Abstract
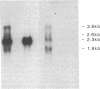
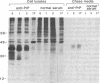
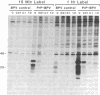
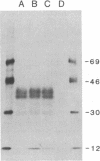
It has been proposed that the causative agent of scrapie represents a class of infectious particle that is devoid of nucleic acid and that an altered form of the endogenous prion protein (PrP) is the agent. However, it has been difficult to exclude the possibility that PrP purified from scrapie tissues might be contaminated with a more conventional viral agent. To obtain PrP uncontaminated by scrapie-infected tissues, PrP cDNA cloned from a scrapie-infected mouse brain was expressed in mouse C127 cells in vitro. mRNA and protein encoded by the cloned PrP gene were identified. The expressed PrP polypeptides appeared to be glycosylated and were released from the cell surface into the medium. Homogenates of the cells expressing the cloned PrP gene were inoculated into susceptible mice but failed to induce clinical signs of scrapie. Thus, either PrP is not the transmissible agent of scrapie or the expressed PrP requires additional modification to be infectious.
Full text
PDF
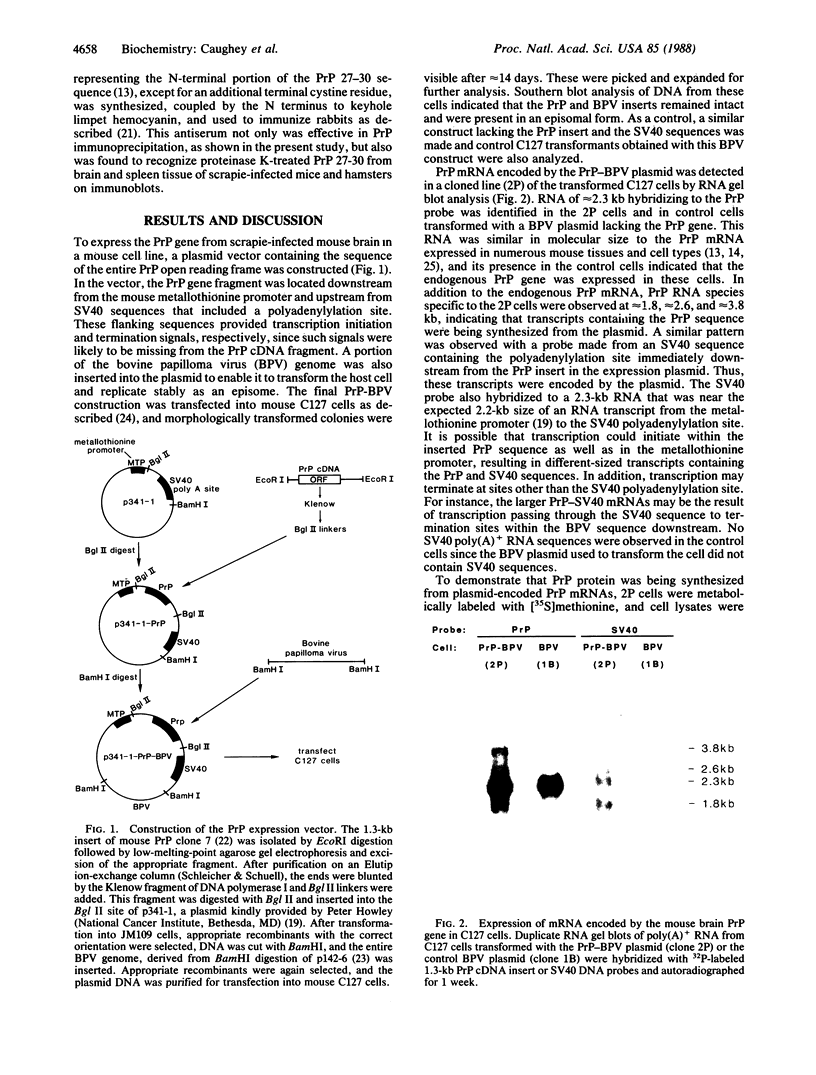

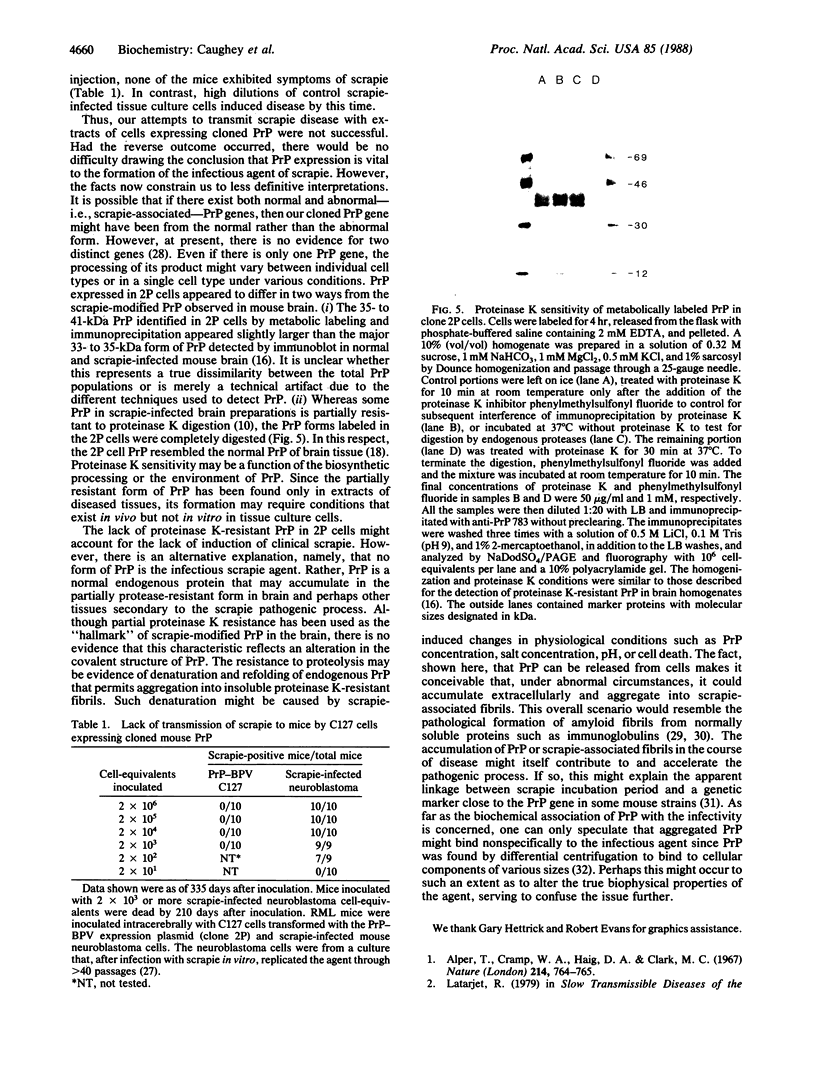

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper T., Cramp W. A., Haig D. A., Clarke M. C. Does the agent of scrapie replicate without nucleic acid? Nature. 1967 May 20;214(5090):764–766. doi: 10.1038/214764a0. [DOI] [PubMed] [Google Scholar]
- Basler K., Oesch B., Scott M., Westaway D., Wälchli M., Groth D. F., McKinley M. P., Prusiner S. B., Weissmann C. Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell. 1986 Aug 1;46(3):417–428. doi: 10.1016/0092-8674(86)90662-8. [DOI] [PubMed] [Google Scholar]
- Bellinger-Kawahara C., Cleaver J. E., Diener T. O., Prusiner S. B. Purified scrapie prions resist inactivation by UV irradiation. J Virol. 1987 Jan;61(1):159–166. doi: 10.1128/jvi.61.1.159-166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton D. C., McKinley M. P., Prusiner S. B. Identification of a protein that purifies with the scrapie prion. Science. 1982 Dec 24;218(4579):1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- Bolton D. C., Meyer R. K., Prusiner S. B. Scrapie PrP 27-30 is a sialoglycoprotein. J Virol. 1985 Feb;53(2):596–606. doi: 10.1128/jvi.53.2.596-606.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braig H. R., Diringer H. Scrapie: concept of a virus-induced amyloidosis of the brain. EMBO J. 1985 Sep;4(9):2309–2312. doi: 10.1002/j.1460-2075.1985.tb03931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson G. A., Kingsbury D. T., Goodman P. A., Coleman S., Marshall S. T., DeArmond S., Westaway D., Prusiner S. B. Linkage of prion protein and scrapie incubation time genes. Cell. 1986 Aug 15;46(4):503–511. doi: 10.1016/0092-8674(86)90875-5. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Race R., Wehrly K., Nishio J., Bloom M., Lechner D., Bergstrom S., Robbins K., Mayer L., Keith J. M. Identification of scrapie prion protein-specific mRNA in scrapie-infected and uninfected brain. Nature. 1985 May 23;315(6017):331–333. doi: 10.1038/315331a0. [DOI] [PubMed] [Google Scholar]
- Cho H. J. Antibody to scrapie-associated fibril protein identifies a cellular antigen. J Gen Virol. 1986 Feb;67(Pt 2):243–253. doi: 10.1099/0022-1317-67-2-243. [DOI] [PubMed] [Google Scholar]
- Dees C., Wade W. F., German T. L., Marsh R. F. Inactivation of the scrapie agent by ultraviolet irradiation in the presence of chlorpromazine. J Gen Virol. 1985 Apr;66(Pt 4):845–849. doi: 10.1099/0022-1317-66-4-845. [DOI] [PubMed] [Google Scholar]
- Diringer H., Gelderblom H., Hilmert H., Ozel M., Edelbluth C., Kimberlin R. H. Scrapie infectivity, fibrils and low molecular weight protein. Nature. 1983 Dec 1;306(5942):476–478. doi: 10.1038/306476a0. [DOI] [PubMed] [Google Scholar]
- Eiden M., Newman M., Fisher A. G., Mann D. L., Howley P. M., Reitz M. S. Type 1 human T-cell leukemia virus small envelope protein expressed in mouse cells by using a bovine papilloma virus-derived shuttle vector. Mol Cell Biol. 1985 Nov;5(11):3320–3324. doi: 10.1128/mcb.5.11.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Griffith J. S. Self-replication and scrapie. Nature. 1967 Sep 2;215(5105):1043–1044. doi: 10.1038/2151043a0. [DOI] [PubMed] [Google Scholar]
- Hope J., Morton L. J., Farquhar C. F., Multhaup G., Beyreuther K., Kimberlin R. H. The major polypeptide of scrapie-associated fibrils (SAF) has the same size, charge distribution and N-terminal protein sequence as predicted for the normal brain protein (PrP). EMBO J. 1986 Oct;5(10):2591–2597. doi: 10.1002/j.1460-2075.1986.tb04539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C., Chesebro B., Race R., Keith J. M. Molecular cloning and complete sequence of prion protein cDNA from mouse brain infected with the scrapie agent. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6372–6376. doi: 10.1073/pnas.83.17.6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Scolnick E. M. Helper-independent transformation by unintegrated Harvey sarcoma virus DNA. J Virol. 1978 May;26(2):291–298. doi: 10.1128/jvi.26.2.291-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley M. P., Bolton D. C., Prusiner S. B. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983 Nov;35(1):57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Manuelidis L., Manuelidis E. E. Scrapie-associated fibrils in Creutzfeldt-Jakob disease. Nature. 1983 Dec 1;306(5942):474–476. doi: 10.1038/306474a0. [DOI] [PubMed] [Google Scholar]
- Meyer R. K., McKinley M. P., Bowman K. A., Braunfeld M. B., Barry R. A., Prusiner S. B. Separation and properties of cellular and scrapie prion proteins. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2310–2314. doi: 10.1073/pnas.83.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch B., Westaway D., Wälchli M., McKinley M. P., Kent S. B., Aebersold R., Barry R. A., Tempst P., Teplow D. B., Hood L. E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Pattison I. H., Jones K. M. The possible nature of the transmissible agent of scrapie. Vet Rec. 1967 Jan 7;80(1):2–9. doi: 10.1136/vr.80.1.2. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Bolton D. C., Groth D. F., Bowman K. A., Cochran S. P., McKinley M. P. Further purification and characterization of scrapie prions. Biochemistry. 1982 Dec 21;21(26):6942–6950. doi: 10.1021/bi00269a050. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Hadlow W. J., Garfin D. E., Cochran S. P., Baringer J. R., Race R. E., Eklund C. M. Partial purification and evidence for multiple molecular forms of the scrapie agent. Biochemistry. 1978 Nov 14;17(23):4993–4999. doi: 10.1021/bi00616a021. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Race R. E., Fadness L. H., Chesebro B. Characterization of scrapie infection in mouse neuroblastoma cells. J Gen Virol. 1987 May;68(Pt 5):1391–1399. doi: 10.1099/0022-1317-68-5-1391. [DOI] [PubMed] [Google Scholar]
- Robakis N. K., Sawh P. R., Wolfe G. C., Rubenstein R., Carp R. I., Innis M. A. Isolation of a cDNA clone encoding the leader peptide of prion protein and expression of the homologous gene in various tissues. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6377–6381. doi: 10.1073/pnas.83.17.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein R., Kascsak R. J., Merz P. A., Papini M. C., Carp R. I., Robakis N. K., Wisniewski H. M. Detection of scrapie-associated fibril (SAF) proteins using anti-SAF antibody in non-purified tissue preparations. J Gen Virol. 1986 Apr;67(Pt 4):671–681. doi: 10.1099/0022-1317-67-4-671. [DOI] [PubMed] [Google Scholar]
- Sarver N., Byrne J. C., Howley P. M. Transformation and replication in mouse cells of a bovine papillomavirus--pML2 plasmid vector that can be rescued in bacteria. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7147–7151. doi: 10.1073/pnas.79.23.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley C. A., Burrola P. G., Buchmeier M. J., Wooddell M. K., Barry R. A., Prusiner S. B., Lampert P. W. Immuno-gold localization of prion filaments in scrapie-infected hamster brains. Lab Invest. 1987 Dec;57(6):646–656. [PubMed] [Google Scholar]