Abstract
Self-renewal of human embryonic stem (ES) cells proceeds by a unique abbreviated cell cycle with a shortened G1 phase and distinctions in molecular cell cycle regulatory parameters. In this study, we show that early lineage-commitment of pluripotent hES cells modifies cell cycle kinetics. Human ES cells acquire a lengthened G1 within 72 h after lineage-programming is initiated, as reflected by loss of the pluripotency factor Oct4 and alterations in nuclear morphology. In hES cells that maintain the pristine pluripotent state, we find that autocrine mechanisms contribute to sustaining the abbreviated cell cycle. Our data show that naive and mitotically synchronized pluripotent hES cells are competent to initiate two consecutive S phases in the absence of external growth factors. We conclude that short term self-renewal of pluripotent hES cells occurs autonomously, in part due to secreted factors, and that pluripotency is functionally linked to the abbreviated hES cell cycle.
Keywords: WA09, H9 human embryonic stem cells, restriction point, growth factor, S phase, pluripotent stem cell, self-renewal
INTRODUCTION
Pluripotency, unlimited proliferative growth potential (Carpenter et al, 2003; Sato et al, 2003; Stojkovic et al, 2004) and a short cell division cycle due to an abbreviated G1 phase (Becker et al, 2006; Becker et al, 2007; Ghule et al, 2007; Ghule et al, 2008) are defining characteristics of human embryonic stem (hES) cells. The undifferentiated phenotype is maintained by a distinct set of transcription factors (e.g., OCT4, SOX2, and NANOG) (Boyer et al, 2005; Mathur et al, 2008; Meissner et al, 2008; Mikkelsen et al, 2007). Pluripotency is preserved in human ES cell culture models by propagating cells on inactivated mouse embryo fibroblasts (MEFs), which secrete growth factors and inhibitors of developmental cell signaling (e.g., noggin and gremlin), as well as by supplementing culture media with recombinant basic Fibroblast Growth Factor (bFGF/FGF2) (Bodnar et al, 2004; Levenstein et al, 2006; Xu et al, 2005; Xu et al, 2008).
Lineage-committed somatic cells exhibit a canonical cell cycle in which G1 phase is longer than S phase, while the G1 phase in pluripotent hES cells is shorter than S phase. The distinction in the duration of cell cycle progression from mitosis to S phase is due in part to molecular differences in cell cycle regulatory parameters including pronounced expression of cyclin D2/CDK4, rather than cyclin E/CDK2, just prior to the G1/S phase transition in hES cells (Becker et al, 2006). Because of differences in cell cycle kinetics between lineage-committed and pluripotent cells, there is a requirement to define developmental transitions that mediate lengthening of the G1 phase and functional linkage with loss of pluripotency.
In somatic cells, the classical Restriction (R) point represents the cell cycle transition at which progression into S phase becomes growth factor independent, reflected by commencement of DNA synthesis even upon growth factor withdrawal (Blagosklonny and Pardee, 2002; Pardee et al, 1978; Pardee, 1974). The unique proliferative properties of human ES cells are attributed in part to the lack of a growth factor dependent R point. For example, cell cycle progression in mouse ES cells occurs independent of the classical release of pocket proteins (i.e., pRB, p107 and p130) from E2F transcription factors to promote S phase entry (White et al, 2005). The presumed absence of the E2F/pRB switch in human ES cells predicts that competency for cell cycle progression may occur independent of external factors and/or regulatory signals.
In this study, we mechanistically addressed functional relationships between the abbreviated G1 period and pluripotency in human ES cells. We investigated the length of G1 in mitotically synchronized hES cells by measuring the onset of DNA synthesis as reflected by BrdU incorporation during initial stages of lineage-commitment in the absence of a MEF feeder layer. In addition, we examined the external growth factor requirements of hES cells during progression from mitosis into two consecutive S phases. Our findings establish that lengthening of the hES cell cycle is associated with loss of the pluripotent state. Furthermore, we demonstrate that hES cells do not require growth factor-related external regulatory cues from either the MEF feeder layer or media components. Rather, upon release from mitotic arrest, hES cells are primed for at least two rounds of cell proliferation. Our results suggest that pluripotent cells are capable of short-term autonomous growth, and that programming of hES cells results in loss of competency for the characteristic abbreviated G1 phase.
MATERIALS AND METHODS
Cell lines and tissue culture
The human ES cell line H9 (WA09) was acquired from WiCell Research Institute (Madison, WI). ES cells were routinely grown on monolayers of inactivated mouse embryonic fibroblasts (MEFs) that were isolated from 13.5-day embryos of CF-1 mice (Charles River Laboratories, Wilmington, MA). MEFs were cultured until passage 3 in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT), 1% L-glutamine and 1% penicillin/streptomycin (both from Invitrogen). MEFs were mitotically inactivated by irradiation at 40 Gy before seeding on a 6-well plate at 1.125 × 106 cells/plate.
Human ES cells were expanded under non-differentiating conditions as recommended by the supplier. The human ES culture medium (see below) was changed daily and cells were replated every 6–7 days. For replating during regular cell maintenance, ES cell suspensions were generated by incubating colonies with collagenase Type IV (1 mg/ml; Invitrogen) for 15–20 min at 37°C and mild mechanical disruption. Cells were maintained in standard human ES cell medium (referred to in this study as ‘Medium 1’) that consists of 80% DMEM/F12, 20% KnockOut-Serum Replacement (KO-SR), 2 mM L-glutamine, 1% non-essential amino acids, 0.1 mM β-mercaptoethanol, 4 ng/ml basic fibroblast growth factor (bFGF/FGF-2) (all from Invitrogen) at 37°C, 5% CO2 and high humidity. To examine the dependence of cell cycle parameters in hES cells on external factors, we also tested two other medium conditions (‘Medium 2’ and ‘Medium 3’). Medium 2 is the same as Medium 1 but bFGF is omitted, whereas Medium 3 represents incomplete DMEM/F12 medium without any other additives. For studies in which hES cells were permitted to initiate early stages of lineage commitment, we cultured cells in differentiating medium (‘Medium 4’) containing 80% Iscove Modified Dulbecco’s Medium (IMDM; Invitrogen), 20% fetal bovine serum (FBS; HyClone), 1% L-glutamine and 1% penicillin/streptomycin (both from Invitrogen).
Cell cycle analysis by mitotic synchronization
For mitotic synchronization studies H9 cells were allowed to establish robust colonies for 4–5 days after replating. H9 cells were then replated on coverslips coated with inactivated mouse feeder cells and synchronized by incubation for 16 h with 200 ng/ml nocodazole (Sigma-Aldrich, St. Louis, MO). In the first set of experiments, cells were maintained on the coverslips with inactivated MEFs and the mitotic inhibitor was removed by repeated washes with incomplete DMEM/F12 medium. Cultures were then supplemented with Medium 1, -2 or -3 as indicated and cells were harvested at hourly intervals. For each condition, progression into S phase at each time point was assessed by monitoring BrdU incorporation for 20 min at 37°C (see below).
In the second set of experiments, the mitotic inhibitor was removed by repeated washes with incomplete DMEM/F12 medium and cells were replated on feeder-free cover slips. Replating was carried out by briefly incubating synchronized H9 cells colonies with Dispase (2,000 catalytic units/ml; BD Bioscience, Bedford, MA) at 37°C to allow separation of ES cells from inactivated mouse feeder layer cells. Colonies were washed out with fresh DMEM/F12 and the resulting suspensions were subjected to low-speed centrifugation (IEC Damon CRU-500 centrifuge equipped with a swing-out bucket rotor for 15 ml tubes at 1,000 rpm for 4 min), followed by a single wash with DMEM/F12 and a repeat of the centrifugation step. The resulting colony pellets were then resuspended in one of three different types of media (i.e., Medium 1, -2 or -3) and seeded on coverslips without the inactivated MEF feeder layer. Cells were allowed to adhere to the coverslips (<1 h) and samples were obtained at hourly intervals to assess BrdU incorporation for 20 min at 37°C for up to 24 hours.
A third set of experiments involved examination of cell cycle parameters under differentiating conditions. H9 human ES cells were plated on plastic coverslips and cultured in complete differentiating media (Medium 4) for 24 h, 48 h and 72 h. As above, cells were then synchronized for 16 h with 200 ng/ml nocodazole, and cells were released from the mitotic block by repeated washes with Medium 4. Cell cycle progression into S phase was monitored every 2 h by BrdU incorporation for up to 24 h. Parallel experiments were performed with cells in which we analyzed absence of BrdU incorporation after treatment with 20 μg/ml aphidicolin (Sigma-Aldrich) at 18 h to block entry into the second S phase.
BrdU incorporation assays and immunofluorescence microscopy
Incorporation of 5-Bromo-2′-deoxyuridine (BrdU; Roche Diagnostics Corporation/Roche Applied Science, Indianapolis, IN) was used to analyze S phase entry and progression. Cells were grown on coverslips with or without feeder cells and incubated for 20 min at 37°C to incorporate BrdU. Cells were then fixed with ethanol and 50 mM glycine (pH 2.0) for 20 min at −20°C. BrdU was detected with antibodies for BrdU (1:10 dilution) and visualized at 488 nm using immunofluorescence microscopy according to the instructions of the manufacturer (Roche).
Maintenance of pluripotency of asynchronous ES cell colonies was assessed by immunofluorescence visualization of Oct4. In brief, cells were rinsed with ice-cold phosphate buffered salines (PBS) and fixed in 3.7% formaldehyde in PBS for 10 min on ice. After rinsing once with PBS, cells were permeabilized in 0.1% Triton X-100 in PBS, and rinsed twice in PBSA (0.5% bovine serum albumin [BSA] in PBS) and stained with goat polyclonal Oct4 (N19) (1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA). For localization of antigen/antibody complexes, the complementary fluorescent secondary antibody (Alexa Fluor 488 conjugated donkey anti-goat IgG) (1:800 dilution; Molecular Probes/Invitrogen) was used.
RESULTS
Lengthening of the self-renewal cell cycle is linked to loss of pluripotency in human ES cells
One defining characteristic of self-renewal in pluripotent human ES cells is the short cell cycle duration (16 h) due to an abbreviated G1 cell cycle phase (Becker et al, 2006; Becker et al, 2007). Because somatic cells typically have a longer cell cycle time (24–30 h), it is important to experimentally establish regulatory parameters of a switch that occurs in the length of the cell cycle coinciding with the loss of pluripotency and/or lineage-commitment. Therefore, we addressed the key question whether the unique self-renewal cell cycle characteristics of hES cells are altered during early stages of differentiation and concomitant loss of pluripotency.
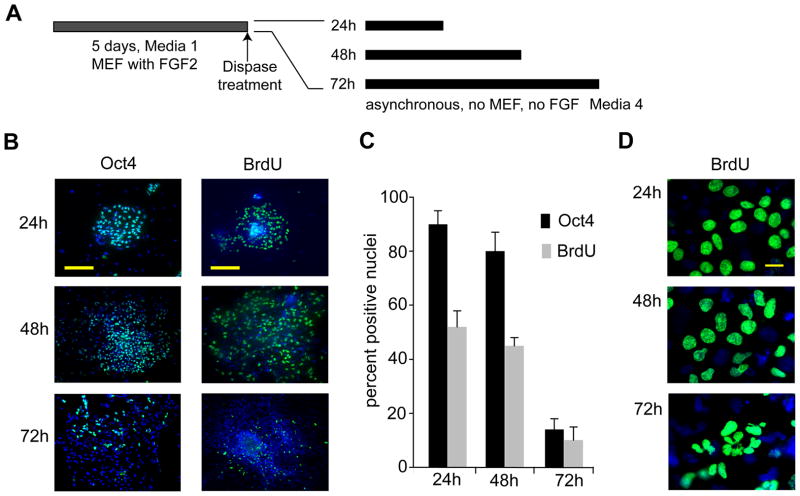
To induce early lineage commitment, undifferentiated H9 hES cells were seeded under feeder-free conditions in serum-containing differentiation medium (‘Medium 4’) (Fig. 1A). At 24 h, 48 h and 72 h, cells were examined for BrdU incorporation (20 min pulse-labeling) as an indicator of the fraction of cells in S phase, as well as for loss of the pluripotency marker Oct4 as an indicator of differentiation. We have previously shown that asynchronously self-renewing hES cells maintained on MEF feeder layers have a BrdU-positive fraction of >50% and an abbreviated ~16 h cell cycle due to a reduced G1 phase (~2.5 h) (Becker et al, 2006). Consistent with these earlier findings, human ES cells growing in feeder-free cultures that permit lineage-commitment show significant BrdU incorporation (>50%) and >90% of nuclei stain positive for Oct4 after 24 h. However, after induction of differentiation for 72 h, BrdU incorporation and Oct4 staining decline drastically to ~10% and ~12%, respectively (Figs. 1B and 1C), together with modifications in nuclear architecture. We detected irregularly shaped, multi-lobulated nuclei that remained competent to proliferate based on BrdU incorporation. These nuclear changes were prominent after 72 h and more frequent in cells at the periphery of the colony (Fig. 1D). The reduction in BrdU-positive and Oct4 cells between 48 h and 72 h of differentiation indicates that a lengthened cell cycle and a lineage-committed state are acquired concomitantly within a limited number of cell generations.
Figure 1. Reduced DNA synthesis in hES cells is linked to loss of pluripotency.
(A) Experimental design. (B) Immunofluorescence microscopy detection of Oct4 and BrdU in asynchronous human ES cells (WA09/H9) at 24 h, 48 h, and 72 h and cultured in differentiating medium under feeder-free conditions (scale bar = 200 μm). (C) Quantitation of results depicted in (B); the error-bars represent the standard error of the mean (SEM) for four independent determinations of 400 each (n=1600). Changes in Oct4 and BrdU signals between 48h and 72 h are statistically significant (P<0.05) (D) Micrographs of nuclei at higher magnification show changes in nuclear morphology after 24 h 48 h, and 72 h under differentiating conditions (scale bar = 20 μm).
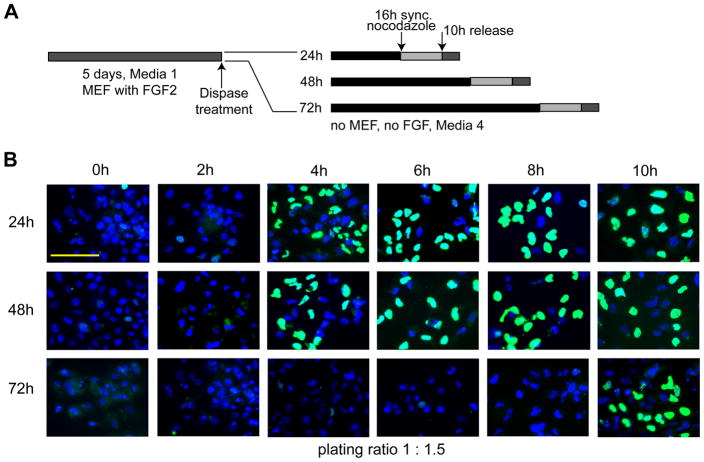
To investigate directly whether changes in cell cycle length occur during early stages of lineage-commitment, we examined BrdU incorporation in cells that were synchronized at successive stages of differentiation. Human ES cells were seeded in feeder-free conditions and after 24 h, 48 h and 72 h, cultures were incubated with nocodazole for an additional 16 h to arrest cells in mitosis (Fig. 2A). Subsequently, cells were released by removal of the inhibitor, and cell cycle progression into the ensuing G1 and S phases was monitored by BrdU incorporation for 10 h at 2 h intervals (Fig. 2B). The results clearly show that BrdU incorporation is initiated within 2 to 4 h after mitotic release of cells that were allowed to differentiate passively for 24 h or 48 h (Fig. 2B). Because onset of BrdU incorporation reflects S phase entry, our results indicate that the G1 phase in these hES cultures is less than 4 h. This finding is consistent with retention of an abbreviated G1 phase of 2.5 to 3 h as we previously reported (Becker et al, 2006). In contrast, cells that were cultured under differentiation conditions for 72 h exhibited incorporation of BrdU only at 10 h after release from mitotic block (Fig. 2B). Taken together, our results suggest that expedited initiation of S phase is restricted to the initial 48 h of differentiation while prolonged induction beyond this period lengthens the G1 phase of the cell cycle.
Figure 2. Extension of the G1 phase in human ES cells during early lineage-programming.
(A) Experimental design. (B) Human ES cells were cultured in differentiating media for 24 h, 48 h, and 72 h under feeder-free conditions (first, second and third rows, respectively). Cell cultures were then synchronized using nocodazole for an additional 16 h and subsequently released. BrdU incorporation in nuclei was detected by immunofluorescent microscopy (scale bar = 100 μm) using cells taken at the time of release (0 h) and at 2 h intervals for up to 10 h (first through sixth column, respectively). Cells were plated at a 1:1.5 split ratio (see Figure 3).
Lengthening of cell cycle duration during lineage-differentiation is cell density dependent
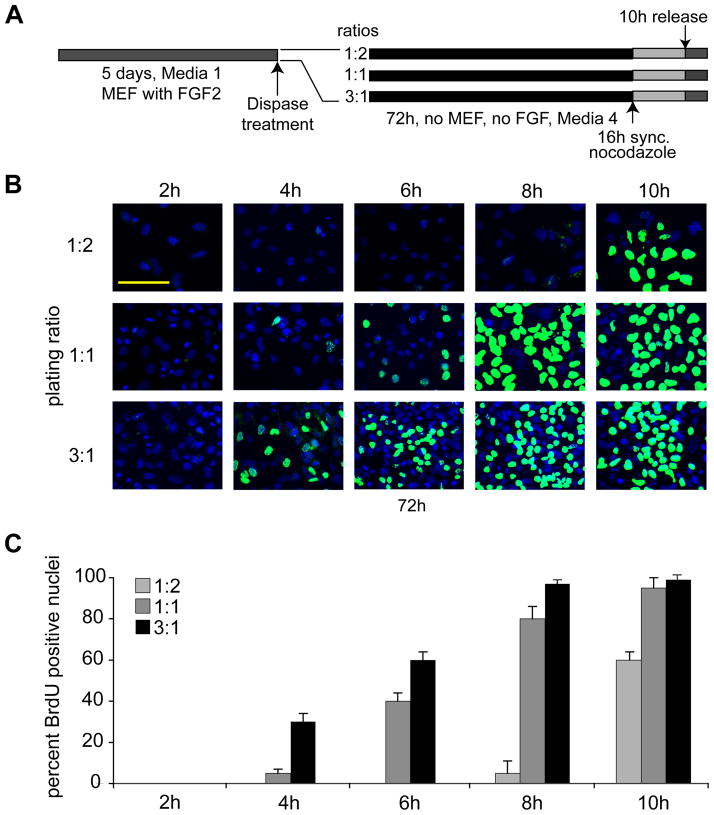
Differentiation of hES and cell cycle lengthening require two to three days. The gradual loss of competency for self-renewal may be related to autocrine and/or paracrine signaling (e.g., related to FGF2 production), or perhaps cell/cell contact. As an initial test of this hypothesis, we seeded hES cells at progressively increasing cell densities by dividing cultures at different ratios. Colonies from one well were split into two wells (1:2) or transferred into one well (1:1) or colonies from three wells were pooled into one (3:1) (Fig. 3A); we note that under our standard conditions, cells are propagated at a 1:1.5 ratio (see Fig. 2). As above, we maintained the resulting hES cultures for 72 h in feeder-free differentiation conditions (Medium 4) to induce early lineage commitment. Cells were synchronized using nocodazole for an additional 16 h to arrest cells at mitosis. Upon release from mitotic arrest, cell cycle progression was monitored by BrdU incorporation for 10 h at 2 h intervals (Fig. 3). Loss of pluripotency as reflected by the absence of Oct4 was established in a control group of asynchronous cells by immunofluorescence microscopy (data not shown; see Fig. 1). Human ES cells seeded at a starting density of 1:2 that were allowed to differentiate for 72 h do not exhibit prominent BrdU incorporation until after 10 h (Fig. 3B), consistent with results above (Fig. 2). However, cells seeded at a higher density (1:1) exhibit modest BrdU labeling at an earlier time (i.e., 6 h), while cells cultured at the highest density show initial BrdU staining at 4 h and robust staining by 6 h (Fig. 3). Thus, the length of G1 phase after 72 h in differentiating media is inversely proportional to the cell density at plating. This finding is consistent with the role of auto- and paracrine factors (e.g., FGF2) and/or cell-cell contact in promoting self-renewal and preserving the pluripotent state of hES cells.
Figure 3. Lengthening of the G1 phase in human ES cells depends on cell density.
(A) Experimental design. (B) Human ES cells were seeded in differentiating media under feeder-free and conditions at low (1:2), medium (1:1), and high density (3:1) for 72 h. These ratios reflect the split of the original culture. In a split of 1:2, colonies from one plate were divided into two, a 3:1 ratio means that colonies from three plates were pooled into one plate. At 72 h, cells were mitotically arrested using nocodazole for an additional 16 h, and cell cycle progression was monitored using immunofluorescence detection of BrdU incorporation at 2 h, 4 h, 6 h, 8 h, and 10 h after mitotic release (Scale bar = 100 μm). (C) Quantitation of the fraction of BrdU labeled nuclei at the indicated time points after culture at different plating densities (as reflected by split ratios). The quantitation is based on two independent experiments performed in duplicate in which 400 nuclei were counted (n=1600; error bars = SEM).
Autocrine signaling maintains the abbreviated self-renewal cell cycle of pluripotent human ES cells
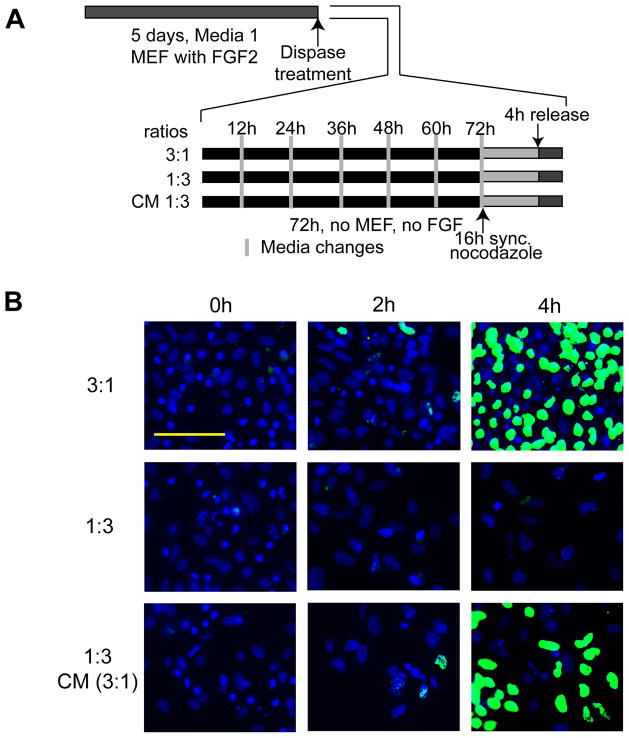
To assess the contribution of autocrine factors in the stem cell microenvironment, we performed experiments with conditioned medium. Human ES cells were seeded under lineage-committing conditions (Medium 4) for 72 h in ratios of either 3:1 (high-density) or 1:3 (low-density), respectively. Conditioned media from high-density cultures were replenished every 12 h after transfer to low-density cultures. After 72 h of conditioning, human ES cells were synchronized in mitosis by nocodazole treatment for 16 h. Subsequently, cells were released from mitotic block and BrdU incorporation was monitored at 2 h intervals to assess the initiation of S phase. As predicted from results presented in Figure 3, human ES cells plated at high density and cultured for 72 h in differentiating conditions (Medium 4), exhibit BrdU incorporation at 4 hr after release, while cells plated at low density do not (Fig. 4). However, human ES cells seeded at low density (1:3) and supplemented with conditioned media from high density cells (3:1) incorporate BrdU at 4 h after mitosis. Thus, autocrine factors secreted by hES cells produce a potent conditioned medium that is capable of maintaining the abbreviated G1 phase (~2.5 – 3 h) characteristic of pluripotent stem cells.
Figure 4. Medium conditioned by hES cells prevents lengthening of the G1 phase.
(A) Experimental design. (B) Human ES cells were plated without feeder layer at high density (3:1) (first row) and low density (1:3) (second and third rows), and maintained with differentiation media for 72 h. Media changes were applied every 12 h. Control cells grown in high density (first row) or low density (second row) received fresh media, but cells in grown in low density (1:3) (third row) received the conditioned medium (CM) supernatant of cells grown in high density (3:1, first row) every 12 h. After 72 h, cultures were synchronized for an additional 16 h using nocodazole. Upon release from mitotic block, BrdU incorporation was determined at 0 h, 2 h and 4 h to determine the duration of G1 and the onset of S phase (scale bar = 100 μm).
Human ES cells are pre-mitotically committed to enter S phase in the absence of external growth factors
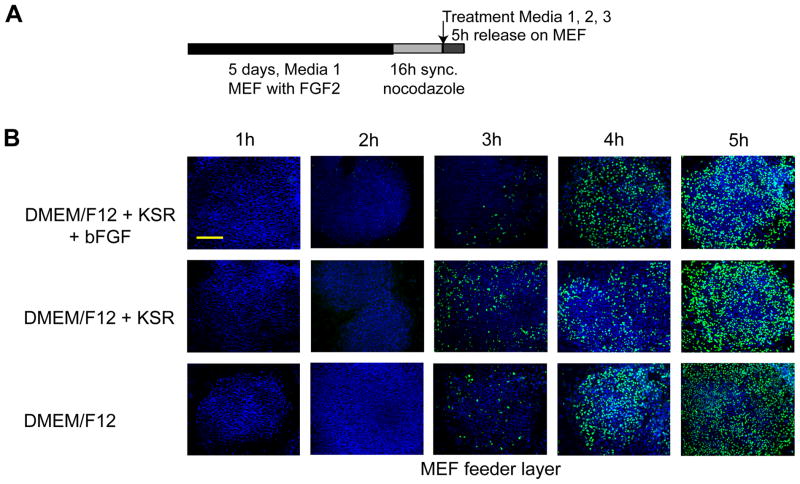
Previous studies (White et al, 2005) and our unpublished data indicate that proliferation of mammalian ES cells is independent of the pRB/E2F switch that operates at the R point and normally responds to growth factor stimulation to establish competency for cell cycle progression. Absence of R-point mechanisms could indicate that cell cycle progression from mitosis and G1 into S phase in hES cells may proceed without external factors. To test this important concept, we released mitotically synchronized hES cells into G1 under three different conditions where growth factor supplementation to the culture media was varied in either the presence (Fig. 5) or absence (Fig. 6) of inactivated MEF feeder layers. Entry into S phase was monitored using BrdU incorporation assays. The results show that H9 cells can progress from mitosis to S phase in 3 to 4 h under culture conditions in which external bFGF and/or knock-out serum replacer (a proprietary formulation of growth factors) are omitted, irrespective of whether a feeder layer is present (Fig. 5) or absent (Fig. 6). Hence, human ES cells appear to be pre-committed at mitosis to initiate a new round of DNA replication and cell division.
Figure 5. G1 phase progression in human ES cells is independent of exogenous FGF2.
(A) Experimental design. (B) Mitotically synchronized WA09/H9 cells were generated in standard hES medium (Medium 1) on a feeder layer of inactivated mouse embryo fibroblasts (MEFs) by mitotic inhibition using nocodazole for 16 h. Cells were then released from mitotic block and allowed to reenter the cell cycle in the presence of fresh complete DMEM/F12 medium (Medium 1) (first row), the same medium but without bFGF/FGF2 (Medium 2) (second row), or incomplete DMEM/F12 medium (Media 3) (third row). Cell cycle progression was examined at the indicated hourly time points after release (columns) by monitoring BrdU incorporation (scale bar = 200 μm).
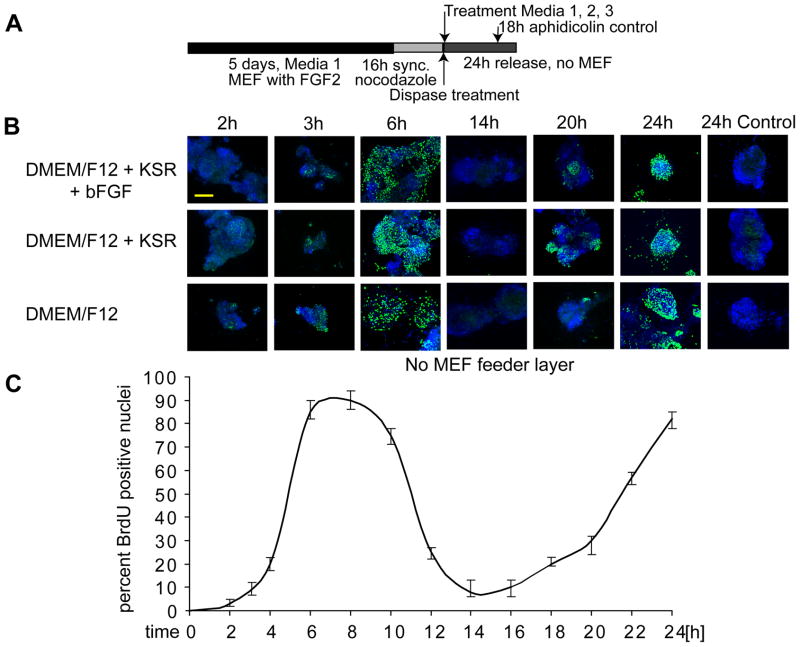
Figure 6. Human ES cells have the intrinsic ability to enter two consecutive S phases.
(A) Experimental design. (B) Human ES cells were synchronized using nocodazole and mitotically blocked colonies were separated from the inactivated MEF feeder layer by gentle mechanical disruption and dispase treatment. Colonies were then seeded on plastic coverslips (i.e., without feeder layer) and cultures were supplemented under different culture conditions: regular human ES cell media (Medium 1) (first row), medium in which FGF2 is omitted (Medium 2) (second row) and medium consisting of DMEM/F12 only (Medium 3) (third row). After adherence (< 1 h), BrdU incorporation was determined for multiple time-points at increasing temporal intervals as indicated (columns) to cover cell cycle progression through two consecutive cell cycles (up to 24 h). In parallel, we treated a control group of cells at18 h after mitotic release (i.e., after one cell cycle) with aphidicolin to prevent the initiation of a second S phase (24 h Control) (scale bar = 200 μm). The bottom panel shows a line graph that quantifies BrdU incorporation as a function of time after mitotic release and reflects the oscillating behavior expected from progression into two consecutive S phases. (C) Shows the quantification of results depicted in (B); the error-bars represent SEM for four independent determinations of 400 each (n=1600).
To investigate whether human ES cells are capable of sustaining a second S phase while maintaining their characteristic short cell cycle, we also determined BrdU incorporation in mitotically synchronized cells that were maintained for more than one cell cycle (Fig. 6). Our results show that cells are in G2 by 14 h (Fig. 6) and are expected to go through mitosis between 15–16 h, because this is the approximate cell cycle duration of ES cells (Becker et al, 2006). Strikingly, our results show that H9 cells can enter a new round of DNA synthesis during a second S phase in the absence of a feeder layer or external factors. Hence, hES cells can autonomously initiate at least two rounds of cell division in short term cultures.
DISCUSSION
Self-renewal of pluripotent human ES cells proceeds through a unique abbreviated cell cycle with a very short G1 phase (< 3 h). In this study, we first examined what biological conditions propel hES cells to acquire the lengthened cell cycle characteristics of somatic cells. One principal finding of the current work is that the G1 phase of hES cells is dramatically lengthened within 3 days after exposure to culture conditions that support early lineage programming. Cell cycle lengthening is observed when hES cultures are propagated at reduced cell densities in the absence of a MEF feeder layer, while increased cell plating densities preclude cell cycle lengthening. Supplying conditioned medium from high density cultures to low density cultures protects hES cells from cell cycle lengthening. Thus, our results show that autocrine mechanisms can sustain R-point independent self-renewal and suggest that pluripotency of hES cells is directly linked to an abbreviated cell cycle.
Human ES cells appear to lack a typical E2F/pRB mediated transition in late G1 that normally supports commitment to S phase entry at the Restriction point, similar to findings with mouse and primate ES cells (White et al, 2005; Fluckiger et al, 2006). The absence of an E2F/pRB switch in pluripotent cells predicts a mechanism that primes hES cells for S phase entry without the requirement for exogenously supplied factors. Importantly, we found that under serum-free conditions and in the absence of MEF feeder layers, human ES cells maintain the abbreviated self-renewal cell cycle for at least two consecutive mitotic divisions. This finding is consistent with the concept that naive human ES cells are epigenetically poised to expand through rapid self-renewal cell cycles.
Self-renewal and pluripotency of mammalian ES cells is preserved by culturing cells on feeder layers of inactivated mouse embryo fibroblasts and supplementation with FGF2 (Thomson et al, 1998; Amit et al, 2000). Mouse ES cells require the additional supplementation with Leukemia Inhibitory Factor (LIF) to retain pluripotent proliferation (Smith et al, 1988; Williams et al, 1988), although LIF is dispensable in human ES cells (Humphrey et al, 2004). These differences are based on distinctions in the signaling pathways required for preserving pluripotency. In human ES cells, noggin and FGF2 inhibit BMP signaling (Pera et al, 2004; Xu et al, 2002), and high levels of FGF2 and conditioned media support self-renewal in the absence of MEFs (Xu et al, 2005). To utilize human ES cells for replacement therapy, xenobiotic cross-contamination by animal-derived products must be eliminated. Ligand mixtures have been developed containing defined supplements including recombinant bFGF/FGF2, Wnt and Insulin that permit retention of pluripotency in long-term cultures (Lu et al, 2006). Our results show that human ES cells are able to maintain the abbreviated pluripotent self-renewal cell cycle for at least 60 hours (approximately four cell division cycles) in the absence of externally supplied ligands.
Pluripotent human ES cells form colonies in vitro comparable to the spatial arrangement of the inner cell mass of a blastocyst. Our data indicate that the abbreviated self-renewal cell cycle and short term autonomous growth require a critical cell density in culture. In contrast, human ES cells cultured at lower densities and in the periphery of colonies show early signs of lineage commitment. Density may promote pluripotency through direct cell-to-cell contacts, deposition of extracellular matrix components or secreted factors that diffuse only over a short range. Because feeder-layers produce FGF2 to preserve pluripotency (Xu et al, 2005), autocrine production of FGF2 by hES cells may delay the onset of cell cycle lengthening. Conversely, cell cycle lengthening may occur when local FGF2 concentrations become rate-limiting.
Our data demonstrate that human ES cells are poised for two consecutive rounds of cell division and enter a second S phase in the absence of external factors. The latter finding is consistent with the absence of a functional R point in human ES cells and independence of S phase commitment from the pRB mediated release of E2F. Human ES cells could perhaps sustain a limited amount of proliferation in the absence of external factors due to continued engagement of growth factors (e.g., FGF2) with cognate receptors or auxiliary proteoglycans at the cell surface (Levenstein et al, 2008; Levenstein et al, 2006; Diecke et al, 2008; Rider et al, 2008; Sasaki et al, 2008; Fluckiger et al, 2006). These cell surface interactions may resist the limited dispase digestion that is performed to re-plate cells after mitosis. However, these growth factor/receptor interactions would need to be sustained over at least two consecutive mitotic divisions. Therefore, we consider it more likely that hES cell cycle progression in short-term cultures is autonomous, at least in part due to the production of autocrine factors.
In conclusion, our studies show that the abbreviated cell cycle of human ES cells is biologically linked to the pluripotent state and that human ES cells are already pre-committed in mitosis to enter the next S phase in the absence of external stimuli.
Acknowledgments
Contract grant sponsor: NIH; Contract grant numbers: R01-CA139322, T32-DK007302 and DK32520. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
LITERATURE CITED
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- Becker KA, Ghule PN, Therrien JA, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Self-renewal of human embryonic stem cells is supported by a shortened G1 cell cycle phase. J Cell Physiol. 2006;209:883–893. doi: 10.1002/jcp.20776. [DOI] [PubMed] [Google Scholar]
- Becker KA, Stein JL, Lian JB, van Wijnen AJ, Stein GS. Establishment of histone gene regulation and cell cycle checkpoint control in human embryonic stem cells. J Cell Physiol. 2007;210:517–526. doi: 10.1002/jcp.20903. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV, Pardee AB. The restriction point of the cell cycle. Cell Cycle. 2002;1:103–110. [PubMed] [Google Scholar]
- Bodnar MS, Meneses JJ, Rodriguez RT, Firpo MT. Propagation and maintenance of undifferentiated human embryonic stem cells. Stem Cells Dev. 2004;13:243–253. doi: 10.1089/154732804323099172. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MK, Rosler E, Rao MS. Characterization and differentiation of human embryonic stem cells. Cloning Stem Cells. 2003;5:79–88. doi: 10.1089/153623003321512193. [DOI] [PubMed] [Google Scholar]
- Diecke S, Quiroga-Negreira A, Redmer T, Besser D. FGF2 signaling in mouse embryonic fibroblasts is crucial for self-renewal of embryonic stem cells. Cells Tissues Organs. 2008;188:52–61. doi: 10.1159/000121282. [DOI] [PubMed] [Google Scholar]
- Fluckiger AC, Marcy G, Marchand M, Negre D, Cosset FL, Mitalipov S, Wolf D, Savatier P, Dehay C. Cell cycle features of primate embryonic stem cells. Stem Cells. 2006;24:547–556. doi: 10.1634/stemcells.2005-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghule PN, Becker KA, Harper JW, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Cell cycle dependent phosphorylation and subnuclear organization of the histone gene regulator p220NPAT in human embryonic stem cells. J Cell Physiol. 2007;213:9–17. doi: 10.1002/jcp.21119. [DOI] [PubMed] [Google Scholar]
- Ghule PN, Dominski Z, Yang XC, Marzluff WF, Becker KA, Harper JW, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Staged assembly of histone gene expression machinery at subnuclear foci in the abbreviated cell cycle of human embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:16964–16969. doi: 10.1073/pnas.0809273105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey RK, Beattie GM, Lopez AD, Bucay N, King CC, Firpo MT, Rose-John S, Hayek A. Maintenance of pluripotency in human embryonic stem cells is STAT3 independent. Stem Cells. 2004;22:522–530. doi: 10.1634/stemcells.22-4-522. [DOI] [PubMed] [Google Scholar]
- Levenstein ME, Berggren WT, Lee JE, Conard KR, Llanas RA, Wagner RJ, Smith LM, Thomson JA. Secreted proteoglycans directly mediate human embryonic stem cell-basic fibroblast growth factor 2 interactions critical for proliferation. Stem Cells. 2008;26:3099–3107. doi: 10.1634/stemcells.2007-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenstein ME, Ludwig TE, Xu RH, Llanas RA, VanDenHeuvel-Kramer K, Manning D, Thomson JA. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 2006;24:568–574. doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Hou R, Booth CJ, Yang SH, Snyder M. Defined culture conditions of human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:5688–5693. doi: 10.1073/pnas.0601383103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur D, Danford TW, Boyer LA, Young RA, Gifford DK, Jaenisch R. Analysis of the mouse embryonic stem cell regulatory networks obtained by ChIP-chip and ChIP-PET. Genome Biol. 2008;9:R126. doi: 10.1186/gb-2008-9-8-r126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner A, Mikkelsen TS, Gu H, Wernig M, Hanna J, Sivachenko A, Zhang X, Bernstein BE, Nusbaum C, Jaffe DB, Gnirke A, Jaenisch R, Lander ES. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454:766–770. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP, Lee W, Mendenhall E, O’Donovan A, Presser A, Russ C, Xie X, Meissner A, Wernig M, Jaenisch R, Nusbaum C, Lander ES, Bernstein BE. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee AB. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974;71:1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee AB, Dubrow R, Hamlin JL, Kletzien RF. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Pera MF, Andrade J, Houssami S, Reubinoff B, Trounson A, Stanley EG, Ward-van OD, Mummery C. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci. 2004;117:1269–1280. doi: 10.1242/jcs.00970. [DOI] [PubMed] [Google Scholar]
- Rider DA, Dombrowski C, Sawyer AA, Ng GH, Leong D, Hutmacher DW, Nurcombe V, Cool SM. Autocrine fibroblast growth factor 2 increases the multipotentiality of human adipose-derived mesenchymal stem cells. Stem Cells. 2008;26:1598–1608. doi: 10.1634/stemcells.2007-0480. [DOI] [PubMed] [Google Scholar]
- Sasaki N, Okishio K, Ui-Tei K, Saigo K, Kinoshita-Toyoda A, Toyoda H, Nishimura T, Suda Y, Hayasaka M, Hanaoka K, Hitoshi S, Ikenaka K, Nishihara S. Heparan sulfate regulates self-renewal and pluripotency of embryonic stem cells. J Biol Chem. 2008;283:3594–3606. doi: 10.1074/jbc.M705621200. [DOI] [PubMed] [Google Scholar]
- Sato N, Sanjuan IM, Heke M, Uchida M, Naef F, Brivanlou AH. Molecular signature of human embryonic stem cells and its comparison with the mouse. Dev Biol. 2003;260:404–413. doi: 10.1016/s0012-1606(03)00256-2. [DOI] [PubMed] [Google Scholar]
- Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- Stojkovic M, Lako M, Strachan T, Murdoch A. Derivation, growth and applications of human embryonic stem cells. Reproduction. 2004;128:259–267. doi: 10.1530/rep.1.00243. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- White J, Stead E, Faast R, Conn S, Cartwright P, Dalton S. Developmental activation of the Rb-E2F pathway and establishment of cell cycle-regulated cyclin-dependent kinase activity during embryonic stem cell differentiation. Mol Biol Cell. 2005;16:2018–2027. doi: 10.1091/mbc.E04-12-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- Xu RH, Chen X, Li DS, Li R, Addicks GC, Glennon C, Zwaka TP, Thomson JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- Xu RH, Sampsell-Barron TL, Gu F, Root S, Peck RM, Pan G, Yu J, Antosiewicz-Bourget J, Tian S, Stewart R, Thomson JA. NANOG is a direct target of TGFbeta/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell. 2008;3:196–206. doi: 10.1016/j.stem.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]