Abstract
The aim of this investigation was to test the hypothesis that skin grafting (5–9 months after surgery) impairs sympathetically mediated cutaneous vasoconstrictor responsiveness. Skin blood flow (laser-Doppler flowmetry) was assessed in grafted skin and adjacent healthy control skin in fourteen subjects (seven male, seven female) during indirect whole-body cooling (ie, cooling the entire body, except the area where skin blood flow was assessed), as well as local cooling (ie, only cooling the area where skin blood flow was assessed). Whole-body cooling was performed by perfusing 5°C water through a water perfusion suit for 3 minutes. Local cooling was performed on a separate visit using a custom Peltier cooling device, which decreased local skin temperature from 39 °C to 19 in 5 °C decrements in 15-minute stages. Cutaneous vascular conductance (CVC) was calculated from the ratio of skin blood flow to mean arterial pressure. Indirect whole-body cooling decreased CVC from baseline (ΔCVC) similarly (P = 0.17) between grafted skin (ΔCVC = −0.23 ± 0.04 au/mm Hg) and adjacent healthy skin (ΔCVC = −0.16 ± 0.02 au/mm Hg). Likewise, decreasing local skin temperature from 39 to 19 °C resulted in similar decreases (P = .82) in CVC between grafted skin (ΔCVC = −1.11 ± 0.18 au/mm Hg) and adjacent healthy skin (ΔCVC = −1.06 ± 0.18 au/mm Hg). Appropriate cutaneous vasoconstriction in grafted skin to both indirect whole-body and local cooling indicates re-innervation of the cutaneous vasoconstrictor system at the graft site. These data suggest that persons with significant skin grafting may have a normal capacity to regulate body temperature during cold exposure by cutaneous vasoconstriction.
Skin blood flow is neurally controlled via two distinct sympathetic pathways: a sympathetic vasoconstrictor system and a nonadrenergic sympathetic active vasodilator system.1,2 The cutaneous active vasodilator system mediates 85 to 95% of the rise in skin blood flow in nonglabrous (ie, hairy) skin during whole-body heating.3,4 Importantly, active cutaneous vasodilation is absent in denervated skin.2,5–7 Thus, an intact functioning cutaneous sympathetic active vasodilator system must be present for the skin to appropriately dilate during exposure to a thermal stress. We have previously reported that split-thickness skin grafts 5 to 9 months after surgery have impaired cutaneous vasodilation and sweating in response to indirect whole-body heating, indicating that grafted skin has an attenuated capability of contributing to thermoregulation during heat stress.8 Further investigation revealed that altered postsynaptic function possibly contributes to these impaired cutaneous vasodilator responses.9 However, these findings did not rule out the possibility that impaired vasodilation may simply be due to disrupted innervation of the cutaneous vasculature (ie, decreased number of functional nerves innervating cutaneous vessels).8
Another approach to determine possible altered cutaneous innervation in grafted skin is to examine the second system governing skin blood flow, the sympathetic vasoconstrictor system. Modulation of the cutaneous vasoconstrictor system is a vital mechanism for thermoregulation in cool, thermoneutral, and moderately warm environments. During cold exposure, cutaneous sympathetic vasoconstrictor nerve activity increases.10 This increased nerve activity leads to the release of norepinephrine and subsequent cutaneous vasoconstriction.1,11,12 Modulation of vasoconstrictor nerve activity also tightly regulates body temperature during thermoneutral conditions.1 Removal of this tonic vasoconstrictor activity leads to increases in skin blood flow at rest even in cool environments. 4,13 Related to this point, previously observed small elevations in skin blood flow in grafted skin during normothermia may be attributed to a lack of tonic sympathetic vasoconstrictor activity.8 A lack of vasoconstrictor activity coupled with previous observations of impaired vasodilation during whole-body heating may together suggest absence of sympathethic innervation to grafted skin.8
Therefore, the primary aim of the present investigation was to test the hypothesis that cutaneous vasoconstrictor responses during indirect whole-body cooling and local cooling would be attenuated in juvenile (5–9 months after surgery) split-thickness grafted skin compared with adjacent control skin. Impairments in the cutaneous vasoconstrictor system could have a significant impact on the ability to thermoregulate in cool, thermoneutral, and moderately warm environments.
METHODS
Protocol 1: Whole-Body Cooling
Human Subjects
Fourteen subjects (seven male, seven female) who had undergone split-thickness autograft application after tangential excision to viable fat (no visible dermis remaining) within the prior 5 to 9 months participated in this study. Patients with superficial and deep dermal excisions were not enrolled. The mean (±SEM) age, height, and weight of the subjects were 32.6 ± 2.6 years, 168.1 ± 2.6 cm, and 82.1 ± 4.8 kg, respectively. Participants provided informed written consent before testing. All protocols were approved by the Institutional Review Boards at the University of Texas Southwestern Medical Center at Dallas and Presbyterian Hospital of Dallas and were conducted in accordance with the Declaration of Helsinki principles. Subjects were not taking any medications that would affect cutaneous vasoconstrictor responses. All subjects refrained from caffeine, alcohol, and exercise for 24 hours before the study.
Instrumentation
Heart rate was obtained from an electrocardiogram (Agilent, Palo Alto, CA), with the signal interfaced with a cardiotachometer (CWE, Ardmore, PA). Arterial blood pressure was measured from the brachial artery using electrosphygmomanometry (SunTech, Raleigh, NC). Internal temperature was measured from an ingestible pill telemetry system (HTI Technologies, Palmetto, FL). The telemetry pill correlates well with other methods of internal temperature measurement.14 Mean skin temperature was measured by the weighted average of six thermocouples attached to the skin.15 Skin blood flux (index of flow) from both grafted and adjacent control skin was measured continuously from integrating laser-Doppler flowmetry probes (model PF413, Perimed, Sweden), each housed in a 3-cm diameter heater element placed on grafted and adjacent control skin. The integrating laser-Doppler flow probes continuously measured skin blood flow over a small area (~0.28 cm2) throughout the entire protocol.16
Laser-Doppler flowmetry derives an index of skin blood flow from both the velocity of blood in cutaneous vessels and a concentration component, which is a derivation of the number of photons that are Doppler shifted. Laser-Doppler flowmetry is widely accepted as the standard for measurement of cutaneous blood flow and has been shown to correlate in a linear fashion with blood flow measured using established techniques including plethysmography and isotope clearance.16–18 This method also provides a noninvasive index of skin blood flow independent of muscle blood flow.19
Protocol
Subjects were dressed in a tube-lined suit that permitted the control of skin and core temperature by changing the temperature of water perfusing the suit (Med-Eng, Ottawa, Canada). The perfusion suit covered the entire body with the exception of the head, hands, feet, and instrumented area (Table 1). Instrumented areas were not in contact with the suit. Therefore, any observed changes in skin blood flow associated with the cold stress were not due to the effects of locally cooling the skin. Data were collected with the subject in the supine position. Baseline normothermic measurements were obtained while perfusing the suit with 34 °C water. Following normothermic data collection, a whole-body cold stress ensued by perfusing 5 °C water through the suit for 3 minutes. On completion of whole-body cold stress, normothermic water was perfused through the suit and local heating was performed by increasing local skin temperature to 42 °C via heating elements housing the laser-Doppler flow probes. Local temperature was held at this level for 30 minutes to elicit maximal cutaneous vasodilation.20 Skin blood flow responses to the cold stress were then normalized relative to maximal cutaneous vasodilation for each site.
Table 1.
Location of split thickness skin graft and skin donor site
| Subject No. | Gender | Graft Site | Donor Site |
|---|---|---|---|
| 1 | Female | Left Forearm | Right Thigh |
| 2 | Male | Right Forearm | Right Calf |
| 3 | Male | Left Thigh | Left Thigh |
| 4 | Male | Right Forearm | Right Thigh |
| 5 | Female | Right Hand/Wrist | Right Thigh |
| 6 | Female | Right Forearm | Right Thigh |
| 7 | Female | Left Calf | Right Thigh |
| 8 | Female | Left Calf | Right Thigh |
| 9 | Male | Right Forearm | Right Thigh |
| 10 | Male | Left Forearm | Right Thigh |
| 11 | Female | Right Arm | Right Thigh |
| 12 | Male | Left Hand/Wrist | Left Thigh |
| 13 | Male | Right Forearm | Right Thigh |
| 14* | Female | Left Calf | Right Thigh |
Individuals who did not complete Protocol 2.
Protocol 2: Local Cooling
Human Subjects
Thirteen subjects (seven male, six female) returned to the laboratory a minimum of 48 hours after completing Protocol 1 to participate in the local cooling protocol (Table 1).
Instrumentation
Subjects were instrumented in the same manner as described above for heart rate and arterial blood pressure. Skin blood flow was measured continuously from both grafted and adjacent control skin using integrating laser-Doppler flowmetry probes (model PF413, Perimed, Sweden), each housed in a custom-designed Peltier heating-cooling device. A thermocouple was placed between the skin and the Peltier device allowing for precise control and recording of local skin temperature at graft sites and adjacent control skin.
Protocol
Local skin temperature was initially increased to 39 °C over a period of 10 minutes and maintained at this level for 15 minutes to reduce tonic vasoconstriction.21 After heating to 39 °C, local skin temperature was decreased to 19 °C in 5 °C decrements (ie, to 34, 29, 24, 19 °C) over 15-minute intervals. For each local temperature, local skin temperature was decreased during the first 10 minutes to the desired temperature and was then maintained at that temperature for 5 minutes.
Data and Statistical Analysis
For both protocols, data were continuously acquired at a sampling rate of 50 Hz using a data collection system (Biopac System, Santa Barbara, CA). Cutaneous vascular conductance (CVC) was calculated from laser-Doppler derived skin blood flow divided by mean arterial blood pressure. During indirect whole-body cooling, 1-minute-averaged responses were calculated from the final minute of the normothermic baseline period and the whole-body cooling period. CVC data were also normalized to maximal vasodilation obtained during the final minute of local heating at 42 °C and expressed as percentage of maximum CVC (CVCmax). Because of the heterogeneity of skin blood flow, coupled with the inability of laser-Doppler flowmetry to provide an absolute blood flow (eg, in milliliters per volume of tissue per minute), laser-Doppler flow measurements are typically expressed as a percentage of CVCmax. However, data are presented as both absolute CVC units (au/mm Hg) and as a percentage of CVCmax, because of potential differences in baseline skin blood flow and differences in maximal skin blood flows during local heating between grafted and adjacent control skin. During local cooling, 1-minute-averaged responses were calculated during the final minute of each local temperature stage.
A two-way repeated-measures analysis of variance (ANOVA) with main factors of skin site and thermal condition was used to compare CVC responses at grafted and adjacent control skin during indirect whole-body cooling. Bonferroni post hoc tests were used if a significant main effect was observed. Student paired t-tests were also used to compare the differences in maximal CVC obtained during local heating. A two-way repeated-measures ANOVA with main factors of skin site and local skin temperature was used to compare CVC responses at grafted and adjacent control skin during local cooling. Bonferroni post hoc tests were used if a significant main effect was observed. Statistical significance was accepted at P < .05. All data are presented as mean ± SEM.
RESULTS
Protocol 1: Whole-Body Cooling
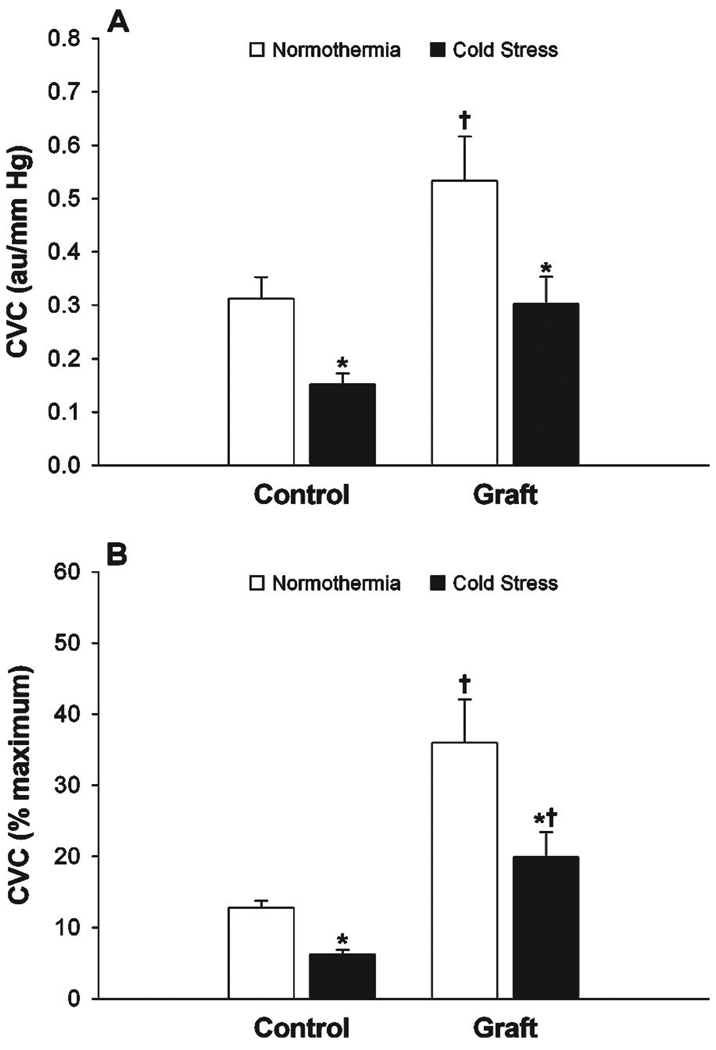
Normothermic baseline CVC was higher (P =.020) in grafted skin (0.53 ± 0.08 au/mm Hg; 36.0 ± 6.1% CVCmax) compared with adjacent control skin (0.31 ± 0.04 au/mm Hg; 12.8 ± 0.9% CVCmax) whether data were expressed in absolute units or as percentages of maximum (Figure 1). Indirect whole-body cooling significantly decreased (P<.001) mean skin temperature from 34.7 ± 0.1 to 31.8 ± 0.3 °C. This perturbation significantly decreased absolute CVC from baseline in grafted skin (0.53 ± 0.08 to 0.30 ± 0.05 au/mmHg; P < .001). A similar decrease in CVC from baseline (0.31 ± 0.04 to 0.15 ± 0.02 au/mmHg; P<.001) was observed in adjacent control skin. The absolute CVC decrease from baseline in grafted skin (ΔCVC = −0.16 ± 0.02 au/mm Hg) tended to be lower (P = .09) than the absolute CVC decrease observed in adjacent control skin (ΔCVC = −0.23 ± 0.04 au/mm Hg). However, when expressed as % of maximum, the decrease in CVC from baseline in grafted skin (ΔCVC = −16.1 ± 3.4% CVCmax) was significantly larger (P =.02) than adjacent control skin (ΔCVC = −6.6 ± 0.06% CVCmax) because of differences in maximal CVC during local heating. Maximal CVC from local heating to 42 °C tended to be lower (P = .11) in grafted skin (1.80 ± 0.28 au/mm Hg) when compared with adjacent control skin (2.46 ± 0.26 au/mm Hg).
Figure 1.
Cutaneous vascular conductance (CVC) expressed as absolute units (panel A) and percentage of CVC maximum (panel B) during normothermia (white bars) and whole-body cooling (black bars) in grafted skin (graft) and adjacent control skin (control). * indicates difference from normothermia (P < .05). † indicates difference from Control (P < .05).
Protocol 2: Local Cooling
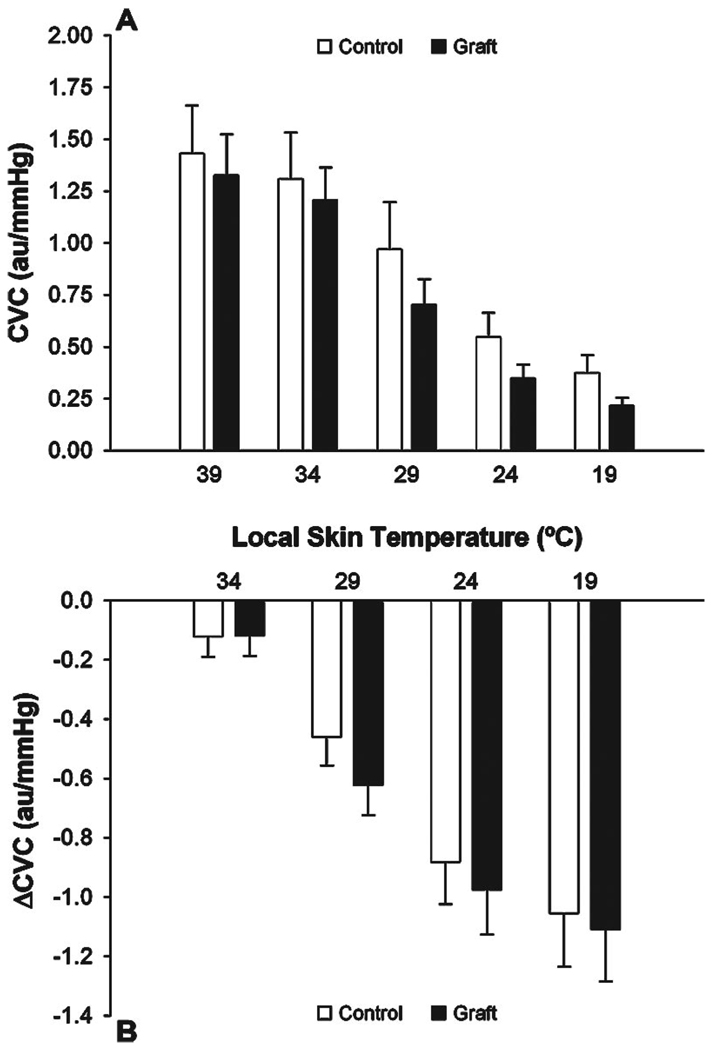
CVC at a local skin temperature of 39 °C was not different in grafted skin (1.33 ± 0.20 au/mm Hg) compared with adjacent control skin (1.43 ± 0.23 au/mm Hg). Cooling 39 to 19 °C caused a similar decrease (P = .82) in CVC in both grafted (ΔCVC = −1.11 ± 0.18 au/mm Hg) and adjacent control skin (ΔCVC = −1.06 ± 0.18 au/mm Hg). No differences were observed in CVC between grafted sites and adjacent control skin at each local cooling temperature stage (Figure 2).
Figure 2.
Cutaneous vascular conductance (CVC) expressed as absolute units (panel A) and as change from 39 °C (panel B) during local cooling in grated skin (graft) and adjacent control skin (control). Local cooling caused clear decreases in CVC at both the graft and adjacent control sites, with the magnitude of the decrease in CVC not being affected by skin grafting.
DISCUSSION
The primary finding of this investigation is that appropriate cutaneous vasoconstriction was observed in grafted skin 5 to 9 months after surgery during indirect whole-body cooling and local cooling when compared with adjacent control skin. These novel findings indicate appropriate innervation of the cutaneous vasoconstrictor system in grafted skin. This is in contrast to previously identified impairments in cutaneous active vasodilation during whole-body heating in grafted skin.8
Control of skin blood flow during cold exposure occurs through both reflex and local mechanisms.22 Reflex vasoconstriction primarily mediates constrictor responses during indirect whole-body cooling through adrenergic and nonadrenergic mechanisms. During such cold exposure, cutaneous sympathetic vasoconstrictor nerve activity increases leading to release of norepinephrine, along with vasoconstrictor co-transmitters, causing subsequent cutaneous vasoconstriction. 5,10–12,23,24 To vasoconstrict in response to indirect whole-body cooling, grafted skin must have functional adrenergic nerves, functional α-adrenergic receptors on the cutaneous vasculature, and normal smooth muscle responses to α-adrenergic receptor stimulation.
Decreasing local skin temperature causes temperature-dependent cutaneous vasoconstriction resulting in decreased cutaneous blood flow.25 Multiple mechanisms are responsible for cutaneous vasoconstriction during local cooling. During early phases of local cooling (less than 10 minutes), the vasoconstrictor response is thought to be dependent on intact noradrenergic cutaneous vasoconstrictor nerves mediating the release of norepinephrine, based on observations that local cooling-induced vasoconstriction is abolished with pretreatment of skin with bretylium tosylate, which inhibits neural transmission of adrenergic nerves.21,26,27 Sensory nerves may also have a role in mediating the early phase response to local cooling.28 Vasoconstriction during longer periods of local cooling (more than 10 minutes) is mediated by adrenergic and nonadrenergic mechanisms, including functional nitric oxide synthase.29 Recent evidence also suggests that Rho kinase, a key mediator in the contraction of vascular smooth muscle, plays an important role in the cutaneous vasoconstrictor response to local cooling.30 The magnitude of cutaneous vasoconstriction was similar between grafted skin and nongrafted skin during local cooling, confirming the observations made during whole-body cooling indicating that mechanisms responsible for cutaneous vasoconstriction are intact and functional. Evidence of re-innervation in grafted skin from responses observed during whole-body and local cooling could lend support to the possibility of re-innervation of the cutaneous vasodilator system. Counter to that hypothesis, we recently found no evidence of a functional active cutaneous vasodilator system in grafted skin during an indirect whole-body heat stress.8
Normothermic baseline CVC was significantly higher in grafted skin when compared with adjacent control skin. We previously suggested that elevated baseline CVC in grafted skin was due to the lack of tonic sympathetic vasoconstrictor activity.8 However, the current findings of appropriate vasoconstriction in grafted skin in response to whole-body and local cooling suggest that the elevated CVC at baseline is not due to altered sympathetic neural function of the vasoconstrictor pathways. Although speculative, elevated CVC at the grafted sites may be associated with continued healing of the 5 to 9-month-old wound.
Previously, we observed small increases in CVC in grafted skin during indirect whole-body heating, although the magnitude of vasodilation was much less compared with adjacent control skin.8 Based on the current findings, these small increases in CVC at the grafted sites could be due to withdrawal of tonic vasoconstrictor tone. However, these subtle increases in CVC, presumably due to withdrawal of vasoconstrictor activity, at the grafted sites may be insufficient to counteract impairments in the cutaneous vasodilator pathways and thereby contribute to the attenuated thermoregulatory capability during heat exposure previously described in burn patients.8,31–34
CONCLUSION
Split-thickness skin grafts 5 to 9 months after surgery demonstrate appropriate cutaneous vasoconstriction in response to indirect whole-body and local cooling. Appropriate cutaneous vasoconstriction in grafted skin to both indirect whole-body cooling and local cooling indicates re-innervation of the cutaneous vasoconstrictor system at the graft site. These data suggest that persons with significant skin grafting may have a normal capacity to regulate body temperature during cold exposure by cutaneous vasoconstriction. Despite re-innervation of the cutaneous vasoconstrictor system, impairments in the cutaneous active vasodilator system in grafted skin are still present, likely impairing thermoregulatory responses to heat stress.
Acknowledgments
We express our appreciation to Marilee Brown, RN and Kimberly Williams, RN for their technical assistance. The considerable time and effort of the participants are greatly appreciated.
This project was supported by National Institute of General Medical Sciences (NIGMS) grant GM68865 (to C.G.C.) and an individual National Research Service Award (GM71092) (to S.L.D.).
REFERENCES
- 1.Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Fregley MJ, Blatteis CM, editors. Handbook of physiology; Section 4: Environmental physiology. New York: Oxford University Press; 1996. pp. 215–243. [Google Scholar]
- 2.Rowell LB. Human circulation: regulation during physical stress. New York: Oxford University Press; 1986. [Google Scholar]
- 3.Roddie IC. Circulation to skin and adipose tissue. In: Shepherd JT, Abboud FM, editors. Handbook of physiology; Section 2: The cardiovascular system. New York: Oxford University Press; 1983. pp. 285–317. [Google Scholar]
- 4.Roddie IC, Shepherd JT, Whelan RF. The contribution of constrictor and dilator nerves to the skin vasodilation during body heating. J Physiol (Lond) 1957;136:489–497. doi: 10.1113/jphysiol.1957.sp005775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edholm OG, Fox RH, Macpherson RK. Vasomotor control of the cutaneous blood vessels in the human forearm. J Physiol (Lond) 1957;139:455–465. doi: 10.1113/jphysiol.1957.sp005904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis T, Pickering GW. Vasodilation in the limbs in response to warming the body; with evidence for sympathetic vasodilator nerves in man. Heart. 1931;16:33–51. [Google Scholar]
- 7.Roddie IC, Shepherd JT, Whelan RF. The vasomotor nerve supply to the skin and muscle of the human forearm. Clin Sci. 1956;16:67–74. [PubMed] [Google Scholar]
- 8.Davis SL, Shibasaki M, Low DA, et al. Impaired thermoregulatory cutaneous vasodilation and sweating in grafted skin 5–9 months post surgery. J Burn Care Res. 2007;28:427–434. doi: 10.1097/BCR.0B013E318053D312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis SL, Shibasaki M, Low DA, et al. Impaired endothelial-dependent cutaneous vasodilation and sweating in grafted skin 5–9 months post surgery. J Burn Care Res. 2007;28:435–441. doi: 10.1097/BCR.0B013E318053D312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bini G, Hagbarth KE, Hynninen P, et al. Thermoregulatory and rhythm-generating mechanisms governing the sudomotor and vasoconstrictor outflow in human cutaneous nerves. J Physiol (Lond) 1980;306:537–552. doi: 10.1113/jphysiol.1980.sp013413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kellogg DL, Jr., Johnson JM, Kosiba WA. Selective abolition of adrenergic vasoconstrictor responses in skin by local iontophoresis of bretylium. Am J Physiol. 1989;257:H1599–H1606. doi: 10.1152/ajpheart.1989.257.5.H1599. [DOI] [PubMed] [Google Scholar]
- 12.Stephens DP, Aoki K, Kosiba WA, et al. Nonnoradrenergic mechanism of reflex cutaneous vasoconstriction in men. Am J Physiol. 2001;280:H1496–H1504. doi: 10.1152/ajpheart.2001.280.4.H1496. [DOI] [PubMed] [Google Scholar]
- 13.Rowell LB. Reflex control of the cutaneous vasculature. J Invest Dermatol. 1977;69:154–166. doi: 10.1111/1523-1747.ep12497938. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien C, Hoyt RW, Buller MJ, et al. Telemetry pill measurement of core temperature in humans during active heating and cooling. Med Sci Sports Exerc. 1998;30:468–472. doi: 10.1097/00005768-199803000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Taylor WF, Johnson JM, Kosiba WA, et al. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol. 1989;66:1586–1592. doi: 10.1152/jappl.1989.66.4.1586. [DOI] [PubMed] [Google Scholar]
- 16.Johnson JM. The cutaneous circulation. In: Shpeherd AP, Öberg PA, editors. Laser-Doppler blood flowmetry. Boston: Kluwer; 1990. pp. 121–140. [Google Scholar]
- 17.Stern MD, Lappe DL, Bowen PD, et al. Continuous measurement of tissue blood flow by laser-Doppler spectroscopy. Am J Physiol. 1977;232:H441–H448. doi: 10.1152/ajpheart.1977.232.4.H441. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JM, Taylor WF, Shepherd AP, et al. Laser-Doppler measurement of skin blood flow: comparison with plethysmography. J Appl Physiol. 1984;56:798–803. doi: 10.1152/jappl.1984.56.3.798. [DOI] [PubMed] [Google Scholar]
- 19.Saumet JL, Kellogg DL, Jr., Taylor WF, et al. Cutaneous laser- Doppler flowmetry: influence of underlying muscle blood flow. J Appl Physiol. 1988;65:478–481. doi: 10.1152/jappl.1988.65.1.478. [DOI] [PubMed] [Google Scholar]
- 20.Taylor WF, Johnson JM, O’Leary D, et al. Effect of high local temperature on reflex cutaneous vasodilation. J Appl Physiol. 1984;57:191–196. doi: 10.1152/jappl.1984.57.1.191. [DOI] [PubMed] [Google Scholar]
- 21.Pergola PE, Kellogg DL, Jr., Johnson JM, et al. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am J Physiol. 1993;265:H785–H792. doi: 10.1152/ajpheart.1993.265.3.H785. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez GE, Zhao K, Kosiba WA, et al. Relative roles of local and reflex components in cutaneous vasoconstriction during skin cooling in humans. J Appl Physiol. 2006;100:2083–2088. doi: 10.1152/japplphysiol.01265.2005. [DOI] [PubMed] [Google Scholar]
- 23.Stephens DP, Saad AR, Bennett LA, et al. Neuropeptide Y antagonism reduces reflex cutaneous vasoconstriction in humans. Am J Physiol. 2004;287:H1404–H1409. doi: 10.1152/ajpheart.00061.2004. [DOI] [PubMed] [Google Scholar]
- 24.Thompson CS, Kenney WL. Altered neurotransmitter control of reflex vasoconstriction in aged human skin. J Physiol (Lond) 2004;558:697–704. doi: 10.1113/jphysiol.2004.065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellogg DL., Jr. In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J Appl Physiol. 2006;100:1709–1718. doi: 10.1152/japplphysiol.01071.2005. [DOI] [PubMed] [Google Scholar]
- 26.Johnson JM, Yen TC, Zhao K, et al. Sympathetic, sensory, and nonneuronal contributions to the cutaneous vasoconstrictor response to local cooling. Am J Physiol. 2005;288:H1573–H1579. doi: 10.1152/ajpheart.00849.2004. [DOI] [PubMed] [Google Scholar]
- 27.Pergola PE, Johnson JM, Kellogg DL, Jr., et al. Control of skin blood flow by whole body and local skin cooling in exercising humans. Am J Physiol. 1996;270:H208–H215. doi: 10.1152/ajpheart.1996.270.1.H208. [DOI] [PubMed] [Google Scholar]
- 28.Hodges GJ, Traeger JA, Tang T, et al. Role of sensory nerves in the cutaneous vasoconstrictor response to local cooling in humans. Am J Physiol. 2007;293:H784–H789. doi: 10.1152/ajpheart.00323.2007. [DOI] [PubMed] [Google Scholar]
- 29.Yamazaki F, Sone R, Zhao K, et al. Rate dependency and role of nitric oxide in the vascular response to direct cooling in human skin. J Appl Physiol. 2006;100:42–50. doi: 10.1152/japplphysiol.00139.2005. [DOI] [PubMed] [Google Scholar]
- 30.Thompson-Torgerson CS, Holowatz LA, Flavahan NA, et al. Cold-induced cutaneous vasoconstriction is mediated by Rho kinase in vivo in human skin. Am J Physiol. 2007;292:H1700–H1705. doi: 10.1152/ajpheart.01078.2006. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Simchon C, Tsur H, Keren G, et al. Heat tolerance in patients with extensive healed burns. Plast Reconstr Surg. 1981;67:499–504. doi: 10.1097/00006534-198104000-00013. [DOI] [PubMed] [Google Scholar]
- 32.McGibbon B, Beaumont WV, Strand J, et al. Thermal regulation in patients after the healing of large deep burns. Plast Reconstr Surg. 1973;52:164–170. doi: 10.1097/00006534-197308000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Roskind JL, Petrofsky J, Lind AR, et al. Quantitation of thermoregulatory impairment in patients with healed burns. Ann Plast Surg. 1978;1:172–176. doi: 10.1097/00000637-197803000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro Y, Epstein Y, Ben-Simchon C, et al. Thermoregulatory responses of patients with extensive healed burns. J Appl Physiol. 1982;53:1019–1022. doi: 10.1152/jappl.1982.53.4.1019. [DOI] [PubMed] [Google Scholar]