Abstract
Insulin promotes muscle anabolism, but it is still unclear whether it stimulates muscle protein synthesis in humans. We hypothesized that insulin can increase muscle protein synthesis only if it increases muscle amino acid availability. We measured muscle protein and amino acid metabolism using stable-isotope methodologies in 19 young healthy subjects at baseline and during insulin infusion in one leg at low (LD, 0.05), intermediate (ID, 0.15), or high (HD, 0.30 mU·min−1·100 ml−1) doses. Insulin was infused locally to induce muscle hyperinsulinemia within the physiological range while minimizing the systemic effects. Protein and amino acid kinetics across the leg were assessed using stable isotopes and muscle biopsies. The LD did not affect phenylalanine delivery to the muscle (−9 ± 18% change over baseline), muscle protein synthesis (16 ± 26%), breakdown, or net balance. The ID increased (P < 0.05) phenylalanine delivery (+63 ± 38%), muscle protein synthesis (+157 ± 54%), and net protein balance, with no change in breakdown. The HD did not change phenylalanine delivery (+12 ± 11%) or muscle protein synthesis (+9 ± 19%), and reduced muscle protein breakdown (−17 ± 15%), thus improving net muscle protein balance but to a lesser degree than the ID. Changes in muscle protein synthesis were strongly associated with changes in muscle blood flow and phenylalanine delivery and availability. In conclusion, physiological hyperinsulinemia promotes muscle protein synthesis as long as it concomitantly increases muscle blood flow, amino acid delivery and availability.
Keywords: metabolism, muscle perfusion
insulin is a potent anabolic stimulus for muscle proteins. Insulin deficiency leads to a protein catabolic state with loss of muscle mass that can only be reversed by insulin therapy (1). Nonetheless, the mechanisms by which insulin enhances muscle protein anabolism are still debated. A stimulatory effect of insulin on protein synthesis has been demonstrated in various tissues, including skeletal muscle (16, 35, 37, 38). Furthermore, in vitro animal studies and a recent human experiment have shown that insulin can acutely stimulate muscle protein synthesis by increasing the initiation of mRNA translation (18, 23-25). On the other hand, if the physiological increase in insulin secretion is pharmacologically suppressed during feeding in rats, the stimulation of translation initiation is abolished and muscle protein synthesis suppressed (40). Insulin can also reduce protein breakdown by stabilizing lysosomes and reducing the activity of the ubiquitin-proteasome pathway (9, 15, 27).
In human subjects, insulin infusion induces net amino acid uptake across a limb (forearm or leg), an indication of net muscle protein anabolism, but the mechanisms are still unclear (4, 5, 14, 17, 19, 20, 28, 32-34, 53). About half of these studies reported that this effect was due to an increase in protein synthesis with no major changes or some reduction in proteolysis (4, 5, 20, 33, 34, 53). Conversely, the other studies found a significant reduction in protein degradation with no significant changes in protein synthesis (14, 17, 19, 28, 32). Because these experiments were performed using comparable stable-isotope arteriovenous balance methodologies, it is unlikely that technical or methodological problems were responsible for the conflicting findings. A review of these studies suggests that these apparent discrepancies on the metabolic mechanisms by which insulin stimulates muscle protein anabolism might be explained by differences in amino acid availability for the muscle tissue (55). Specifically, all studies in which muscle protein synthesis had been stimulated by insulin also had an increased amino acid delivery to the muscle tissue (amino acid concentration × blood flow) (4, 5, 18, 20, 33, 34, 53), whereas most studies reporting a decrease in muscle protein breakdown with no increase in synthesis during insulin infusion also had a decrease or no change in amino acid delivery (14, 19, 28, 32). The differences in amino acid delivery were mainly due to differences in amino acid concentrations, which, in turn, were determined by the modality of insulin infusion (systemic or local) and/or the concomitant infusion of exogenous amino acids. This is because systemic insulin infusion decreases blood amino acid concentrations (13, 14, 29, 32, 34, 45) unless amino acids are replaced by exogenous infusion (18-20, 32-34). Conversely, local insulin infusion in a leg or a forearm allows for the exposure of the muscle tissue to relatively high insulin levels while avoiding a major reduction in blood amino acid concentration (5, 28).
The current study was conducted to determine whether the response of muscle protein synthesis to insulin depends on insulin-induced changes in amino acid delivery and availability for the muscle tissue. To test this hypothesis, we induced local hyperinsulinemia in one leg in the absence of amino acid infusion. We used three physiological insulin doses (low, intermediate, and high) selected to either avoid a significant reduction in the systemic amino acid concentrations (the two lower doses) (5) or allow amino acids to decrease due to systemic hyperinsulinemia (higher dose) (13). Because the reduction in amino acid concentration was expected to occur at the highest insulin dose, this design allowed us to determine the relative contribution of insulin dose and amino acid availability on the response of muscle protein synthesis to insulin. This design was preferred to a fixed systemic insulin infusion with and without amino acid replacement because previous data indicate that available amino acid solutions for intravenous infusion cannot exactly replace all essential amino acids and maintain the baseline physiological pattern (12, 18). This is a major problem because it is known that essential amino acids can independently stimulate muscle protein synthesis (3, 49). Thus, if the amino acid replacement infusion delivered excessive amounts of certain essential amino acids, it would be impossible to distinguish between the effect of insulin and that of the amino acid infusion. In contrast, incomplete replacement of some amino acids could be responsible for an incomplete muscle protein synthesis response, again making data interpretation very difficult. Finally, the amino acid dose necessary to maintain the baseline concentrations would be significant (∼0.8 mg·kg−1·min−1) (12), so that over 3 h it could reach ∼10−11 g per subject, an amount that can stimulate muscle protein synthesis even in the absence of hyperinsulinemia (36).
SUBJECTS AND METHODS
Subjects
We studied 19 young subjects (11 men and 8 women) from the Los Angeles metropolitan area. All subjects were healthy and physically active, but they were not engaged in an exercise training program. Screening of subjects was performed with clinical history, physical examination, and laboratory tests, including complete blood count with differential, liver, and kidney function tests, coagulation profile, fasting blood glucose and oral glucose tolerance test (OGTT), hepatitis B and C screening, HIV test, TSH, lipid profile, pregnancy test in women, urinalysis, drug screening test, and electrocardiogram. Only subjects with screening results within the normal limits were randomly assigned to one of three groups according to the dose of insulin infused during the experiment: low dose (LD), intermediate dose (ID), and high dose (HD). The doses were chosen to increase insulin concentrations in the femoral vein within the physiological postprandial range (5, 13, 50). We did not include a fourth group infused with saline because we have found in a separate experiment that muscle protein metabolism is unaffected by time alone over the short term (6 h; data not shown). The subjects’ characteristics are summarized in Table 1.
Table 1.
Physical characteristics of the subjects
| LD | ID | HD | P | |
|---|---|---|---|---|
| n | 6 | 7 | 6 | |
| Sex | 4 M 2 F | 3 M 4 F | 4 M 2 F | 0.60 |
| Age, yr | 29±3 | 25±2 | 28±2 | 0.51 |
| Height, cm | 169±5 | 169±4 | 170±2 | 0.99 |
| Body weight, kg | 72±7 | 63±6 | 74±5 | 0.44 |
| Body mass index, kg/m2 | 25±2 | 22±1 | 26±2 | 0.19 |
| Fat-free mass, kg | 50±4 | 45±5 | 54±3 | 0.30 |
| Fat mass, kg | 18±4 | 11±1 | 17±3 | 0.21 |
| Leg volume, liters | 9.5±1.1 | 8.4±5.9 | 9.9±3.5 | 0.33 |
| Leg muscle mass, kg | 8.6±8.9 | 7.3±8.9 | 9.9±3.3 | 0.21 |
Values are means ± SE. Subjects were randomized to 3 groups receiving a low (LD), intermediate (ID), or high dose (HD) insulin infusion. P, significance level for differences between groups.
All subjects gave informed, written consent before participating in the study, which was approved by the Institutional Review Board of the University of Southern California (Los Angeles, CA).
Study design
The protocol was designed to measure muscle protein and amino acid and glucose kinetics in the postabsorptive basal state (0−240 min) and during insulin infusion (240−420 min).
The night before the study, each subject was admitted to the General Clinical Research Center of the University of Southern California. At admission, a pregnancy test was repeated in women, and a dual-energy X-ray absorptiometry (DEXA) scan (QDR 4500W; Hologic, Bedford, MA) was performed to measure muscle mass. The subjects were then fed a standard dinner, and a snack was given at 2200. After 2200, the subjects were allowed only water ad libitum until the end of the experiment. The morning of the study, polyethylene catheters were inserted into a forearm vein for tracer and dextrose infusion, in a contralateral hand or wrist vein for arterialized blood sampling, and in the femoral artery and vein of one leg for blood sampling. The arterial catheter was also used for the infusion of insulin and indocyanine green (ICG; Akorn, Buffalo Grove, IL).
At ∼0730, after drawing of a blood sample for the measurement of background phenylalanine and glucose enrichments and ICG concentration, a primed continuous infusion of l-[ring-13C6]phenylalanine and d-[6,6-2H2]glucose (Cambridge Isotope Laboratories, Andover, MA) was started and maintained at a constant rate until the end of experiment. The following priming doses (PD) and infusion rates (IR) were used: l-[ring-13C6]phenylalanine: PD 2 μmol/kg, IR 0.05 μmol·kg−1·min−1; d-[6,6-2H2]glucose: PD 19 μmol/kg, IR 0.22 μmol·kg−1·min−1.
At 120 min, a first muscle biopsy was taken from the lateral portion of the vastus lateralis of the leg with the femoral catheters, using a 5-mm Bergström biopsy needle, using sterile procedure and local anesthesia with 1% Lidocaine injected subcutaneously and on the fascia. The muscle sample (150−400 mg) was rinsed with ice-cold saline and blotted, any visible fat or connective tissue was quickly removed, and it was immediately frozen in liquid nitrogen and stored at −80°C until analysis.
At 170 min, the continuous infusion of ICG was started in the femoral artery (0.5 mg/min) and maintained until 210 min. During ICG infusion, blood samples were taken four times at 10-min intervals from the femoral vein and the hand vein to measure ICG concentration. Subsequently, between 210 and 240 min, four blood samples were taken from the femoral artery and vein and from the hand vein to measure femoral arterial and venous phenylalanine and glucose concentrations and enrichments, femoral vein insulin concentration, and systemic insulin concentration (hand vein samples). At 240 min, a second muscle biopsy was taken as previously described.
Immediately after the second biopsy at 240 min, an insulin infusion was initiated into the femoral artery. The following infusion rates were used: LD 0.05 mU·min−1·100 ml−1 (0.06 mU·kg−1·min−1); ID 0.15 mU·min−1·100 ml−1 (0.2 mU·kg−1·min−1); and HD 0.30 mU·min−1·100 ml−1 (0.4 mU·kg−1·min−1). After the start of the insulin infusion, blood samples (0.5 ml) were taken every 5−10 min to monitor the plasma glucose concentration. Dextrose (20%) containing 2% d-[6,6-2H2]glucose was then infused at variable rate as necessary to clamp the plasma glucose concentration at the basal value. Glucose tracer was added to the dextrose to maintain the plasma glucose enrichment constant during the euglycemic hyperinsulinemic clamp.
Between 350 and 390 min, ICG was again infused to measure leg blood flow, and blood samples were taken between 350 and 390 min and 390 and 420 min, as described for the basal period. At 420 min, before the tracer and insulin infusion were stopped, a third muscle biopsy was taken, as described above.
Analytic methods
Plasma insulin concentrations were determined by radioimmunoassay (Linco Research, St. Charles, MO).
Serum ICG concentration for the determination of leg blood flow was measured spectrophotometrically (Beckman Coulter, Fullerton, CA) at λ = 805 nm (21, 22).
Plasma glucose concentration was measured using an automated glucose analyzer (YSI, Yellow Springs, OH). Enrichment of plasma glucose was determined on its pentaacetate derivative using gas-chromatography mass-spectrometry (GC-MS; 6890 Plus GC, 5973N MSD/DS, 7683 autosampler; Agilent Technologies, Palo Alto, CA) as previously described (54).
Concentrations and enrichments of blood phenylalanine were determined on its tert-butyldimethylsilyl (t-BDMS) derivative using l-[15N]phenylalanine as an internal standard and GC-MS as previously described (54). We did not measure the concentrations of all essential amino acids, since previous studies have shown that insulin-induced changes in phenylalanine can predict the behavior of all essential amino acids (14).
Muscle tissue samples were ground in sulfosalycilic acid, and the intracellular free phenylalanine and muscle proteins were extracted as previously described (54). Intracellular free concentrations and enrichments of phenylalanine were determined by GC-MS after t-BDMS derivatization and using l-[15N]phenylalanine as an internal standard (54). Mixed muscle protein-bound phenylalanine enrichment was analyzed by GC-MS after protein hydrolysis and amino acid extraction (54), using the external standard curve approach (11).
Calculations
The kinetics of muscle phenylalanine were calculated using two different arteriovenous balance methods: the two-pool model (54) and the three-pool model (6). We used both models because each of them provides unique information regarding muscle amino acid kinetics. Additionally, although the three-pool model provides more detailed information regarding intracellular amino acid kinetics, it is a fairly new method and is used only by a few groups. On the other hand, the two-pool model has been used by a number of research groups, thus allowing for a comparison of our results with data collected by others.
Phenylalanine was chosen because it is not oxidized by skeletal muscle, and therefore its utilization is a direct measure of muscle protein synthesis. With the two-pool model, phenylalanine enrichments and concentrations in the femoral artery and vein were used to estimate muscle protein synthesis, breakdown, and net balance. These parameters are based on the extraction of the labeled phenylalanine from the femoral artery, the appearance of unlabeled phenylalanine from the muscle in the femoral vein, and the net arteriovenous difference of the phenylalanine concentrations, respectively (54). Thus this model provides data regarding the kinetics of plasma phenylalanine across the leg with no consideration for intracellular recycling of the amino acid from breakdown to synthesis. In other words, this method allows for the measurement of the effect of our treatments on the net kinetics of plasma phenylalanine across the leg while not offering any insight into its intracellular kinetics.
The three-pool model is an expansion of the two-pool model and relies not only on the measurement of the amino acid enrichments and concentrations in the femoral artery and vein but also on the direct measurement of the amino acid enrichment in the free tissue water. This allows for the direct measurement of phenylalanine intracellular utilization for protein synthesis and release from protein breakdown. In addition, it is possible to calculate the rate of phenylalanine transport from the artery into the tissue and from the tissue into the venous blood.
The two- and three-pool models shared the following parameters:
| (1) |
| (2) |
| (3) |
The other kinetic parameters of the two-pool method were calculated as follows:
| (4) |
| (5) |
| (6) |
where CA and CV are the plasma phenylalanine concentrations in the femoral artery and vein, respectively; EA and EV are phenylalanine enrichments, expressed as tracer-to-tracee ratio, in the femoral arterial and venous plasma, respectively; and BF is leg blood flow as calculated from the steady-state ICG concentration values in the femoral and wrist veins, as previously described (21, 22). Data were expressed per 100 ml of leg volume.
The specific parameters of the three-pool model were calculated as follows:
| (7) |
| (8) |
| (9) |
| (10) |
| (11) |
where EM is phenylalanine enrichment, expressed as tracer-to-tracee ratio, in the muscle.
Additionally, we calculated the intracellular phenylalanine availability as the sum of transport into the muscle FM,A and the intracellular Ra from breakdown FM,O:
| (12) |
We also calculated the fractional synthetic rate (FSR) of mixed muscle proteins by measuring the incorporation rate of the phenylalanine tracer into the proteins (ΔEP/t) and using the precursor-product model to calculate the synthesis rate as follows (47):
| (13) |
where ΔEp is the increment in protein-bound phenylalanine enrichment between two sequential biopsies, t is the time between the two sequential biopsies, and EM(1) and EM(2) are the phenylalanine enrichments in the free tissue pool in the two sequential biopsies. Data were expressed as percent per hour.
Although the use of free tissue enrichment may lead to an underestimation of protein synthesis compared with tRNA (46), Miller et al. (31) have shown, using the microdialysis technique, that the rate-limiting factor for the exchange of amino acids between extra- and intracellular compartments is the transport from the blood into the extracellular compartment. Thus tissue enrichment can be safely used to estimate muscle protein FSR.
Steady-state whole body glucose Ra, which is equal to its utilization rate (Rd), was calculated using the single-pool model (54):
| (14) |
where I is the tracer infusion rate and EgA is the arterial glucose enrichment. During the clamp, the endogenous glucose Ra was calculated by subtracting the exogenous glucose infusion from the total Ra. Data were reported per kilogram of body weight.
Leg glucose utilization was calculated as net glucose uptake across the leg:
| (15) |
where CgA and CgV are the glucose arterial and venous concentrations.
To determine the degree of muscle tissue exposure to insulin, we calculated the insulin delivery rate to the leg. This is because a small portion of the insulin directly infused into the leg was recycled through the systemic circulation back into the leg, thus increasing the amount of insulin delivered to the muscle. Additionally, since changes in leg blood flow can significantly affect insulin concentration when the exogenous infusion is constant, insulin concentration alone may not reflect the actual insulin availability for the muscle tissue. Because the arterial insulin concentration was not measurable during insulin infusion because the infusion was administered through the arterial catheter, insulin delivery to the leg was estimated by multiplying the insulin concentration in the femoral vein (InsFV) by the blood flow:
| (16) |
Although this method may slightly underestimate the insulin delivery rate because some insulin is taken up by the muscle cells after binding the insulin receptor and does not return in the venous blood, for the reasons listed above we found it preferable to relying only on the calculated insulin dose as assessed at the time of infusion.
Statistical analysis
Subjects’ characteristics were analyzed using one-way analysis of variance (ANOVA) with the exception of sex, a categorical variable, which was analyzed using a χ2 test. Differences between baseline values for all measured variables were analyzed using one-way ANOVA. The effects of each insulin dose on the response variables were assessed using the paired t-test. The effect of the three insulin doses on the response variables was assessed on the changes from baseline values by one-way ANOVA. Post hoc tests were performed using the Tukey-Kramer test. The effect of the treatments on the changes in leg blood flow was analyzed using the Kruskal-Wallis test (using exact methods) because the change scores failed a test for normality. The Pearson product-moment correlation was used to assess associations between continuous variables reported. Stepwise regression analysis was performed using the step forward method. Differences were considered significant at P < 0.05. P for trend was set at <0.10 and >0.05. Data are expressed as means ± SE.
RESULTS
Subjects’ characteristics
The demographic and physical characteristics of the subjects were not different among the groups (LD, ID, and HD; Table 1).
Blood flow
Baseline leg blood flow was not different between the groups (LD 3.31 ± 0.45, ID 4.03 ± 0.43, and HD 3.56 ± 0.40 ml·min−1·100 ml leg volume−1). During insulin infusion, leg blood flow was LD 2.90 ± 0.29, ID 6.69 ± 1.27, HD 4.73 ± 0.56 ml·min−1·100 ml leg volume−1. After the (post/pre) change scores failed Shapiro-Wilk's test of normality (P = 0.001), the Kruskal-Wallis test indicated differences between the groups (P = 0.04). Pairwise comparisons indicated significant differences between LD and HD (P = 0.026) and between LD and ID (P = 0.05) but not between ID and HD (P = 0.83).
Insulin and glucose concentrations and kinetics
The results of insulin and glucose concentrations and kinetics are summarized in Table 2. There were no basal differences in femoral or systemic (hand) vein insulin concentrations among the three groups. Insulin infusion significantly increased both femoral and systemic vein insulin concentrations in all groups (P < 0.05), with the largest changes occurring in the HD group and the smallest changes in the LD group (P < 0.05).
Table 2.
Insulin and glucose concentrations and kinetics
| LD |
ID |
HD |
|||||
|---|---|---|---|---|---|---|---|
| Basal | Insulin | Basal | Insulin | Basal | Insulin | P | |
| Insulin concentrations, μU/ml | |||||||
| Femoral vein | 10.6±1.5 | 30.9±7.7a* | 10.6±1.2 | 45.8±6.3a* | 6.7±0.9 | 84.5±5.0b* | <0.0001 |
| Hand vein | 9.9±1.6 | 15.0±2.1a* | 12.1±0.4 | 17.4±2.5a,b* | 6.4±1.1 | 19.1±2.4b* | 0.03 |
| Leg insulin kinetics, μU·min−1·100 ml leg volume−1 | |||||||
| Leg delivery | 33.6±4.7 | 86.9±20.5a* | 42.3±6.8 | 282.1±25.4b* | 24.5±4.2 | 411.7±57.6c* | <0.0001 |
| Glucose concentrations, mmol/l | |||||||
| Artery | 5.13±0.23 | 4.98±0.18 | 4.88±0.13 | 4.84±0.13 | 4.76±0.08 | 4.74±0.14 | 0.59 |
| Vein | 5.02±0.24 | 4.84±0.19 | 4.79±0.13 | 4.55±0.15 | 4.66±0.08 | 4.27±0.14* | 0.42 |
| Glucose enrichments (tracer/tracer ratio) | |||||||
| Artery | 2.3±0.2 | 2.9±0.2a* | 2.0±0.1 | 2.9±0.1b* | 2.3±0.2 | 3.1±0.1b* | 0.0181 |
| Whole body glucose kinetics, μmol·kg−1·min−1 | |||||||
| Endogenous Ra | 9.95±0.68 | 7.93±0.52* | 11.24±0.75 | 7.71±0.32* | 10.08±0.87 | 7.07±0.31* | 0.12 |
| Rd | 9.95±0.68 | 11.76±1.08a* | 11.24±0.75 | 18.35±0.88a,b* | 10.08±0.87 | 22.16±3.76b* | 0.004 |
| Leg glucose kinetics, μmol·min−1·100 ml leg volume−1 | |||||||
| Net balance | 0.36±0.11 | 0.39±0.13a | 0.29±0.09 | 1.57±0.35b* | 0.33±0.06 | 2.21±0.34b* | 0.0003 |
Values are means ± SE. Insulin concentrations in the femoral and hand vein and delivery to the leg; glucose concentrations in the femoral artery and vein, enrichment in the femoral artery, whole body endogenous rate of appearance (endogenous Ra), utilization rate (Rd), and leg uptake (net balance) at baseline and during LD, ID, and HD insulin infusions in the leg. P, significance value for 1-way ANOVA on effect of insulin dose on changes from baseline.
P < 0.05 vs. basal (paired t-test). Common letter denotes that changes from baseline do not differ between groups (Tukey's test).
Insulin delivery to the leg was not different among the three groups during the basal period. Insulin infusion increased the insulin delivery to the leg in all groups but to a different extent according to the dose: ∼3-fold in the LD group, ∼7-fold in ID group, and ∼17-fold in HD group.
Plasma arterial and venous glucose concentrations were not different in the three groups during the basal period. During insulin infusion, the arterial glucose concentration did not change from baseline as a result of the glucose clamp, whereas the venous glucose concentrations significantly decreased in the HD group; however, the (post/pre) change scores did not differ between groups.
Arterial glucose enrichments were at steady state during the last hour of each study period and were not different among the three groups in the basal period. During insulin infusion, arterial glucose enrichments increased significantly (P < 0.01) in all three groups, with a larger change in the ID and HD groups (P < 0.05).
Whole body glucose utilization (Rd) and endogenous glucose Ra were not different among the three groups during the basal period. During insulin infusion with euglycemic clamp, glucose Rd increased in a dose-dependent manner (P < 0.01), whereas the endogenous Ra was suppressed in all three groups, with no differences among the groups.
Net leg glucose uptake (net balance) was not different among the groups during the basal period. With insulin infusion net balance increased significantly only in the ID and HD groups (P < 0.01).
Phenylalanine concentrations and enrichments
The average phenylalanine concentrations in the femoral artery and vein and in the muscle are reported in Table 3. During the basal period, phenylalanine concentrations in the femoral artery and vein and in the muscle tissue were not different among the groups. Phenylalanine arterial concentrations slightly but significantly (P < 0.05) decreased in all groups, with significant differences between groups (P < 0.05), the largest change occurring in the HD group. Phenylalanine venous concentrations also decreased significantly in all groups with significant differences between groups (P < 0.05), the smallest change observed in the LD group. The muscle concentrations of free phenylalanine decreased significantly only in HD group.
Table 3.
Leg phenylalanine concentrations and kinetics
| LD |
ID |
HD |
|||||
|---|---|---|---|---|---|---|---|
| Basal | Insulin | Basal | Insulin | Basal | Insulin | P | |
| Phenylalanine concentrations, μmol/l | |||||||
| Artery | 62±5 | 58±5a* | 55±2 | 50±2a* | 63±5 | 53±5b* | 0.04 |
| Vein | 68±6 | 63±6a* | 60±2 | 48±2b* | 70±5 | 53±6b* | 0.001 |
| Muscle | 101±10 | 90±7 | 89±9 | 82±6 | 104±9 | 69±7* | 0.08 |
| Phenylalanine kinetics, nmol·min−1·100 ml leg volume−1 | |||||||
| Net balance | −25±9 | −14±4a | −22±5 | 15±4b* | −25±6 | −3±4a,b* | 0.04 |
| Fin | 208±37 | 168±18 | 221±25 | 338±65 | 221±28 | 234±13 | 0.09 |
| Fout | 233±44 | 182±20 | 243±29 | 322±63 | 245±32 | 237±14 | 0.19 |
| Leg Ra | 65±17 | 49±8 | 57±13 | 56±16 | 68±9 | 44±8* | 0.44 |
| Leg Rd | 41±9 | 36±7a | 35±8 | 71±19b* | 43±6 | 41±7a | 0.04 |
| FM,A | 147±42 | 94±20 | 144±35 | 158±41 | 112±19 | 98±25 | 0.37 |
| FV,M | 172±49 | 108±21 | 166±37 | 142±38 | 137±24 | 101±26 | 0.72 |
| FV,A | 61±20 | 73±17 | 77±28 | 180±39* | 109±24 | 136±20 | 0.17 |
| FM,0 | 72±18 | 60±8 | 63±13 | 78±19 | 85±11 | 68±14 | 0.28 |
| F0,M | 47±10 | 47±7 | 40±8 | 93±21* | 60±8 | 65±15 | 0.04 |
| IC availability | 219±55 | 155±23 | 207±45 | 235±57 | 197±26 | 166±31 | 0.32 |
Values are means ± SE. Phenylalanine concentrations in femoral artery and vein and in muscle tissue, net balance, leg Ra, leg Rd, delivery to the leg (Fin) and leg outflow (Fout), transport into (FM,A) and from the muscle (FV,M), a–v shunting (FV,A), release from proteolysis (FM,0), utilization for protein synthesis, and intracellular availability (IC availability) at baseline and during LD, ID, and HD insulin infusion in a leg. P, significance value for 1-way ANOVA on effect of insulin dose on changes from baseline. Common letter denotes that changes from baseline do not differ between groups (Tukey's test).
P < 0.05 vs. basal (paired t-test).
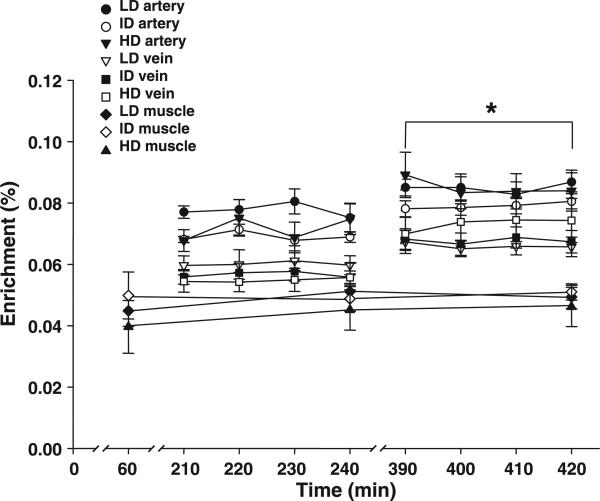
Phenylalanine enrichments in the femoral artery and vein were at steady state during both sampling periods (Fig. 1). Enrichments in the femoral artery and vein and in the muscle tissue were not different among the groups during the basal period (Fig. 1). With insulin infusion, phenylalanine enrichment in the artery significantly increased in all groups, with no group differences. Phenylalanine enrichment in the vein increased significantly in a dose-dependent manner, with the largest changes occurring in the HD group and the smallest changes occurring in the LD group (P < 0.05). Phenylalanine enrichments in the muscle tissue did not change significantly in any of the groups.
Fig. 1.
Phenylalanine enrichments in the femoral artery and vein and in the free tissue water in the basal and postabsorptive state (0−240 min) and during the infusion of a low (LD), intermediate (ID) and high (HD) physiological dose of insulin in one leg. Values are means ± SE. *P < 0.05 vs. basal values for arterial and venous enrichments in all groups.
Phenylalanine kinetics
Leg and muscle phenylalanine kinetics are shown in Table 3. All kinetic parameters were not different between groups in the basal period.
Phenylalanine delivery to the leg (Fin) and the release from the leg (Fout) were not significantly affected by insulin, although Fin tended to increase in the ID group. Phenylalanine net balance increased significantly only in the ID and HD group. Net balance became positive only in the ID group, indicating a shift from net muscle protein loss to net muscle protein deposition in this group. Net balance improved also in the HD group, although it did not reach positive values.
With the two-pool method, the leg Ra decreased significantly with insulin infusion only in the HD group (P < 0.05), whereas no significant change was detected in the LD and ID groups. Insulin infusion significantly increased the leg Rd only in the ID group (P < 0.05).
With the three-pool model, phenylalanine transport into the muscle (FM,A) and out of the muscle (FV,M) did not significantly change during insulin infusion in any of the three groups. With insulin infusion, phenylalanine arteriovenous shunting (FV,A) increased only in the ID group. Phenylalanine release from proteolysis (FM,0) was unaffected by insulin infusion in any of the three groups. Phenylalanine F0,M, which is a direct measure of phenylalanine utilization for muscle protein synthesis, increased significantly only in the ID group and remained unchanged in the LD and HD groups. The ID group had a slightly, but not significantly, lower BMI due to a larger, but not significantly, proportion of female subjects. To assess whether the differences between groups could be due to the subjects’ sex or to differences in body composition, we performed an analysis of covariance, using BMI and sex as covariates, and found that FO,M was still significantly higher in the ID group compared with the others.
Mixed muscle FSR
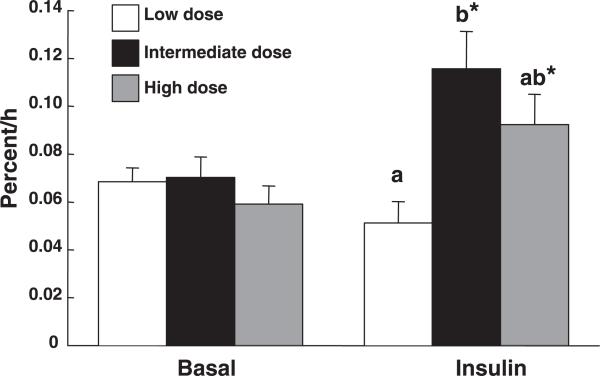
The mixed muscle protein FSR (Fig. 2) was not different among the three groups during the basal period. Insulin infusion affected FSR according to the dose (P < 0.05): it significantly increased FSR in the ID group and, to a lesser extent, in the HD group, whereas FSR remained unchanged in the LD group.
Fig. 2.
Muscle protein fractional synthetic rates in the basal postabsorptive state and during low, intermediate, and high insulin infusion in one leg. Values are means ± SE. A common letter denotes that the changes from the baseline do not differ between groups (Tukey's test). *P < 0.05 vs. basal (paired t-test).
Correlations and stepwise regression
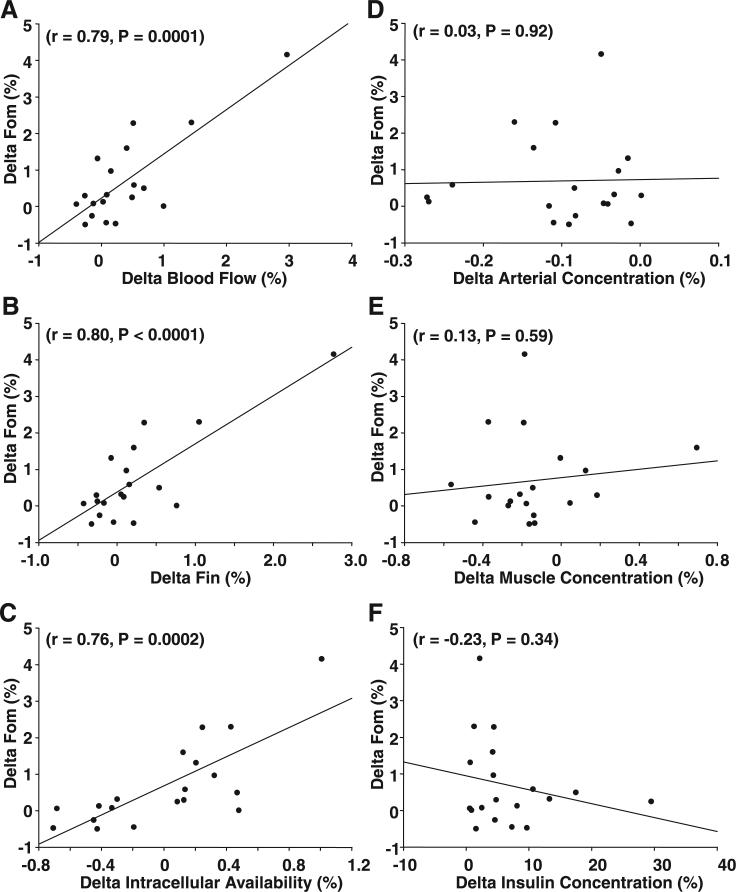
The relative changes above baseline in the rate of protein synthesis (ΔF0,M) were highly associated with the changes in blood flow (r = 0.79, P = 0.0001) as well as with the changes in phenylalanine delivery to the leg (r = 0.80, P < 0.0001), phenylalanine transport into the muscle (FM,A) (r = 0.54, P = 0.016), and intracellular phenylalanine availability (r = 0.76, P = 0.0002; Fig. 3). However, no association was found between the change in protein synthesis and changes in free arterial phenylalanine concentration (r = 0.03, P = 0.92), free muscle phenylalanine concentration (r = 0.13, P = 0.59), femoral insulin concentration (r = −0.23, P = 0.34), or delivery (r = 0.02, P = 0.92).
Fig. 3.
Correlations between the relative changes in muscle protein synthesis (ΔF0,M) and changes in blood flow (A), phenylalanine delivery to the muscle (ΔFin; B), intracellular phenylalanine availability (C), arterial phenylalanine concentration (D), muscle phenylalanine concentration (E), and arterial insulin concentration (F). Values are shown as relative change over the basal postabsorptive period.
The relationships between ΔF0,M and the concomitant changes in several potentially relevant variables were analyzed using two different stepwise regression models. In the first model, we entered as regressors all variables that could potentially affect muscle protein synthesis regardless of collinearity. With this model we found that ΔF0,M was significantly associated with changes in Fin (ΔFin; P < 0.0001), partially associated with changes in phenylalanine concentration in the muscle (ΔCM; P = 0.10), and negatively associated with changes in inward transport (ΔFM,A; P = 0.0054). The final regression equation (R2 = 0.85) with this model was:
However, some potentially meaningful regressors were collinear with others. Thus we developed a second model using only one of the collinear regressors. The choice between collinear regressors was primarily based on ease of measurement and degree of physiological significance. Thus we used blood flow, insulin concentration, phenylalanine arterial concentration, and intracellular phenylalanine availability, and we excluded Fin because it was collinear with blood flow, insulin delivery because it was collinear with insulin concentration, FM,A because it was collinear with intracellular phenylalanine availability, and phenylalanine concentration in the muscle because it was collinear with arterial phenylalanine concentration. In the final model, ΔF0,M was significantly associated with the changes in blood flow (ΔBF; P = 0.0001) and intracellular phenylalanine availability (ΔICPhe; P = 0.12), although the latter did not reach the statistical significance. The final regression equation was:
The R2 for this multiple regression model was 0.72.
DISCUSSION
The results of our experiment indicate that, in healthy young individuals, isolated physiological hyperinsulinemia can stimulate skeletal muscle protein synthesis provided that it increases blood flow and amino acid delivery to the muscle. Specifically, using the local insulin infusion technique in one leg at three physiological doses in the absence of exogenous amino acid replacement, we found that, whereas the insulin effect on muscle glucose uptake was dose dependent, as expected, its effect on muscle amino acid and protein turnover was more complex and significantly relied on the insulin modulation of blood flow and amino acid delivery and availability.
The lower insulin dose induced a twofold increase in leg insulin concentration and insulin delivery to the leg muscle; yet it did not significantly affect blood flow or the arterial phenylalanine delivery to the leg and did not change either muscle protein synthesis or breakdown; nor did it improve net muscle protein anabolism. Despite a slight decrease in the arterial phenylalanine concentration, the intermediate insulin dose tended to increase phenylalanine delivery via increased blood flow. These changes were associated with a significant increase in muscle protein synthesis, no change in breakdown, and the achievement of a net muscle protein anabolic state, confirming previous reports obtained with similar insulin dose and experimental design (5, 8). Finally, the high insulin dose increased blood flow, induced a larger decrease in phenylalanine concentration, and, consequently, did not change phenylalanine delivery. These effects, in turn, did not affect muscle protein synthesis (when the arteriovenous balance methods were used), or they increased it but to a lesser extent than in the intermediate dose group (when the precursor-product model was used) and tended to reduce muscle protein breakdown. As a result, the high dose improved net muscle protein balance, albeit to a lesser extent than the intermediate dose. Interestingly, the change in muscle protein synthesis was predicted mostly by changes in blood flow and phenylalanine availability, both extracellular (delivery to the leg) and intracellular (concentration and intracellular flux), whereas insulin concentrations and delivery did not significantly predict the response of protein synthesis.
All together, these data indicate that, although insulin can directly stimulate initiation of translation (23-25), its stimulatory effect on human skeletal muscle protein synthesis is modulated by increases in muscle perfusion and amino acid delivery and availability for the muscle tissue. When muscle perfusion increases as a consequence of hyperinsulinemia, more tissue is exposed to the nutrients contained in the blood. However, if arterial amino acid concentrations decrease too much (as they did in the HD group), the increase in blood flow may be insufficient to overcome the consequent decline in amino acid delivery. Because amino acids, particularly leucine, can directly stimulate initiation of mRNA translation via pathways partially shared with insulin (2), it is possible that their contribution is necessary for an adequate response of muscle protein synthesis to insulin. From a teleological standpoint, this is not surprising if we consider that insulin is normally secreted during meal absorption, which increases amino acid availability.
Our findings may allow for the reconciliation of the conflicting studies previously published on the effects of insulin on human skeletal muscle protein synthesis (4, 5, 20, 33, 34, 53). In light of the results of the present study, these discrepancies may be explained by differences in muscle amino acid availability, as the studies finding a positive effect of insulin on protein synthesis also reported an increase in amino acid delivery to the muscle (4, 5, 20, 33, 34, 53). Conversely, those reporting no change or a decrease in protein synthesis also reported a decrease in amino acid concentration and a decrease or no change in amino acid delivery (14, 19, 28, 32, 34). In only one experiment, increased amino acid delivery was not associated with a change in muscle protein synthesis (17). It is possible that this was due to either increased intracellular amino acid recycling, not detectable with the two-pool model employed in that study, and/or the use of the forearm as opposed to the leg as a sampling site, because of the potential differential contribution of skin and/or bone marrow protein turnover to leg and forearm protein turnover.
The considerations above underscore the difficulties encountered when trying to isolate the physiological effects of insulin on skeletal muscle protein turnover apart from the context of a meal. The problem is much more complicated than that offered by the insulin regulation of muscle glucose turnover, because glucose alone cannot significantly stimulate its own utilization, whereas amino acids, particularly leucine, can stimulate muscle protein synthesis by stimulating initiation of translation (2, 41, 42, 52). Thus, when insulin is infused to reach physiological postprandial concentrations, the infusion route is a very important variable, because a systemic infusion will decrease blood amino acid concentrations unless they are replaced by exogenous infusion. On the other hand, a local insulin infusion will not decrease amino acid concentrations unless the dose is large enough to exert systemic effects, as seen in our HD group. Additionally, if a systemic or a large local insulin infusion is chosen, then the decision as to whether or not to concomitantly provide exogenous amino acids during hyperinsulinemia is a crucial step that can significantly affect the outcome of the experiment. Either way, there will be an additional variable to account for: decreased amino acid concentration and delivery or increased amino acid delivery and availability as a result of the exogenous infusion, which may per se increase muscle protein synthesis (7).
Furthermore, the sampling site (leg or forearm) is another critical factor because of the possible differential contribution of the nonmuscle tissues (mostly skin and bone) to each limb protein turnover. Skin protein breakdown is decreased and skin protein synthesis is unaffected by insulin (56), whereas bone marrow proteins are likely to turn over faster than muscle proteins, given the high turnover rate of bone marrow cells, but no data are available on the effects of insulin on these cells. The choice of the model to calculate muscle protein turnover can also significantly affect the conclusions of the study. In our experiment, we used all of the three models available to measure human muscle protein synthesis: the two arteriovenous balance methods [two-pool (54) and three-pool (6) models] and the precursor-product model (47). This decision was made because most earlier studies had been performed using the two-pool arteriovenous balance method (14, 17, 19, 20, 28, 32-34, 53). Thus the use of that method allowed us to compare previous results with ours. However, that method cannot detect increases in intracellular amino acid recycling from breakdown into synthesis because it measures the utilization of plasma amino acids for synthesis and the release in the plasma of amino acids deriving from proteolysis. The three-pool model allows for the measurement of the total synthesis and breakdown rates, including recycling (6), and the precursor-product model provides a measure of the overall effect of the treatment over the entire experimental period.
All three methods indicated that the intermediate insulin dose induced a significant increase in muscle protein synthesis, whereas the low dose did not significantly affect any of the muscle protein metabolic parameters. However, although the precursor-product model indicated that the high insulin dose could stimulate muscle protein synthesis, albeit less than the intermediate dose, both the two-pool and the three-pool models did not confirm this result. This is likely due to the timing of the measurements. The parameters of the two arteriovenous balance models were measured during the last hour of each study period, whereas the FSR was measured over the last 3 h of each period, thus providing an average protein synthesis rate for each incorporation period. We hypothesize that the high insulin dose initially stimulated muscle protein synthesis during the early stages of the infusion period and that by the third hour, when the samples for the arteriovenous balance techniques were collected, this effect had vanished, probably due to the decrease in amino acid concentrations and/or availability. Nonetheless, the overall effect of the higher insulin dose on muscle protein synthesis was less pronounced than that of the intermediate dose and therefore does not contradict the general interpretation of our data. However, further studies on the earlier effects of high insulin infusions on skeletal muscle protein synthesis are warranted. Also, animal data suggest that insulin may exert protein-specific effects (10) that were not measured in the present and previous human studies (4, 5, 14, 17-20, 28, 32-34, 53). Thus future studies should also assess the role of insulin modulation of muscle perfusion and amino acid availability on the regulation of the synthesis rates of specific muscle proteins.
Finally, the insulin infusion significantly increased leg blood flow in the ID and HD groups, although no difference was detected between the two groups. Thus there was a dose-response effect of blood flow that apparently reached a maximum with the intermediate dose. Because the blood flow response to insulin is nitric oxide dependent (43), it is possible that the relatively smaller vasodilatory response in the HD group was due to reduced arginine availability for nitric oxide production. We did not measure arginine concentration, but it has been shown that blood arginine declines with insulin to approximately the same extent as phenylalanine (14, 29). The lower blood flow response to insulin in the HD group might have been partially responsible for the blunted response of protein synthesis in that group. Had blood flow increased in the high-insulin dose group more than in the intermediate-dose group, thus increasing amino acid delivery, on the basis of our regression models we would have expected a larger increase in muscle protein synthesis with the high insulin dose. Further studies are necessary to test this hypothesis.
In summary, our study indicates that, in healthy young individuals, physiological hyperinsulinemia can stimulate muscle protein synthesis provided that it increases blood flow and amino acid delivery to and availability for the muscle tissue. Thus muscle perfusion seems to be a critical factor in the insulin regulation of muscle protein turnover. This may be an important issue in certain conditions such as diabetes or aging, since amino acid delivery may not increase during hyperinsulinemia due to a decreased vasodilatory response to insulin (26, 30, 44, 51).
ACKNOWLEDGMENTS
We thank Jeanine Cordero for technical assistance, the study volunteers for their patience and dedication, and all the nurses and personnel of the General Clinical Research Center of the University of Southern California for help with the conduct of the clinical portion of this study.
Footnotes
GRANTS
This study was supported by National Institutes of Health Grants R01-AG-18311 from the National Institute on Aging, S10-RR-16650 from the Shared Instrumentation Grant Program, National Center for Research Resources, and M01-RR-43 from the General Clinical Research Branch, National Center for Research Resources.
REFERENCES
- 1.Abu-Lebdeh HS, Nair KS. Protein metabolism in diabetes mellitus. Baillières Clin Endocrinol Metab. 1996;10:589–601. doi: 10.1016/s0950-351x(96)80741-5. [DOI] [PubMed] [Google Scholar]
- 2.Anthony JC, Anthony TG, Kimball SR, Jefferson LS. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J Nutr. 2001;131:856S–860S. doi: 10.1093/jn/131.3.856S. [DOI] [PubMed] [Google Scholar]
- 3.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 4.Bennet WM, Connacher AA, Scrimgeour CM, Jung RT, Rennie MJ. Euglycemic hyperinsulinemia augments amino acid uptake by leg tissues during hyperaminoacidemia. Am J Physiol Endocrinol Metab. 1990;259:E185–E194. doi: 10.1152/ajpendo.1990.259.2.E185. [DOI] [PubMed] [Google Scholar]
- 5.Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest. 1995;95:811–819. doi: 10.1172/JCI117731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biolo G, Fleming RY, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol Endocrinol Metab. 1995;268:E75–E84. doi: 10.1152/ajpendo.1995.268.1.E75. [DOI] [PubMed] [Google Scholar]
- 7.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol Endocrinol Metab. 1997;273:E122–E129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- 8.Biolo G, Williams BD, Fleming RY, Wolfe RR. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes. 1999;48:949–957. doi: 10.2337/diabetes.48.5.949. [DOI] [PubMed] [Google Scholar]
- 9.Blommaart EF, Luiken JJ, Meijer AJ. Autophagic proteolysis: control and specificity. Histochem J. 1997;29:365–385. doi: 10.1023/a:1026486801018. [DOI] [PubMed] [Google Scholar]
- 10.Boirie Y, Short KR, Ahlman B, Charlton M, Nair KS. Tissue-specific regulation of mitochondrial and cytoplasmic protein synthesis rates by insulin. Diabetes. 2001;50:2652–2658. doi: 10.2337/diabetes.50.12.2652. [DOI] [PubMed] [Google Scholar]
- 11.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002−0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom. 1992;6:421–424. doi: 10.1002/rcm.1290060704. [DOI] [PubMed] [Google Scholar]
- 12.Chevalier S, Gougeon R, Kreisman SH, Cassis C, Morais JA. The hyperinsulinemic amino acid clamp increases whole-body protein synthesis in young subjects. Metabolism. 2004;53:388–396. doi: 10.1016/j.metabol.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 13.De Feo P, Volpi E, Lucidi P, Cruciani G, Reboldi G, Siepi D, Mannarino E, Santeusanio F, Brunetti P, Bolli GB. Physiological increments in plasma insulin concentrations have selective and different effects on synthesis of hepatic proteins in normal humans. Diabetes. 1993;42:995–1002. doi: 10.2337/diab.42.7.995. [DOI] [PubMed] [Google Scholar]
- 14.Denne SC, Liechty EA, Liu YM, Brechtel G, Baron AD. Proteolysis in skeletal muscle and whole body in response to euglycemic hyperinsulinemia in normal adults. Am J Physiol Endocrinol Metab. 1991;261:E809–E814. doi: 10.1152/ajpendo.1991.261.6.E809. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez C, Sainz RD. Pathways of protein degradation in L6 myotubes. Proc Soc Exp Biol Med. 1997;214:242–247. doi: 10.3181/00379727-214-44092. [DOI] [PubMed] [Google Scholar]
- 16.Garlick PJ, Grant I. Amino acid infusion increases the sensitivity of muscle protein synthesis in vivo to insulin. Effect of branched-chain amino acids. Biochem J. 1988;254:579–584. doi: 10.1042/bj2540579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelfand RA, Barrett EJ. Effect of physiologic hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man. J Clin Invest. 1987;80:1–6. doi: 10.1172/JCI113033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillet C, Prod'homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 2004;18:1586–158. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- 19.Heslin MJ, Newman E, Wolf RF, Pisters PW, Brennan MF. Effect of hyperinsulinemia on whole body and skeletal muscle leucine carbon kinetics in humans. Am J Physiol Endocrinol Metab. 1992;262:E911–E918. doi: 10.1152/ajpendo.1992.262.6.E911. [DOI] [PubMed] [Google Scholar]
- 20.Hillier TA, Fryburg DA, Jahn LA, Barrett EJ. Extreme hyperinsulinemia unmasks insulin's effect to stimulate protein synthesis in the human forearm. Am J Physiol Endocrinol Metab. 1998;274:E1067–E1074. doi: 10.1152/ajpendo.1998.274.6.E1067. [DOI] [PubMed] [Google Scholar]
- 21.Jorfeldt L, Juhlin-Dannfelt A. The influence of ethanol on splanchnic and skeletal muscle metabolism in man. Metabolism. 1978;27:97–106. doi: 10.1016/0026-0495(78)90128-2. [DOI] [PubMed] [Google Scholar]
- 22.Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci. 1971;41:459–473. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- 23.Kimball SR, Horetsky RL, Jefferson LS. Signal transduction pathways involved in the regulation of protein synthesis by insulin in L6 myoblasts. Am J Physiol Cell Physiol. 1998;274:C221–C228. doi: 10.1152/ajpcell.1998.274.1.C221. [DOI] [PubMed] [Google Scholar]
- 24.Kimball SR, Jefferson LS, Fadden P, Haystead TA, Lawrence JC., Jr Insulin and diabetes cause reciprocal changes in the association of eIF-4E and PHAS-I in rat skeletal muscle. Am J Physiol Cell Physiol. 1996;270:C705–C709. doi: 10.1152/ajpcell.1996.270.2.C705. [DOI] [PubMed] [Google Scholar]
- 25.Kimball SR, Jurasinski CV, Lawrence JC, Jr, Jefferson LS. Insulin stimulates protein synthesis in skeletal muscle by enhancing the association of eIF-4E and eIF-4G. Am J Physiol Cell Physiol. 1997;272:C754–C759. doi: 10.1152/ajpcell.1997.272.2.C754. [DOI] [PubMed] [Google Scholar]
- 26.Laakso M, Edelman SV, Brechtel G, Baron AD. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes. 1992;41:1076–1083. doi: 10.2337/diab.41.9.1076. [DOI] [PubMed] [Google Scholar]
- 27.Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE. Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol. 2004;15:1537–1545. doi: 10.1097/01.asn.0000127211.86206.e1. [DOI] [PubMed] [Google Scholar]
- 28.Louard RJ, Fryburg DA, Gelfand RA, Barrett EJ. Insulin sensitivity of protein and glucose metabolism in human forearm skeletal muscle. J Clin Invest. 1992;90:2348–2354. doi: 10.1172/JCI116124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNurlan MA, Essen P, Thorell A, Calder AG, Anderson SE, Ljungqvist O, Sandgren A, Grant I, Tjader I, Ballmer PE. Response of protein synthesis in human skeletal muscle to insulin: an investigation with l-[2H5]phenylalanine. Am J Physiol Endocrinol Metab. 1994;267:E102–E108. doi: 10.1152/ajpendo.1994.267.1.E102. [DOI] [PubMed] [Google Scholar]
- 30.Meneilly GS, Elliot T, Bryer-Ash M, Floras JS. Insulin-mediated increase in blood flow is impaired in the elderly. J Clin Endocrinol Metab. 1995;80:1899–1903. doi: 10.1210/jcem.80.6.7775638. [DOI] [PubMed] [Google Scholar]
- 31.Miller S, Chinkes D, MacLean DA, Gore D, Wolfe RR. In vivo muscle amino acid transport involves two distinct processes. Am J Physiol Endocrinol Metab. 2004;287:E136–E141. doi: 10.1152/ajpendo.00092.2004. [DOI] [PubMed] [Google Scholar]
- 32.Moller-Loswick AC, Zachrisson H, Hyltander A, Korner U, Matthews DE, Lundholm K. Insulin selectively attenuates breakdown of nonmyofibrillar proteins in peripheral tissues of normal men. Am J Physiol Endocrinol Metab. 1994;266:E645–E652. doi: 10.1152/ajpendo.1994.266.4.E645. [DOI] [PubMed] [Google Scholar]
- 33.Newman E, Heslin MJ, Wolf RF, Pisters PW, Brennan MF. The effect of systemic hyperinsulinemia with concomitant amino acid infusion on skeletal muscle protein turnover in the human forearm. Metabolism. 1994;43:70–78. doi: 10.1016/0026-0495(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 34.Nygren J, Nair KS. Differential regulation of protein dynamics in splanchnic and skeletal muscle beds by insulin and amino acids in healthy human subjects. Diabetes. 2003;52:1377–1385. doi: 10.2337/diabetes.52.6.1377. [DOI] [PubMed] [Google Scholar]
- 35.O'Connor PM, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2003;285:E40–E53. doi: 10.1152/ajpendo.00563.2002. [DOI] [PubMed] [Google Scholar]
- 36.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–E328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 37.Pain VM, Albertse EC, Garlick PJ. Protein metabolism in skeletal muscle, diaphragm, and heart of diabetic rats. Am J Physiol Endocrinol Metab. 1983;245:E604–E610. doi: 10.1152/ajpendo.1983.245.6.E604. [DOI] [PubMed] [Google Scholar]
- 38.Pain VM, Garlick PJ. Effect of streptozotocin diabetes and insulin treatment on the rate of protein synthesis in tissues of the rat in vivo. J Biol Chem. 1974;249:4510–4514. [PubMed] [Google Scholar]
- 39.Reeds PJ, Fjeld CR, Jahoor F. Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states? J Nutr. 1994;124:906–910. doi: 10.1093/jn/124.6.906. [DOI] [PubMed] [Google Scholar]
- 40.Sinaud S, Balage M, Bayle G, Dardevet D, Vary TC, Kimball SR, Jefferson LS, Grizard J. Diazoxide-induced insulin deficiency greatly reduced muscle protein synthesis in rats: involvement of eIF4E. Am J Physiol Endocrinol Metab. 1999;276:E50–E61. doi: 10.1152/ajpendo.1999.276.1.E50. [DOI] [PubMed] [Google Scholar]
- 41.Smith K, Barua JM, Watt PW, Scrimgeour CM, Rennie MJ. Flooding with [1-13C]leucine stimulates human muscle protein incorporation of continuously infused l-[1-13C]valine. Am J Physiol Endocrinol Metab. 1992;262:E372–E376. doi: 10.1152/ajpendo.1992.262.3.E372. [DOI] [PubMed] [Google Scholar]
- 42.Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol Endocrinol Metab. 1998;275:E73–E78. doi: 10.1152/ajpendo.1998.275.1.E73. [DOI] [PubMed] [Google Scholar]
- 43.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94:1172–1179. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tack CJ, Ong MK, Lutterman JA, Smits P. Insulin-induced vasodilatation and endothelial function in obesity/insulin resistance. Effects of troglitazone. Diabetologia. 1998;41:569–576. doi: 10.1007/s001250050948. [DOI] [PubMed] [Google Scholar]
- 45.Tessari P, Inchiostro S, Biolo G, Vincenti E, Sabadin L. Effects of acute systemic hyperinsulinemia on forearm muscle proteolysis in healthy man. J Clin Invest. 1991;88:27–33. doi: 10.1172/JCI115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toffolo G, Albright R, Joyner M, Dietz N, Cobelli C, Nair KS. Model to assess muscle protein turnover: domain of validity using amino acyl-tRNA vs. surrogate measures of precursor pool. Am J Physiol Endocrinol Metab. 2003;285:E1142–E1149. doi: 10.1152/ajpendo.00106.2003. [DOI] [PubMed] [Google Scholar]
- 47.Toffolo G, Foster DM, Cobelli C. Estimation of protein fractional synthetic rate from tracer data. Am J Physiol Endocrinol Metab. 1993;264:E128–E135. doi: 10.1152/ajpendo.1993.264.1.E128. [DOI] [PubMed] [Google Scholar]
- 48.Utriainen T, Malmstrom R, Makimattila S, Yki-Jarvinen H. Methodological aspects, dose-response characteristics and causes of inter-individual variation in insulin stimulation of limb blood flow in normal subjects. Diabetologia. 1995;38:555–564. doi: 10.1007/BF00400724. [DOI] [PubMed] [Google Scholar]
- 49.Volpi E, Kobayashi H, Mittendorfer B, Sheffield-Moore M, Wolfe RR. Essential amino acids are primarily responsible for the amino acid-stimulation of muscle protein anabolism in healthy older adults. Am J Clin Nutr. 2003;78:250–258. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Volpi E, Lucidi P, Monacchia F, Cruciani G, Reboldi G, Santeusanio F, Brunetti P, Bolli GB, De Feo P. Contribution of amino acids and insulin to protein anabolism during meal absorption. Diabetes. 1996;45:1245–1252. doi: 10.2337/diab.45.9.1245. [DOI] [PubMed] [Google Scholar]
- 51.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–4490. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first pass splanchnic extraction. Am J Physiol Endocrinol Metab. 1999;277:E513–E520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 53.Wolf RF, Heslin MJ, Newman E, Pearlstone DB, Gonenne A, Brennan MF. Growth hormone and insulin combine to improve whole-body and skeletal muscle protein kinetics. Surgery. 1992;112:284–292. [PubMed] [Google Scholar]
- 54.Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine. Principles and Practice of Kinetic Analysis. Wiley-Liss; New York: 1992. [Google Scholar]
- 55.Wolfe RR, Volpi E. Insulin and protein metabolism. In: Jefferson LS, Cherrington AD, editors. The Endocrine Pancreas and Regulation of Metabolism. Oxford Univ. Press; New York: 2000. pp. 733–755. [Google Scholar]
- 56.Zhang XJ, Chinkes DL, Wolf SE, Wolfe RR. Insulin but not growth hormone stimulates protein anabolism in skin wound and muscle. Am J Physiol Endocrinol Metab. 1999;276:E712–E720. doi: 10.1152/ajpendo.1999.276.4.E712. [DOI] [PubMed] [Google Scholar]