Abstract
The transition from acute to chronic graft versus host disease (GVHD) is characterized by the progressive loss of self tolerance and the development of autoimmune manifestations. Interleukin 17 (IL-17) is a proinflammatory cytokine that has been shown to play a prominent role in autoimmune disorders in the nontransplant setting, but the extent to which IL-17 is necessary for the autoimmunity that occurs as a consequence of GVHD is not known. In this study, we demonstrate using a combination of antibody-based and genetic approaches that IL-17 is not required for the loss of self tolerance and resulting CD4+ T cell-dependent pathological damage which occurs during the evolution from acute to chronic GVHD. Rather, TH1 cells and other proinflammatory cytokines are fully competent to promote autoimmune-mediated tissue damage. Thus, the selective targeting of IL-17 may not be a viable clinical strategy for preventing the autoimmune manifestations that develop during chronic GVHD.
INTRODUCTION
Graft versus host disease (GVHD) is characterized by the over production of inflammatory cytokines that mediate tissue damage, activate secondary effector cells and induce the differentiation of proinflammatory T cells from naïve T cells [1-4]. Interleukin 17 (IL-17) is one such inflammatory cytokine that has been identified in the serum and tissues of allograft recipients with GVHD [5,6], although the extent to which this cytokine is required for the induction of pathology during GVHD remains unresolved. Recent studies have demonstrated that transplantation of a highly purified TH17 cell population can cause acute GVHD-related pathological damage with tissue predilection for the skin and lung [7]. However, studies using IL-17-deficient mice as donors in transplantation experiments have yielded divergent results with respect to whether IL-17 has protective or deleterious effects during acute GVHD [6,8]. The role of IL-17 in chronic GVHD where autoimmune-like manifestations are a defining characteristic of the disease [9-11], has not been examined even though IL-17 has been directly implicated in the pathophysiology of other autoimmune disorders [12,13]. In previous studies, we [5] and others [14], using different murine models, have demonstrated that TH17 cells are present in target organs of animals that develop GVHD-associated autoimmune-mediated pathological damage. However, whether IL-17 is required for the induction of pathology or whether this cytokine is dispensable was not resolved. In the current report, we employed a previously described BMT model [5,15] that is characterized by loss of self tolerance and the development of autoimmunity that are hallmarks of chronic GVHD in human allogeneic stem cell transplant recipients [9-11] to examine whether IL-17 is required for the development of autoimmune-mediated pathological damage.
METHODS
Mice
C57BL/6 (B6) (H-2b), Balb/cJ (H-2d) and B6.129S7-Rag 1 (B6 Rag) mice were bred in the Animal Resource Center at the Medical College of Wisconsin (MCW) or purchased from Jackson Laboratories (Bar Harbor, ME). IL-17 deficient mice (IL-17−/−) on a B6 background were obtained from Dr. Yoichiro Iwakura (University of Tokyo, Tokyo, Japan). All animals were housed in the Association for the Assessment and Accreditation of Laboratory Animal Care-accredited Animal Resource Center of the MCW. Experiments were all carried out under protocols approved by the MCW Institutional Animal Care and Use Committee. Mice received regular mouse chow and acidified tap water ad libitum.
Bone Marrow Transplantation
Bone marrow (BM) was flushed from donor femurs and tibias with Dulbecco's modified media (DMEM) and passed through sterile mesh filters to obtain single cell suspensions. Host mice were conditioned with total body irradiation administered as a single exposure at a dose rate of 67 cGy using a Shepherd Mark I Cesium Irradiator (J.L. Shepherd and Associates, San Fernando, CA). Irradiated recipients received a single intravenous injection in the lateral tail vein of BM and spleen cells in a total volume of 0.4 ml. In adoptive transfer experiments, nonirradiated B6 Rag mice received splenocytes from previously irradiated, fully donor-engrafted B6→Balb/c chimeras 19-21 days post transplantation. Typically, 30-40% of transferred spleen cells were comprised of donor T cells.
Flow Cytometry
Monoclonal antibodies (mAb) conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), phycoerythrin-Cy5 (PE-Cy5) were used to assess cell populations and were purchased from BD Biosciences Pharmingen (San Diego, CA). For intracellular staining, lymphocytes isolated from spleen, liver and colon were stimulated with 50 ng/ml PMA (Sigma, St Louis, MO) and 750 ng/ml ionomycin (Calbiochem, La Jolla, CA) for 2 1/2 hours and then incubated with GolgiStop (BD Pharmingen) for an additional 2 1/2 hours. Cells were analyzed on a FACSCalibur flow cytometer with Cellquest software (Becton-Dickenson). Data were analyzed using FlowJo (Treestar, Ashland, Oregon).
Reagents
Anti-IL-17 antibody (MM17F3) is a mouse IgG1 that has been previously described [16,17]. Animals received 200 micrograms of antibody twice weekly by intraperitoneal injection. Antibody was re-suspended in phosphate-buffered saline (PBS) prior to injection. Mouse IgG1 (Sigma, St Louis, MO) was used as a control and administered at the same dose and schedule as MM17F3.
Histological Analysis
Representative samples of liver, colon and lung were obtained from transplanted recipients and fixed in 10% neutral-buffered formalin. Samples were then embedded in paraffin, cut into five micron thick sections and stained with hematoxylin and eosin. A semiquantitative scoring system was employed to account for histological changes in the colon, liver and lung as previously described [18]. All slides were coded and read in a blinded fashion. Images were visualized with an Olympus BX45 microscope (Tokyo, Japan). Image acquisition was performed with an Olympus DP70 digital camera and software package.
Serum Cytokine Analysis
Serum was collected from mice by retroorbital bleeds and analyzed on a Bioplex System (BioRad Laboratories, Hercules, CA) according to the manufacturer's instructions. All samples were run in duplicate.
Cell Isolation
To isolate lamina propria lymphocytes (LPLs), pooled colons were incubated with EDTA for 30 minutes at 37°C and cells were then passed through a 100 micron strainer to remove cellular debris. The remaining colon tissue was cut into smaller pieces and incubated in PBS containing 2% fetal bovine serum and 0.15 mg/ml collagenase D (Roche Diagnostics, Mannheim, Germany) for 75 minutes at 37°C. The cell suspension was then filtered through a 100 micron strainer and layered on a 44%/67% Percoll gradient. Liver lymphocytes were isolated by collagenase D digestion followed by layering on a Percoll gradient.
Statistics
Group comparisons of T cell populations, cytokine levels, and pathology scores were performed using the Mann Whitney U test or student t test. A p value ≤ 0.05 was deemed to be significant in all experiments.
RESULTS AND DISCUSSION
Blockade of IL-17 signaling has no protective effect on the autoimmune-mediated pathological damage that occurs during chronic GVHD
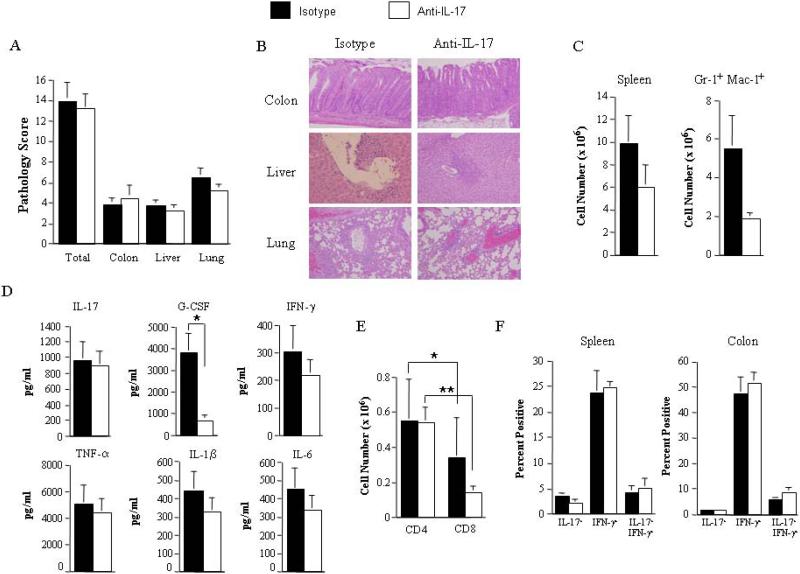
To define the role of IL-17 in chronic GVHD, lethally irradiated Balb/c mice were first transplanted with B6 BM along with 0.4-0.5 × 106 B6 spleen cells to induce acute GVHD. Approximately 19-21 days post transplantation, spleen cells (0.45-0.5 × 106) from fully donor-engrafted animals were adoptively transferred into nonirradiated syngeneic B6 Rag mice. These mice typically develop autoimmune-mediated tissue damage in the colon, liver and lung within 45-60 days. Cohorts of these animals were treated with either isotype control or anti-IL-17 antibody twice weekly beginning at the time of transfer. This antibody administration schedule was based on published studies which showed that this dose and schedule was sufficient to protect animals from developing experimental allergic encephalomyelitis [16]. All mice survived the duration of these studies and were evaluable for analysis. Examination of target organ tissues revealed no significant differences in pathology scores in the colon, liver or lung in mice treated with isotype versus anti-IL-17 antibody (Figures 1A and 1B). Furthermore, there was no statistically significant difference in overall spleen cellularity and myeloid expansion (Figure 1C) which are hallmarks of autoimmunity in this model [15]. Examination of serum proinflammatory cytokine levels also revealed no differences with the exception of G-CSF which was significantly reduced in animals treated with anti-IL-17 antibody consistent with the previously described effects of IL-17 on granulopoiesis [19-21] (Figure 1D). Studies were then done to determine whether blockade of IL-17 affected the generation of TH1 and TH17 cells in the spleen and colon of these same animals since pathological damage in this model is CD4+ T cell dependent [5]. The absolute number of splenic CD4+ T cells, although significantly increased relative to CD8+ T cells, did not differ between groups (Figure 1E). Moreover, no difference was observed in the percentage of CD4+IL-17+ or CD4+IFN-γ+ T cells in either the spleen or the colon of anti-IL-17 versus isotype antibody-treated mice (Figure 1F), indicating that anti-IL-17 antibody administration did not appear to alter the differentiation of naïve T cells into IL-17 or IFN-γ-secreting CD4+ T cells. The fact that antibody administration did not result in a decrease in the absolute numbers of TH17 cells was not unexpected given previous data which has shown that TGF-β and IL-6, but not IL-17 itself, are required for the differentiation of naïve T cell into TH17 cells [22,23].
Figure 1. Blockade of IL-17 signaling has no protective effect on the development of autoimmunity during chronic GVHD.
Lethally irradiated (900 cGy) Balb/c mice were transplanted with B6 bone marrow (BM) (10 × 106) plus 0.4-0.5 × 106 B6 spleen cells. On day 19-21 post transplantation, mice were sacrificed and spleen cells from fully donor-engrafted animals (0.45-0.5 × 106) were transferred into nonirradiated B6 Rag mice. Cohorts of B6 Rag animals were then treated with either IgG1 isotype control or anti-IL-17 antibody (200 micrograms/dose) twice weekly. Mice were sacrificed 48-75 days post transfer. (A). Pathological damage in the colon, liver and lung of mice treated with isotype control (■, n=12) or anti-IL-17 antibody (□, n=12) using a semiquantitative scoring system as detailed in Methods. (B). Histology of colon, liver and lung from representative recipients treated with either isotype or anti-IL-17 antibody. In isotype control animals, colon shows inflammation in the lamina propria, and goblet cell depletion, liver reveals portal triad inflammation with mononuclear cells and endothelialitis, and lung demonstrates perivascular cuffing with mononuclear cells. In anti-IL-17 antibody-treated mice, colon also shows extensive inflammation in the lamina propria, goblet cell depletion and crypt cell destruction, liver has portal triad inflammation and lung demonstrates perivascular cuffing. (C). Total cellularity and absolute number of Gr-1+ Mac-1+ cells in the spleen are depicted. (D). Serum proinflammatory cytokine levels from B6 Rag mice treated with either isotype (n=9) or anti-IL-17 antibody (n=9) immediately prior to sacrifice of animals. (E). Absolute number of CD4+ and CD8+ T cells in the spleens of mice administered isotype (n=9) or anti-IL-17 antibody (n=9) 48-69 days post transfer. (F). Spleens and colon tissue from animals that received either control or anti-IL-17 antibody (n=3/group) were pooled and the percentage of CD4+ T cells secreting IL-17, IFN-γ or both cytokines in each organ is shown. Data are presented as the mean ± SEM and are the cumulative results from three to four independent experiments for all studies depicted. Statistics: **p ≤ 0.01; * p ≤ 0.05.
Autoimmune-mediated pathology is not abrogated in the absence of IL-17 secreted by donor-derived CD4+ T cells
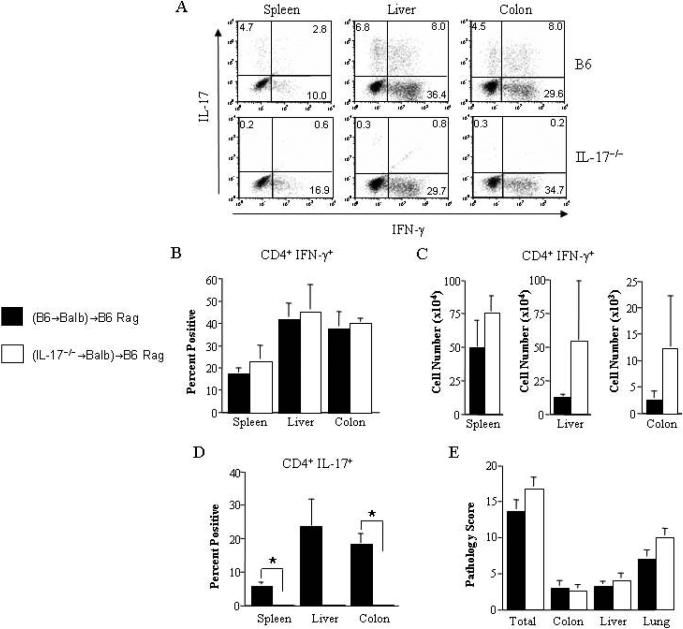
To confirm these findings, we employed a complementary approach to examine this question using mice that were incapable of secreting IL-17 as donors in adoptive transfer studies. In these experiments, lethally irradiated Balb/c mice were transplanted with either B6 or IL-17−/− BM and spleen cells to induce acute GVHD. Approximately three weeks post transplantation, spleen cells from donor-engrafted B6→Balb or IL-17−/−→Balb chimeras were transferred into nonirradiated B6 Rag mice. We observed that CD4+IFN-γ+ T cells were present in both groups of mice, although there was no statistically significant difference in the percentage (Figures 2A and 2B) or absolute number (Figure 2C) of these cells in the spleen, liver or colon. In contrast, examination of these tissues in Rag animals that received splenocytes from B6→Balb chimeras mice revealed defined populations of CD4+IL-17+ T cells in all sites, whereas these cells were essentially absent in mice reconstituted with splenocytes from IL-17−/−→Balb chimeras (Figures 2A and 2D). The absence of CD4+IL-17+ T cells, however, did not abrogate pathological damage, as individual tissue-specific pathology scores were similar in both groups of mice (Figure 2E).
Figure 2. Absence of donor-derived IL-17 has no protective effect on the development of autoimmunity.
Lethally irradiated Balb/c mice were transplanted with BM (10 × 106) and spleen cells (0.4 × 106) from wild type B6 or IL-17−/− animals to induce GVHD. Mice from each group were sacrificed 19-21 days after transplantation and pooled spleen cells (0.5 × 106) were transferred into non irradiated B6 Rag mice. (A). Representative dot plot depicting the percentage of IL-17 and/or IFN-γ-secreting cells within the gated CD4+ T cell population obtained from the spleen, liver or colons of mice 60-65 days after transfer of spleen cells from B6→Balb/c or IL-17−/−→Balb/c chimeras. (B,C). Individual spleens, liver and colon tissue from B6 Rag mice that received adoptive transfer of spleen cells from B6→Balb/c (■) or IL-17−/−→Balb/c (□) chimeras (n=2-4/group) were pooled and the percentage (panel B) and absolute number (panel C) of CD4+ T cells secreting IFN-γ in each organ is depicted. Data are the cumulative results from three independent experiments. Each experiment had the same number of mice per group. (D). Individual spleens, livers and colon tissue from B6 Rag mice that received adoptive transfer of spleen cells from B6→Balb/c or IL-17−/−→Balb/c chimeras (n=2-4/group) were pooled and the percentage of CD4+ T cells secreting IL-17 in each organ is depicted. (E). Pathological damage in the colon, liver and lung of mice 60-65 days after adoptive transfer of spleen cells from B6→Balb/c (n=9) or IL-17−/−→Balb/c (n=9) chimeras. Data are the cumulative results from three independent experiments. Data are presented as the mean ± SEM. Statistics: * p ≤ 0.05.
In summary, using a combination of antibody-based and genetic approaches, these studies demonstrate that IL-17 is not required for the induction of autoimmune-mediated pathological damage that is a defining characteristic of chronic GVHD. These results support recent studies by Yi and colleagues [6] who observed that the absence of donor-derived IL-17 had no protective effect during acute GVHD and actually exacerbated the disease. Our data are also consistent with work by Imanguli et al implicating TH1 cells as major effectors in human chronic GVHD [23]. In this study, the authors demonstrated the presence of Tbet+ T cells with expression of cytotoxic molecules such as granzyme B in the oral lesions of patients with chronic GVHD and concluded that a TH1-mediated process appeared to be primarily responsible for pathological damage. Finally, these studies suggest that a clinical strategy designed to specifically target IL-17 is unlikely to significantly modulate the severity of chronic GVHD as remaining TH1 cells and other proinflammatory cytokines remain fully capable of inducing pathological damage.
ACKNOWLEDGMENTS
This research was supported by grants from the National Institutes of Health (HL064603 and HL081650) and by an award from the Midwest Athletes Against Childhood Cancer Fund (Milwaukee, WI).
This research was supported by grants from the National Institutes of Health (HL64603 and 081650) and by awards from the Midwest Athletes Against Childhood Cancer Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Ann Rev Immunol. 2007;25:139–170. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 2.Piguet P-F, Grau GE, Allet B, Vassalli P. Tumor necrosis factor/cachectin is an effector of the skin and gut lesions of the acute phase of graft versus host disease. J Exp Med. 1987;166:1280–1289. doi: 10.1084/jem.166.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nestel FP, Price KS, Seemayer TA, Lapp WS. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor α during graft versus host disease. J Exp Med. 1992;175:405–413. doi: 10.1084/jem.175.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, Das R, Komorowski R, Beres A, Hessner MJ, Mihara M, Drobyski WR. Blockade of interleukin 6 signaling augments regulatory T cell reconstitution and attenuates the severity of graft versus host disease. Blood. 2009;114:891–900. doi: 10.1182/blood-2009-01-197178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Vodanovic-Jankovic S, Johnson B, Keller M, Komorowski R, Drobyski WR. Absence of regulatory T cell control of TH1 and TH17 cells is responsible for the autoimmune-mediated pathology in chronic graft versus host disease. Blood. 2007;110:3804–3813. doi: 10.1182/blood-2007-05-091074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi T, Zhao D, Lin CL, et al. Absence of donor TH17 leads to augmented TH1 differentiation and exacerbated acute graft versus host disease. Blood. 2008;112:2101–2110. doi: 10.1182/blood-2007-12-126987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson MJ, West ML, Coghill JM, Panoskaltsis-Mortari A, Blazar BR, Serody JS. In vitro differentiated TH17 cells mediate lethal acute graft versus host disease with severe cutaneous and pulmonary pathologic manifestations. Blood. 2009;113:1365–1374. doi: 10.1182/blood-2008-06-162420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kappel LW, Goldberg GL, King CG, et al. IL-17 contributes to CD4-mediated graft versus host disease. Blood. 2009;113:945–952. doi: 10.1182/blood-2008-08-172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of health Consensus Development Project on criteria for clinical trails in chronic graft versus host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–955. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Rouquette-Gally AM, Boyeldieu D, Gluckman E, Abuaf N, Combrisson A. Autoimmunity in 28 patients after allogeneic bone marrow transplantation: comparison with Sjogren's syndrome and scleroderma. Br J Haematol. 1987;66:45–47. doi: 10.1111/j.1365-2141.1987.tb06888.x. [DOI] [PubMed] [Google Scholar]
- 11.Rouquette-Gally AM, Boyeldieu D, Prost AC, E. Gluckman E. Autoimmunity after allogeneic bone marrow transplantation. A study of 53 long-term-surviving patients. Transplantation. 1988;46:238–240. doi: 10.1097/00007890-198808000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17 deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 13.Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 14.Zhao D, Zhang C, Yi T, et al. In vivo activated CD103+ CD4+ regulatory T cells ameliorate ongoing chronic graft versus host disease. Blood. 2008;112:2129–2138. doi: 10.1182/blood-2008-02-140277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tivol EA, Komorowski R, Drobyski WR. Emergent autoimmunity in graft versus host disease. Blood. 2005;105:4887–4893. doi: 10.1182/blood-2004-12-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uyttenhove C, Van Snick J. Development of an anti-IL-17A auto-vaccine that prevents experimental auto-immune encephalomyelitis. Eur J Immunol. 2006;36:2868–2874. doi: 10.1002/eji.200636662. [DOI] [PubMed] [Google Scholar]
- 17.Uyttenhove C, Sommereyns C, Theate I, Michiels T, van Snick J. Anti-IL-17A autovaccination prevents clinical and histological manifestations of experimental autoimmune encephalomyelitis. Ann NY Acad Sci. 2007;1110:330–336. doi: 10.1196/annals.1423.035. [DOI] [PubMed] [Google Scholar]
- 18.Das R, Chen X, Komorowski R, Hessner MJ, Drobyski WR. Interleukin 23 secretion by donor antigen presenting cells is critical for organ-specific pathology in graft versus host disease. Blood. 2009;113:2352–2362. doi: 10.1182/blood-2008-08-175448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fossiez F, Djossou O, Chomarat P, et al. T cell interleukin 17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarzenberger P, La Russa V, Miller A, et al. IL-17 stimulates granulopoiesis in mice: Use of an alternate novel gene therapy-derived method for in vivo evaluation of cytokines. J Immunol. 1998;161:6383–6389. [PubMed] [Google Scholar]
- 21.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17 produced by lymphocytes and neutrophils is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170:2106–2112. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- 22.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 23.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGF in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Imanguli M, Swaim WD, League SC, et al. Increased Tbet+ cytotoxic effectors and type 1 interferon-mediated processes in cGVHD of the oral mucosa. Blood. 2009;113:3620–3630. doi: 10.1182/blood-2008-07-168351. [DOI] [PMC free article] [PubMed] [Google Scholar]