Advances in imaging technologies have led to a much better appreciation of the role of intercellular physical contacts in enabling cells to efficiently communicate with one another. These technologies have advanced our understanding of events that take place during the formation of immunological synapses, first described over a decade ago when the imaging of live cells was in its infancy.1 More recently, several studies have shown that viruses take advantage of these structured interfaces, in this case termed virological synapses, to enhance their cell-to-cell spread.2 There is also growing evidence that pathogens take advantage of these structures to interfere with immune responses. In a recent study published in Nature Immunology, Cerutti and colleagues3 used a series of elegant tissue-imaging approaches to demonstrate how the HIV accessory protein Nef induces the formation of nanotubule-like structures in infected macrophages, ultimately resulting in its selective transfer into neighboring B cells. The presence of Nef in B cells that otherwise remain uninfected interfered with their ability to produce certain classes of antibodies. These findings add to the long list of potential explanations of why antibody responses are ineffective in the majority of HIV-infected individuals.
A plethora of properties have been attributed to Nef ever since it was first discovered almost a quarter century ago,4 and somewhat misnamed ‘negative factor’. The true properties of Nef have been difficult to determine and many of its proposed attributes remain a source of contention.5,6 Nonetheless, several recent studies have made convincing arguments that Nef can both enhance HIV replication by inducing CD4+ T-cell activation and dampen the immune response directed against HIV by interfering with the events that lead to the formation of immunological synapses.6 It is this latter line of investigation that Cerutti and colleagues7 have been pursuing, by first showing in 2006 that Nef, but not HIV capsid and matrix proteins, is taken up by B cells. The presence of Nef in B cells led to the disruption of class-switch recombination (CSR), an essential component of the antibody diversification process that occurs in germinal centers (GCs), whereby the effector portion of immunoglobulins (Igs) switch from IgD or IgM to any one of the subclasses IgG, IgA or IgE. CSR disruptions were associated with a decrease in the levels of activation-induced cytidine deaminase (AID), an enzyme that is essential for the induction of CSR and somatic hypermutation of Ig genes. In their most recent study, Cerutti and colleagues extend their previous findings by demonstrating a paucity of IgA and IgG2 in the GCs of HIV-infected individuals when compared with those of HIV-uninfected individuals, and that these Ig-deficient GCs were in close proximity to areas of high Nef expression. Furthermore, the uptake of Nef by B cells co-cultured with HIV-infected macrophages in the presence of CD40 ligand, an essential factor in the induction of AID activity that is provided by cognate CD4+ T cells, diminished the secretion of IgG and IgA when compared with B cells co-cultured with uninfected macrophages. Although the antigen specificity of these tissue- and culture-derived Igs was not determined, the assumption is that this inhibition would target HIV-specific B cells while receiving help from cognate CD4+ T cells.
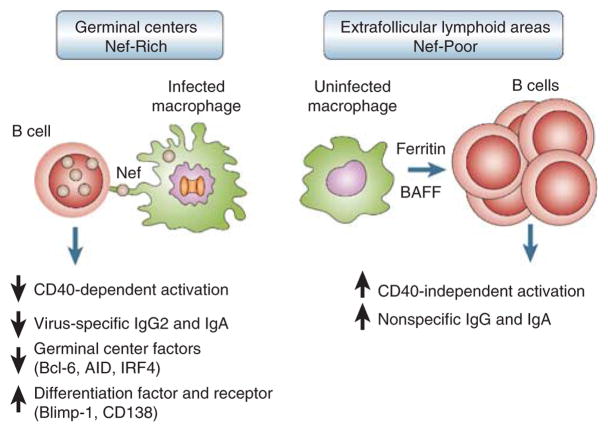
After demonstrating that Nef alone could inhibit CSR through cell-to-cell contacts between macrophages and B cells, Cerutti and colleagues went on to generate several Nef mutants, and with the use of various inhibitors of subcellular structures and elegant imaging techniques, elucidated several features of the Nef delivery process. Multiple motifs in Nef contribute both to the formation of actin-dependent long-range conduits within macrophages and to the delivery of Nef to B cells. The remarkable images and videos provide compelling evidence that Nef alters both macrophages and the formation of GCs. Of note, these alterations lead to a decrease in the expression of B-cell transcription factors BCL-6 and IRF4, both of which are involved in maintaining GCs and promoting class switching, and an increase in the expression of transcription factor Blimp-1 and receptor CD138, both of which are associated with terminal differentiation of activated B cells into Ig-secreting plasma cells.8 Furthermore, in analyses of lymphoid tissue sections obtained from both intestinal and peripheral sites, the investigators observed increased B-cell hyperactivity in extrafollicular sites where Nef was largely absent. These findings are consistent with the longstanding notion that HIV infection leads to extensive hyperplasia in these extra-follicular sites, although hyperplasia within GCs is also generally observed.9,10 As summarized in Figure 1, Cerutti and colleagues propose that Nef interferes with CD40-dependent B-cell stimulation in GCs, whereas B-cell hyperactivity in Nef-poor extrafollicular areas is driven by ferritin and the B-cell activating factor of the tumor necrosis factor family, two stimulators of B cells that are found to be overexpressed in HIV-infected individuals.11,12 However, this concept of a compartmentalized inhibition of B-cell activity in Nef-rich areas is somewhat at odds with the recent observation linking Nef to B-cell hyperactivity via the induction of ferritin in infected macrophages.12
Figure 1.
Schematic of the inhibitory effects of Nef on follicular B cells. Nef delivered to B cells from infected macrophages in germinal center (GC) inhibits CD40-mediated class switching to immunoglobulin (Ig)A and IgG2. In contrast, B cells in extrafollicular areas of low Nef expression undergo polyclonal activation in response to increased levels of ferritin and the B-cell activating factor of the tumor necrosis factor family.
Polyclonal B-cell activation and terminal differentiation, lymph node hyperplasia of areas enriched in B cells, and hypergamma-globulinemia are all the hallmarks of HIV infection and disease.13 Typically, activation of the immune response is an important step in the expression of normal immune function. Yet, as first described more than 25 years ago for B cells,14 HIV infection leads to loss of immune function in a majority of untreated individuals in association with increased immune activation. Although numerous scenarios have been proposed to explain this apparent paradox that characterizes almost all components of the immune system in HIV infection,13,15–18 the underlying causes of HIV-induced immunopathogenesis still remain a matter of intense debate. In this regard, the findings of Cerutti and colleagues provide a scenario that can help explain both the hyperactivity and the dysfunction of B cells observed in HIV-infected individuals. Their study also has physiological relevance in that many of the observations were made by comparing intestinal and peripheral lymphoid tissues, in which most of the B cells of our body reside, of HIV-infected and uninfected individuals and, most remarkably, by tapping into the unique cohort of individuals in Australia who were infected with a Nef-defective variant of HIV.19 This cohort has been carefully characterized and followed for almost 15 years. Most individuals in the cohort remain classified as long-term non-progressors (LTNPs) in that they maintain high CD4+ T-cell counts, low viral loads and normal serum levels of Ig. In the recent study by Cerutti and colleagues, the sera of LTNPs in the Nef-defective cohort were found to contain higher levels of HIV-specific IgG2 and IgA than those of LTNPs which harbor wild-type HIV but otherwise have a similar virological and immunological profile. These findings help solidify the notion that the presence of Nef may interfere with virus-specific antibody responses in HIV-infected individuals.
In summary, the study by Cerutti and colleagues provides new and compelling insight into the pathogenesis of B-cell dysfunction in HIV infection. Their findings also raise several questions. Although there is general agreement that B-cell dysregulation in HIV infection and the factors that feed the dysregulation are largely the result of direct and indirect effects of ongoing viral replication, there is one important exception. The loss of memory B cells and associated responses are likely to be irreversible, especially in chronically HIV-infected individuals.13,20,21 Although there is evidence that HIV structural proteins persist in the follicular dendritic cell networks of GC for months to years after effective antiretroviral therapy, there is currently little evidence that macrophages would continue to harbor Nef and form conduits that would lead to sustained inhibition of B-cell responses against HIV and other pathogens. In addition, although the cohort of individuals infected with Nef-defective HIV provides compelling evidence that Nef inhibits HIV-specific IgA and IgG2 responses, there is no reason why such a deficiency would not extend to other Ag-specific responses given that the transfer of Nef to B cells is unlikely to be restricted to HIV-specific B cells. In this regard, it would be interesting to compare IgA and IgG2 responses with that of a panel of vaccines in the Nef-deficient and Nef-proficient LTNP cohorts.
References
- 1.Wulfing C, Davis MM. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science. 1998;282:2266–2269. doi: 10.1126/science.282.5397.2266. [DOI] [PubMed] [Google Scholar]
- 2.Sattentau Q. Avoiding the void: cell-to-cell spread of human viruses. Nat Rev Microbiol. 2008;6:815–826. doi: 10.1038/nrmicro1972. [DOI] [PubMed] [Google Scholar]
- 3.Xu W, Santini PA, Sullivan JS, He B, Shan M, Ball SC, et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol. 2009;10:1008–1017. doi: 10.1038/ni.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muesing MA, Smith DH, Cabradilla CD, Benton CV, Lasky LA, Capon DJ. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retro-virus. Nature. 1985;313:450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- 5.Roeth JF, Collins KL. Human immunodeficiency virus type 1 Nef: adapting to intracellular trafficking pathways. Microbiol Mol Biol Rev. 2006;70:548–563. doi: 10.1128/MMBR.00042-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fackler OT, Alcover A, Schwartz O. Modulation of the immunological synapse: a key to HIV-1 pathogenesis? Nat Rev Immunol. 2007;7:310–317. doi: 10.1038/nri2041. [DOI] [PubMed] [Google Scholar]
- 7.Qiao X, He B, Chiu A, Knowles DM, Chadburn A, Cerutti A. Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nat Immunol. 2006;7:302–310. doi: 10.1038/ni1302. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5:230–242. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- 9.Racz P, Tenner-Racz K, van Vloten F, Schmidt H, Dietrich M, Gluckman JC, et al. Lymphatic tissue changes in AIDS and other retrovirus infections: tools and insights. Lymphology. 1990;23:85–91. [PubMed] [Google Scholar]
- 10.Pantaleo G, Graziosi C, Demarest JF, Cohen OJ, Vac-carezza M, Gantt K, et al. Role of lymphoid organs in the pathogenesis of human immunodeficiency virus (HIV) infection. Immunol Rev. 1994;140:105–130. doi: 10.1111/j.1600-065x.1994.tb00867.x. [DOI] [PubMed] [Google Scholar]
- 11.Stohl W, Cheema GS, Briggs WS, Xu D, Sosnovtseva S, Roschke V, et al. B lymphocyte stimulator protein-associated increase in circulating autoantibody levels may require CD4+ T cells: lessons from HIV-infected patients. Clin Immunol. 2002;104:115–122. doi: 10.1006/clim.2002.5238. [DOI] [PubMed] [Google Scholar]
- 12.Swingler S, Zhou J, Swingler C, Dauphin A, Greenough T, Jolicoeur P, et al. Evidence for a pathogenic determinant in HIV-1 Nef involved in B cell dysfunction in HIV/AIDS. Cell Host Microbe. 2008;4:63–76. doi: 10.1016/j.chom.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309:453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 15.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med. 2006;12:289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 16.Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat Immunol. 2006;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 17.Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol. 2005;5:835–843. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- 18.Herbeuval JP, Shearer GM. HIV-1 immunopathogenesis: how good interferon turns bad. Clin Immunol. 2007;123:121–128. doi: 10.1016/j.clim.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deacon NJ, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker DJ, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 20.Cagigi A, Nilsson A, De Milito A, Chiodi F. B cell immunopathology during HIV-1 infection: lessons to learn for HIV-1 vaccine design. Vaccine. 2008;26:3016–3025. doi: 10.1016/j.vaccine.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 21.Haynes BF, Shattock RJ. Critical issues in mucosal immunity for HIV-1 vaccine development. J Allergy Clin Immunol. 2008;122:3–9. doi: 10.1016/j.jaci.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]