Abstract
Preclinical studies have shown the inhibition of ornithine decarboxylase (ODC) by α-difluoromethylornithine (DFMO) and resultant decreases in tissue concentrations of polyamines (putrescine & spermidine) prevents neoplastic developments in many tissue types. Clinical studies of oral DFMO at 500 mg/m2/day revealed it to be safe and tolerable and resulted in significant inhibition of phorbol ester-induced skin ODC activity. Two hundred and ninety-one participants (mean 61 y.o., 60% male) with a history of prior non-melanoma skin cancer (mean 4.5 skin cancers) were randomized to oral DFMO (500 mg/m2/day) or placebo for 4–5 years. There was a trend toward a history of more prior skin cancers in subjects randomized to placebo, but all other characteristics including sunscreen and NSAID use were evenly distributed. Evaluation of 1200-person years of follow-up revealed a new non-melanoma skin cancer (NMSC) rate of 0.5 events/person/year. The primary endpoint, new NMSC’s, was not significantly different between subjects taking DFMO and placebo (260 vs. 363 cancers, p=0.069, two-sample t test). Evaluation of basal cell (BCC) and squamous cell (SCC) cancers separately revealed very little difference in SCC between treatment groups but a significant difference in new BCC (DFMO 163 cancers; Placebo 243 cancers; expressed as event rate 0.28 BCC/person/year vs. 0.40 BCC/person/year, p=0.03). Compliance with DFMO was >90% and it appeared to be well tolerated with evidence of mild ototoxicity as measured by serial audiometric examination when compared to placebo subjects. Analysis of normal skin biopsies revealed a significant (p<0.05) decrease in 12-0 – tetradecanoylphorbol-13-acetate-induced ODC activity (month 24, 36 and 48) and putrescine concentration (month 24 and 36 only) in DFMO subjects. Subjects with a history of skin cancer taking daily DFMO had an insignificant reduction (p=0.069), in new NMSC that was predominantly due to a marked reduction in new BCC. Based on these data, the potential of DFMO, alone or in combination, to prevent skin cancers should be explored further.
Keywords: DFMO, Chemoprevention, Clinical Trial, Skin Cancer
INTRODUCTION
Skin cancer is the most common malignancy in the US, with more than 1 million new diagnoses of non-melanoma skin cancer (predominantly basal cell and squamous cell carcinomas) expected in 2008 (1). While mortality due to non-melanoma skin cancer (NMSC) is low relative to other malignancies, it still poses significant public health concerns. Twenty percent of US residents will develop non-meloma skin cancer (NMSC), which translates into an annual cost to Medicare that are estimated to exceed $400–500 million (2). Certain vulnerable populations, e.g. organ transplant recipients, have extraordinarily high rates of NMSC (>50%) with markedly worse morbidity and mortality as compared to the general public (3–5). The most important risk factor for NMSC is chronic exposure to UV radiation from sunlight (6). Exposure to UV radiation is expected to increase with ongoing depletion of the ozone layer, and the impact of NMSC on health and healthcare costs will likely increase. Although education on the risks of excessive sun exposure and increasing use of photoprotection can be helpful, new strategies to decrease the burden of NMSC are sorely needed. (7). To date, attempts at the chemoprevention of skin cancer have been unsuccessful (5, 7–12).
Increased levels of polyamines have been implicated in epithelial tumorigenesis, starting with early work by O’Brien et al showing induction of polyamine biosynthetic enzymes in response to tumor promoting agents (13). Putrescine, spermidine, and spermine are polyamines that are derived from amino acids (arginine and methionine) and are present in all mammalian cells. The first and rate-limiting step in polyamine biosynthesis is the formation of putrescine from ornithine by the action of ornithine decarboxylase (ODC). Putrescine is further converted to spermidine and spermine through the consecutive action of two distinct aminopropyltransferases. The three key enzymes that regulate polyamine biosynthesis are ODC, S-adenosyl-L-methionine decarboxylase (which insures the availability of the aminopropyl donor for spermidine/spermine synthesis), and spermidine/spermine N-acetyltransferase (the enzyme that initiates polyamine catabolism) (14, 15). In epithelial tumorigenesis, Verma has shown potent initiator/promoters like UV irradiation strongly induces ODC activity and this induction correlates with tumor formation (15–17). Conversely, inhibition of ODC induction correlates with an agent’s ability to prevent tumor formation (18). The ability of non-specific ODC inhibitors to prevent tumor formation led to the development of a specific, irreversible inhibitor, difluoromethylornithine (DFMO, eflornithine) (16).
Chemoprevention studies of DFMO have been ongoing for many years at the University of Wisconsin and other institutions with systematic emphasis on the prevention of skin, colon, bladder, prostate, cervix and breast cancers (19–26). The side effects of DFMO have been gastrointestinal upset and mild reversible hearing changes at doses of 1–3 gm/m2/day and minimal to no toxicity at doses < 0.5 gm/m2/day (16). Multiple studies have reported on the tolerability and safety of DFMO dosed as high as 1.0 gm/day for as long as one year (19–26).
DFMO has also been examined in combination with other potential chemoprevention agents. A UW Phase 1 study examined the combination of DFMO and piroxicam in subjects at risk for cancer and observed good tolerability (27). More recently Meyskens and colleagues reported excellent tolerability and significant reduction in the risk of colonic adenomas among subject receiving DFMO + sulindac as compared to subjects receiving placebo (28).
The strong link between 12-0-tetradecanolphorbol-13-acetate (TPA)-induced ODC, polyamines and mouse skin carcinogenesis has led us to focus on the effects of DFMO upon skin ODC induction. To determine the dose and schedule of DFMO that would effectively inhibit skin ODC activity in human skin prevention trials, we developed an assay to measure ODC activity in 3mm punch skin biopsies (29). One of our first clinical trials was a randomized Phase I study of DFMO to determine the lowest daily oral dose that could achieve at least 50% inhibition of TPA-induced skin ODC activity with minimal clinical toxicity (19). Subjects with a history of colon, prostate, or bladder cancer received oral DFMO in varying doses four times a day or once daily and underwent skin biopsies and assessment of DFMO pharmacokinetics. Although dose-related reductions in TPA-induced ODC activity were observed, ototoxicity occurred with doses or schedules exceeding 0.5 gm/m2/day of DFMO. Additional subjects were enrolled and received 0.5 gm/m2 once a day of DFMO for 10–12 months. Serial skin biopsies to assess TPA-induced ODC activity revealed ≥ 50% reduction in ODC activity throughout 10 months of therapy. When 0.5 gm/m2 was given once a day, peak plasma concentration was 47 ± 5 uM (mean ± SEM) at 3–4 hours post-ingestion and plasma concentration 12 hours post-dose was 15 ± 5 uM. The area under the curve for plasma concentration × time was 311 ± 39 uM × hour with a half-life of 3.5 hours. DFMO pharmacokinetics did not significantly change from day 1 through month 10 of therapy.
On the strength of these pre-clinical and clinical data, we performed a phase 3 study of daily DFMO (0.5 g/m2/day) compared to placebo for up to 5 years in men and women with a history of non-melanoma skin cancer to determine whether daily DFMO would lead to a significant reduction in the number of new non-melanoma skin cancers.
METHODS
Study Design
This double-blind, placebo-controlled phase 3 trial planned to enroll 334 subjects with a prior history of skin cancer, either basal or squamous, and randomize them to 0.5 g/m2/day PO of DFMO or matching placebo. In order to improve compliance, there was a 4-week run-in phase in which all subjects received placebo. Participants who returned as scheduled on day 28 and had taken 80% or more of the run-in medication were then randomized to receive DFMO or placebo for 3 to 5 years (average 4 years), depending on when they entered the study.
Subjects
Eligible participants were previously treated for basal or squamous cell cancers (stage 0–2), older than 21 years of age, had an ECOG performance status 0 or 1, and were more than four weeks from prior major surgery, or chemo-, radio- or hormonal therapy for cancer. Subjects needed to have adequate organ function, defined as white blood cells ≥ 3,500/mm3, platelets ≥ 100,000/mm3, hemoglobin ≥ 11.0 g/dL, bilirubin ≤ 2 mg%, SGOT < 3× normal, and creatinine < 2.0 mg%. All study participants underwent informed consent (including signed documentation) per institutional, state and federal standards.
Subjects with pre-existing hearing loss, defined as significant clinical hearing loss or requiring the use of a hearing aid were excluded from the trial, as were pregnant or lactating females. Women of childbearing potential needed to have a pregnancy test before entering the study and agree to use an adequate form of contraception for the duration of the study. Subjects must not have used topical medications for treatment of skin cancers in the previous four weeks, including retin A, acutane, PUVA and 5-FU. Subjects could not have a family history of early retinal blindness or ornithine diaminotransferase deficiency, nor could they receive cytotoxic chemotherapy (e.g., methotrexate for arthritis), tamoxifen or hormonal therapy for cancer treatment/prophylaxis, or anti-seizure medication or corticosteroids of any kind. Bone marrow transplant recipients were also excluded.
Study Drug
DFMO and placebo medications were supplied by ILEX Oncology Services, Inc. The drugs were dispensed by the University of Wisconsin research pharmacists to the appropriate clinics. All trial participants and staff were blinded to the study medication, with the exception of the dispensing pharmacists. The initial dose of study medication, dispensed as a liquid formulation of 0.2 g/ml DFMO in aqueous solution, was determined for each subject using the calculated BSA with 0.5 g DFMO/m2. Liquid medication was to be taken once daily, after mixing the assigned amount with fruit juice or another liquid to mask the flavor. There were no dose escalations. During the run-in period, all subjects received placebo in liquid form by mouth for four weeks. Subjects who returned as scheduled on day 28 and had taken 80% or more of the run-in medication were randomized in a 1:1 ratio to receive blinded study medication, liquid DFMO or placebo, at the same volume. Subjects would receive blinded treatment for up to five years. Dose levels were recorded as the volume of liquid medication aliquotted by study participants, who were assigned doses ranging from 2.2 ml to 7.0 ml.
After DFMO became available in tablet form and was shown to have satisfactory bioequivalence to the liquid formulation (30), subjects were switched to receive tablets (250 mg DFMO per tablet or matching placebo). The dose level for each subject was rounded up or down to the nearest 250 mg, with tablets to be taken once a day by mouth with liquid.
Schedule of Events
Potential subjects underwent baseline screening as described above, including a history and physical exam, vitals, safety blood work (hemoglobin, white blood cell and platelet counts, serum creatinine, total bilirubin and aspertate aminotransferase) and dermatologic skin assessment. Subjects who took >80% of run-in study drug were registered and randomized to study drug assignment, underwent an audiogram and biopsies of normal skin. Every 6 months on study subjects were seen for study drug compliance assessment (calendar and pill count) and concomitant medications, history and physical exam, dermatologic assessment and an assessment of adverse events. Safety blood work was repeated after one year on study and again at the end of the study. Biopsies of normal skin were repeated in randomly selected patients after 24, 36 and 48 months on study.
Skin biopsies
Three punch skin biopsies were obtained at baseline in all subjects and a random sample of 60 subjects in each treatment arm underwent two punch skin biopsies after 24, 36 and 48 months of study drug.
Dose Modification
If subjects developed any drug-related toxicity of grade >1 according to the NCI Common Toxicity Criteria Version 2, study medication was discontinued. If toxicity resolved within four weeks, subjects could be considered for further therapy at a 50% dose. Drug dose would be maintained at that level. If the subject continued to experience clinical toxicity at the reduced dose, the drug was stopped.
Audiometry
Audiograms were performed on all subjects at baseline, year one and again at the end of the study. Audiograms were also performed at any time when grade 2 ototoxicity was observed, subjects self-reported hearing changes, and 6 months following any abnormal audiogram (≥ 15 dB hearing loss across two consecutive octaves) to assess for reversibility. Audiograms consisted of behavioral threshold determination at octave intervals between 250 and 8000 Hz and determination of speech discrimination. If a hearing loss was detected, in addition to air-borne stimuli, bone conducted stimuli was also used to determine whether the hearing loss was sensorineural or conductive. Study audiometry was based on the recommendations of Shotland et al. (31).
Statistical Considerations
The primary objective was to evaluate the effectiveness of DFMO in preventing new non-melanoma skin cancers in a population of individuals who already had at least one non-melanoma skin cancer. The primary efficacy endpoint used to address this was the rate of new non-melanoma skin cancer among the subjects. For each subject we computed the number of new cancers adjusted for time on study, i.e., number of new cancers/years on study. The DFMO and placebo arms were then to be compared by a two-sample Student t-test of these rates. By the central limit theorem, this statistic should be asymptotically standard normal when the null hypothesis holds. However, for greater precision, efficacy was evaluated using the exact probability value from the permutation test obtained from the randomization distribution. Note that this was done on an intent-to-treat basis, without adjusting for compliance.
A prior large study of similar subjects found that new skin cancers were observed in the placebo group at a rate of 0.25 cancers per person-year of follow-up (32). In order to estimate the number of events that would be observed, the number of person-years was estimated allowing for drop-out and staggered enrollment times. It was anticipated that the lengths of time on study for subjects in each arm would be almost evenly distributed across three, four and five years. Using this information, the expected number of new skin cancers in the placebo group would be approximately equal to the size of the group. Treatment effect was assumed to increase with time on treatment, to allow for the possibility that at the time of entry onto the study, undetectable tumors might already exist in a state of progression against which DFMO would have limited effectiveness. It was assumed that the number of new cancers in the DFMO group would ultimately reach 0.125 new cancers per person-year, a 50% decrease, by the fifth year of treatment. However, taking into account the possibility that DFMO might be less effective early in treatment, the anticipated cumulative rate over the entire study was assumed to be 0.65 new cancers per subject, resulting in a 35% decrease. We used Poisson distributions to approximate the probability distributions of the total number of new cancers in the two treatment arms and concluded that a sample size of 284 compliant subjects would be needed to achieve 90% power to reject the null hypothesis (i.e., equal rates for incident cancers under assumptions described above at the 0.05 level of significance using a two-sided test). It was assumed that about 15% of subjects would not meet the compliance requirement during the run-in period. Under this assumption, a target sample size was adjusted to a total of 334 subjects.
Secondary endpoints, e.g. biomarkers, were analyzed by Wilcoxon Rank Sum tests or Chi square analyses.
Interim analyses were performed during the course of the trial to ensure that the trial would be stopped early if sufficient evidence of efficacy were obtained or if excessive toxicity were observed. A Lan-DeMets alpha-spending function of a two-sided O’Brien-Fleming boundary was used for monitoring the primary efficacy endpoint. A data monitoring committee consisting of a clinical oncologist, a dermatologist and a statistician met at regular intervals during the active enrollment and follow-up periods to provide appropriate oversight and monitoring of the conduct of the trial and to ensure the safety of the trial participants and the validity and integrity of the data
Assays
Three mm punch biopsies were obtained from the volar surface of the forearm and transferred to a container of serum free MEM medium and placed on ice. One skin sample was immediately processed for TPA-induced ODC activity on the day of biopsy. The second sample was processed and flash frozen for determination of polyamine concentrations at a later date. At baseline only, a third sample was obtained for polyamine concentration determination. The method for in vitro induction of human skin ODC by TPA has been reported (29). The medium is gassed with 95% oxygen and 5% CO2 for 1 minute; the appropriate additions (TPA or ethanol) are made, and the flask is sealed and incubated in a shaking water bath at 37°C. Immediately after incubation, the skin biopsies are removed to assay ODC activity in whole 3 mm skin biopsy by measuring the release of 14CO2 from DL-[1-14C] ornithine hydrochloride.
The polyamines putrescine, spermidine and spermine were analyzed in the acid extract by the method of Kabra (23, 33). The standard curve for each polyamine was linear from 0–50 nmoles/ml of extract (r2 > 0.99 in each case). The intra-day coefficient of variation in duplicate determinations was less than 2% for low and high standards of each polyamine. The inter-day coefficient of variation was less than 5% for high standards of each polyamine (n=4, putrescine = 10 nmoles/ml, spermidine and spermine = 50 nmoles/ml). The inter-day coefficient of variation for low standards of each polyamine was less than 10% for each polyamine (n=4, putrescine = 0.625 nmoles/ml, spermidine and spermine = 3.12 nmoles/ml).
RESULTS
A total of 334 subjects were enrolled over two years into the placebo run-in phase. After 28 days of the placebo run-in, 291 subjects (87%) met the minimum compliance rate (≥ 80%) and were randomized to continue with blinded study treatment between September 1998 and July 2000. The mean age at enrollment was 60.9 years, with a median of 61.9 years. Among randomized subjects 175 (60%) were male and 116 (40%) were female. For performance status, most randomized subjects (267, or 92%) were level 0 at enrollment, with 23 subjects at level 1 and one at level 2. Nearly all subjects (290, or 99.7%) were white, non-Hispanic with one Hispanic subject.
Baseline characteristics are summarized in Table 1. Body surface area, which was derived from height and weight, was used to determine the dose of study medication. The resulting mean and median values for randomized subjects were both 1.96 m2. Baseline variables across the two treatment groups appeared reasonably well balanced and consistent with randomization, with the possible exception of weight (Wilcoxon p-value=0.060) and body surface area (Wilcoxon p-value=0.063). At each study visit, subjects were asked if they had used any of five specific medications since their prior visit: sunscreen, vitamin A, vitamin C, vitamin E, or any non-steroidal anti-inflammatory drug (NSAID). The observed differences in baseline concomitant medication use between treatment groups were small and appeared to be consistent with randomization.
TABLE 1.
Randomized Subject Baseline Characteristics
| DFMO | Placebo | ||
|---|---|---|---|
| Number of patients | 144 | 147 | |
| Age (years) | 61.6 | 60.2 | |
| Gender | |||
| Female (%) | 39.6 | 40.1 | |
| Male (%) | 60.4 | 59.9 | |
| Race | |||
| White (%) | 100 | 100 | |
| Performance status (ECOG) | |||
| Zero (%) | 92.4 | 91.2 | |
| One (%) | 7.6 | 8.2 | |
| Body Surface Area (m2) | 1.94 ±0.24 | 1.99 ±0.23 | |
| Baseline Concomitant Medications | |||
| Sunscreen (%) | 79.9 | 82.3 | |
| Vitamin A (%) | 6.9 | 6.1 | |
| Vitamin C (%) | 38.9 | 31.3 | |
| Vitamin E (%) | 45.1 | 42.2 | |
| NSAIDS (%) | 52.8 | 53.1 | |
| Prior Non-melanoma Skin Cancers | 4.2 ±7.7 | 4.9 ±5.7 | p=0.10 |
| Time Since First Diagnosis of Skin Cancer | p=0.002 | ||
| 0–6 months (%) | 23.6 | 11.6 | |
| 7–12 months (%) | 9.7 | 9.5 | |
| 13–18 months (%) | 7.6 | 4.1 | |
| 19–24 months (%) | 7.6 | 6.1 | |
| >24 months (%) | 51.4 | 68.7 | |
| Prior Tumor Rate (NMSC/year) | 2.3 ±3.3 | 2.1 ±3.5 | p=0.08 |
Mean values with and without Standard Deviation, p-values were calculated with chi-square tests for dichotomous data, and Wilcoxon rank-sum test for ordinal data. Prior tumor rate is defined as the number of prior skin cancers divided by the time from the initial diagnosis to randomization.
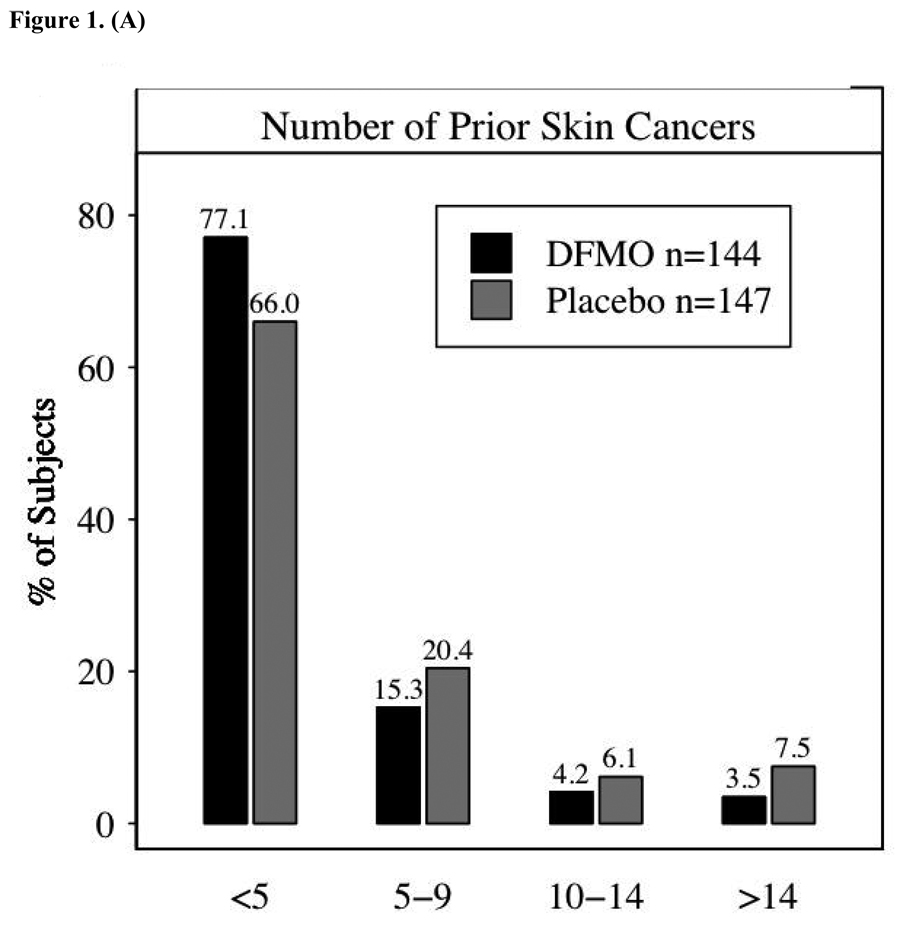
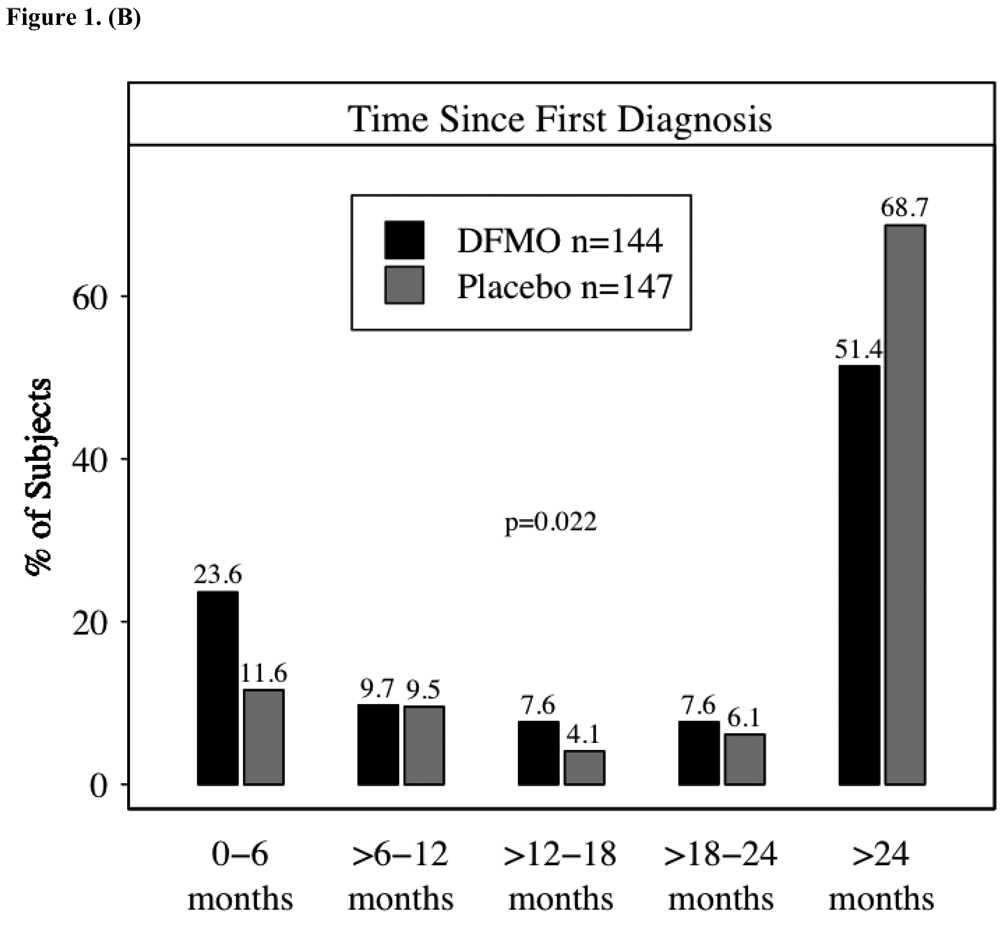
Figure 1A presents information on skin cancer history, the number of prior skin cancers, and the time from the subject’s first skin cancer diagnosis to study enrollment. Time since first diagnosis was summarized in 6-month intervals, with Figure 1B showing the percent of subjects falling within each interval. Most subjects (208, or 71%) reported fewer than five prior skin cancers; and 16 subjects (5%) had 15 or more prior skin cancers. The mean number of prior cancers among randomized subjects was 4.5, with a median of 2; values ranged from 1 prior skin cancer to 74. Although somewhat unbalanced between the treatment groups, a mean of 4.23 prior skin cancers occurred in subjects randomized to DFMO as compared to a mean of 4.91 among placebo subjects. A Wilcoxon rank-sum test comparing the distribution among randomized subjects in the two groups did not achieve statistical significance (p-value of 0.10). Time since first diagnosis differed slightly between groups: more subjects randomized to placebo had a first diagnosis more than 24 months prior to enrollment (69% vs. 51%), while more subjects on DFMO had their first diagnosis within the previous six months. The p-value from a Wilcoxon test of the distribution of ordered time categories between groups was 0.002. Consistent with the longer history of skin cancers in the placebo group the skin cancer rate (Table 2) was slightly higher in the DFMO group.
Figure 1.
1a – Number of prior skin cancers per subject; 1b – Duration in months from first skin cancer to study enrollment. The difference between the groups was assessed using the Wilcoxon rank- sum test.
Table 2.
Skin Cancer Results
| Treatment | Group | ||
|---|---|---|---|
| DFMO (n = 143) |
Placebo (n = 147) |
Overall (n = 290) |
|
| Subject with new cancer (p=0.475) | 86 (60%) | 95 (65%) | 181 |
| Total new cancer (p=0.069) | 260 | 363 | 623 |
| Time under observation (years) | 599.8 | 603.5 | 1202.3 |
| New cancer/year (SE) | 0.43 (0.05) | 0.60 (0.07) | 0.52 |
| Subject with basal cancer | 69 | 77 | 146 |
| Total basal cancer | 163 | 245 | 408 |
| Subject with squamous cancer | 40 | 50 | 90 |
| Total squamous cancer | 95 | 116 | 211 |
P-values were calculated with chi-square tests for dichotomous data, and Wilcoxon rank-sum test for ordinal data. The exception to this is total new cancers – as specified in the protocol for the efficacy analysis, the DFMO and placebo arms were compared by a two-sample t-statistic testing the difference in rates of new cancers/years on study using the exact probability value from the permutation test obtained from the statistic’s randomization distribution.”
Of the 291 subjects randomized to study treatment, 168 subjects completed four years of study drug treatment and 6 months of follow-up after stopping study drug. One hundred twenty-three subjects (42% of randomized subjects) discontinued study drug treatment early (90 subjects stopped secondary to adverse events and 21 withdrew consent). However, many of those who discontinued were evaluated for follow-up status and new skin cancers after stopping treatment. When the trial ended, there were a total of 1,202 observed person-years of follow-up for all randomized subjects. There was a mean follow-up time of 4.1 years per subject (median 4.5 years) and a maximum of 5.3 years.
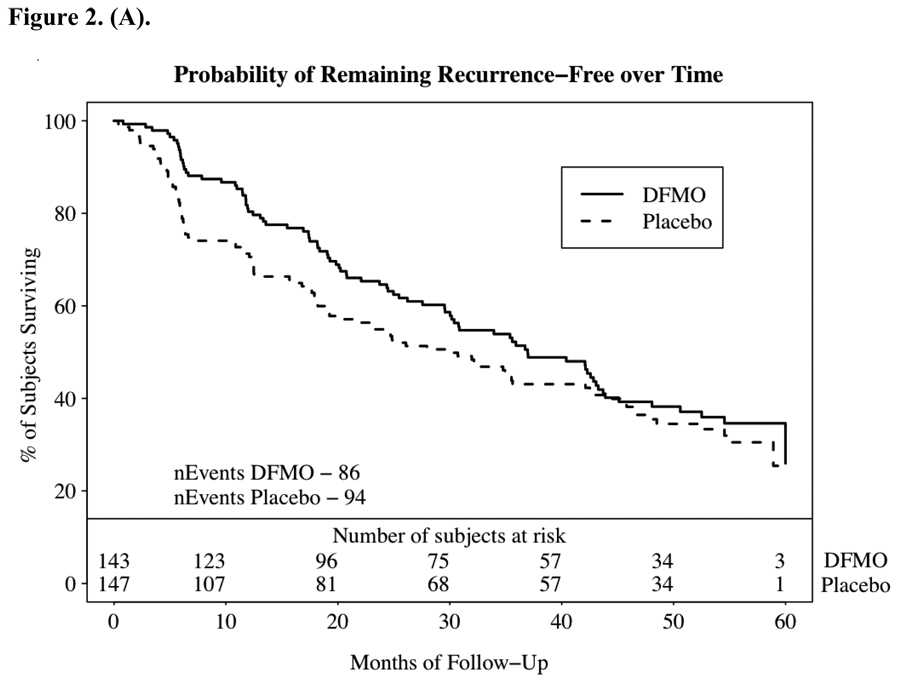
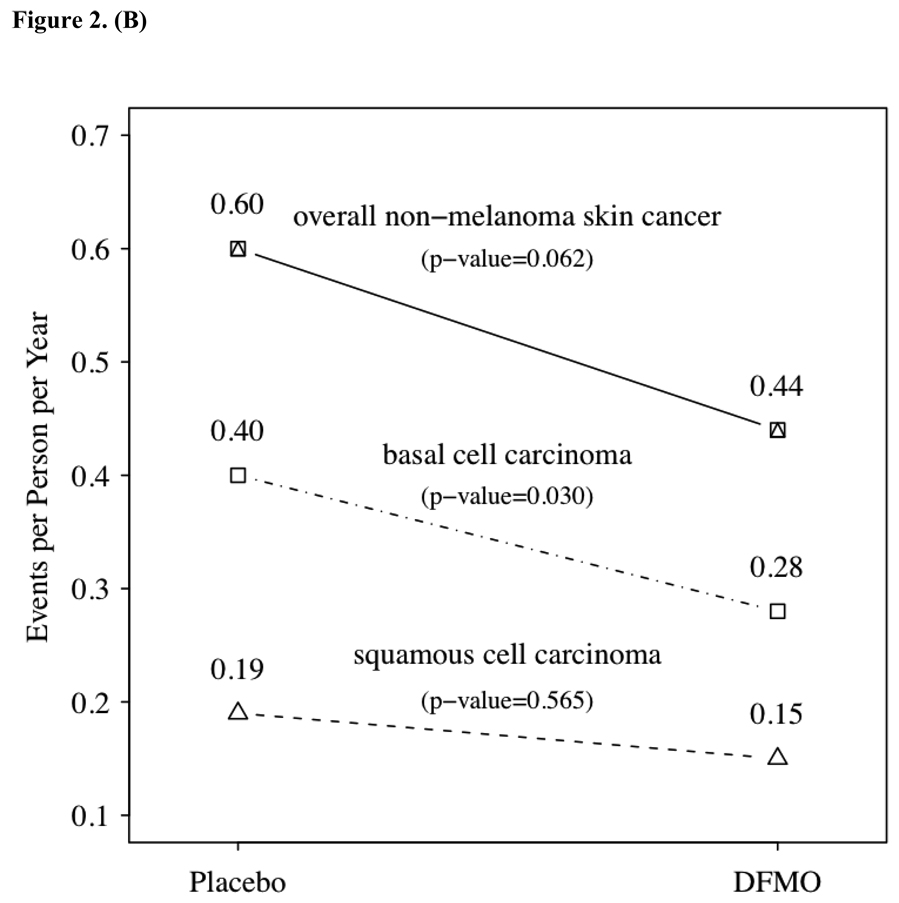
The primary endpoint of the study was the number of new NMSC events observed between subjects randomized to DFMO or placebo. Table 2 summarizes the NMSC events and fig. 2A graphs the probability of developing a NMSC. Over the course of approximately 1200 subject-years of follow-up 623 new NMSC observed, 260 in the DFMO group, with an event rate of 0.44 cancers per year of follow-up, and 363 in the placebo group, for an event rate of 0.61 (two sample t test comparing cancer incidence rates, p = 0.069). The number of NMSC per subject ranged from 0–33 with an event-rate of 0.5 NMSC’s per subject-year. Consistent with the expected pattern for NMSC in immune-competent patients, basal cell cancers were predominant. Examining the subsets of basal cell and squamous cell cancers the majority of difference in NMSC events between the two groups was within the basal cell cancer category (see Figure 2.B). Subjects receiving DFMO had a significantly lower (p=0.03) rate of basal cell cancers per year of follow-up than subjects on placebo (0.28 vs. 0.40). This significant difference was maintained when controlling for differences in prior skin cancer history between the groups.
Figure 2.
2a – Probability of remaining recurrence free over time as indicated by the Kaplan-Meier estimator. Time to first non-melanoma skin cancer (NMSC) in study. Time to first diagnosis was computed from the date of first new skin cancer diagnosis after randomization, and the randomization date. Panel displays a Kaplan-Meier curve of the probability of remaining recurrence-free over time. Numbers at the bottom are the number of subjects per treatment group still at risk. Patients with no new skin cancers reported were censored at the date of the last study visit in the follow-up database: 2b – non-melanoma skin cancer event rate by treatment group and histology. P values were obtained from over dispersed Poisson models considering the cumulative counts of new cancer over time within each subject, with treatment as the independent variable.
Biomarkers
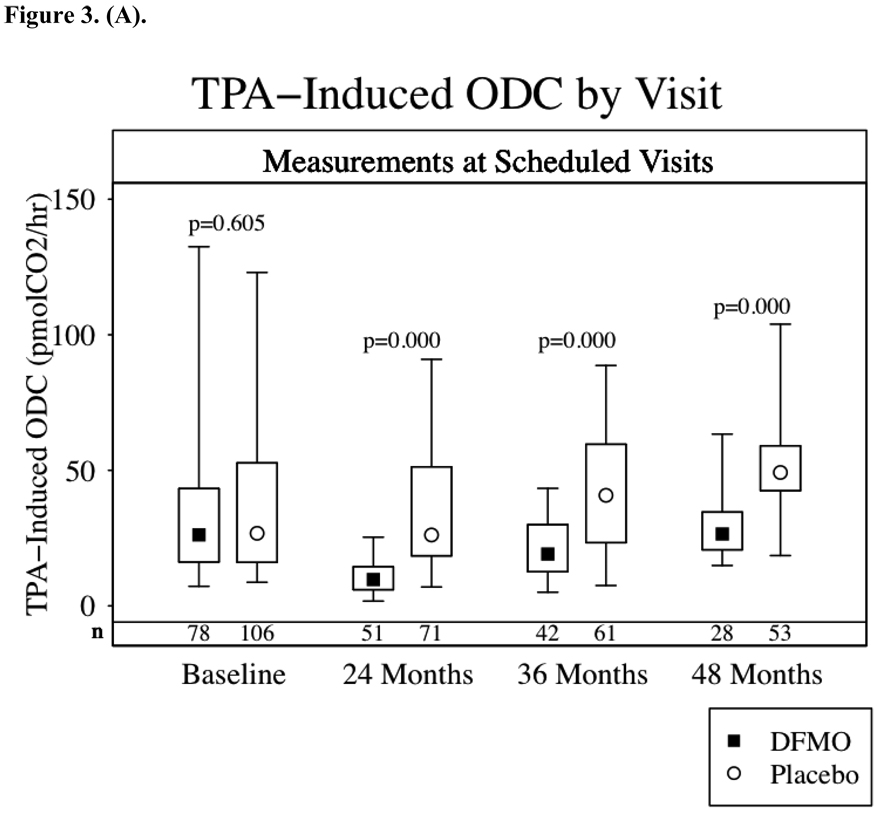
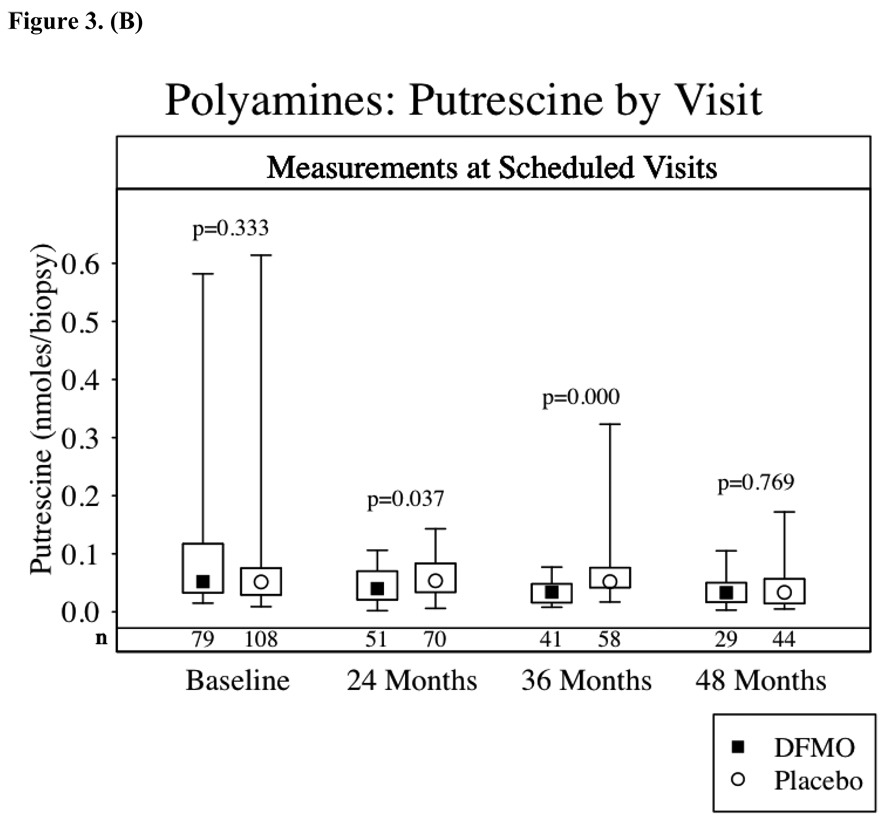
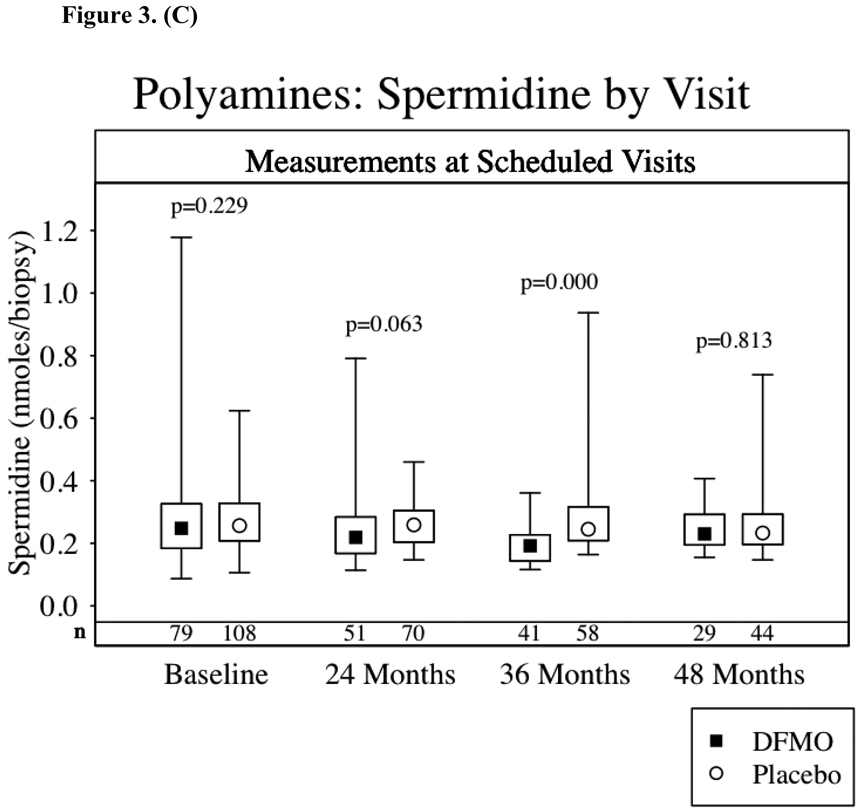
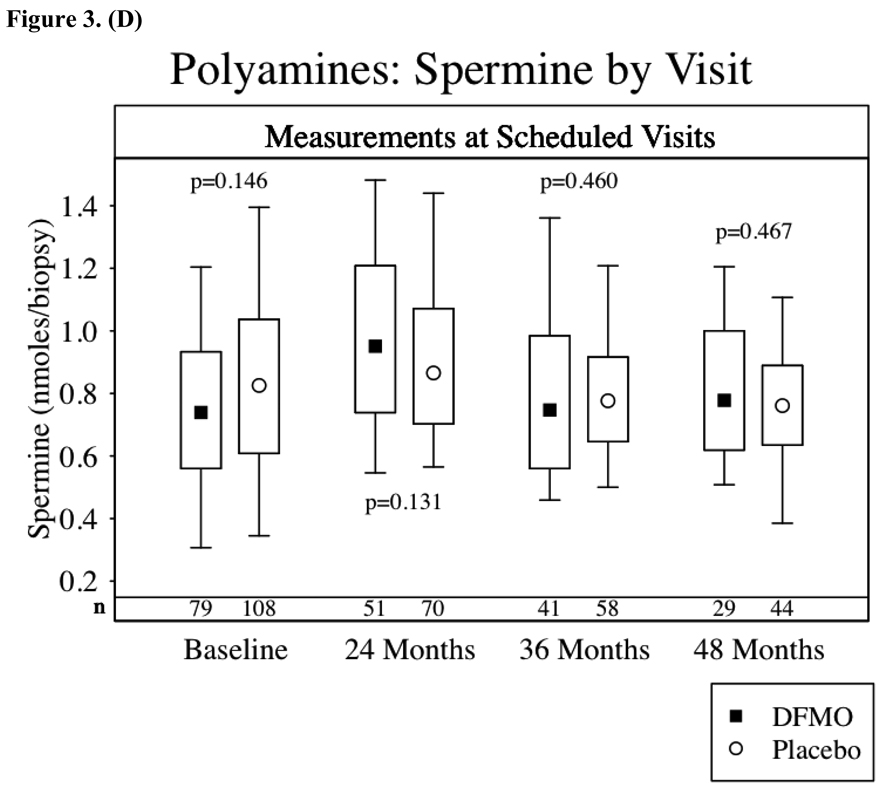
Figures 3a–d show the results of TPA-induced ODC activity and putrescine, spermidine and spermine levels from normal skin at baseline and again 24, 36 and 48 months after starting study drug treatment. Subjects receiving DFMO had a significant (p<0.001) reduction in TPA-induced skin ODC activity throughout study participation. Skin putrescine concentrations were significantly lower in subjects on DFMO at 24 and 36 months but not at 48 months. Despite a trend toward lower skin spermidine concentrations in subjects randomized to DFMO at month 24 (p=0.06) and significantly lower concentrations at month 36 (p<0.001), there was no difference at month 48. There was no apparent difference in skin spermine concentrations at any time point. When comparing the above parameters across treatment groups only in the subset of subjects with or without recurrence of NMSC, the same presence or absence of a significant difference between treatment groups was observed.
Figure 3.
Skin Biomarkers Box Plots. Median values ▪ ◦, boxes denote first (lower border) and third (upper border) quartiles of the data distribution, and lines/whiskers denote 5th (lower) and 95th (upper) percentile of the distribution. The differences between the groups were assessed using the Wilcoxon rank-sum test. Lower numbers (n) are the number of subjects with skin samples for analysis per treatment group. 3a-TPA-Induced skin ODC activity, 3b-skin putrescine concentrations, 3c-skin spermidine concentrations and 3d-skin spermidine.
Compliance
Compliance with study drug was excellent throughout the study. Overall compliance (expressed as percent of days taking the study drug as instructed) was 91.9±14.5% (mean±S.D.) or a median of 98.4% for the DFMO group and 93.5±11.6% or a median of 98.3% for the placebo group. The compliance was nearly identical when comparing the liquid formulation versus pills. The liquid formulation (DFMO or placebo) was taken by 271 patients for a median time of 1.56 years. The tablet formulation (DFMO or placebo) was taken by 207 patients for a median time of 2.53 years.
Toxicity
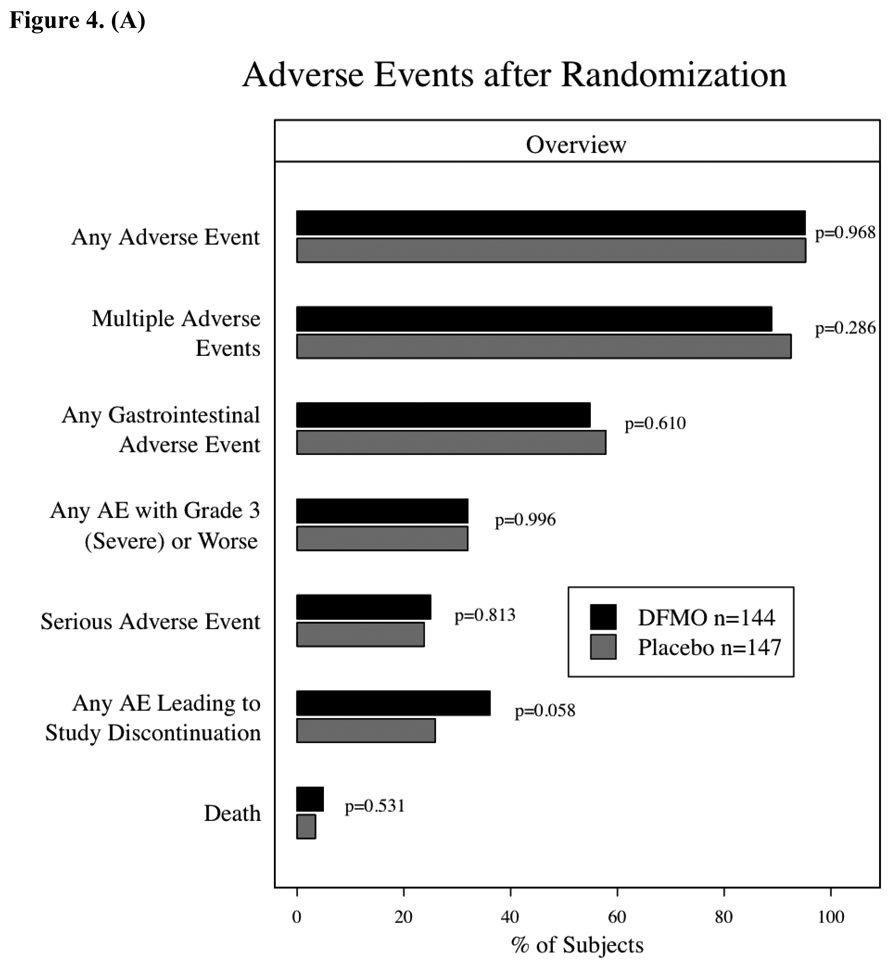
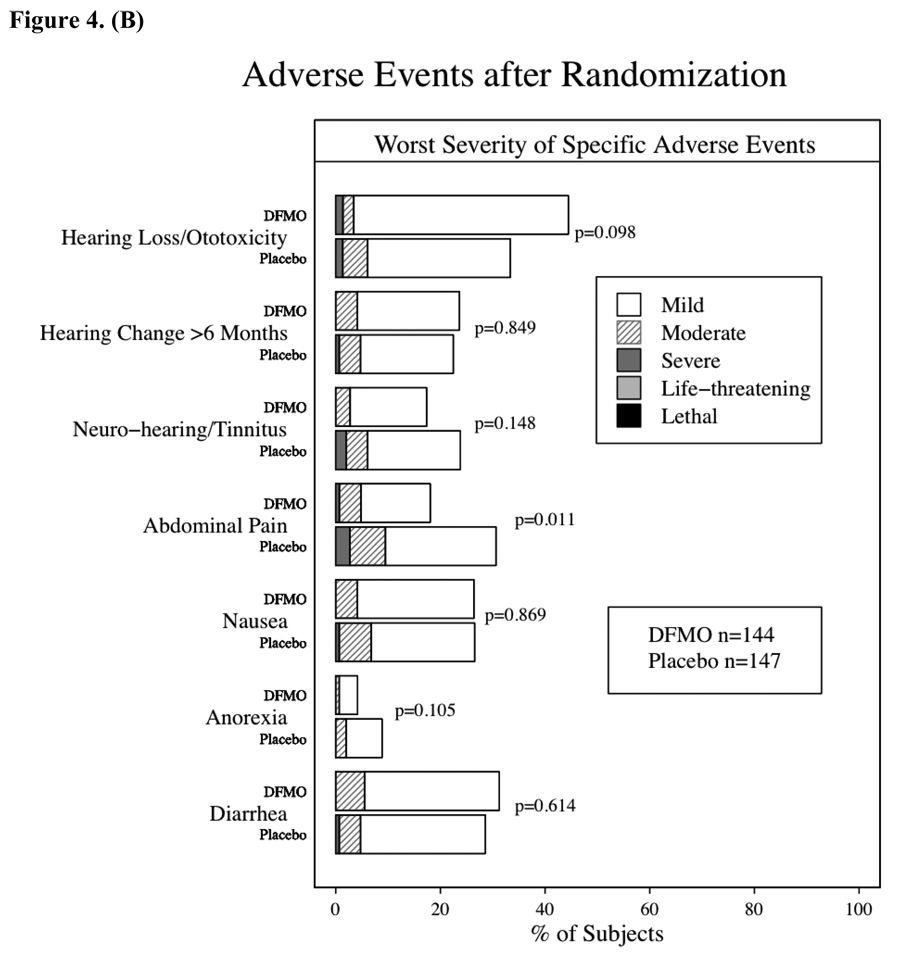
Adverse events during the placebo run-in phase (28 days) were uncommon except for gastrointestinal toxicity, which was reported by 20% of participants and primarily consisted of grade 1 nausea or diarrhea. After randomization, adverse events were common throughout the study with 95% of subjects in each group reporting at least one. Figures 4A & B compare the DFMO and placebo groups relative to general toxicity events and the most common specific adverse events, respectively. Gastrointestinal adverse events were the most commonly observed toxicity, and nausea or diarrhea were often attributed as possibly related to study drug. As shown in Figure 4B, gastrointestinal pain was more commonly observed in the placebo group. With regard to serious adverse events (i.e., hospitalizations, surgeries, grade 3 or 4 toxicities), 36 occurred in DFMO subjects and 35 in placebo subjects. Among these 71 events, 20 of the 36 subjects with grade 4 or life-threatening events had been randomized to DFMO. Ten subjects/events were attributed as probably related with 26 events described as possibly or unlikely related to study drug. Attribution descriptions (definitely, probably, possibly, unlikely or not related) were the same for both groups. The timing of serious or not serious adverse events was evaluated, and there were no differences in time-to-event or freedom-from events between the DFMO or placebo groups. Greater than or equal to grade 2 adverse events required study drug discontinuation, which occurred in approximately 30% (90 subjects) of randomized subjects, Despite the lack of any obvious differences in adverse events or rate between the two groups, significantly more subjects taking DFMO (36%) (p=0.058) had to discontinue study treatment due to ≥ grade 2 adverse event than subjects taking placebo (26%) (fig. 4A). This was predominately due to the increased rate of grade 2 audiometric abnormalities in subjects taking DFMO.
Figure 4.
Adverse events after randomization. The differences between the groups were assessed using chi-square tests. 4a – Overview of Adverse Events after randomization. 4b – Incidence of specific, more common toxicities by treatment group and severity (mild – grade 1, moderate – grade 2, severe – grade 3, life-threatening – grade 4, lethal – grade 5).
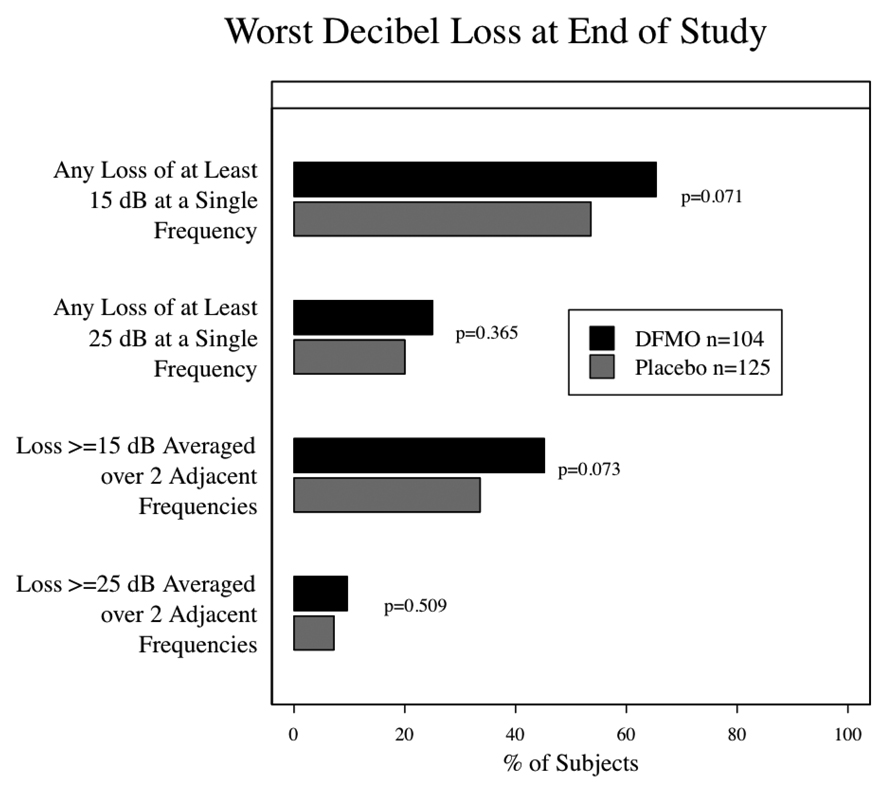
An audiogram was performed before beginning study treatment to assess baseline hearing status; of the 289/291 randomized subjects who had audiogram results recorded from that visit, 190 (66%) were reported as having normal hearing and 99 (34%) as abnormal. 244 subjects had an audiogram after one year on study. While there was no difference between groups in self-reported hearing loss or tinnitus, the audiograms reveal significant differences between the groups. While the overall average decibel loss at one year was approximately 1 dB in the DFMO group and zero in the placebo group, this was statistically significant (p=0.0001). Greater ototoxicity (resulting in study drug discontinuation) was found in the DFMO group as measured by the proportion of subjects with ≥ 15 dB hearing loss at 2 adjacent frequencies, but this did not achieve statistical significance (10.8% in the DFMO group and 4.5% in the placebo group, p=0.06). At the end of study (fig. 5) the overall average hearing loss was significantly greater in the DFMO arm (p=0.003) with approximately 4 dB of loss in the DFMO group and 2 dB of loss in the placebo group. When comparing proportions of subjects with potentially significant hearing changes, the differences were not significant but trended toward a greater proportion in the DFMO group, 45.2% of DFMO subjects versus 33.6% of placebo subjects had ≥ 15 dB hearing loss at 2 adjacent frequencies (p=0.07). Whenever feasible, subjects with significant hearing changes on audiogram underwent repeat audiograms 6 months later to assess reversibility. Thirty-one (19%) DFMO subjects and 33 (18%) placebo subjects had persistent abnormalities 6 months after stopping study drug. No additional audiometric testing was done.
Figure 5.
End of Study Audiometry results by treatment group. The panel summarizes the worst change for each subject according to certain thresholds. The differences between the groups were assessed using the Wilcoxon rank-sum test.
Twelve study subjects died during study participation or follow-up, 7 on the DFMO arm (age 69 to 78 y.o.) and 5 on the placebo arm (age 62 to 78 y.o.). While no deaths were felt to be possibly or probably related to the study drug 4 deaths on the DFMO arm are described: (1) a 69 y.o. took drug for 18 months and stopped due to cardiac disease and died 6 months later from congestive heart failure; (2) a 70 y.o. on drug for 30 months died 10 days after the last dose with a ruptured spleen and congestive heart failure; (3) a 69 y.o. took drug for 12 months and died 2 months later related to a cerebrovascular accident; and (4) a 76 y.o. took drug for 24 months and died from acute renal failure 4 months later.
DISCUSSION
The increasing burden of cancer and especially the increasing incidence and societal cost of skin cancer mandate continued pursuit of effective preventive agents in conjunction with appropriate lifestyle changes. Therefore we performed a single institution phase 3 skin cancer prevention study assessing the ability of daily DFMO to prevent the occurrence of new non-melanoma skin cancers. Daily dosing of 500 mg/m2 of DFMO for up to 5 years was safe and subject compliance was high. However the primary endpoint of observing a statistically significant reduction (p<0.05) in the number of new NMSC in subjects receiving DFMO as compared to subjects receiving placebo was not met. While there was a trend (p=0.069) toward overall prevention of NMSC in subjects taking DFMO this must be viewed in light of the incongruity in skin cancer history between the DFMO and placebo arms (placebo subjects were more likely to have multiple skin cancers prior to study registration). What is even more striking about the difference in skin cancer history between the two groups is the placebo group had a significantly longer history of NMSC (initial skin cancers occurring > 5 years prior to study enrollment). Another way to examine risk is by calculating the event rate prior to study enrollment, especially within 5 years of enrollment. When comparing “tumor burden” or annual event rates since the advent of their first NMSC, the groups are equivalent, with a slight increased rate in the DFMO group.
In order to better account for the apparent imbalance in skin cancer history a series of exploratory analyses of the number of new skin cancers over follow-up visits were performed using longitudinal models. More specifically we performed exploratory analyses using generalized estimating equations (GEE) approach and generalized linear Poisson regression models with over-dispersion. When the imbalance in the prior tumor burden and history is accounted for in the form of the prior tumor rate, both longitudinal models showed statistically significant effect of DFMO (p=0.026 for GEE and p=0.033 for Poisson model) in reducing recurrence of NMSC in this patient population.
Despite the differences in skin cancer history, the study groups were well matched in all other important categories including sunscreen and NSAID use, and the observed incidence of NMSC (0.5 skin cancers per person/year) equaled or slightly exceeded our pre-study predictions.
The apparent reduction in NMSC incidence in subjects taking DFMO was largely driven by significant reductions in the number of basal cell carcinoma. Sixty-nine subjects taking DFMO developed 163 new basal cell carcinomas, as compared to 77 subjects taking placebo who developed 245 new basal cell carcinomas. The incidence of basal cell carcinomas in the placebo arm (0.40 events/patient-years) was significantly greater (p=0.03) than the rate in subjects taking DFMO (0.28 events/patient-years). This difference is noteworthy regardless of whether the two treatment groups are considered equitable due to similar historical skin cancer rates or slightly inequitable due to differences in total number of skin cancers preceding study entry. An exploratory analysis mentioned above further demonstrates the DFMO effect in reducing the development of new BCC. As with any subset analysis, is it more consistent with biologically plausible evidence of greater subgroup sensitivity or an artifact of repeated statistical measures? This observation of disparate results between basal cell and small squamous cell carcinomas is consistent with findings in other skin cancer prevention studies (7, 9, and 12).
While basal and squamous cell carcinomas share the common risk factor of UV exposure and are increasing in incidence, they are two histologically distinct entities that develop along different carcinogenic pathways (34–35). The pivotal step in basal cell carcinogenesis is inappropriate activation of the Hedgehog (Hh) signaling pathway. In humans Sonic hedgehog (Shh), when not bound to its receptor Patched 1 (Ptch 1), activates its downstream components Smoothened (Smo) and the Glioma-associated (Gli) family of transcription factors that play an important role in multiple malignancies (34). Ptch 1 functions as a tumor suppressor of basal cell cancers, in fact inheritance of Ptch 1 mutation (loss of function) leads to basal cell nevus syndrome and the majority of sporadic basal cell cancers have Ptch 1 mutations (35). Consistent with our findings, treatment of the murine basal cell cancer model (Ptch +/− mice) with DFMO not only significantly inhibited basal cell cancer formation but also reduced mRNA expression of Shh and Gli transcription factors (36).
TPA-induced skin ODC activity and skin polyamine concentrations were assessed as potential biomarkers for DFMO skin cancer prevention. Since our study did not significantly lower the overall incidence of NMSC, we cannot establish these biomarkers as surrogate endpoints for NMSC prevention. TPA-induced skin ODC activity was significantly repressed throughout the study in subjects taking DFMO. We found no correlation between inhibition of skin ODC activity and NMSC occurrence. Clearly TPA-induced skin ODC activity demonstrates a DFMO biological effect, but this effect did not lead to significant NMSC prevention. However, inhibition of TPA-induced skin ODC activity might still correlate with prevention of basal cell carcinomas or it may be that greater inhibition of TPA-induced ODC activity correlates with prevention of NMSC. Inhibition of skin concentrations of putrescine and spermidine in subjects taking DFMO was observed at 24 and 36 months but not at 48 months. These findings most likely reflect a modest decrease in skin putrescine and spermidine concentrations, when taking oral DFMO at 500 mg/m2/day, which was only detected with larger sample sizes. The number of subjects undergoing skin biopsies decreased steadily from month 24 (122 subjects) to 36 (102 subjects) to month 48 (73 subjects).
Another important aspect of testing a potential chemopreventive agent is likelihood of clinical viability if proven effective. Two critical determinants of clinical viability are safety and compliance. While more subjects on DFMO discontinued study treatment secondary to AE’s, examination of all other parameters (AE relationship to study drug, duration on study, safety blood work) did not reveal any trends toward greater incidence of adverse events. The increased incidence of stopping study drug for AE appeared related to the increased rate of audiometric abnormalities in DFMO subjects. While there was no difference in self-reporting of ototoxicity between the groups (tinnitus, decreased hearing), examination of serial audiograms observed a trend toward hearing loss in the DFMO arm (p=0.06 at one year, p=0.10 at study end). The observed audiometric abnormalities were usually reversible 19% and 18% of DFMO and placebo subjects having persistent abnormal audiograms 6 months after stopping study drug. These data suggest that daily DFMO (500 mg/m2) for 4–5 years is reasonably safe with the only apparent concern being mild ototoxicity. Compliance was high throughout the study including when DFMO/placebo were administered as self-measured liquid rather than capsules. These data support the potential clinical viability of DFMO.
Until recently reported results from Meyskens et al. (28) documenting a significant reduction in new colonic adenomatous polyps in subjects taking DFMO plus sulindac as compared to placebo, most reports from larger phase 2 or 3 studies of DFMO had been negative (22,25, 26). Even though the Meyskens study administered a lower dose of DFMO (500 mg) in combination with sulindac, our study is most similar to it given the extended duration of study treatment (3–5 years) whereas other larger DFMO studies (22, 26) in breast and bladder only administered study drug for 6–12 months. The Meyskens study also did not observe a significant difference in toxicity between active and placebo treatment groups. Similar to our observations, the Meyskens study noted little to no difference between treatment groups in clinical ototoxicity and an insignificant trend toward greater audiometric evidence of hearing loss in the treatment group.
Our study did not achieve the primary objective of observing a statistically significant reduction in new NMSC’s in subjects taking DFMO as compared to placebo. However, the significant reduction in basal cell carcinomas and the biological plausibility of different sensitivity to ODC inhibition between squamous and basal cell carcinogenesis are compelling. Other than mild, usually reversible ototoxicity, daily oral DFMO at doses ≤ 500 mg/m2 appears safe and well tolerated. Our data coupled with the recent results from Meyskens et al. (28) support the continued study of DFMO as a chemopreventive agent. We hope to perform a confirmatory study of daily DFMO (500 mg/m2) for skin cancer prevention with the primary endpoint being reduction in basal cell carcinomas and a secondary objective of total non-melanoma skin cancer incidence.
ACKNOWLEDGEMENTS
We thank the following for their contributions: Independent Data and Safety Monitoring Board members- Thomas Louis, PhD, Charles Loprinzi, MD, and Robert McDonald, MD; Data and Statistical Management- Abhik Bhattacharya, PhD, Melissa Schultz, MS, and Li-Yin Lee, PhD.
Supported by NCI awards R01 CA77158, U01 CA77158, P30 CA 14520, and NCRR CTSA awards UL1 RR025011.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Fleisher AB, Jr, Smith ED, et al. Cost of non-melanoma skin cancer treatment in the United States. Dermatol Surg. 2001;27:1035–1038. doi: 10.1046/j.1524-4725.2001.01004.x. [DOI] [PubMed] [Google Scholar]
- 3.Lindelof B, Sigurgeirsson B, Gabel H, Stern RS. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143(3):513–519. [PubMed] [Google Scholar]
- 4.Ramsay HM, Reece SM, Fryer AA, et al. Seven-year prospective study of non-melanoma skin cancer incidence in U.K. renal transplant recipients. Transplantation. 2007;84:437–439. doi: 10.1097/01.tp.0000269707.06060.dc. [DOI] [PubMed] [Google Scholar]
- 5.Bath-Hextall FJ, Leonardi-Bee J, Somchand N, Webster AC, Delitt J, Perkins W. Interventions for preventing non-melanoma skin cancers in high-risk groups. Cochrane Database Syst Rev. 2007;(Issue 4) doi: 10.1002/14651858.CD005414.pub2. Art. No.: CD005414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boukamp P. Non-melanoma skin cancer: what drive tumor development and progression. Carcinogenesis. 2005;26:1657–1667. doi: 10.1093/carcin/bgi123. [DOI] [PubMed] [Google Scholar]
- 7.van der Pols JC, Williams GM, Pandeya N, et al. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev. 2006;15:2546–2548. doi: 10.1158/1055-9965.EPI-06-0352. [DOI] [PubMed] [Google Scholar]
- 8.Tangrea JA, Edwards BK, Taylor PR, et al. Isotretoin-basal cell study group. Long term therapy with low-dose isotretoin for prevention of basal cell carcinoma: A multicenter clinical trial. J Natl Cancer Inst. 1992;5:328–332. doi: 10.1093/jnci/84.5.328. [DOI] [PubMed] [Google Scholar]
- 9.Moon TE, Levine N, Cartmel B, et al. Southwest Skin Cancer Prevention Study Group. Effect of retinol in preventing squamous cell skin cancer in moderate-risk subjects: a randomized, double-blind, controlled trial. Cancer Epidemiol Biomarkers Prev. 1997;6:949–956. [PubMed] [Google Scholar]
- 10.Levine N, Moon TE, Cartmel B, et al. Southwest Skin Cancer Prevention Study Group. Trial of retinol and isotretinoin in skin cancer prevention: a randomized, double-blind, controlled trial. Cancer Epidemiol Biomarkers Prev. 1997;6:957–961. [PubMed] [Google Scholar]
- 11.Frieling UM, Schaumberg DA, Kupper TS, Muntwyler J, Hennekens CH. A randomized, 12-year primary-prevention trial of beta carotene supplementation for non-melanoma skin cancer in the physician's health study. Arch Dermatol. 2000;136:179–184. doi: 10.1001/archderm.136.2.179. [DOI] [PubMed] [Google Scholar]
- 12.Duffield-Lillico AJ, Slate EH, Reid ME, et al. Selenium supplementation and secondary prevention of non-melanoma skin cancer in a randomized trial. J Natl Cancer Inst. 2003;95:1477–1481. doi: 10.1093/jnci/djg061. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien TG, Simsiman RC, Boutwell RK. Induction of the polyamine biosynthetic enzymes in mouse epidermis by tumor-promoting agents. Cancer Res. 1975;35:1662–1670. [PubMed] [Google Scholar]
- 14.Morgan DML. Polyamines: an introduction. Methods Mol Biol. 1998;79:3–30. doi: 10.1385/0-89603-448-8:3. [DOI] [PubMed] [Google Scholar]
- 15.Pegg AE. Polyamine metabolism and its importance in neoplastic growth and as a target for chemotherapy. Cancer Res. 1988;48:759–774. [PubMed] [Google Scholar]
- 16.Meyskens FL, Gerner EW. Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin Cancer Res. 1999;5:945–951. [PubMed] [Google Scholar]
- 17.Verma A, Lowe N, Boutwell R. Induction of mouse epidermal ornithine decarboxylase activity and DNA synthesis by ultraviolet light. Can Res. 1979;39:1035–1040. [PubMed] [Google Scholar]
- 18.Verma AK, Shapas BG, Rice HM, Boutwell RK. Correlation of the inhibition by retinoids of tumor promoter-induced mouse epidermal ornithine decarboxylase activity and of skin tumor promotion. Cancer Res. 1979;39:419–425. [PubMed] [Google Scholar]
- 19.Love RR, Carbone PP, Verma AK, et al. Randomized phase I chemoprevention dose-seeking study of alpha-difluoromethylomithine. J Natl Cancer Inst. 1993;85:732–737. doi: 10.1093/jnci/85.9.732. [DOI] [PubMed] [Google Scholar]
- 20.Love RR, Jacoby R, Newton MA, et al. A randomized, placebo-controlled trial of low-dose α-difluoromethylornithine in individuals at risk for colorectal cancer. Cancer Epid Bio Prev. 1998;7:989–992. [PubMed] [Google Scholar]
- 21.Meyskens FL, Gerner EW, Emerson S, et al. A randomized double-blind placebo controlled phase IIb trial of difluoromethylornithine for colon cancer prevention. J Natl Cancer Inst. 1998;90:1212–1218. doi: 10.1093/jnci/90.16.1212. [DOI] [PubMed] [Google Scholar]
- 22.Messing E, Kim KM, Sharkey F, Schultz M, Parnes H, Kim D, et al. Randomized prospective phase III trial of difluoromethylornithine vs. placebo in preventing recurrence of completely resected low risk superficial bladder cancer. J Urol. 2006;176:500–504. doi: 10.1016/j.juro.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 23.Messing EM, Love RR, Tutsch KD, et al. Low-dose difluoromethylornithine and polyamine levels in human prostate tissue. J Natl Cancer Inst. 1999;16:1416–1417. doi: 10.1093/jnci/91.16.1416. [DOI] [PubMed] [Google Scholar]
- 24.Simoneau AR, Gerner EW, Phung M, McLaren CE, Meyskens FL. α-difluoromethylornithine and polyamine levels in the human prostate: Results of a Phase IIa trial. J Natl Cancer Inst. 2001;93:57–59. doi: 10.1093/jnci/93.1.57. [DOI] [PubMed] [Google Scholar]
- 25.Vlastos AT, West LA, Atkinson EN, et al. Results of a phase II double-blinded randomized clinical trial of difluoromethylornithine for cervical intraepithelial neoplasia grades 2 to 3. Clin Cancer Res. 2005;11:390–396. [PubMed] [Google Scholar]
- 26.Fabian CJ, Kimler BF, Brady DA, et al. A phase II breast cancer chemoprevention trial of oral α-difluoromethylornithine: breast tissue, imaging, and serum and urine biomarkers. Clin Cancer Res. 2002;8:3105–3117. [PubMed] [Google Scholar]
- 27.Carbone PP, Douglas JA, Larson PO, et al. Phase I chemoprevention study of piroxicam and α-difluoromethylornithine. Cancer Epid Bio Prev. 1998;7:907–912. [PubMed] [Google Scholar]
- 28.Meyskens FL, McLaren CE, Pelot D, et al. Difluoromethylornithine Plus Sulindac for the Prevention of Sporadic Colorectal Adenomas: A Randomized Placebo-Controlled, Double-Blind Trial. Cancer Prev Res. 2008;1:9–11. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verma AK, Loprinzi CL, Boutwell RK, Carbone PP. In vitro induction of human skin ornithine decarboxylase by the tumor promoter 12-O-tetradecanoylphorbol-13-acetate. J Natl Cancer Inst. 1985;75:85–90. [PubMed] [Google Scholar]
- 30.Carbone PP, Douglas JA, Thomas J, et al. Bioavailability study of oral liquid and tablet forms of α-difluoromethylornithine. Clin Cancer Res. 2000;6:3850–3854. [PubMed] [Google Scholar]
- 31.Shotland LI, Ondrey FG, Mayo KA, Viner JL. Recommendations for Cancer Prevention Trials Using Potentially Ototoxic Agents. J Clin Oncol. 2001;19:1658–1663. doi: 10.1200/JCO.2001.19.6.1658. [DOI] [PubMed] [Google Scholar]
- 32.Greenberg ER, Baron JA, Stukel TA, et al. A clinical trial of beta carotene to prevent basal-cell and squamous-cell cancers of the skin. N Engl J Med. 1990;323:789–795. doi: 10.1056/NEJM199009203231204. [DOI] [PubMed] [Google Scholar]
- 33.Kabra P, Lee HK, Lubich WP, Marton LJ. Solid-phase extraction and determination of dansyl derivatives of unconjugated and acetylated polyamines by reversed-phase liquid chromatography: improved separation systems for polyamines in cerebral spinal fluid, urine and tissue. J. Chrom. 1986;380:19–32. doi: 10.1016/s0378-4347(00)83621-x. [DOI] [PubMed] [Google Scholar]
- 34.Rubin AI, Chen EH, Ratner D. Current Concepts Basal Cell Carcinoma. N Engl J Med. 2005;353:2262–2269. doi: 10.1056/NEJMra044151. [DOI] [PubMed] [Google Scholar]
- 35.Tang JY, So P-L, Epstein EH., Jr Novel Hedgehog pathway targets against basal cell carcinoma. Toxicol Appl Pharmacol. 2007;224:257–264. doi: 10.1016/j.taap.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang X, Kim AL, Feith DJ, et al. Ornithine Decarboxylase is a target for chemoprevention of basal and Squamous cell carcinomas in Ptch+/− mice. J Clin Invest. 2004;13:867–875. doi: 10.1172/JCI20732. [DOI] [PMC free article] [PubMed] [Google Scholar]