Abstract
Intranasal insulin administration raises central nervous system (CNS) insulin levels in humans and acutely facilitates verbal memory in patients with Alzheimer’s disease (AD), an effect that may differ by APOE genotype. The purpose of this study was to examine the cognitive dose response curves for intranasal insulin administration, and determine whether the effects of insulin differ between participants with (ε4+) and without (ε4−) the APOE- ε4 allele. On separate mornings, 33 memory-impaired adults with AD or amnestic mild cognitive impairment and 59 normal adults each underwent five intranasal treatment conditions consisting of insulin (10, 20, 40, or 60 IU) or placebo. Cognition was tested 15-minutes post-treatment, and blood was acquired at baseline and 45-minutes post-treatment. Plasma insulin and glucose levels were unaffected by treatment. Insulin administration facilitated recall on two measures of verbal memory in memory-impaired ε4− adults, with performance generally peaking at 20 IU. In contrast, memory-impaired ε4+ subjects demonstrated a relative decline in verbal memory. Insulin also differentially modulated plasma β-amyloid for memory-impaired subjects and normal controls, effects that again differed by APOE genotype. These findings suggest that groups with different genetic risks for AD may show differential dose-response curves following intranasal insulin administration.
Keywords: Intranasal administration, insulin, memory, β-amyloid, Alzheimer’s disease, mild cognitive impairment
1. Introduction
Insulin plays a role in central nervous system (CNS) energy metabolism, and perturbation of this role may contribute to cognitive deficits in Alzheimer’s disease (AD). Insulin receptors and insulin-sensitive glucose transporters are co-localized in medial temporal structures that support memory and are compromised early in the course of AD [3,24,51]. In addition, peripheral insulin abnormalities, such as peripheral hyperinsulinemia and insulin resistance (reduced insulin efficiency), are associated with an increased risk of AD [9,35,36,41], and alter CNS functioning. For example, insulin resistance down-regulates transport of insulin to the brain [20,27,53], and increases neuropathological features of AD [5]. Reduced cerebrospinal fluid (CSF) insulin levels and insulin signaling molecules have been described in patients with AD [9,13], and intravenous (IV) insulin administration while maintaining euglycemia dose-dependently improves memory in AD patients [8,10,12], possibly by augmenting low brain insulin levels or by overcoming insulin resistance.
Several mechanisms have been identified through which insulin administration may facilitate cognition including regionally specific CNS effects on glucose metabolism [6,14,37], modulation of long-term potentiation (LTP), and modulation of CNS concentrations of neurotransimitters, such as norepinephrine and acetylcholine [16,30]. However, peripherally administered insulin is not a viable treatment, in part due to risks associated with hypoglycemia.
The existence of direct pathways from the nasal cavity to the CNS raises the possibility that intranasal drug administration may circumvent risks associated with peripheral administration, and/or allow drugs that cannot penetrate the blood brain barrier (BBB) to rapidly access the CNS. Recent evidence supports bulk flow transport through several extracellular pathways from the nasal cavity to the CNS. Olfactory sensory neurons are exposed in the upper nasal cavity while their axons extend through the cribriform plate to the olfactory bulb. Insulin can access the CSF along the olfactory neurons through the cribriform plate, or it can enter the CNS parenchyma through perivascular channels associated with the olfactory or trigeminal systems [57,58]. In addition, a slower axonal transport pathway has been identified along which insulin can access the CNS several hours after intranasal administration [4].
A number of compounds, including insulin and insulin-like growth factor-I, have been successfully delivered to the brain or CSF following intranasal administration [18,25]. Intranasal insulin administration increases CSF insulin levels in humans 10-minutes after administration with peak levels achieved in about 30-minutes [7]; blood glucose and insulin levels do not change after intranasal administration [5,7,28,31] demonstrating that the changes in CSF are not due to transport from the nasal cavity to the systemic circulation.
Functional and cognitive studies of the acute and chronic effects of intranasal insulin administration also reveal CNS effects. Sixty minutes of intranasal insulin treatment induced changes in auditory-evoked brain potentials (AEPs) compared to placebo [28]. In addition, two months of daily insulin administration significantly improved verbal memory and enhanced mood in young healthy adults [5]. In a previous study, we examined the acute cognitive effects of intranasal insulin administration in participants with AD [31]. Subjects received placebo, 20, or 40 IU of intranasally administered insulin in randomized order on separate mornings; cognitive testing began 15-minutes post-insulin administration on each study visit. Based on studies with intravenous insulin administration [11,12], we examined whether treatment response would differ between subjects with and without the apolipoprotein (APOE) ε4 allele (ε4+ or ε4−), a genetic risk factor for late-onset AD. Insulin treatment facilitated recall on two measures of verbal memory in memory-impaired ε4− adults. In contrast, memory-impaired ε4+ adults did not benefit from treatment and demonstrated decreased memory performance on one memory test at the higher insulin dose. One possible explanation for these results is that dose-response curves differ by APOE genotype. A prior study of intravenous insulin dose response effects suggested that ε4+ subjects may show cognitive facilitation at very low doses of insulin [12], whereas ε4− carriers required higher doses or insulin to show similar facilitation.
The purpose of our study was to examine cognitive dose-response curves associated with intranasal insulin administration in adults with AD or amnestic mild cognitive impairment (MCI). Since peripheral insulin administration alters plasma amyloid β (Aβ) levels [31], we also examined the effects of intranasal insulin treatment on plasma Aβ. We hypothesized that intranasal administration of insulin would facilitate memory and modulate Aβ, and that dose response curves would differ by APOE genotype.
2. Materials and Methods
2.1 Subjects
This study was approved by the Human Subjects Review Committee of the University of Washington. Written informed consent was obtained from all subjects and the legal representatives of patients with AD. Participants included 59 cognitively normal older control subjects (48 ε4− and 11 ε4+) and 33 memory-impaired participants (11 ε4− and 22 ε4+) with either probable AD (NINCDS/ADRDA criteria [39] (n=13) or amnestic mild cognitive impairment (MCI) or multiple domain MCI with amnestic features [44] (n=20), a disorder widely believed to represent a prodromal stage of AD [43]. Diagnoses and study eligibility were determined by neuropsychologist and physician consensus following cognitive testing, medical history, physical exam, electrocardiogram, and clinical laboratory screening as previously described [31]. All subjects were free from psychiatric disorders, alcoholism, severe head trauma, hypoxia, neurological disorders other than AD, renal or hepatic disease, diabetes, chronic obstructive pulmonary disease, congestive heart failure, or unstable cardiac disease. Subject characteristics are presented in Table 1. Normal and memory-impaired groups did not differ in education or body mass index. Adults with memory impairment were slightly older than normal adults (mean age of about 77 versus 74); thus age was used as a covariate in all analyses. No differences were observed between ε4 carriers and non-carriers within the diagnostic groups on age, BMI, education or cognitive status as indexed by the Mattis Dementia Rating Scale (DRS).
Table 1.
Subject Characteristics
| Normal | Memory-Impaired | |||
|---|---|---|---|---|
| E4− | E4+ | E4− | E4+ | |
| Age | 73.8 (1.0) | 72.5 (2.0) | 76.3 (2.0) | 76.8 (1.4) |
| Education(yrs) | 14.6 (0.4) | 15.2 (0.8) | 14.5 (0.8) | 14.7 (0.6) |
| Body Mass Index (kg/m2) | 26.1 (0.6) | 25.6 (1.2) | 26.4 (1.2) | 26.5 (0.8) |
| Dementia Rating Scale | 139.3 (1.1) | 138.4 (2.3) | 130.7 (2.3) | 129.9 (1.6) |
2.2 Procedure
Fasting subjects came on five separate mornings, one to six weeks apart, to receive saline or one of four insulin doses (10, 20, 40, or 60 IU) in randomized counterbalanced order. An initial blood sample was obtained and subjects were placed in a supine position with the head tilted back. One-hundred μL of insulin (Novolin R, Novo Nordisk, Princeton, NJ, USA) or saline were administered with a needle-less syringe into alternating nostrils for a total volume of 600 μL at each visit. One-hundred μL of insulin corresponded to 10 IU; saline was administered as needed at insulin visits to achieve the total study drug volume of 600 μL. Subjects were instructed to sniff following administration to facilitate transport of insulin into the nasal cavity. Subjects rested for 15-minutes. A brief cognitive battery was administered and blood was subsequently collected 45-minutes post-nasal administration for glucose, insulin, and Aβ analyses.
2.3 Assays
Plasma glucose was measured with a glucose oxidase method using a HemoCue glucose analyzer (HemoCue, Lake Forest, CA). Insulin was assayed as previously described [10]. The APOE genotypes were determined using the PCR conditions described by Emi et al. [15] and the Hhai restriction digest method of Hixson and Vernier [23]. Plasma Aβ40 levels were measured by sandwich ELISA using a kit with 6E10-coated plates and a biotinylated anti-Aβ40 detection antibody (Signet Laboratories, Dedham, MA). Aβ42 levels were measured using 6E10 as a capture antibody (1.5 μg/well; Signet) and biotinylated anti-Aβ42 for detection as previously described [40]. Detergents were added to the plasma at final concentrations of 0.05% Tween-20 (both assays) and 1% sodium dodecyl sulfate (Aβ40 assay only) to improve detection of Aβ from plasma. Horseradish peroxidase–avidin was used for detection, and ELISAs were developed with tetramethylbenzidene as a peroxidase substrate. Initial reaction rate was determined by absorbance measured every 15 seconds at 650 nm, and the limit of detection for both assays was 10 pg/mL.
2.4 Cognitive protocol
Five comparable versions of the cognitive protocol were constructed and randomly assigned in counterbalanced order to the five treatment conditions. Each protocol included verbal declarative memory measures (Story Recall and Hopkins Verbal Learning Test), a test of selective attention (Stroop Color-Word test) [21], a visual working memory measure (Self-Ordered Pointing Task) [45], and a test of psychomotor processing speed (Digit Symbol). On the story recall test [12], subjects heard a brief narrative containing 44 informational bits, and were asked to recall as much as possible both immediately and after a 10-minute delay. Subjects received credit for verbatim recall and accurate paraphrases.
During the Hopkins Verbal Learning Test [54], subjects heard a list of 12 words drawn from 3 semantic categories and were asked to recall as many words as possible across three learning trials and after a 10-minute delay. Immediate recall was calculated as the number of correctly recalled words summed across the learning trials.
The Stroop Color-Word Test is a frontal-executive test of selective attention and response inhibition that was administered via computer according to methods described by Spieler et. al [56]. In two types of trial blocks, color names were presented one at a time, via computer equipped with a voice key, with concordant or discordant font colors (e.g., the word “red” presented in red or green font). At the beginning of each trial, a color word was presented on the screen and subjects were instructed to either read the word or name the font color. A vocalized response terminated each trial and initiated the next, and response content and voice onset response time were recorded. Trial blocks were distinguished only by the different task instructions.
The Self-Ordered Pointing Task (SOPT) is a test of visual working memory comprised of two subtests in which either 10 or 12 abstract designs appeared on a computer touch screen. For each subtest, subjects were asked to touch any one design that they had not touched previously. After each response, the designs in the array were rearranged. Trials continued until subjects had had one opportunity to touch each design. Each subtest was repeated three times with the same designs. Numbers of errors on each trial were recorded.
Digit Symbol is a test of visual scanning and motor speed [59]. Numbers were paired with an abstract symbol on a legend. Subjects were asked to copy the appropriate symbol from the legend below a series of randomly presented numbers for 120 seconds. Correctly completed items were scored.
2.5 Statistical Analysis
Memory scores were subjected to a repeated measures analysis of variance with insulin dose (0, 10, 20, 40, and 60 IU) and recall condition (immediate or delayed) as within subjects factors. Diagnostic category (normal or memory-impaired) and APOE-ε4 carriage (ε4+ or ε4−) were between subjects factors. Age, education, BMI, and DRS score were entered as covariates. Non-contributing covariates were dropped. Data from the Stroop interference test were treated similarly, with errors and reaction times during color naming used as dependent variables, interference condition (match vs. non-match) and insulin dose as within subjects factors, and diagnostic group and APOE status as between subjects factors. Tests of working memory (Self-Ordered Pointing Test) and psychomotor speed were similarly analyzed.
3. Results
3.1 Intranasal insulin did not affect peripheral glucose or insulin levels
Blood glucose and insulin levels did not differ between memory-impaired and normal subjects, and intranasal insulin administration did not affect these values (data not shown).
3.2 Insulin modulated verbal memory for the memory-impaired group in an APOE-dependent manner
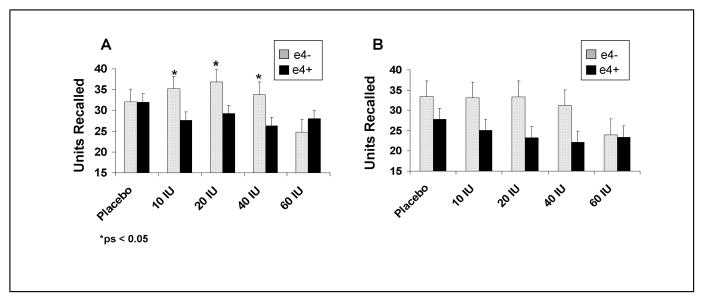
For story recall, adults with memory impairment showed a different dose-response pattern with intranasal insulin than did normal adults, as reflected in an interaction between diagnostic group and insulin dose [F(4,340)= 4.06, p=0.0031]. For the memory-impaired group, response to insulin differed according to APOE-ε4 status [F(4,116)=2.67, p=0.0354]. As shown in Fig. 1, the memory-impaired/ε4− group’s immediate recall improved at the 10, 20, and 40 IU doses, whereas the ε4 carriers performance declined [F(1,29)=4.25, 5.09, 5.40, ps=0.0484, 0.0317, 0.0273]. A similar trend of reduced performance was observed for delayed recall for ε4 carriers, who had lower delayed recall compared to non-ε4 carriers at the 10, 20, and 40 IU doses (ps=0.1061, 0.0739, and 0.0595). As has been previously reported, no changes were observed in story recall in response to intranasally administered insulin for normal adults (data not shown).
Figure 1.
(A) Immediate and (B) delayed story recall scores for memory-impaired adults with (E4+) and without (E4−) the APOE-ε4 allele. The memory-impaired/ε4− group’s immediate recall improved at the 10, 20, and 40 IU doses, whereas the ε4 carriers performance declined (ps=0.0484, 0.0317, 0.0273). A similar trend of reduced performance was observed for delayed recall for ε4 carriers, who had lower delayed recall compared to non-ε4 carriers at the 10, 20, and 40 IU doses (ps=0.1061, 0.0739, and 0.0595).
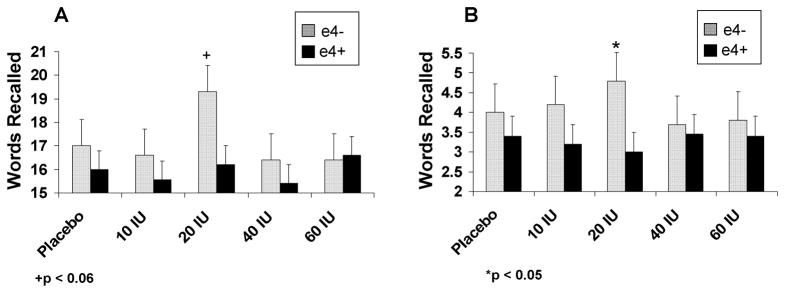
Adults with memory impairment also showed a different dose-response pattern with intranasal insulin for list learning than did normal adults, as reflected in an interaction between diagnostic group and insulin dose [Fig. 2; F(4,336)= 2.52, p=0.0409]. As with story recall, the memory-impaired/ε4− group showed maximal benefit at the 20 IU dose relative to the memory-impaired/ε4+ group (ps=0.0637and 0.0416 for between group comparisons for immediate and delayed recall). Normal adults’ performance was unaffected by intranasal insulin at any dose. No effects of intranasal insulin were observed for the other cognitive tests.
Figure 2.
(A) Immediate and (B) delayed list learning scores for memory-impaired adults with (E4+) and without (E4−) the APOE-ε4 allele. The memory-impaired/ε4− group showed maximal benefit at the 20 IU dose relative to the memory-impaired/ε4+ group (ps=0.0637and 0.0416 for between group comparisons for immediate and delayed recall).
3.3 Intranasal insulin modulated plasma amyloid β levels
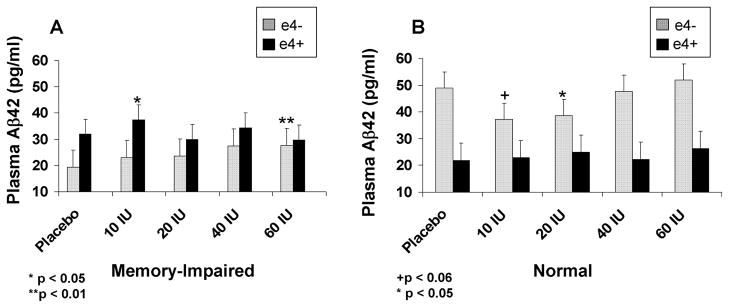
Plasma Aβ levels were measured at each of the five insulin doses for a randomly-selected subset of normal (n=13) and memory-impaired (n=15) participants. For the longer specie of amyloid (Aβ42), insulin produced differential and APOE-ε4 related effects for memory-impaired adults compared with normal adults [Fig. 3; F(4,92)=3.03, p=0.0213]. Aβ42 levels increased for memory-impaired adults from saline to 10 IU regardless of ε4 status. For memory-impaired adults without the APOE-ε4 allele, Aβ42 levels continued to rise with increasing dose, an effect that reached significance at the highest insulin dose [F(1,12)=10.51, p=0.0071], whereas no further increases were observed for memory-impaired ε4 carriers. In contrast, normal adults without the APOE-ε4 allele showed a U-shaped dose response curve, in which Aβ42 levels decreased in the 10 and 20 IU dose conditions compared with saline, whereas normal adults with the APOE-ε4 allele showed no significant changes. [F(1,10)=4.56 and 6.55, ps=0.0585 and 0.0285]. Non-ε4 carriers’ Aβ42 levels returned to baseline levels at the 40 and 60 IU doses. No effects were observed for diagnostic group, APOE-ε4 carriage, or insulin dose for Aβ40 levels.
Figure 3.
Plasma Aβ42 levels (pg/ml) for (A) memory-impaired and (B) normal adults. Aβ42 levels increased for memory-impaired adults from saline to 10 IU regardless of ε4 status (p=.0213). For memory-impaired adults without the APOE-ε4 allele, Aβ42 levels at the highest insulin dose were greater than levels observed following saline administration (p=0.0071). Normal adults without the APOE-ε4 allele showed a U-shaped dose response curve, in which Aβ42 levels decreased in the 10 and 20 IU dose conditions compared with saline (ps=0.0585 and 0.0285).
4. Discussion
Intranasal insulin administration facilitated verbal memory in adults with AD and MCI who were not APOE-ε4 carriers. For memory-impaired ε4− adults, 10, 20, and 40 IU of insulin improved declarative memory. The cognitive dose-response curves for this group showed that memory facilitation generally peaked at the 20 IU dose. In contrast, 60 IU of insulin did not facilitate memory. Relative to the benefits of treatment in the memory-impaired ε4− group, several memory scores in the memory-impaired ε4+ group declined following treatment. These findings are consistent with the results of our prior study, which found that intranasal insulin administration acutely facilitated verbal memory in ε4− memory-impaired subjects, whereas theε4+ subjects showed no benefits or a decline in memory.
Differential cognitive responses to insulin treatment by APOE genotype may result from differences in insulin sensitivity. There is growing evidence that insulin resistance may be a more common occurrence in adults with AD who are not APOE-ε4 carriers. Insulin resistance is a condition in which muscle, fat, and hepatic cell responses to insulin are impaired; higher doses of insulin are required to trigger biological responses normally observed at lower insulin doses in healthy adults. We have previously reported that AD patients without the ε4 allele show reduced insulin-mediated glucose disposal compared to ε4+ patients [10], and ε4 non-homozygous AD patients have higher plasma insulin levels and lower CSF-to-plasma insulin ratios compared to ε4 homozygotes [9]. In addition, the association between AD and syndromes related to peripheral hyperinsulinemia and insulin resistance has been strongest in ε4- groups in some epidemiological studies [1,34], although some inconsistency has been noted [42,60]. Consistent with the results of the current study, a prior dose-response study showed that the cognitive effects of intravenously administered insulin differed by APOE genotype such that non-ε4 homozygous AD patients required higher insulin doses than ε4 homozygotes to induce memory facilitation [12].
Other studies examining energy metabolism in AD have also reported results that differ by APOE genotype. A recent clinical trial showed that the insulin sensitizing thiazolidinedione, rosiglitazone, improved cognition and functional skills in ε4− AD patients but not in ε4+ patients. A prospective cohort study of older adults reported an inverse relationship between physical activity and risk for AD among ε4− individuals, but not among ε4+ older adults [46]. In addition, we found that raising plasma levels of the ketone body, beta-hydroxybutyrate, an alternative energy substrate for the brain, facilitated cognitive performance in ε4− AD and MCI patients, but not for ε4+ subjects [47].
Of interest, intranasal insulin reduced memory performance in the memory-impaired ε4+ adults. One possible explanation to account for this pattern is that the insulin doses employed in the present study were too high for these patients. Our prior study showed that following intravenous insulin infusion, ε4+ patients demonstrated memory facilitation at only very low insulin doses [12]. It is possible that even the lowest intranasal insulin dose in the current study was still too high for ε4+ subjects. Insulin can impair cognition when hypoglycemia is induced [29] or when insulin abnormalities exert inflammatory effects in the CNS [17]. It is also possible that insulin treatment may have exacerbated the reduction in CNS energy metabolism that has been associated with the ε4+ genotypes [48,49], a possibility that may have been further exacerbated by the fasting status of study participant. Alternatively, treatment response differences between APOE groups may reflect differences in the amount of insulin transported to the CNS following intranasal administration. Future research is required to explore these possibilities.
Intranasal insulin treatment acutely modulated plasma Aβ42 levels. For AD and MCI groups, plasma Aβ42 was increased following administration of 10 IU of insulin; however higher insulin doses resulted in additional relative increases in Aβ42 among memory impaired ε4− subjects, while the ε4+ group showed no further elevations. In contrast, 10 and 20 IU of insulin reduced Aβ42 levels in ε4− normal adults. These results are generally consistent with a recent study in which plasma Aβ42 was measured in AD and control subjects after a 120 minute intravenous infusion of one of three insulin doses or placebo [31]. For AD subjects in that study, insulin raised plasma Aβ42 at all insulin doses, with the greatest increase observed at the highest dose (1.76-mU · kg−1). For normal controls, however, Aβ42 was reduced during moderate hyperinsulinemia (1.0-mU · kg−1), with attenuated effects observed at lower and higher insulin doses.
The clinical implications of altered plasma Aβ42 levels require additional study. Although differential memory effects were observed by APOE status, both ε4− and ε4+ patients showed increased plasma Aβ42 with treatment (although differential dose response curves were noted). The relationship between plasma Aβ42 levels and cognitive functioning is complex. WhileCSF Aβ levels are reliably lower in patients with AD [2], mixed results have been reported for plasma Aβ levels [19,26,40,50,52]. Longitudinal studies suggest that plasma Aβ levels may increase early in the disease and decrease as AD progresses [22,38].
The precise mechanisms through which insulin alters plasma Aβ levels are unknown, and may include release from systemic sources, efflux from the CNS, binding to lipoproteins and other carrier molecules, and peripheral clearance. One of the advantages of intranasal administration methods is that it provides an opportunity to dissociate central and peripheral effects of insulin. Although intranasal insulin administration raises CNS insulin levels [7], our findings replicate prior research showing that plasma insulin and glucose levels remain unchanged. Thus, increased plasma Aβ can not be attributed to peripheral insulin elevations. It is possible that the increase in plasma Aβ for the memory-impaired group was derived from efflux from the CNS. We have previously demonstrated that insulin infusion produced an age-related increase in CSF Aβ42 levels in normal older adults [9], and Aβ has been shown to rapidly cross the blood-brain-barrier in animal studies [9,55]. It is also possible that CNS insulin signals to peripheral systems may invoke mechanisms that affect Aβ production, binding, or clearance.
In contrast to patterns observed in the memory-impaired group, for normal adults without the ε4 allele, Aβ42 decreased following the 10 and 20 IU insulin doses. Although the mechanisms causing this decrease are unknown, Benedict and colleagues [5] recently demonstrated that intranasal insulin administration reduced plasma cortisol levels in healthy adults. It is therefore possible that increased CNS insulin may facilitate clearance of Aβ in brain by affecting levels of cortisol which in turn regulates activity of the Aβ-degrading protease, insulin degrading enzyme (IDE). [32]
Future research should also clarify mechanisms related insulin’s selective effects on memory. Positron emission tomography (PET) may be a useful tool for determining whether intranasal insulin administration increases cerebral glucose metabolism. In addition, research is needed to determine the chronic effects of intranasal insulin administration on cognition and CSF biomarkers. Clarification of insulin transport mechanisms, pathways, and variables that affect nose-to-brain transport is needed. Finally, although available evidence suggests that intranasal insulin administration is safe [33], additional information about the risks of chronic intranasal insulin administration in an older population that may be more vulnerable to adverse events is needed to assess the potential therapeutic value of this approach.
The results of this study provide further support for the view that intranasal insulin administration can improve verbal memory, and that insulin dose-response curves differ by APOE genotype. The acute clinical benefits of treatment were greatest at 20 IU, but large clinical trials would be helpful to clarify clinical dosing regimens for chronic treatment approaches. To our knowledge, these results also provide the first evidence that intranasal insulin administration acutely modulates plasma Aβ. This exciting field of research may provide a novel treatment approach for central insulin abnormalities associated with neurodegenerative disorders.
Acknowledgments
This study was supported by the Department of Veterans Affairs and the Institute for the Study of Aging.
References
- 1.Akomolafe A, Beiser A, Meigs JB, Au R, Green RC, Farrer LA, Wolf PA, Seshadri S. Diabetes mellitus and risk of developing Alzheimer disease: results from the Framingham Study. Arch Neurol. 2006;63:1551–1555. doi: 10.1001/archneur.63.11.1551. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen N, Minthon L, Davidsson P, Vanmechelen E, Vanderstichele H, Winblad B, Blennow K. Evaluation of CSF-tau and CSF-Abeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol. 2001;58:373–379. doi: 10.1001/archneur.58.3.373. [DOI] [PubMed] [Google Scholar]
- 3.Apelt J, Mehlhorn G, Schliebs R. Insulin-sensitive GLUT4 glucose transporters are colocalized with GLUT3-expressing cells and demonstrate a chemically distinct neuron-specific localization in rat brain. J Neurosci Res. 1999;57:693–705. [PubMed] [Google Scholar]
- 4.Balin BJ, Broadwell RD, Salcman M, el-Kalliny M. Avenues for entry of peripherally administered protein to the central nervous system in mouse, rat, and squirrel monkey. J Comp Neurol. 1986;251:260–280. doi: 10.1002/cne.902510209. [DOI] [PubMed] [Google Scholar]
- 5.Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Bingham EM, Hopkins D, Smith D, Pernet A, Hallett W, Reed L, Marsden PK, Amiel SA. The role of insulin in human brain glucose metabolism: an 18fluoro-deoxyglucose positron emission tomography study. Diabetes. 2002;51:3384–3390. doi: 10.2337/diabetes.51.12.3384. [DOI] [PubMed] [Google Scholar]
- 7.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- 8.Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y, Luby J, Dagogo-Jack A, Alderson A. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol Aging. 1996;17:123–130. doi: 10.1016/0197-4580(95)02002-0. [DOI] [PubMed] [Google Scholar]
- 9.Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D., Jr Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164–168. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- 10.Craft S, Asthana S, Newcomer JW, Wilkinson CW, Matos IT, Baker LD, Cherrier M, Lofgreen C, Latendresse S, Petrova A, Plymate S, Raskind M, Grimwood K, Veith RC. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch Gen Psychiatry. 1999;56:1135–1140. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]
- 11.Craft S, Asthana S, Schellenberg G, Baker L, Cherrier M, Boyt AA, Martins RN, Raskind M, Peskind E, Plymate S. Insulin effects on glucose metabolism, memory, and plasma amyloid precursor protein in Alzheimer’s disease differ according to apolipoprotein-E genotype. Ann N Y Acad Sci. 2000;903:222–228. doi: 10.1111/j.1749-6632.2000.tb06371.x. [DOI] [PubMed] [Google Scholar]
- 12.Craft S, Asthana S, Cook DG, Baker LD, Cherrier M, Purganan K, Wait C, Petrova A, Latendresse S, Watson GS, Newcomer JW, Schellenberg GD, Krohn AJ. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer’s disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28:809–822. doi: 10.1016/s0306-4530(02)00087-2. [DOI] [PubMed] [Google Scholar]
- 13.de la Monte SM, Wands JR. Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer’s disease. J Alzheimers Dis. 2006;9:167–181. doi: 10.3233/jad-2006-9209. [DOI] [PubMed] [Google Scholar]
- 14.Doyle P, Cusin I, Rohner-Jeanrenaud F, Jeanrenaud B. Four-day hyperinsulinemia in euglycemic conditions alters local cerebral glucose utilization in specific brain nuclei of freely moving rats. Brain Res. 1995;684:47–55. doi: 10.1016/0006-8993(95)00402-c. [DOI] [PubMed] [Google Scholar]
- 15.Emi M, Wu LL, Robertson MA, Myers RL, Hegele RA, Williams RR, White R, Lalouel JM. Genotyping and sequence analysis of apolipoprotein E isoforms. Genomics. 1988;3:373–379. doi: 10.1016/0888-7543(88)90130-9. [DOI] [PubMed] [Google Scholar]
- 16.Figlewicz DP, Szot P, Israel PA, Payne C, Dorsa DM. Insulin reduces norepinephrine transporter mRNA in vivo in rat locus coeruleus. Brain Res. 1993;602:161–164. doi: 10.1016/0006-8993(93)90258-o. [DOI] [PubMed] [Google Scholar]
- 17.Fishel MA, Watson GS, Montine TJ, Wang Q, Green PS, Kulstad JJ, Cook DG, Peskind ER, Baker LD, Goldgaber D, Nie W, Asthana S, Plymate SR, Schwartz MW, Craft S. Hyperinsulinemia provokes synchronous increases in central inflammation and beta-amyloid in normal adults. Arch Neurol. 2005;62:1539–1544. doi: 10.1001/archneur.62.10.noc50112. [DOI] [PubMed] [Google Scholar]
- 18.Frey WH. 2nd, Intranasal delivery: bypassing the blood-brain barrier to deliver therapeutic agents to the brain and spinal cord. Drug Deliv Technol. 2002;2:46–49. [Google Scholar]
- 19.Fukumoto H, Tennis M, Locascio JJ, Hyman BT, Growdon JH, Irizarry MC. Age but not diagnosis is the main predictor of plasma amyloid beta-protein levels. Arch Neurol. 2003;60:958–964. doi: 10.1001/archneur.60.7.958. [DOI] [PubMed] [Google Scholar]
- 20.Gerozissis K, Orosco M, Rouch C, Nicolaidis S. Basal and hyperinsulinemia-induced immunoreactive hypothalamic insulin changes in lean and genetically obese Zucker rats revealed by microdialysis. Brain Res. 1993;611:258–263. doi: 10.1016/0006-8993(93)90511-k. [DOI] [PubMed] [Google Scholar]
- 21.Golden CJ. Stroop Color and Word Test. Stoelting; Chicago: 1978. [Google Scholar]
- 22.Graff-Radford NR, Crook JE, Lucas J, Boeve BF, Knopman DS, Ivnik RJ, Smith GE, Younkin LH, Petersen RC, Younkin SG. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64:354–362. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- 23.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 24.Ibberson M, Uldry M, Thorens B. GLUTX1, a novel mammalian glucose transporter expressed in the central nervous system and insulin-sensitive tissues. Journal of Biological Chemistry. 2000;275:4607–4612. doi: 10.1074/jbc.275.7.4607. [DOI] [PubMed] [Google Scholar]
- 25.Illum L. Nasal drug delivery: new developments and strategies. Drug Discov Today. 2002;7:1184–1189. doi: 10.1016/s1359-6446(02)02529-1. [DOI] [PubMed] [Google Scholar]
- 26.Iwatsubo T. Amyloid beta protein in plasma as a diagnostic marker for Alzheimer’s disease. Neurobiol Aging. 1998;19:161–163. doi: 10.1016/s0197-4580(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 27.Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 2000;49:1525–1533. doi: 10.2337/diabetes.49.9.1525. [DOI] [PubMed] [Google Scholar]
- 28.Kern W, Born J, Schreiber H, Fehm HL. Central nervous system effects of intranasally administered insulin during euglycemia in men. Diabetes. 1999;48:557–563. doi: 10.2337/diabetes.48.3.557. [DOI] [PubMed] [Google Scholar]
- 29.Kopf SR, Boccia MM, Baratti CM. AF-DX 116, a presynaptic muscarinic receptor antagonist, potentiates the effects of glucose and reverses the effects of insulin on memory. Neurobiol Learn Mem. 1998;70:305–313. doi: 10.1006/nlme.1998.3855. [DOI] [PubMed] [Google Scholar]
- 30.Kopf SR, Baratti CM. Effects of posttraining administration of insulin on retention of a habituation response in mice: participation of a central cholinergic mechanism. Neurobiol Learn Mem. 1999;71:50–61. doi: 10.1006/nlme.1998.3831. [DOI] [PubMed] [Google Scholar]
- 31.Kulstad JJ, Green PS, Cook DG, Watson GS, Reger MA, Baker LD, Plymate SR, Asthana S, Rhoads K, Mehta PD, Craft S. Differential modulation of plasma beta-amyloid by insulin in patients with Alzheimer disease. Neurology. 2006;66:1506–1510. doi: 10.1212/01.wnl.0000216274.58185.09. [DOI] [PubMed] [Google Scholar]
- 32.Kupfer SR, Wilson EM, French FS. Androgen and glucocorticoid receptors interact with insulin degrading enzyme. J Biol Chem. 1994;269:20622–20628. [PubMed] [Google Scholar]
- 33.Kupila A, Sipila J, Keskinen P, Simell T, Knip M, Pulkki K, Simell O. Intranasally administered insulin intended for prevention of type 1 diabetes--a safety study in healthy adults. Diabetes Metab Res Rev. 2003;19:415–420. doi: 10.1002/dmrr.397. [DOI] [PubMed] [Google Scholar]
- 34.Kuusisto J, Koivisto K, Mykkanen L, Helkala EL, Vanhanen M, Hanninen T, Kervinen K, Kesaniemi YA, Riekkinen PJ, Laakso M. Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: cross sectional population based study. British Medical Journal. 1997;315:1045–1049. doi: 10.1136/bmj.315.7115.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O’Brien PC, Palumbo PJ. The risk of dementia among persons with diabetes mellitus: a population-based cohort study. Ann N Y Acad Sci. 1997;826:422–427. doi: 10.1111/j.1749-6632.1997.tb48496.x. [DOI] [PubMed] [Google Scholar]
- 36.Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 37.Marfaing-Jallat P, Portha B, Penicaud L. Altered conditioned taste aversion and glucose utilization in related brain nuclei of diabetic GK rats. Brain Research Bulletin. 1995;37:639–643. doi: 10.1016/0361-9230(95)00060-r. [DOI] [PubMed] [Google Scholar]
- 38.Mayeux R, Honig LS, Tang MX, Manly J, Stern Y, Schupf N, Mehta PD. Plasma A[beta]40 and A[beta]42 and Alzheimer’s disease: relation to age, mortality, and risk. Neurology. 2003;61:1185–1190. doi: 10.1212/01.wnl.0000091890.32140.8f. [DOI] [PubMed] [Google Scholar]
- 39.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 40.Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1–40 and 1–42 in Alzheimer disease. Arch Neurol. 2000;57:100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- 41.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 42.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 43.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 44.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 45.Petrides M, Milner B. Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1982;20:249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- 46.Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, Lyketsos CG. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 47.Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, Hyde K, Chapman D, Craft S. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging. 2004;25:311–314. doi: 10.1016/S0197-4580(03)00087-3. [DOI] [PubMed] [Google Scholar]
- 48.Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98:3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nature medicine. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 51.Schulingkamp RJ, Pagano TC, Hung D, Raffa RB. Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci Biobehav Rev. 2000;24:855–872. doi: 10.1016/s0149-7634(00)00040-3. [DOI] [PubMed] [Google Scholar]
- 52.Schupf N, Patel B, Silverman W, Zigman WB, Zhong N, Tycko B, Mehta PD, Mayeux R. Elevated plasma amyloid beta-peptide 1–42 and onset of dementia in adults with Down syndrome. Neuroscience letters. 2001;301:199–203. doi: 10.1016/s0304-3940(01)01657-3. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz MW, Figlewicz DF, Kahn SE, Baskin DG, Greenwood MR, Porte D., Jr Insulin binding to brain capillaries is reduced in genetically obese, hyperinsulinemic Zucker rats. Peptides. 1990;11:467–472. doi: 10.1016/0196-9781(90)90044-6. [DOI] [PubMed] [Google Scholar]
- 54.Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol. 1999;13:348–358. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- 55.Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer’s amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spieler DH, Balota DA, Faust ME. Levels of selective attention revealed through analyses of response time distributions. J Exp Psychol Hum Percept Perform. 2000;26:506–526. doi: 10.1037//0096-1523.26.2.506. [DOI] [PubMed] [Google Scholar]
- 57.Thorne RG, Frey WH. 2nd, Delivery of neurotrophic factors to the central nervous system: pharmacokinetic considerations. Clin Pharmacokinet. 2001;40:907–946. doi: 10.2165/00003088-200140120-00003. [DOI] [PubMed] [Google Scholar]
- 58.Thorne RG, Pronk GJ, Padmanabhan V, Frey WH. 2nd, Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 59.Wechsler D. Wechsler Adult Intelligence Scale. 3. Harcourt Assessment; San Antonio: 2003. [Google Scholar]
- 60.Xu WL, Qiu CX, Wahlin A, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology. 2004;63:1181–1186. doi: 10.1212/01.wnl.0000140291.86406.d1. [DOI] [PubMed] [Google Scholar]