) of the isolated proteins were determined to be mitochondrial. One of these 12 genes was studied more closely and proved to be homologous to human mitochondrial succinate-CoA ligase. Succinate-CoA ligase is a component of the succinyl CoA synthetase complex that functions in the citric acid cycle to convert succinyl CoA to succinate. Existing interaction data suggested that this mitochondrial MAP was able to interact directly with the proteins SkpA and the Drosophila CDK2 ortholog, cdc2c. SkpA is homologous to Skp1, the S-phase kinase associated protein 1 that mediates protein degradation [22]. This association led the group to rename the mitochondrial protein SkAP or SkpA Associated Protein even though it shares significant homology with the mitochondrial protein Succinate-CoA ligase.
Validation of the SkpA and SkAP interaction was carried out using a co-immunoprecipitation assay with Drosophila embryo extracts. In addition, to confirm that the two proteins were bona fide MAPs, MT co-sedimentation assays with embryo extracts were performed. To address localization, an affinity-purified antibody that recognizes SkAP/Succinate-CoA ligase stained both the centrosomes and mitochondria in Drosophila spermatocytes and larval neuroblasts. In addition, in vivo studies with cultured S2 cells expressing a GFP::SkAP fusion protein also revealed centrosomal localization of SkAP. To determine if SkAP and SkpA had similar functions in the cell, each protein was RNAi depleted in cultured S2 cells; these depletions lead to spindle morphology defects. Interestingly, depletion of either SkAP or SkpA resulted in a supernumerary centrosome phenotype. The increased number of centrosomes appeared to be due to defects in centrosome duplication and not due to defects in cytokinesis. These data strongly suggest that SkAP/Succinate-CoA ligase is a centrosomal MAP that plays a role in centrosome duplication. It will be interesting to determine after further analysis and validation whether the other 11 mitochondrial proteins identified in their microtubule co-sedimentation assay also localize to spindle or centrosomal structures and also influence centrosomal number or function. Perhaps localization to centrosomes, coupled with abnormal centrosomal phenotypes upon RNAi depletion, will lend credence to the hypothesis that other mitochondrial proteins are bifunctional and can function in other cellular processes outside the mitochondria.
B. Mitochondria proteins and the Spindle Assembly Checkpoint
The Spindle Assembly Checkpoint (SAC) is a complex pathway that serves as a “checks and balances” system to protect the chromosomal integrity of the cell. The checkpoint triggers a cell cycle delay by inhibiting the activity of the Anaphase Promoting Complex (APC) and thus allows the cell the opportunity to ensure that chromosomes will be segregated properly during mitosis. During this delay, proper attachment of microtubules at the kinetochores, proper position of the spindle, and correct chromosome alignment at the metaphase plate are all assessed for proper functioning.
The APC is a multi-subunit E3 ubiquitin ligase that drives chromosome separation during the metaphase to anaphase transition. Prior to this transition, securin, an inhibitor of anaphase, is bound to the protease separase. When anaphase is triggered, the APC ubiquitinates securin, which frees separase. Active separase is now able to sever the cohesin molecules that hold sister chromatids together during metaphase. The cleavage of cohesin complexes allows for proper segregation of chromosomes to opposite poles of the cellat anaphase.
When mitochondrial proteins are isolated in spindle/centrosome proteomic studies, they are considered to be contamination and ignored, discarded, or remain unpublished [23]. For example, proteomic studies with components of the spindle assembly checkpoint, conducted by S. van der Sar and K. Hardwick, have identified a number of mitochondrial proteins. Spindle checkpoint components, such as the Mad and Bub proteins, were tagged and purified using a tandem affinity purification strategy and analyzed by tandem mass spectrometry. In addition to previously characterized proteins pulled down in this assay, approximately 5–10 mitochondrial proteins were unexpectedly found to interact with these components of the SAC (S. van der Sar and K. Hardwick, personal communication). Even though the SAC components are well characterized, novel accessory proteins that may be auxiliary to spindle checkpoint function can still be elucidated. The results from this group suggest that mitochondrial proteins might play a previously undetected role in the spindle assembly checkpoint.
C. Mitochondrial proteins in yeast 2 hybrid assays
Numerous RNAi screens have been performed in C. elegans to elucidate the number of genes essential for embryogenesis. A screen carried out by Sonnichsen et al. [17] found approximately 2000 genes that, when depleted by RNAi, cause embryonic lethality. Many of the embryos that failed to hatch had severe developmental defects prior to the two-cell stage of development. Utilizing 800 genes in this database, Boxem et al. [24] took a yeast 2 hybrid approach in order to determine novel interactions with centrosome components. The goal of the work was to define a comprehensive map of the centrosome interactome. The novel approach for this assay was the generation of an additional fragment library that allowed for determination of discreet domains that may interact in vivo in yeast 2 hybrid assays. In many instances, a fragment of one gene product was able to interact with another gene product, yet the interactions between two full-length constructs did not exist. In the initial screen, 705 full-length bait proteins were generated and screened against a cDNA library. Subsequently, these same baits were screened against 749 genes in the fragment prey library with each of these genes represented by as many as 40 fragments. Of more than 800 interactions that were determined, a number were between mitochondrial proteins (that were thought to be solely involved in the TCA or the ETC) and various proteins that function outside of the mitochondria, such as Microtubule Associated Proteins and centrosomal components. For example, the KLP-7 kinesin protein that is required to pull mitotic spindles asymmetrically to the posterior of 1-cell embryos interacts with the mitochondrial chaperone DNJ-10. Also, the mitochondrial FoF1 type ATP synthase gamma interacts with a microtubule associated bicaudal protein. One must be cautious in the interpretation of yeast 2 hybrid studies due to the observations that the methodology is notorious for producing non-specific interactions. However, given the examples presented in this Commentary, many of the interactions observed in the Boxem et al. study beg for further validation. Confirmation of these interactions would support our view that mitochondrial proteins play roles in processes distinct from energy generation. This conclusion is based on the above observations that mitochondrial proteins have the ability to interact directly with proteins that function at the centrosome or spindle. Most of these yeast 2 hybrid interactions remain to be validated in vivo from C. elegans extracts, but the data presented in this review combined with results from other studies suggests that some of these interactions will likely pan out in vivo. We are currently examining these interactions more closely and will validate which of these mitochondrial proteins do indeed directly interact with known components that function in the centrosome or spindle.
IV. Conclusions
We introduced a number of studies in this review in order to support our idea of a paradigm shift. Is it possible that a select group of nuclear encoded mitochondrial proteins function in a least two locations in the cell? Could these proteins translocate to the mitochondria and also play a role in processes outside of this organelle?
There are numerous examples of “moonlighting” proteins in the cell. For example, Histone H1 binds chromatin in the nucleus but at the cell membrane histone H1 is a receptor for thyroglobulin [25, 26]. PMS2, a mouse mismatch repair gene, is involved in both mismatch repair and antibody somatic hypermutation [26, 27]. During conditions where iron is high in the cell, cytosolic aconitase is an active enzyme that converts isocitrate from citrate. When iron is low in the cell, aconitase acts as an iron response element binding protein that can bind directly to ferritin mRNA [28]. Perhaps the most active multi-function protein in the cell is glyceraldehyde-3-phosphate dehydrogenase, which is involved in at least seven different processes in the cell ranging from roles in glycolysis, export of RNA from the nucleus, transcription, translation, Uracil DNA glycosylase activity, endocytosis, and membrane transport [29].
Although few, there are also examples of proteins that function in both the mitochondria and other locations in the cell. For example, the protein cytochrome C falls into the category of being a bifunctional protein in the cell. Cytochrome C is nuclear encoded and is transported into the mitochondria. Once in the mitochondria, cytochrome C serves as an electron acceptor in the electron transport chain where it donates electrons to oxygen in complex IV. During times of cellular stress, cytochrome C is released from the mitochondria and translocates to the surface of the ER where it binds to specific surface proteins and triggers the release of calcium. The presence of increased levels of free calcium in the mitochondria triggers the release of more cytochrome C that activates other processes in the cell. If cytochrome C is responding to a cell death signal, the cofactor binds to the WD40 repeat sequences of the pro-apoptotic factor APAF-1/CED-4 [30]. The binding of cytochrome C to this protein triggers the association of a number of other proteins, which together form the apoptosome that is able to trigger the activation of caspases, which initiate the multi-step process of apoptosis. Another mitochondrial enzyme that has dual roles in the cell is citrate synthase that catalyzes the production of citrate from acetyl coA and oxaloacetate in the organelle during the TCA. Outside of the mitochondria, in Tetrahymena thermophila, citrate synthase forms 14nm filaments that are involved in binary fission and in germ cell movement [26, 29, 28, 31]. Since examples do exist of mitochondrial genes that have dual roles in the cell, it is not unreasonable to hypothesize that mitochondrial genes might also have additional roles in the cell that have yet to be elucidated.
Without additional evidence, we cannot rule out though, that known mitochondrial proteins only function in the mitochondria. It is possible that the observed phenotypes described in this review are due to alterations in ATP levels in the cell. There are many processes that could be potentially altered by changes in ATP levels. For example, all ATPases in the cell could potential be effected, motors might not function properly to move chromosomes, and microtubules might not properly polymerize. In our studies in C. elegans, we have observed a very tight stage specific arrest when numerous individual mitochondrial proteins are depleted by RNAi. The specific arrest that we observe, if these defects are due to altered levels of ATP, would suggest that there is a point in the cell cycle or in early embryonic development that is particularly sensitive to a decrease in ATP levels. We do not think this is the case because reports have suggested that the C. elegans embryo actually requires very low levels of ATP to complete fundamental processes during the early stages of development [16]. Alternatively, there might be a mitocheckpoint in C. elegans shortly after fertilization of the oocyte. Upon fertilization, the embryo may evaluate its mitochondrial functionality. If there are any deficits in mitochondrial proteins or mitochondrial function, a checkpoint may exist that prevents further development of the embryo. Our observations that RNAi of numerous nuclear encoded mitochondrial genes all cause an early embryonic arrest suggest that such a checkpoint might exist.
We must also ask why more mitochondrial proteins have not been found at the midbody [32], bound to components of the SAC/APC ([33], and S. van der Sar, personal communication), or associated with centrosome clustering ([18], and M. Kwon, personal communication)? It seems unlikely that all of these observations of mitochondrial proteins in these various assays could be due to spurious contamination of mitochondrial proteins. If mitochondria were contaminating these different assays, one should anticipate the identification of many more mitochondrial proteins in these assays. Why is it that only a small subset of mitochondrial proteins is found? Why in some cases do we find only that depletion of one subunit of a multi-subunit complex causes a stage-specific arrest? We believe that these observations are too compelling to be ignored. We also believe there is valuable unpublished data sitting in notebooks in a few laboratories that support our hypothesis that some mitochondrial proteins are bifunctional. One of these functions includes a centrosomal role. We anticipate the publication of data in support of this hypothesis. Push the paradigm.
Acknowledgments
We are grateful to all those who shared unpublished data with us for this review, including S. van der Sar and K. Hardwick (Wellcome Trust Centre for Cell Biology, University of Edinburgh, Edinburgh, Scotland, UK), M. Kwon and D. Pellman (Dana-Farber Cancer Institute, Boston, Massachusetts), and J. Hanover (NIDDK/NIH, Bethesda, Maryland). We also thank H. Smith (University of Maryland Biotechnology Institute, Rockville, Maryland) for discussions concerning a C. elegans mitocheckpoint, K. O’Connell (NIDDK/NIH, Bethesda, Maryland) for the SPD-2 antibody staining used in Figure 3, and Dr. Clive Shiff (Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland) where this work started.
References
- 1.Okimoto R, et al. The mitochondrial genomes of two nematodes, Caenorhabditis elegans and Ascaris suum. Genetics. 1992;130(3):471–98. doi: 10.1093/genetics/130.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kakkar P, Singh BK. Mitochondria: a hub of redox activities and cellular distress control. Mol Cell Biochem. 2007;305(1–2):235–53. doi: 10.1007/s11010-007-9520-8. [DOI] [PubMed] [Google Scholar]
- 3.Singh KK. Mitochondria damage checkpoint, aging, and cancer. Ann N Y Acad Sci. 2006;1067:182–90. doi: 10.1196/annals.1354.022. [DOI] [PubMed] [Google Scholar]
- 4.Sweet S, Singh G. Changes in mitochondrial mass, membrane potential, and cellular adenosine triphosphate content during the cell cycle of human leukemic (HL-60) cells. J Cell Physiol. 1999;180(1):91–6. doi: 10.1002/(SICI)1097-4652(199907)180:1<91::AID-JCP10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Mandal S, et al. Mitochondrial regulation of cell cycle progression during development as revealed by the tenured mutation in Drosophila. Dev Cell. 2005;9(6):843–54. doi: 10.1016/j.devcel.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Hinchcliffe EH, et al. Requirement of Cdk2-cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts. Science. 1999;283(5403):851–4. doi: 10.1126/science.283.5403.851. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan W, Fogarty P, Theurkauf W. Mutations affecting the cytoskeletal organization of syncytial Drosophila embryos. Development. 1993;118(4):1245–54. doi: 10.1242/dev.118.4.1245. [DOI] [PubMed] [Google Scholar]
- 8.Kidd T, et al. The epsilon-subunit of mitochondrial ATP synthase is required for normal spindle orientation during the Drosophila embryonic divisions. Genetics. 2005;170(2):697–708. doi: 10.1534/genetics.104.037648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connell KF, Leys CM, White JG. A genetic screen for temperature-sensitive cell-division mutants of Caenorhabditis elegans. Genetics. 1998;149(3):1303–21. doi: 10.1093/genetics/149.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinkelmann MV, et al. SPD-3 is required for spindle alignment in Caenorhabditis elegans embryos and localizes to mitochondria. Genetics. 2007;177(3):1609–20. doi: 10.1534/genetics.107.078386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonczy P, et al. Cytoplasmic dynein is required for distinct aspects of MTOC positioning, including centrosome separation, in the one cell stage Caenorhabditis elegans embryo. J Cell Biol. 1999;147(1):135–50. doi: 10.1083/jcb.147.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skop AR, White JG. The dynactin complex is required for cleavage plane specification in early Caenorhabditis elegans embryos. Curr Biol. 1998;8(20):1110–6. doi: 10.1016/s0960-9822(98)70465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burkhardt JK, et al. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139(2):469–84. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helfand BT, et al. A requirement for cytoplasmic dynein and dynactin in intermediate filament network assembly and organization. J Cell Biol. 2002;157(5):795–806. doi: 10.1083/jcb.200202027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varadi A, et al. Cytoplasmic dynein regulates the subcellular distribution of mitochondria by controlling the recruitment of the fission factor dynamin-related protein-1. J Cell Sci. 2004;117(Pt 19):4389–400. doi: 10.1242/jcs.01299. [DOI] [PubMed] [Google Scholar]
- 16.Feng J, Bussiere F, Hekimi S. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev Cell. 2001;1(5):633–44. doi: 10.1016/s1534-5807(01)00071-5. [DOI] [PubMed] [Google Scholar]
- 17.Sonnichsen B, et al. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature. 2005;434(7032):462–9. doi: 10.1038/nature03353. [DOI] [PubMed] [Google Scholar]
- 18.Kwon M, et al. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev. 2008;22(16):2189–203. doi: 10.1101/gad.1700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayley JP, et al. Mutation analysis of SDHB and SDHC: novel germline mutations in sporadic head and neck paraganglioma and familial paraganglioma and/or pheochromocytoma. BMC Med Genet. 2006;7:1. doi: 10.1186/1471-2350-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato T, et al. The human prohibitin (PHB) gene family and its somatic mutations in human tumors. Genomics. 1993;17(3):762–4. doi: 10.1006/geno.1993.1402. [DOI] [PubMed] [Google Scholar]
- 21.Hughes JR, et al. A microtubule interactome: complexes with roles in cell cycle and mitosis. PLoS Biol. 2008;6(4):e98. doi: 10.1371/journal.pbio.0060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai C, et al. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86(2):263–74. doi: 10.1016/s0092-8674(00)80098-7. [DOI] [PubMed] [Google Scholar]
- 23.Andersen JS, et al. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426(6966):570–4. doi: 10.1038/nature02166. [DOI] [PubMed] [Google Scholar]
- 24.Boxem M, et al. A protein domain-based interactome network for C. elegans early embryogenesis. Cell. 2008;134(3):534–45. doi: 10.1016/j.cell.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brix K, et al. Extracellularly occurring histone H1 mediates the binding of thyroglobulin to the cell surface of mouse macrophages. J Clin Invest. 1998;102(2):283–93. doi: 10.1172/JCI1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeffery CJ. Multifunctional proteins: examples of gene sharing. Ann Med. 2003;35(1):28–35. doi: 10.1080/07853890310004101. [DOI] [PubMed] [Google Scholar]
- 27.Cascalho M, et al. Mismatch repair co-opted by hypermutation. Science. 1998;279(5354):1207–10. doi: 10.1126/science.279.5354.1207. [DOI] [PubMed] [Google Scholar]
- 28.Basilion JP, et al. The iron-responsive element-binding protein: localization of the RNA-binding site to the aconitase active-site cleft. Proc Natl Acad Sci U S A. 1994;91(2):574–8. doi: 10.1073/pnas.91.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piatigorsky J. Gene Sharing and Evolution The Diversity of Protein Functions. Harvard University Press; 2007. [Google Scholar]
- 30.Zou H, et al. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90(3):405–13. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 31.Jeffery CJ. Moonlighting proteins. Trends Biochem Sci. 1999;24(1):8–11. doi: 10.1016/s0968-0004(98)01335-8. [DOI] [PubMed] [Google Scholar]
- 32.Skop AR, et al. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science. 2004;305(5680):61–6. doi: 10.1126/science.1097931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon HJ, et al. Proteomics analysis identifies new components of the fission and budding yeast anaphase-promoting complexes. Curr Biol. 2002;12(23):2048–54. doi: 10.1016/s0960-9822(02)01331-3. [DOI] [PubMed] [Google Scholar]


