Abstract
Sexual dimorphism in skeletal muscle mass is apparent, with men having more muscle mass and larger individual muscle cells. However, no sex-based differences have been detected in blood forearm phenylalanine turnover, although whole body leucine oxidation has been reported to be greater in men than in women. We hypothesized that sex differences in intracellular amino acid turnover may account for these discrepancies, with men having a higher intracellular turnover than women. We studied young, healthy women (women, n = 8) and men (men, n = 10) following an overnight fast. Phenylalanine, leucine, and alanine muscle intracellular kinetics were assessed using stable isotope methodologies, femoral arteriovenous blood sampling, and muscle biopsies. Muscle intracellular amino acid kinetics were reported relative to both leg volume and lean leg mass because of sex differences in leg volume and in muscle and fat distribution. When expressed per leg volume (nmol · min−1 100 ml leg volume−1), phenylalanine net balance (women: −16 ± 4, men: −31 ± 5), release from proteolysis in the blood (women: 46 ± 9, men: 75 ± 10) and intracellular availability (women: 149 ± 23, men: 241 ± 35), and alanine production, utilization, and intracellular availability were higher in men (P < 0.05). However, when the kinetic parameters were normalized per unit of lean leg mass, all differences disappeared. Muscle fractional synthetic rate was also not different between women and men. We conclude that there are no sex-based differences in basal muscle intracellular amino acid turnover when the data are normalized by lean mass. It remains to be determined if there are sex differences in intracellular amino acid metabolism following anabolic or catabolic stimuli.
Keywords: protein metabolism, muscle, sex, stable isotopes, gender
Sexual differences in human skeletal muscle mass are apparent. Muscle fiber type distribution is not different between women and men; however, cross-sectional area is larger for all fiber types in men (27, 35). Interestingly, the largest muscle fibers in men appear to be fast-twitch type IIa fibers, whereas in women the slow-twitch type I fibers have the largest cross-sectional area (27). Furthermore, during short-term limb immobilization, men and women have a similar reduction in muscle cell size, although women have larger reductions in strength (35).
The sexual dimorphism in muscle mass is mainly attributed to endocrine differences between women and men because testosterone concentrations are usually ~10-fold higher in men (35). It has been shown that castration reduces muscle mass while testosterone administration at replacement or supraphysiological doses increases muscle mass in hypogonadal (3, 5) and normal men (2), respectively. These effects are likely due to a stimulation of muscle protein synthesis by testosterone (7).
Muscle mass and cell size is determined by the balance between muscle protein synthesis and muscle protein breakdown (i.e., muscle protein turnover), with net deposition of protein occurring when the synthesis rates exceed the breakdown rates (23). When basal postabsorptive muscle protein turnover was measured in men and women using the traditional two-pool arteriovenous (AV) isotope dilution technique across the forearm, estimates of muscle protein synthesis and breakdown were reported to be higher in men (9), suggesting a greater muscle plasticity. A greater basal plasticity in men would favorably affect the response to anabolic stimuli, in terms of both speed and magnitude of the response. However, because adult women have an increased proportion of peripheral fat mass, when the kinetic data were expressed per amount of forearm muscle, rather than forearm volume, these differences disappeared (9). Nonetheless, several investigators have reported significant differences in whole body leucine kinetics, with men exhibiting higher rates of leucine oxidation both at rest and during aerobic exercise, even after correction of the data by lean body mass (13, 14, 16, 19, 30). Because leucine is mainly oxidized in the muscle, a higher leucine oxidation indicates a greater net muscle amino acid/protein catabolism in men, which contrasts with the muscle kinetic data previously reported (9).
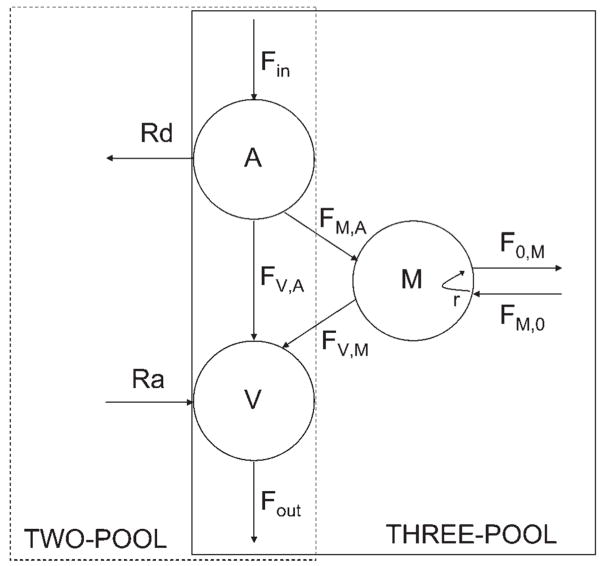
One possible explanation for these discrepancies is that the traditional two-pool AV model used to estimate muscle protein breakdown and synthesis cannot detect the actual rates of muscle protein synthesis and breakdown occurring inside the muscle cells (Fig. 1) because it measures blood amino acid kinetics, i.e., disappearance of amino acids from the blood in the muscle (Rd) and appearance in the blood of amino acids released from breakdown (Ra). However, it cannot measure differences in intracellular amino acid utilization for synthesis and oxidation if the amino acids directly derive from protein breakdown without first appearing in the circulation. Thus it is possible that men have an overall greater muscle protein turnover and/or a lower intracellular amino acid recycling into synthesis with a consequent increased oxidation of amino acids directly deriving from proteolysis. This hypothesis can be tested using a three-pool AV model, which expands upon the two-pool model, and enables the measurement of amino acid kinetics in the intracellular muscle pool. Additionally, it is also possible to determine differences in muscle protein synthesis by directly measuring the incorporation of labeled amino acids into proteins using the precursor-product method (i.e., fractional synthetic rate of muscle proteins), which is independent of muscle size or body composition.
Fig. 1.
Two- and three-pool models of leg amino acid kinetics. Free amino acid pools in femoral artery (A), femoral vein (V), and muscle (M) are connected by arrows indicating unidirectional amino acid flow between each compartment. With both models, amino acids enter the leg via the femoral artery (Fin) and leave the leg via the femoral vein (Fout). Two-pool model: Rd, rate of amino acid disappearance (an estimate of muscle protein synthesis when using the phenylalanine tracer, and synthesis and oxidation when using the leucine and alanine tracers); Ra, rate of amino acid appearance in blood from breakdown (with phenylalanine and leucine), or breakdown and de novo synthesis (with alanine). Three-pool model: FV,A, direct amino acid flow from artery to vein without entering intracellular pool; FM,A and FV,M, inward and outward amino acid transport from artery to muscle and from muscle to vein, respectively; FM,0, rate of intracellular appearance from proteolysis (with phenylalanine and leucine), or proteolysis and de novo synthesis (with alanine); F0,M, intracellular amino acid utilization for protein synthesis (with phenylalanine), or synthesis and oxidation (with leucine and alanine); r, amino acid recycling from breakdown into synthesis (with phenylalanine).
Thus we tested whether basal intracellular amino acid turnover is greater and/or intracellular amino acid recycling into synthesis is lower in men then in women by measuring the kinetics of two essential (phenylalanine, leucine) and one nonessential amino acid (alanine) and muscle protein synthesis in the skeletal muscle of young women and men using three different models.
SUBJECTS AND METHODS
Subjects
We studied 18 young subjects (8 women and 10 men) from the Los Angeles metropolitan area. All subjects were healthy and physically active, but they were not engaged in an exercise training program. All subjects had a stable body weight for the past 3 mo before the experiment. All women were studied in the early follicular phase since it has been reported that progesterone may affect protein kinetics as it increases whole body leucine flux and oxidation (15). Screening of subjects was performed with clinical history, physical exam, and laboratory tests, including complete blood count with differential, liver, and kidney function tests, coagulation profile, fasting blood glucose and oral glucose tolerance test, hepatitis B and C screening, human immunodeficiency virus test, thyroid-stimulating hormone (thyrotropin), lipid profile, pregnancy test in women, urinalysis, drug screening, and electrocardiogram. Only subjects with normal screening results were admitted to the experiment. The subjects’ characteristics are summarized in Table 1.
Table 1.
Physical characteristics of the women and men
| Subjects’ Characteristics | Women | Men | P Value |
|---|---|---|---|
| No. of subjects | 8 | 10 | |
| Age, yr | 26±2 | 27± 2 | 0.59 |
| Height, m | 1.61±0.03 | 1.75±0.02 | 0.001 |
| Body wt, kg | 60±5 | 75±4 | 0.03 |
| Body mass index, kg/m2 | 23.0±1.3 | 24.5±1.3 | 0.40 |
| Lean body mass, kg | 41±3 | 57±2 | < 0.001 |
| Fat mass, kg | 17±2 | 14±3 | 0.50 |
| Leg volume, liters | 8.4±0.7 | 9.7±0.5 | 0.14 |
| Lean leg mass, kg | 6.7±0.6 | 9.7±0.3 | < 0.001 |
| Leg fat mass, kg | 3.4±0.4 | 2.3±0.4 | < 0.001 |
Values are means ± SE.
All subjects gave informed written consent before participating in the study, which was approved by the Institutional Review Board of the University of Southern California (Los Angeles, CA).
Study design
The protocol was designed to measure muscle protein and amino acid kinetics in the postabsorptive basal state. The night before the study, each subject was admitted to the General Clinical Research Center of the University of Southern California. At admission, a pregnancy test was repeated in women, and a dual-energy X-ray absorptiometry (DEXA) scan (model QDR 4500W; Hologic, Bedford, MA) was performed to measure lean body mass and lean leg mass (25). The subjects were then fed a standard dinner, and a snack was given at 2200. After 2200, the subject was allowed only water ad libitum until the end of the experiment. The morning of the study, polyethylene catheters were inserted in a forearm vein for tracer infusion, in a contralateral hand or wrist vein for arterialized blood sampling, and in the femoral artery and vein of one leg for blood sampling. The arterial catheter was also used for the infusion of indocyanine green (ICG; Akorn, Buffalo Grove, IL).
At ~0730, after drawing a blood sample for the measurement of background amino acid enrichments and ICG concentration, a primed continuous infusion of L-[ring-13C6]phenylalanine, L-[5,5,5-2H3]leucine and L-[1-13C]alanine (Cambridge Isotope Laboratories, Andover, MA) was started and maintained at a constant rate until the end of the experiment. The following priming doses (PD) and infusion rates (IR) were used: L-[ring-13C6]phenylalanine: PD = 2 μmol/kg, IR = 0.05 μmol · kg−1 · min−1; L-[5,5,5- 2H3]leucine: PD = 4.8 μmol/kg, IR = 0.08 μmol · kg−1 · min−1; L-[1-13C]alanine: PD = 35 μmol/kg, IR = 0.35 nmol · kg−1·min−1.
At 1 h, the first muscle biopsy was taken from the lateral portion of the vastus lateralis of the leg with the femoral catheters, using a 5-mm Bergström biopsy needle, sterile procedure, and local anesthesia with 1% lidocaine injected subcutaneously and on the fascia. The muscle sample was rinsed with ice-cold saline and blotted, any visible fat or connective tissue was quickly removed, and it was immediately frozen in liquid nitrogen and stored at −80°C until analysis. Unpublished data from our laboratory indicate that the time from biopsy to freezing does not affect the tissue amino acid enrichment if the time frame is contained within a few seconds.
Ten minutes prior to 3 h, the continuous infusion of ICG was started in the femoral artery (0.5 mg/min) and maintained for 40 min. During ICG infusion, blood samples were taken four times, at 10-min intervals, from the femoral vein and the hand vein to measure ICG concentration. Subsequently, during the next 30 min, four blood samples were taken from the femoral artery and vein to measure femoral arterial and venous amino acid and glucose concentrations and enrichments and insulin concentrations. At 4 h, a second muscle biopsy was taken as previously described. The blood draws and the last biopsy for the measurement of intracellular muscle amino acid kinetics were taken ~12 h after the last meal.
Analysis
Serum ICG concentration for the determination of leg blood flow was measured spectrophotometrically (Beckman Coulter, Fullerton, CA) at λ = 805 nm (10, 11).
Concentrations and enrichments of blood phenylalanine, leucine, and alanine were determined on their tert-butyldimethylsilyl derivatives using appropriate internal standards (L-[15N]phenylalanine, L-[2H10]leucine, L-[2H4]alanine) and gas chromatography/mass spectrometry (GCMS; 6890 Plus GC, 5973N MSD/DS, 7683 autosampler; Agilent Technologies, Palo Alto, CA) as previously described (33).
Muscle tissue samples were ground, and intracellular free amino acids and muscle proteins were extracted as previously described (33). Intracellular free concentrations and enrichments of phenylalanine, leucine, and alanine were determined by GCMS using appropriate internal standards (L-[15N]phenylalanine, L-[2H10]leucine, and L-[2H4]alanine; see Ref. 33). Mixed-muscle protein-bound phenylalanine enrichment was analyzed by GCMS after protein hydrolysis and amino acid extraction (33), using the external standard curve approach (6).
Calculations
The kinetics of muscle phenylalanine, leucine, and alanine were calculated using two different methods as follows: the two-pool model and the three-pool model (34). Each model provides unique information regarding muscle amino acid kinetics (Fig. 1). Specifically, the two-pool model allows for the measurement of the net kinetics of blood amino acids across the leg, although it does not offer any insight into the intracellular amino acid kinetics. The three-pool model allows for the direct measurement of the amino acid intracellular turnover, including utilization for protein synthesis (only when using phenylalanine) or synthesis plus oxidation (when using leucine and alanine), and release from protein breakdown (with phenylalanine and leucine) or breakdown plus de novo synthesis when using alanine. It is important to underscore that an accurate estimation of muscle protein turnover (simultaneous measurement of synthesis and breakdown) can be obtained only with the phenylalanine tracer, since phenylalanine is not oxidized or synthesized by the muscle. Therefore, the three-pool model also allows for the calculation of amino acid recycling from breakdown into synthesis from phenylalanine data.
The two- and the three-pool models share most of the assumptions, which are (34): 1) an isotopic steady state exists; 2) for calculation of breakdown, there is no de novo synthesis of tracee. This is true for phenylalanine and leucine; 3) for calculation of synthesis, there is no tracee oxidation. This is true for phenylalanine; 4) muscle accounts for leg metabolism. This is not really accurate. However, the contribution of skin metabolism is lower in the leg than in the forearm (the only other region available to measure muscle amino acid kinetics with these methods) because of the significant differences in the ratio between the limb surface area and muscle mass; 5) if plasma enrichment is measured, equilibration between plasma and red blood cells is assumed; 6) individual amino acid tracers are representative of the fate of other amino acids. In our case, this is relevant only for the estimates of muscle protein synthesis and breakdown with phenylalanine, since we measured the kinetics of three amino acids to determine their independent fates; and 7) differences in enrichment between compartments are large enough to be measured.
Assumptions specific to the three-pool model are as follows: 1) the intracellular free pool is the precursor for synthesis; and 2) tissue measurements reflect homogeneous intracellular space.
Assumptions that are specific to the two-pool model are as follows: 1) Rd is a measure of the muscle protein synthesis rate. This is not exact, since Rd measures the irreversible uptake of tracer from blood, which is a composite measure of transport into the muscle and protein synthesis. For example, an increase in Rd could occur with no increase in protein synthesis if inward transport is increased. On the other hand, protein synthesis may increase with no changes in Rd if inward transport does not change, but intracellular amino acid recycling increases; 2) Ra represents muscle protein breakdown. This is not entirely true since Ra represents the rate of release in the blood of amino acid deriving from protein breakdown. Thus it is another composite measure of breakdown and outward transport. If transport does not change but breakdown increases, then the following two scenarios could occur: 1) increased intracellular recycling or 2) increased intracellular amino acid pool size.
Thus the overall number of assumptions for the two- and the three-pool models is similar, and they share most, but not all, assumptions.
The two- and the three-pool model shared the following parameters:
| (1) |
| (2) |
| (3) |
The other kinetic parameters of the two-pool method were calculated as follows:
| (4) |
| (5) |
| (6) |
CA and CV are the plasma amino acid concentrations in the femoral artery and vein, respectively. EA and EV are the amino acid enrichments, expressed as tracer-to-tracee ratio, in the femoral arterial and venous plasma, respectively. BF is leg blood flow as calculated from the steady-state ICG concentration values in the femoral and wrist veins, as previously described (10, 11), and NB is net balance. Data are expressed per 100 ml of leg volume or 100 mg of lean leg mass. Leg Ra represents the flux of amino acids deriving from protein breakdown (phenylalanine and leucine) or breakdown plus de novo synthesis (alanine) that appear in the venous blood. Leg Rd estimates the amount of blood amino acids that are taken up by the muscle for protein synthesis (phenylalanine, which is not oxidized in the muscle) or synthesis plus oxidation (leucine and alanine).
The specific parameters of the three-pool model were calculated as follows:
| (7) |
| (8) |
| (9) |
| (10) |
EM is the amino acid enrichment, expressed as tracer-to-tracee ratio, in the muscle. FM,0 is the amount of amino acids that appear in the muscle tissue from protein breakdown (phenylalanine and leucine) or protein breakdown plus de novo synthesis (alanine). F0,M is the total amount of amino acids utilized for muscle protein synthesis (phenylalanine) or synthesis plus oxidation (leucine and alanine). A recent study using microdyalisis and applying a four-pool model, including the interstitium as an independent amino acid pool, has highlighted that the transport rates as calculated with the three-pool model (see above) are in fact composite transport rates. Specifically, the FM,A includes transport from the arterial blood to the interstitium and from the interstitium to the muscle cells, and the FV,M includes both the transport from the muscle to the interstitium and from the interstitium to the venous blood. However, the same study has also underscored that the addition of the interstitium as an independent pool does not affect the synthesis and breakdown estimates (17).
Additionally, the intracellular amino acid availability was calculated as the sum of transport into the muscle FM,A and the intracellular rate of appearance FM,O.
| (11) |
Using phenylalanine, the efficiency of amino acid utilization for muscle protein synthesis was calculated as follows:
| (12) |
Intracellular recycling of phenylalanine from breakdown to synthesis without appearing in the circulation was calculated as follows:
| (13) |
We also calculated the fractional synthetic rate (FSR) of mixed muscle proteins by measuring the incorporation rate of the phenylalanine tracer into the proteins (ΔEP/t) and using the precursor-product model to calculate the synthesis rate as follows (28):
| (14) |
ΔEp is the increment in protein-bound phenylalanine enrichment between the two biopsies, t is the time between the two biopsies, and EM(1) and EM(2) are the phenylalanine enrichments in the free intra-cellular pool in the two biopsies, respectively. Data are expressed as percentage per hour.
Statistical analysis
Primary outcomes were measures of muscle protein synthesis and breakdown. Subjects’ characteristics and basal amino acid kinetics were analyzed using an unpaired two-tailed Student’s t-test. Differences were considered significant at P ≤ 0.05. P for trend was set at P < 0.10 and P > 0.05. Data are expressed as means ± SE.
RESULTS
Subjects’ characteristics
The subjects’ demographic and physical characteristics are shown in Table 1. There were no differences in age, total fat mass, body mass index, and leg volume between men and women. However, men had significantly greater body weight, height, lean body mass, and lean leg mass. The proportion of lean leg mass relative to the total leg mass was greater in men as well (64 ± 1 and 78 ± 2% for women and men, respectively; P < 0.05).
Blood flow
Blood flow to the leg was not different between women and men whether expressed per 100 ml leg volume (women: 3.42 ± 0.37, men: 3.74 ± 0.35 ml · min−1 · 100 ml leg volume−1) or per 100 g of lean leg mass (women: 4.30 ± 0.45, men: 3.71 ± 0.36 ml · min−1 · 100 g leg muscle−1).
Amino acid enrichments and concentrations
Amino acid concentrations and enrichments in the femoral artery and vein and in the muscle are reported in a table available only electronically. (The online version of this article contains supplemental data.) The average concentrations of phenylalanine, alanine, and leucine in femoral artery and vein and muscle were not different between women and men. Enrichments of phenylalanine, alanine, and leucine were at steady state during the sampling period (data not shown) and were not different between groups except for venous phenylalanine and alanine enrichments, which were lower (P < 0.05) in men.
Leg amino acid kinetics
Basal leg and muscle phenylalanine, leucine, and alanine kinetics expressed per 100 ml leg volume are shown in Table 2.
Table 2.
Basal leg phenylalanine, leucine, and alanine kinetics in women and men expressed per unit of leg volume
| Women | Men | P Value | |
|---|---|---|---|
| Phenylalanine kinetics | |||
| Parameters common to both models | |||
| Net balance | −16±4 | −31±5 | 0.05 |
| Delivery to the leg | 198±21 | 228±26 | 0.40 |
| Release from the leg | 214±24 | 259±31 | 0.28 |
| Two-pool model | |||
| Ra (release in blood from proteolysis) | 46±9 | 75±10 | 0.05 |
| Rd (disappearance from the blood) | 30±7 | 45±5 | 0.10 |
| Three-pool model | |||
| Transport into muscle free pool | 94±14 | 155±29 | 0.10 |
| Transport from muscle free pool | 110±16 | 185±32 | 0.07 |
| Release from proteolysis | 54±11 | 86±11 | 0.06 |
| Utilization for protein synthesis | 39±9 | 56±7 | 0.13 |
| Efficiency of amino acids used for synthesis, % | 25±2 | 26±3 | 0.94 |
| Intracellular availability | 149±23 | 241±35 | 0.05 |
| Recycling | 8±2 | 11±4 | 0.64 |
| Leg leucine kinetics | |||
| Parameters common to both models | |||
| Net balance | −30±8 | −33±6 | 0.80 |
| Delivery to the leg | 535±62 | 594±72 | 0.56 |
| Release from the leg | 566±67 | 627±77 | 0.57 |
| Two-pool model | |||
| Ra (release in blood from proteolysis) | 145±21 | 190±21 | 0.15 |
| Rd (disappearance from the blood) | 115±18 | 157±17 | 0.11 |
| Three-pool model | |||
| Transport into muscle free pool | 257±56 | 299±53 | 0.59 |
| Transport from muscle free pool | 287±57 | 332±56 | 0.59 |
| Release from proteolysis | 208±39 | 295±48 | 0.19 |
| Utilization for protein synthesis + oxidation | 177±36 | 262±45 | 0.18 |
| Intracellular availability | 465±72 | 594±74 | 0.23 |
| Alanine kinetics | |||
| Parameters common to both models | |||
| Net balance | −244±57 | −274±38 | 0.66 |
| Delivery to the leg | 853±88 | 1,034±114 | 0.24 |
| Release from the leg | 1,097±129 | 1,308±130 | 0.27 |
| Two-pool model | |||
| Ra (release in blood from proteolysis + de novo synthesis) | 688±95 | 981±97 | 0.05 |
| Rd (disappearance from the blood) | 444±59 | 707±67 | 0.01 |
| Three-pool model | |||
| Transport into muscle free pool | 344±33 | 461±45 | 0.06 |
| Transport from muscle free pool | 588±72 | 734±71 | 0.17 |
| Release from proteolysis + de novo synthesis | 1,306±305 | 2,873±364 | 0.04 |
| Utilization for protein synthesis + oxidation | 1,539±281 | 2,599±348 | 0.04 |
| Intracellular availability | 2,127±310 | 3,333±389 | 0.03 |
Values are means ± SE in nmol·min−1·100 ml leg volume−1 unless otherwise indicated. Ra, rate of appearance; Rd, rate of disappearance.
Parameters common to both models
Delivery of phenylalanine, leucine, and alanine to the leg and release from the leg were not different between groups. Phenylalanine, leucine, and alanine net balance were negative in all subjects. However, phenylalanine net balance was significantly lower in men than women (P < 0.05), whereas there were no significant differences in alanine and leucine net balance between groups.
Two-pool model
Leg Ra for phenylalanine (P= 0.05) and alanine (P= 0.05) were greater in men, whereas leucine Ra was not different between the groups. Alanine Rd was significantly higher in men than women (P = 0.01), whereas phenylalanine and leucine Rd were not different between groups.
Three-pool model
Alanine transport in the muscle tended to be greater in men than women (P = 0.06), whereas phenylalanine and leucine transport in the muscle was not different between groups. The transport of phenylalanine out of the muscle tended to be greater in men (P = 0.07), whereas alanine and leucine outward transport was not different between groups. Phenylalanine release from protein breakdown tended to be greater in men (P = 0.06), and alanine release from protein breakdown plus de novo synthesis was significantly greater (P = 0.04) in men than women. There was no difference in leucine release from protein breakdown between groups. Alanine utilization for protein synthesis and oxidation was significantly greater in men than women (P = 0.04), whereas the utilization of phenylalanine for protein synthesis and the utilization of leucine for synthesis plus oxidation were not different between women and men. Intracellular availability of phenylalanine was higher in men (P = 0.05), whereas intracellular availability of alanine and leucine were not different between women and men. Phenylalanine recycling from breakdown into synthesis without appearance in the circulation was not different between women and men.
Given the significant difference in lean body mass and in the fraction of lean mass per leg volume between sexes, the parameters of amino acid kinetics were normalized per 100 g of lean leg mass (Table 3). When the kinetic parameters were expressed relative to lean leg mass, all values became closely matched between women and men, and all significant differences were no longer observed. Because the between-subject variability was somewhat large, we performed a power analysis on the intracellular amino acid utilization and release parameters (normalized by lean leg mass) that indicates that we would have needed a much larger sample size to detect significant sex differences in intracellular amino acid metabolism: >70 subjects for phenylalanine, >145 subjects for leucine, and >46 subjects for alanine.
Table 3.
Basal leg phenylalanine, leucine, and alanine kinetics in women and men expressed per unit of lean leg mass
| Women | Men | P Value | |
|---|---|---|---|
| Leg phenylalanine kinetics | |||
| Parameters common to both models | |||
| Net balance across the leg | −19±4 | −30±5 | 0.14 |
| Delivery to the leg | 247±24 | 227±27 | 0.60 |
| Release from the leg | 266±27 | 257±32 | 0.84 |
| Two-pool model | |||
| Ra (release in blood from proteolysis) | 55±8 | 75±10 | 0.15 |
| Rd (disappearance from the blood) | 36±6 | 45±5 | 0.26 |
| Three-pool model | |||
| Transport into muscle free pool | 115±15 | 155±29 | 0.27 |
| Transport from muscle free pool | 134±16 | 185±33 | 0.21 |
| Release from proteolysis | 65±10 | 86±11 | 0.17 |
| Utilization for protein synthesis | 46±8 | 56±7 | 0.32 |
| Efficiency of amino acids used for synthesis, % | 25±2 | 26±3 | 0.94 |
| Intracellular availability | 180±20 | 241±35 | 0.18 |
| Recycling | 10±2 | 11±5 | 0.86 |
| Leg leucine kinetics | |||
| Parameters common to both models | |||
| Net balance across the leg | −37±10 | −32±6 | 0.66 |
| Delivery to the leg | 667±73 | 591±74 | 0.48 |
| Release from the leg | 705±80 | 623±79 | 0.48 |
| Two-pool model | |||
| Ra (release in blood from proteolysis) | 176±17 | 188±21 | 0.66 |
| Rd (disappearance from the blood) | 138±17 | 156±18 | 0.48 |
| Three-pool model | |||
| Transport into muscle free pool | 314±58 | 300±54 | 0.86 |
| Transport from muscle free pool | 351±57 | 332±57 | 0.81 |
| Release from proteolysis | 248±34 | 291±47 | 0.50 |
| Utilization for protein synthesis + oxidation | 211±31 | 259±44 | 0.42 |
| Intracellular availability | 562±62 | 590±75 | 0.79 |
| Leg alanine kinetics | |||
| Parameters common to both models | |||
| Net balance | −292±60 | −268±38 | 0.73 |
| Delivery to the leg | 1,068±107 | 1,028±113 | 0.80 |
| Release from the leg | 1,360±133 | 1,296±130 | 0.74 |
| Two-pool model | |||
| Ra (release in blood from proteolysis + de novo synthesis) | 843±88 | 961±95 | 0.39 |
| Rd (disappearance from the blood) | 551±64 | 693±65 | 0.15 |
| Three-pool model | |||
| Transport into muscle free pool | 432±46 | 454±44 | 0.73 |
| Transport from muscle free pool | 724±68 | 723±70 | 0.99 |
| Release from proteolysis + de novo synthesis | 2,187±331 | 2,836±374 | 0.22 |
| Utilization for protein synthesis + oxidation | 1,894±316 | 2,568±356 | 0.19 |
| Intracellular availability | 2,618±330 | 3,291±399 | 0.23 |
Values are means ± SE in nmol·min−1·100 g lean leg mass−1 unless otherwise indicated.
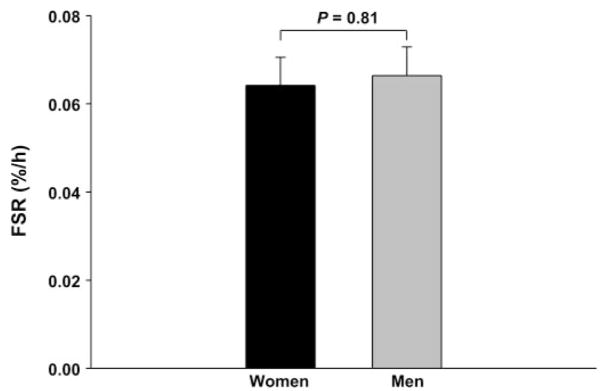
Mixed muscle FSR
The mixed muscle protein FSR is shown in Fig. 2. During the basal postabsorptive state, there was no difference in FSR between women and men, confirming the amino acid kinetic data normalized by muscle mass.
Fig. 2.
Mixed muscle protein fractional synthesis rate (FSR). Error bars indicate SE.
DISCUSSION
Our experiment indicates that the basal muscle intracellular kinetics of one nonessential and two essential amino acids, as well as plasma amino acid kinetics, muscle protein synthesis, and breakdown, are not different between women and men when the data are normalized by lean mass. This finding rejects the hypothesis that muscle intracellular amino acid turnover is greater or recycling into synthesis lower in men. Consequently, our finding suggests that the greater whole body leucine oxidation rates reported in men in the basal state must be due to sex differences in leucine oxidation at the level of other tissues, for example, the splanchnic tissues. More importantly, these data suggest that the metabolic mechanisms that cause and sustain the sexual dimorphism in muscle mass should be mostly active during puberty and, in the adult years, during acute anabolic stimulation, such as nutrient intake and/or muscle contraction.
On the other hand, the current study also indicates that there were significant sex-based differences in postabsorptive amino acid kinetics when the data were expressed in the conventional manner (per 100 ml leg volume). In particular, phenylalanine net balance across the leg, leg Ra and intracellular availability, and muscle alanine intracellular appearance and alanine utilization for protein synthesis and oxidation were significantly higher in men than women. Furthermore, measures of muscle protein turnover, using phenylalanine and alanine tracers, tended to be greater in men. Additionally, we found a discrepancy between leg Ra measured with phenylalanine (higher in men) and the same parameter measured with leucine (no sex difference) in the presence of concordant results relative to leg Rd (no sex difference). As previously mentioned, the leg Ra is a measure of the rate at which amino acids released from proteolysis reach the circulation. Thus this result would suggest that either the leucine released from proteolysis was preferentially oxidized in men rather than transported in the blood (which would have confirmed our original hypothesis) or that phenylalanine outward transport was relatively greater than leucine transport in men but not in women. The second option appears more likely since there was a trend for phenylalanine outward transport to be greater in men, whereas leucine transport did not differ between women and men.
Morphological analysis by DEXA indicated that men had significantly larger lean leg mass and a smaller leg fat mass than women. Because the subjects’ leg volume was not different between women and men, the proportion of leg muscle was greater in men than women. This led us to recalculate the muscle amino acid kinetics relative to lean leg mass. Using this approach, all significant differences and trends in muscle intracellular amino acid kinetics calculated by either the two-pool or the three-pool model disappeared. These results provide evidence that, despite differences in hormonal milieu, basal intracellular muscle protein turnover is not different between women and men when differences in body compositions are accounted for.
Our data are in agreement with a recent study reporting no sex-related differences in basal whole body leucine flux, oxidation, and nonoxidative disposal when the data are expressed per kilogram of fat-free mass (18). In addition, others have shown that basal muscle FSR are not different between women and men (1, 18). However, in these studies, muscle protein breakdown and net balance were not measured; thus, intracellular turnover and recycling from breakdown into synthesis could not be estimated. Our data are also consistent with a previous report in which forearm protein synthesis and breakdown, as estimated with the two-pool model, was greater in men that in women when the data were normalized by total forearm volume, but these differences were no longer detected when the data were expressed by unit of forearm muscle mass (9). Again, from these data, it was not possible to determine if women had a higher intracellular amino acid recycling into synthesis or lower turnover, which could have explained the lower amino acid oxidation rate previously observed (13, 14, 16, 19, 30), because the model could not account for intracellular amino acid kinetics. Our current study demonstrates for the first time that the muscle intracellular kinetics (including incorporation into proteins, release from breakdown, recycling from breakdown into synthesis, and transport) of three different amino acids are not different in women and men and provides strong evidence of a lack of sex differences in baseline muscle protein and amino acid kinetics.
However, Phillips et al. (19) have shown that women have a significantly lower resting energy expenditure and metabolic rate even when adjusted for body composition. Therefore, one would expect to observe a sexual dimorphism in the metabolism of protein and/or other substrates, particularly in the muscle. In other words, the greater energy consumption per unit of active metabolic tissue observed in men would suggest that the turnover and/or oxidation of some substrates are higher in men. Several studies have reported that the whole body rate of leucine appearance (an index of whole body proteolysis) and nonoxidative rate of disappearance (an index of whole body protein synthesis) were not different between women and men (13, 14, 16, 19, 30). Nevertheless, the basal rate of leucine oxidation has been shown to be significantly lower in women even when the data are expressed per unit of lean mass (13, 14, 16, 19, 30). Furthermore, it is well known that leucine oxidation is higher in men than women during aerobic exercise (13, 14, 16, 19). Thus, because of these known sex differences in whole body leucine oxidation, we hypothesized that the higher amino acid oxidation in men could be the result of an increased intracellular amino acid turnover and/or reduced recycling of amino acids from breakdown into synthesis not detectable with the traditional AV balance method. However, we did not observe any significant sex differences in muscle intracellular amino acid turnover or in the efficiency by which amino acids are used for muscle protein synthesis. Therefore, the whole body differences in leucine oxidation between women and men do not appear to be the result of differences in muscle amino acid utilization and suggest differences in amino acid metabolism at the level of other tissues, such as the splanchnic tissues. This hypothesis must be confirmed with appropriate studies.
A potential limitation of this study may derive from the physiological between-subject variability in muscle amino acid kinetics. Although in line with that reported in previous studies, the between-subject variability might have reduced our ability to detect small sex differences in muscle intracellular amino acid kinetics (utilization and release). However, we and others have seen changes in response to treatments such as insulin (4, 8, 22), nutritional stimuli (29, 31, 32), exercise (20, 21, 24, 26), and testosterone (7) that were much larger (typically >100%) than the differences seen between sexes (which ranged between 14 and 26%). As a result, sex is apparently a relatively unimportant determinant of intracellular amino acid kinetics compared with other physiological stimuli of interest when the data are normalized by lean leg mass.
A variable that can influence amino acid kinetics in women is the menstrual phase (12, 15). Increases in progesterone levels, as observed in the luteal phase, have been shown to increase leucine oxidation (15). We accounted for this variable by studying each female subject during the follicular phase. This was verified by measuring the circulating progesterone concentrations. However, future studies are required to determine if basal muscle intracellular amino acid kinetics are altered during the luteal phase.
In summary, despite a greater muscle mass in men, muscle intracellular amino acid kinetics (including muscle protein synthesis and breakdown) per unit of lean mass are not different in men and women. This also suggests that the muscle protein kinetic parameters should be expressed per unit of lean or muscle mass when comparing groups including both women and men. Because men have undoubtedly larger muscles than women, and because basal muscle protein turnover is not different, it is likely that men respond to anabolic stimuli more effectively than women. Thus it will be important to determine if there are sex differences in response to nutrients or other physiological anabolic factors such as contraction. Finally, it is also likely that large sex differences in amino acid and protein kinetics occur during puberty, when the muscle sexual dimorphism becomes evident.
Acknowledgments
We thank Jeanine Cordero for technical assistance, Dr. David L. Chinkes for excellent suggestions, the study volunteers for patience and dedication, and all the nurses and personnel of the General Clinical Research Center of the University of Southern California for help with the conduct of the clinical portion of this study.
GRANTS
This study was supported by National Institutes of Health Grants R01-AG-18311, P30-AG-17231, R01-AR-049877, S10-RR-16650, and M01-RR-43.
References
- 1.Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol Endocrinol Metab. 1997;273:E790–E800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- 2.Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R. The effects of supra-physiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 3.Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger Phillips J, Lee WP, Bunnell TJ, Casaburi R. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab. 1997;82:407–413. doi: 10.1210/jcem.82.2.3733. [DOI] [PubMed] [Google Scholar]
- 4.Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest. 1995;95:811–819. doi: 10.1172/JCI117731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodsky IG, Balagopal P, Nair KS. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men—a clinical research center study. J Clin Endocrinol Metab. 1996;81:3469–3475. doi: 10.1210/jcem.81.10.8855787. [DOI] [PubMed] [Google Scholar]
- 6.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–009 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Sp. 1992;6:421–424. doi: 10.1002/rcm.1290060704. [DOI] [PubMed] [Google Scholar]
- 7.Ferrando AA, Tipton KD, Doyle DJ, Phillips SM, Cortiella J, Wolfe RR. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol Endocrinol Metab. 1998;275:E864–E871. doi: 10.1152/ajpendo.1998.275.5.E864. [DOI] [PubMed] [Google Scholar]
- 8.Fujita S, Rasmussen BB, Cadenas JG, Grady JJ, Volpi E. The effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab. 2006;291:E745–E754. doi: 10.1152/ajpendo.00271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jahn LA, Barrett EJ, Genco ML, Wei L, Spraggins TA, Fryburg DA. Tissue composition affects measures of postabsorptive human skeletal muscle metabolism: comparison across genders. J Clin Endocrinol Metab. 1999;84:1007–1010. doi: 10.1210/jcem.84.3.5522. [DOI] [PubMed] [Google Scholar]
- 10.Jorfeldt L, Juhlin-Dannfelt A. The influence of ethanol on splanchnic and skeletal muscle metabolism in man. Metabolism. 1978;27:97–106. doi: 10.1016/0026-0495(78)90128-2. [DOI] [PubMed] [Google Scholar]
- 11.Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci. 1971;41:459–473. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- 12.Lamont LS, Lemon PW, Bruot BC. Menstrual cycle and exercise effects on protein catabolism. Med Sci Sports Ex. 1987;19:106–110. [PubMed] [Google Scholar]
- 13.Lamont LS, McCullough AJ, Kalhan SC. Gender differences in leucine, but not lysine, kinetics. J Appl Physiol. 2001;91:357–362. doi: 10.1152/jappl.2001.91.1.357. [DOI] [PubMed] [Google Scholar]
- 14.Lamont LS, McCullough AJ, Kalhan SC. Gender differences in the regulation of amino acid metabolism. J Appl Physiol. 2003;95:1259–1265. doi: 10.1152/japplphysiol.01028.2002. [DOI] [PubMed] [Google Scholar]
- 15.Lariviere F, Moussalli R, Garrel DR. Increased leucine flux and leucine oxidation during the luteal phase of the menstrual cycle in women. Am J Physiol Endocrinol Metab. 1994;267:E422–E428. doi: 10.1152/ajpendo.1994.267.3.E422. [DOI] [PubMed] [Google Scholar]
- 16.McKenzie S, Phillips SM, Carter SL, Lowther S, Gibala MJ, Tarnopolsky MA. Endurance exercise training attenuates leucine oxidation and BCOAD activation during exercise in humans. Am J Physiol Endocrinol Metab. 2000;278:E580–E587. doi: 10.1152/ajpendo.2000.278.4.E580. [DOI] [PubMed] [Google Scholar]
- 17.Miller S, Chinkes D, MacLean DA, Gore D, Wolfe RR. In vivo muscle amino acid transport involves two distinct processes. Am J Physiol Endocrinol Metab. 2004;287:E136–E141. doi: 10.1152/ajpendo.00092.2004. [DOI] [PubMed] [Google Scholar]
- 18.Parise G, Mihic S, MacLennan D, Yarasheski KE, Tarnopolsky MA. Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed-muscle protein synthesis. J Appl Physiol. 2001;91:1041–1047. doi: 10.1152/jappl.2001.91.3.1041. [DOI] [PubMed] [Google Scholar]
- 19.Phillips SM, Atkinson SA, Tarnopolsky MA, MacDougall JD. Gender differences in leucine kinetics and nitrogen balance in endurance athletes. J Appl Physiol. 1993;75:2134–2141. doi: 10.1152/jappl.1993.75.5.2134. [DOI] [PubMed] [Google Scholar]
- 20.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1997;273:E99–E107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- 21.Phillips SM, Tipton KD, Ferrando AA, Wolfe RR. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am J Physiol Endocrinol Metab. 1999;276:E118–E124. doi: 10.1152/ajpendo.1999.276.1.E118. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20:768–769. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen BB, Phillips SM. Contractile and nutritional regulation of human muscle growth. Exerc Sport Sci Rev. 2003;31:127–131. doi: 10.1097/00003677-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol. 2000;88:386–392. doi: 10.1152/jappl.2000.88.2.386. [DOI] [PubMed] [Google Scholar]
- 25.Schroeder ET, Jaque SV, Hawkins SA, Olson C, Terk M, Wiswell RA, Sattler FR. Regional DXA and MRI in assessment of muscle adaptation to anabolic stimuli. Clin Ex Physiol. 2001;3:199–206. [Google Scholar]
- 26.Sheffield-Moore M, Yeckel CW, Volpi E, Wolf SE, Morio B, Chinkes DL, Paddon-Jones D, Wolfe RR. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am J Physiol Endocrinol Metab. 2004;287:E513–E522. doi: 10.1152/ajpendo.00334.2003. [DOI] [PubMed] [Google Scholar]
- 27.Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, Crill MT, Ragg KE, Toma K. Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem. 2000;48:623–629. doi: 10.1177/002215540004800506. [DOI] [PubMed] [Google Scholar]
- 28.Toffolo G, Foster DM, Cobelli C. Estimation of protein fractional synthetic rate from tracer data. Am J Physiol Endocrinol Metab. 1993;264:E128–E135. doi: 10.1152/ajpendo.1993.264.1.E128. [DOI] [PubMed] [Google Scholar]
- 29.Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101:2000–2007. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volpi E, Lucidi P, Bolli GB, Santeusanio F, De Feo P. Gender differences in basal protein kinetics in young adults. J Clin Endocrinol Metab. 1998;83:4363–4367. doi: 10.1210/jcem.83.12.5330. [DOI] [PubMed] [Google Scholar]
- 31.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–4490. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first pass splanchnic extraction. Am J Physiol Endocrinol Metab. 1999;277:E513–E520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 33.Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine. Principles and Practice of Kinetic Analysis. New York: Wiley-Liss; 1992. [Google Scholar]
- 34.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. Hoboken, NJ: Wiley; 2004. [Google Scholar]
- 35.Yasuda N, Glover EI, Phillips SM, Isfort RJ, Tarnopolsky MA. Sex-based differences in skeletal muscle function and morphology with short-term limb immobilization. J Appl Physiol. 2005;99:1085–1092. doi: 10.1152/japplphysiol.00247.2005. [DOI] [PubMed] [Google Scholar]